Abstract
The methanolic extract of Languas galanga rhizomes was investigated for antimalarial activity against Plasmodium berghei (NK65) infections in mice. The median lethal dose was determined to ascertain the safety of the extract in ICR mice of both sexes. The antimalarial activities during early and established infections, as well as the prophylactic activity were evaluated. Phytochemical screening and radical scavenging activity of the extract were also investigated to elucidate the possible mechanism of the antimalarial properties. The acute oral toxicity (LD50) of Languas galanga extract in mice was established to be 4,998 mg/kg. The extract of Languas galanga rhizomes demonstrated significant antiplasmodial activity in all the three models of the antimalarial evaluations. Phytochemical screening revealed the presence of some vital antiplasmodial constituents such as terpenoids and flavonoids. The extract also exhibited a moderate capacity to scavenge the free radicals. The rhizome extract of Languas galanga thus possesses antimalarial activity, which explains the rational usage of this plant in traditional Malaysian medicine.
Keywords: Languas galanga, methanolic extract, antimalarial activity
1. Introduction
Languas galanga Syn. Alpinia galanga (Linn.) Stuntz (Zingiberaceae) is native to grassland areas of Southeast Asia and Southern China [1]. In Malaysia, the rhizomes (Langkuas) of this plant are used as a spice for flavouring food. Ethnobotanically, the rhizomes are used to treat a variety of sicknesses including coughs, headache, asthma, bronchitis, inflammation, rheumatoid arthritis and colic [2,3]. Previous biochemical studies on Languas galanga have shown that the rhizomes contain essential antimicrobial components [4,5,6,7,8]. The rhizomes exhibit many pharmacological properties, including antitumour [9], antiallergic [10], antiulcer [11], antifungal [12], antibacterial [13] and antiviral activities [14].
Currently, multi-drug resistance has become one of the most important problems impeding malaria control efforts [15,16]. This has led to attempts to discover other antimalarial agents, mainly from plant sources. Medicinal plants may provide antimalarial drugs directly, as in the case of quinine from cinchona bark, or they may supply template molecules on which to base further new structures by organic synthesis–artemisinin from Artemisia annua. The selection of candidate plants for clinical trials depends largely on ethnopharmacological information, which has appeared to be more predictive compared to the random screening approach [17].
In view of the reported antitrypanosomal [18], antileishmanial [19] and antihelmithic [20] antiparasitic effects of Languas galanga rhizomes, in the present study we evaluated the in vivo antimalarial activity of the methanolic extract of Languas galanga rhizomes against early, established and residual malaria infections. Median lethal dose, phytochemical screening and radical scavenging were also studied to ascertain the validity of its folkloric standing.
2. Results and Discussion
2.1. Median lethal dose
The mortality rate and the acute toxicity symptoms of orally administered Languas galanga rhizome extract increased as the dose increased from 2,000 to 5,000 mg/kg (Table 1). The main observed behavioural signs of toxicity were asthenia, piloerection, ataxia, anorexia, urination, diarrhea, lethargy and coma. Asthenia, piloerection and urination were noticed after dosing with 2,000 mg/kg and were more marked at the highest dose and continued until death, in particular among the male subjects, which showed a lethal median dose lower than that of the females. The acute oral toxicity (LD50) of Languas galanga extract in mice was calculated by the probit analysis to be 4,998 mg/kg. According to Horn [21] and Rhiouani et al. [22], plants or plant products with LD50 values higher than 2,000–3,000 mg/kg are considered free of any toxicity. This supports the logical usage of this plant in folk medicine practices.
Table 1.
Acute oral toxicity of the methanolic extract of Languas galanga rhizomes administered orally to mice.
| Dose (mg/kg) | Mortality | Toxic symptoms | |
|---|---|---|---|
| D/T | Latency (h) | ||
| 0 | 0/10 | - | None |
| 300 | 0/10 | - | None |
| 2,000 | 1/10 | >36, <60 | Asthenia, piloerection, anorexia, urination, diarrhea |
| 5,000 | 5/10 | >24, <60 | Asthenia, piloerection, ataxia, anorexia, urination, diarrhea, lethargy, coma |
All the treated mice were carefully observed for 14 days for any signs of toxicity (behavioural changes and mortality). D/T: dead/treated mice; none: no toxic symptoms were recorded during the observation period; latency: time to death (in hours) after the dose administration.
