Abstract
Oxidative stress causes damage to multiple cellular components such as DNA, proteins, and lipids, and is implicated in various human diseases including cancer, neurodegeneration, inflammatory diseases, and aging. In response to oxidative attack, cells have developed an antioxidant defense system to maintain cellular redox homeostasis and to protect cells from damage. The thiol-containing small molecules (e.g. glutathione), reactive oxygen species-inactivating enzymes (e.g. glutathione peroxidase), and phase 2 detoxifying enzymes (e.g. NAD(P)H: quinine oxidoreductase 1 and glutathione-S-transferases) are members of this antioxidant system. NF-E2-related factor 2 (Nrf2) is a CNC-bZIP transcription factor which regulates the basal and inducible expression of a wide array of antioxidant genes. Following dissociation from the cytosolic protein Keap1, a scaffolding protein which binds Nrf2 and Cul3 ubiquitin ligase for proteasome degradation, Nrf2 rapidly accumulates in the nucleus and transactivates the antioxidant response element in the promoter region of many antioxidant genes. The critical role of Nrf2 has been demonstrated by various animal studies showing that mice with a targeted disruption of the nrf2 gene are prone to develop lesions in response to environmental toxicants/carcinogens, drugs, and inflammatory insults. In this review, we discuss the role of the Nrf2 system, with particular focus on Nrf2-controlled target genes and the potential pleiotropic effects of Nrf2 activation of indirect antioxidants.
Keywords: indirect antioxidants, oxidative stress, Nrf2, Keap1
1. Introduction: Reactive Oxygen Species and Oxidative Stress
Reactive oxygen species (ROS) are constantly produced in aerobic organisms as by-products of normal oxygen metabolism and include free radicals such as superoxide anion (O2−) and hydroxyl radical (OH-), and non-radical hydrogen peroxide (H2O2). Superoxide anion is a common precursor of ROS and is involved in two pathways: i) rapid conversion into hydrogen peroxide and oxygen by superoxide dismutase (SOD) and ii) generation of highly toxic peroxynitrite via reaction with nitric oxide [1,2]. Further, hydrogen peroxide can be converted into hydroxyl radicals, particularly in the presence of transition metals such as iron and cobalt [3]. The mitochondrial respiratory chain and enzymatic reactions by NAD(P)H oxidase, xanthine oxidase, cyclooxygenases, and lipoxygenase, are endogenous sources of ROS [4]. Exogenous ROS-inducing stressors include ionizing radiation, UV light, and divergent oxidizing chemicals.
At low concentrations, ROS serve as an important second messenger in cell signaling; however, at higher concentrations and long-term exposure, ROS can damage cellular macromolecules such as DNA, proteins, and lipids, which leads to necrotic and apoptotic cell death [4]. To restrict the potential toxicity of ROS, cells developed the antioxidant system. A nonenzymatic system involving thiol-containing small molecules such as glutathione (GSH) and thioredoxin (Txn) neutralizes ROS via direct interactions. An enzymatic system, including catalase, glutathione peroxidase (GPx), and peroxiredoxins (Prdx) reduce hydrogen peroxide to water. However, excess ROS can overwhelm the capacity of the antioxidant system, which leads to perturbation of cellular redox balance. Oxidative stress is a condition of imbalance between ROS formation and cellular antioxidant capacity due to enhanced ROS generation and/or dysfunction of the antioxidant system. Protein carbonyls, 8-hydroxyguanosine adducts, and lipid peroxides including 4-hydroxy-2-nonenal and isoprostane are biochemical markers of oxidative stress representing ROS-mediated damage to proteins, nucleic acids, and lipids [5]. Biochemical alterations in these macromolecular components can lead to various pathological conditions and human diseases: cancer, neurodegeneration, atherosclerosis, inflammation, and aging. In particular, ROS can stimulate cell proliferation, invasion, migration, and angiogenesis, and can evade apoptosis in cancer cells [6].
Recent discoveries in the cell biology of the cellular antioxidant system gave rise to the novel concept of “indirect antioxidants”. Indirect antioxidants act through the augmentation of cellular antioxidant capacity by enhancing gene expression. This review describes the role of the transcription factor Nrf2 in antioxidant gene regulation and its implications in various pathology and disease models. We suggest that small molecule Nrf2 activators may be a promising class of indirect antioxidants for the prevention/treatment of a wide array of human diseases.
2. Cellular Antioxidant System
2.1. Directly Acting Antioxidant Proteins
Several proteins are directly involved in ROS removal. These include SOD, catalase, GPx, and small thiol molecules GSH and Txn. Among these, catalase, SOD, and GPx directly neutralize ROS. Mammalian catalase, with a molecular weight of 240 kDa is a tetramer of four identical subunits containing a porphyrin heme group [7] and is expressed in all tissues, but at particularly high concentrations in the liver, erythrocytes, and kidneys [8]. Catalase catalyzes the conversion of two molecules of hydrogen peroxide into two molecules of water and one molecule of oxygen in a decomposition reaction that can prevent the formation of highly reactive hydroxyl radicals from hydrogen peroxide. Human GPx is a selenoprotein which takes the form of 5 isotypes (GPx 1, 2, 3, 4, and 6), and can reduce hydrogen peroxide and soluble fatty acid hydroperoxides using two molecules of GSH as a co-substrate [9]. The antioxidative role of GPx has been demonstrated by gene knock-out studies in animal models: GPx1 deficiency in mice led to abnormalities in endothelial and cardiomyocyte function due to severe oxidative stress [10].
GSH and Txn serve as substrates for GPx and Prdx. GSH is highly abundant (at millimolar concentrations) cellular tripeptide L-γ-glutamyl-L-cysteinyl-glycine and its biosynthesis occurs in the cytoplasm of most tissues via the action of γ-glutamate cysteine ligase (GCL) and GSH synthetase [11,12]. GCL is a heterodimer comprised of a catalytic subunit (GCLC) and a modulatory subunit (GCLM) [13]. Because of its high reactivity with free radicals, GSH is easily oxidized: biosynthesis and enzymatic reduction of disulfide by GSH reductase can rapidly supply GSH. Therefore, the ratio of GSH to oxidized GSH (GSSG) has been used as a marker of cellular redox status. Txn is located in the inner mitochondrial membrane and is involved in the reduction of hydrogen peroxide, lipid peroxide, and proteins with oxidatively modified sulfhydryl residues [14]. Txn reductase catalyzes the reduction of oxidized Txn using NAD(P)H, thereby maintaining a stable ratio of reduced to oxidized Txn.
2.2. Phase 2 Detoxifying Enzymes as Antioxidant Proteins
Phase 2 detoxifying enzymes were originally recognized as xenobiotic metabolizing enzymes. Xenobiotics, which include various environmental chemicals, carcinogens, and drugs, undergo sequential two-step metabolism. Phase 1 enzymes primarily catalyze the introduction of functional groups into hydrophobic organic molecules through the action of cytochrome P450 enzymes [15]. Phase 2 enzymes are largely responsible for the elimination of xenobiotics by