2.2. Antimalarial activity
During early malaria infection, the rhizomes extract of Languas galanga produced a dose-dependent chemosuppression activity at the different doses employed. 4-day suppressive effects of 29, 49, 63 and 65% were shown, respectively, for the corresponding dose of the extract (50, 100, 200 and 400 mg/kg/day). The antimalarial activity produced by the extract was statistically significant (P < 0.05) when related to control (Table 2). However, the 400 mg/kg demonstrated only a marginal increase in activity over 200 mg/kg, suggesting that further increases in the dose produced only marginal increases in activity [23].
Table 2.
The antimalarial activities of the methanolic extract of Languas galanga rhizomes during early, established and residual malaria infections.
| Drug/extract | Dose | Parasitaemia | %Chemo-suppression | Significance |
|---|---|---|---|---|
| Suppressive activity | ||||
| Control (dist. water) | 0.2 mL | 5.10 ± 0.33 | ||
| Rhizome extract | 50 mg/kg | 3.60 ± 0.68 | 29 | ns |
| 100 mg/kg | 2.60 ± 0.37 | 49 | P < 0.05 | |
| 200 mg/kg | 1.91 ± 0.91 | 63 | P < 0.01 | |
| 400 mg/kg | 1.81 ± 0.89 | 65 | P < 0.05 | |
| Chloroquine | 20 mg/kg | 0 | 100 | |
| Curative activity | ||||
| Control (dist. water) | 0.2 mL | 9.60 ± 0.93 | ||
| Rhizome extract | 50 mg/kg | 5.80 ± 1.21 | 40 | ns |
| 100 mg/kg | 3.40 ± 0.22 | 65 | P < 0.05 | |
| 200 mg/kg | 3.40 ± 0.78 | 65 | P < 0.05 | |
| 400 mg/kg | 3.20 ± 0.77 | 67 | P < 0.001 | |
| Chloroquine | 20 mg/kg | 0 | 100 | |
| Prophylactic activity | ||||
| Control (dist. water) | 0.2 mL | 4.60 ± 0.51 | ||
| Rhizome extract | 50 mg/kg | 4.00 ± 0.55 | 13 | ns |
| 100 mg/kg | 3.40 ± 0.93 | 26 | ns | |
| 200 mg/kg | 2.80 ± 0.49 | 39 | P < 0.01 | |
| 400 mg/kg | 2.20 ± 0.37 | 52 | P < 0.001 | |
| Pyrimethamine | 1.2 mg/kg | 1.24 ± 0.47 | 73 | P < 0.05 |
In the established malaria infection, the plant extract exhibited dose-dependent curative antimalarial activity, which was statistically significant (P < 0.05) when compared to control. The mean parasitaemia for the treated groups on the sixth day of infection were 5.80, 3.40, 3.40 and 3.20 for 50, 100, 200, and 400 mg/kg/day, respectively. A marginal increase in activity over 100 mg/kg was produced by the 200 and 400 mg/kg doses. The mean parasitaemia for the control group was 9.60 (Table 2).
As summarized in Table 2, the results of prophylactic activity of the rhizomes extract during the residual malaria infection showed dose-dependent chemosuppression at 50, 100, 200 and 400 mg/kg/day dose levels, exerting 13, 26, 39 and 52% suppressions, respectively. It showed that though there was a significant prophylactic activity (P < 0.05) at the 200 and 400 mg/kg doses, the chemosuppressive activity was rather low and not significant at the 100 and 50 mg/kg doses. The rhizomes extract of Languas galanga exhibited some level of chemosuppression of parasitaemia. The 100 and 200 mg/kg appeared to be effective therapeutic doses in the early and curative tests. On the other hand, the suppression of parasitaemia by chloroquine at 20 mg/kg/day appeared to be in agreement with a previous study conducted on the chloroquine efficacy in Plasmodium berghei NK65-infected ICR mice [24].
The results of the anti-malarial screening assays of the test extract, standard drugs and control groups at different doses in mice parasitized with P. berghei are expressed as percentage (%) for the suppression of parasitaemia. ns: non-significant (P > 0.05); P values ≤ 0.05 were considered to be statistically significant (effective). Parasitaemia are expressed as mean ± S.E.M. (n = 5). Significance compared to control.
2.3. Phytochemical screening
Phytochemical screening of the methanolic extract of Languas galanga revealed that the rhizomes extract contains terpenoids, flavonoids, tannins, saponins, steroids and glycosides (Table 3).
Table 3.
Phytochemical profile of the methanolic extract of Languas galanga rhizomes.
| Chemical constituents | Tests/Reagents | Results |
|---|---|---|
| Alkaloids | Dragendorff’s Reagent | - |
| Anthraquinones | Sulphuric acid-chloroform layer test | - |
| Flavonoids | Ammonium Test | ++ |
| Glycosides | Fehling’s reagent | + |
| Saponins | Frothing Test | ++ |
| Steroids | Salkowski’s test | ++ |
| Tannins | Lead sub-acetate test | +++ |
| Terpenoids | Salkowski’s test | ++ |
+++ = abundance; ++ = moderately present; + = present; - = absent
Phytochemical compounds such as terpenoids are commonly implicated in the antiprotozal and antiplasmodial activity of many plants [25,26,27,28]. An example of common terpenoids is artemisinin, the main active ingredient in the traditional Chinese antimalarial qinghaosu. Flavonoids are the other form of Languas galanga phenolic structures. Flavonoids showed significant antiparasitic activities against different strains of malaria, trypanosome and leishmania [29,30,31]. These chemical compounds may be acting singly or in synergy with one another to exert the observed antimalarial activity of Languas galanga.
2.4. Radical scavenging activity
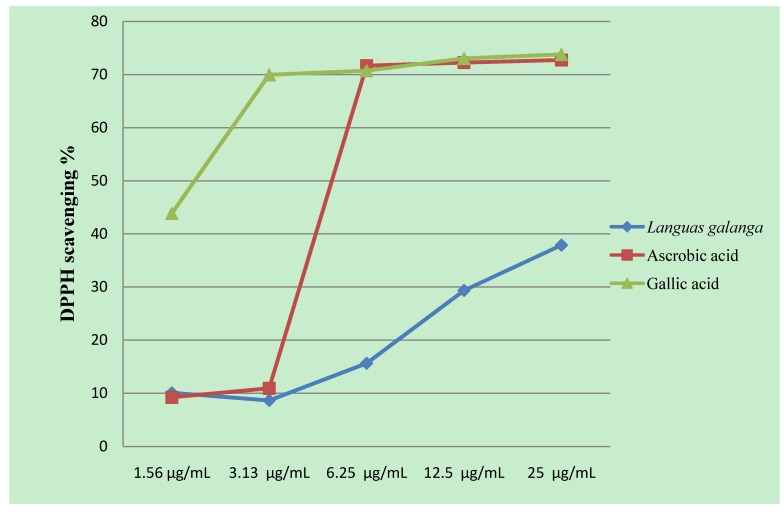
The methanolic rhizome extract of Languas galanga showed moderate DPPH radical scavenging activity. At 1.56–25 µg/mL, the scavenging abilities of the methanol extract were 10.08, 8.63, 15.64, 29.34 and 37.88, respectively, as shown in Figure 1. At 25 µg/mL, ascorbic acid and gallic acid showed strong scavenging abilities reaching 72.74 and 73.79%, respectively. The antioxidant effect of the Languas galanga rhizomes extract may represent another mechanism that contributes to its anti-malarial activity. Rhizomes extract of Languas galanga showed nitric oxide (NO) production inhibitory activities in mouse peritoneal macrophages [32]. NO is a potent intracellular parasite-killing mechanism in macrophages which are crucial in innate immune response. These inhibitory activities assist in providing a favourable environment for the multiplication of intracellular parasites; however, the inhibition of one killing mechanism can cause the up regulation of secondary mechanisms against which the parasite cannot protect itself [33]. The inhibition of NO starves the parasite of an essential amino acid, leading to its death by increasing tryptophan degradation through indolamine deoxygenase induction in human peritoneal macrophages [34].
Figure 1.
DPPH radical scavenging activities of the methanolic extract of Languas galanga rhizomes, ascorbic acid and gallic acid at different concentrations of µg/mL. Absorbance values represent means of triplicates of different concentrations analyzed.
3. Experimental
3.1. Plant materials
The plant part (rhizomes) of Languas galanga (Linn.) was selected based on our ethnobotanical survey conducted in two malaria endemic communities, forest-aboriginal and rural communities, in the Lipis district of Pahang state, Peninsular Malaysia [35]. The plant has been mentioned as a curative antimalarial remedy by the two communities. The voucher specimen was collected and identified by the University of Malaya Herbarium (KLU) and deposited under the reference number KLU 046619.
3.2. Preparation of plant extract
The rhizomes of Languas galanga were dried in a hot air oven at 40 °C and milled. Five hundred grams of the powdered materials were soaked in absolute methanol (3.5 L, Merck, Germany) for 72 h. The extracts were concentrated in vacuo to dryness at 40 °C using a rotary evaporator and were freeze-dried. The percentage yield was 3.27%. The freeze-dried extract was stored in a refrigerator at −4 °C until used.
3.3. Animals
The 6–7 weeks old male (27 ± 2 g) and female (22 ± 2 g) ICR mice that were used for these experiments were obtained from the Laboratory Animal Centre of the Faculty of Medicine, University of Malaya. The mice were housed under standard conditions and were maintained on a standard pelleted feed and water ad libitum. Permission and approval for animal studies were obtained from the Animal Ethics Committee of the Faculty of Medicine, University of Malaya dated 05 June 2009 (Ref. No. PAR/05/6/2009/AHAA-R).
3.4. Median Lethal Dose
Different doses of the extract (300–5,000 mg/kg) were inoculated to evaluate the acute oral toxicity according to OECD guideline No 423; “Acute oral toxicity – acute toxic class method” [36]. The extract doses were divided into equal parts and given to the animals within a period not exceeding 24 hours. A total of 10 mice (five females and five males) were tested. The mice were fasted overnight prior to administration and returned to feeding 3 hrs later. On the day of administration, all the mice were observed for mortality and signs of toxicity at 1, 3 and 4 hrs following administration and thereafter they were observed twice a day for 14 days. The LD50 was calculated using probit regression analysis in the SPSS statistical package (version 13, 2004).
3.5. Parasite inoculation
The blood of a donor female ICR mouse infected with Plasmodium berghei (NK65) was used for inoculum preparation. The desired blood volume was drawn from the donor mouse by heart puncture and diluted serially in Alsever’s solution. The final suspension would contain about 1 × 106 infected RBC’s in every 0.2 mL suspension. This 0.2 mL suspension was injected into mice intraperitoneally to initiate infection [37]. The inoculated animals were then randomized into five mice per cage and maintained in the Animal Room, Department of Parasitology, Faculty of Medicine, University of Malaya, in accordance with the internationally accepted principles for laboratory animal use and care.
3.6. In vivo antimalarial assays
A series of experiments were carried out to evaluate the in vivo anti-malarial activities of the methanolic extract of Languas galanga rhizomes at 50, 100, 200 and 400 mg/kg doses as compared to control groups treated with distilled water (containing 10% DMSO, the solvent of the test extracts) and reference groups treated with standard drugs (chloroquine 20 mg/kg or pyrimethamine 1.2 mg/kg/day). Malaria infection was first established in female mice by the intraperitoneal (i.p.) administration of donor female ICR mouse blood containing about 1 × 106 parasites. The three different methods of treating malaria infections, i.e., 4-Day suppressive test, curative and prophylactic methods were applied according to Peters and Robinson [38], Saidu et al. [39] and Peters [40], respectively. The laboratory tests were started with oral administrations of the extracts in the 4-day suppressive tests (early malaria infection) and further screened for their curative (established malaria infection) and prophylactic (residual malaria infection) activities. Thin blood films were prepared from the tail blood of each mouse. The films were then stained with Giemsa’s stain to determine parasitized erythrocytes. The percentage of parasitaemia was determined by counting the parasitized red blood cells out of 9,000 in random fields of the microscope:
| % Parasitaemia = [No. of parasitized RBC/Total no. of RBC counted] 100 |
Average percentage chemosuppression was calculated as: 100 [A-B/A] where, A is the mean percentage parasitaemia in the negative control group and B is the mean percentage parasitaemia in the test group.
3.7. Phytochemical screening
The methanolic extract of Languas galanga rhizomes was screened for the presence of phytochemical constituents such as alkaloids, terpenoids, anthraquinones, flavonoids, tannins, saponins, steroids and glycosides using qualitative phytochemical screening tests described by Sofowora [41] and Trease [42].
3.8. DPPH radical scavenging activity
The radical scavenging activities of the plant extract against 2,2-diphenyl-1-picrylhydrazyl radical were determined photometrically at 515 nm. Radical scavenging activity was measured by using the method of Gerhäuser et al. [43]. In brief, DPPH in methanol (195 μM) was added to different concentrations of the extract (5 μL) in a 96-well microplate, incubated for 3 hrs and read at 20-min intervals. Ascorbic acid and gallic acid were used for comparison. The free radical-scavenging activity was expressed as percentage scavenging of the DPPH by the extract and was calculated as follows:
| % inhibition = {[Ab-Aa]/Ab} × 100 |
where Ab is the absorption of the blank sample, and Aa is the absorption of the sample.
Each test was carried out three times and the mean ± SEM were calculated. The DPPH % was presented as μg /mL of concentration.
3.9. Statistical analysis
Data obtained by this study were analyzed using SPSS (version 13, 2004). The Student’s t-test and ANOVA (one- or two-way) were used to test the differences between groups. Differences between means at 1 and 5% level (P ≤ 0.01 and 0.05) were considered significant.
4. Conclusions
The antimalarial activities shown by the rhizome extract of Languas galanga could have been produced as a result of the active antiplasmodial components contained in the extract, mainly terpenoids and/or flavonoids constituents. Antioxidant activity may represent another mechanism that contributes to its anti-malarial activity. Although the bioactive components and mechanism are yet to be identified, the results of this study provide the basis for further studies. This study also confirms the rational usage of this plant in Malaysian medicinal traditions.
Acknowledgements
The financial support of the University of Malaya (Grant code PS204/2009C) is gratefully acknowledged. The authors thank the staff of the University of Malaya Herbarium for their fruitful help in the collection and identification of the plant species. We would also like to thank Mahmood Ameen Abdulla and Mr. Pouya Darvish from the Department of Molecular Medicine, University of Malaya, and his students for their useful help in the extraction and antioxidant evaluations.
Supplementary Materials
Footnotes
Samples Availability: Samples of the plant are available at the University of Malaya Herbarium (KLU) on the instruction from the authors.
References
- 1.Chevallier A. The Encyclopedia of Medicinal Plants. Dorling Kindersley Limited; London, UK: 1996. [Google Scholar]
- 2.Burkill I.H. A Dictionary of the Economic Products of the Malay Peninsula. II. Crown Agents for the Colonies; London, UK: 1996. pp. 1327–1332. [Google Scholar]
- 3.Latha C., Shriram D., Jahagirdar S., Dhakephalkar P., Rojatkar S. Antiplasmid activity of 1'-acetoxychavicol acetate from Alpinia galanga against multi-drug resistant bacteria. J. Ethnopharmacol. 2009;25:522–525. doi: 10.1016/j.jep.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Jantan I.B., Ahmad F.B., Ahmad A.S. Constituents of the rhizome and seed oils of greater galangal Alpinia galanga (L) from Malaysia. J. Essent. Oil Res. 2004;16:174–176. doi: 10.1080/10412905.2004.9698687. [DOI] [Google Scholar]
- 5.De Pooter H.L., Omar M.N., Coolsaet B.A., Schamp N.M. The essential oil of greater galangal (Alpinia galanga) from Malaysia. Phytochemistry. 1985;24:93–96. [Google Scholar]
- 6.Scheffer J.J.C., Gani A., Baerheim-Svenden A. Monoterpenes in the essential rhizome oil of Alpinia galanga. Sci Pharm. 1981;49:337–346. [Google Scholar]
- 7.Mori H., Kubota K., Kobayashi A. Potent aroma components of rhizomes of Alpinia galanga Willd. L. Nippon Shokuhin Kagaku Kogaku Kaishi. 1995;42:989–995. doi: 10.3136/nskkk.42.989. [DOI] [Google Scholar]
- 8.Charles D.J., Simon J.E., Singh N.K. The essential oil of Alpinia Galanaga Willd. J. Essent. Oil Res. 1999;11:719–723. doi: 10.1080/10412905.1999.9712004. [DOI] [Google Scholar]
- 9.Itokawa H., Morita H., Sumitomo T., Totsuka N., Takeya K. Antitumour principles from Alpinia galanga. Planta Med. 1987;53:32–33. doi: 10.1055/s-2006-962611. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda H., Morikawa T., Managi H., Yoshikawa M. Antiallergic principles from Alpinia galanga: Structural requirements of phenylproponoids for inhibition of degranulation and release of TNF-α and lL-4 in RBL-2H3 cells. Bioorg. Med. Chem. Lett. 2003;13:3197–3202. doi: 10.1016/S0960-894X(03)00710-8. [DOI] [PubMed] [Google Scholar]
- 11.Mitsui S., Kobayashi S., Nagahori H., Ogiso A. Constituents from seeds of Alpinia galanga WILD and their anti-ulcer activities. Chem. Pharm. Bull. 1976;24:2377–2382. doi: 10.1248/cpb.24.2377. [DOI] [PubMed] [Google Scholar]
- 12.Janssen A.M., Scheffer J.J.C. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Planta Med. 1985;6:507–511. [PubMed] [Google Scholar]
- 13.Oonmetta-aree J., Suzuki T., Gasaluck P., Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT Food Sci. Tech. 2006;39:1214–1220. [Google Scholar]
- 14.Ye Y., Li B. 1'S-1'-Acetoxychavicol acetate isolated from Alpinia galangal inhibits human immunodeficiency virus type 1 replication by blocking Rev transport. J. Gen. Virol. 2006;87:2047–2053. doi: 10.1099/vir.0.81685-0. [DOI] [PubMed] [Google Scholar]
- 15.Htut Z.W. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009;361:1807–1808. doi: 10.1056/NEJMc091737. [DOI] [PubMed] [Google Scholar]
- 16.Sendagire H., Kyabayinze D., Swedberg G., Kironde F. Plasmodium falciparum: Higher incidence of molecular resistance markers for sulfadoxine than for pyrimethamine in Kasangati, Uganda. Trop. Med. Int. Health. 2005;10:537–543. doi: 10.1111/j.1365-3156.2005.01414.x. [DOI] [PubMed] [Google Scholar]
- 17.Saxena S., Pant N., Jain D.C., Bhakuni R.S. Antimalarial agents from plant sources. Curr. Sci. 2003;85:1314–1329. [Google Scholar]
- 18.Nahoko U. Antichagasic activities of natural products against Trypanosoma cruzi. J. Health Sci. 2009;55:31–39. doi: 10.1248/jhs.55.31. [DOI] [Google Scholar]
- 19.Amandeep K., Ranvir S., Sankar D.C., Sharma S.S., Kamlesh K.B., Singh I.P. Antileishmanial phenylpropanoids from Alpinia galanga (Linn.) Willd. Indian J. Exp. Biol. 2010;48:314–322. [PubMed] [Google Scholar]
- 20.Raj R.K. Screening of indigenous plants for anthelmintic action against human Ascaris lumbricoides: Part-II. Indian J. Physiol. Pharmacol. 1975;19:47–49. [PubMed] [Google Scholar]
- 21.Horn H.J. Simplified LD50 (ED50) calculation. Biometrics. 1956;12:312–322. [Google Scholar]
- 22.Rhiouani H., El-Hilaly J., Israili Z.H., Lyoussi B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J. Ethnopharmacol. 2008;118:378–386. doi: 10.1016/j.jep.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Elufioye T.O., Agbedahunsi J.M. Antimalarial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J. Ethnopharmacol. 2004;93:167–1671. doi: 10.1016/j.jep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Ishih A., Suzuki T., Muregi F.W., Kachi S., Matsui K., Terada M. Chloroquine efficacy in Plasmodium berghei NK65-infected ICR mice, with reference to the influence of initial parasite load and starting day of drug administration on the outcome of treatment. Southeast Asian J. Trop. Med. Public Health. 2006;37:13–17. [PubMed] [Google Scholar]
- 25.Philipson J.D., Wright C.W. Antiprotozoal compounds from plants sources. Planta Med. 1991;57:553–559. [PubMed] [Google Scholar]
- 26.Francois G., Passreiter C.M., Woerdenbag H.J., Looveren M.V. Antimalarial activities and cytotoxic effects of aqueous extracts and sesquiterpene lactones from Neurolaena lobata. Planta Med. 1996;2:126–129. doi: 10.1055/s-2006-957833. [DOI] [PubMed] [Google Scholar]
- 27.Ghoshal S., Prasad BN., Lakshmi V. Antiamoebic activity of Piper longum fruits against Entamoeba histolytica in vitro and in vivo. J. Ethnopharmacol. 1996;3:167–170. doi: 10.1016/0378-8741(96)01382-7. [DOI] [PubMed] [Google Scholar]
- 28.Asase A., Akwetey G.A., Achel D.l.G. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J. Ethnopharmacol. 2010;129:367–376. doi: 10.1016/j.jep.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.C., Kim H., Wataya Y., Sohn D.H., Kang T.H., Kim M.S., Kim Y.M., Lee G., Chang J., Park H. Antimalarial activity of lavandulyl flavanones isolated from the roots of Sophora flavescens. Biol. Pharm. Bull. 2004;27:748–750. doi: 10.1248/bpb.27.748. [DOI] [PubMed] [Google Scholar]
- 30.Monbrison F., Maitrejean M., Latour C., Bugnazet F., Peyron F., Barron D., Picot S. In vitro antimalarial activity of flavonoid derivatives dehydrosilybin and 8-(1;1)-DMA-kaempferide. Acta Trop. 2006;97:102–107. doi: 10.1016/j.actatropica.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Tasdemir D., Kaiser D., Brun R., Yardley V., Schmidt T.J., Tosun F., Ruedi P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006;50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morikawa T., Ando S., Matsuda H., Kataoka S., Muraoka O., Yoshikawa M. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: Structures of new 8-9' linked neolignans and sesquineolignan. Chem. Pharm. Bull. 2005;53:625–630. doi: 10.1248/cpb.53.625. [DOI] [PubMed] [Google Scholar]
- 33.Antony P.J., Fyfe L., Smith H. Plant active components-a source for anti-parasitic agents? Trend Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Däubener W., Hucke C., Seidel K., Hadding U., MacKenzie C.R. Interleukin-1 inhibits gamma interferon-induced bacteriostasis in human uroepithelial cells. Infect. Immun. 1999;67:5615–5620. doi: 10.1128/iai.67.11.5615-5620.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdulelah H. A., Zurainee M.N., Hesham M.A., Rohela M. Ethnobotanical study on some Malaysian anti-malarial plants: A community based survey. J. Ethnopharmacol. 2010 doi: 10.1016/j.jep.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Guideline for the testing of chemicals (OECD): Acute oral toxicity - Acute toxic class method. Section 423. 2001 [Google Scholar]
- 37.Ishih A., Suzuki T., Hasegawa T., Kachi S., Wang H.H., Terada M. In vivo evaluation of combination effects of chloroquine with cepharanthin or minocycline hydrochloride against blood-induced choloquine-resistant Plasmodium berghei NK65 infections. Trop. Med. Health. 2004;32:15–19. doi: 10.2149/tmh.32.15. [DOI] [Google Scholar]
- 38.Peters W., Robinson B.L. The chemotherapy of rodent malaria XLVII: studies on pyronaridine and other Mannich base antimalarials. Ann. Trop. Med. Parasitol. 1992;86:455–465. doi: 10.1080/00034983.1992.11812694. [DOI] [PubMed] [Google Scholar]
- 39.Saidu K., Onah J., Orisadipe A., Olusola A., Wambebe C., Gamaniel K. Antiplasmodial, analgesic, and anti-inflammatory activities of the aqueous extract of Erythrina senegalensis. J. Ethnopharmacol. 2000;71:275–280. doi: 10.1016/S0378-8741(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 40.Peters W. Drug resistance in Plasmodium berghei. Vinke and Lips 1948. I. Chloroquine resistance. Exp. Parasitol. 1965;17:80–89. doi: 10.1016/0014-4894(65)90012-3. [DOI] [PubMed] [Google Scholar]
- 41.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. 2nd. Spectrum Books Limited; Ibadan, Nigeria: 1993. [Google Scholar]
- 42.Trease G.E., Evans W.C. Pharmacognosy. 13th. Bailliere Tindall; London, UK: 1989. pp. 683–684. [Google Scholar]
- 43.Gerhäuser C., Klimo K., Heiss E., Neumann I., Gamal-Eldeen A., Knauft J., Liu J.U., Sitthimonchai S., Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mut. Res. 2003;523–524:163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.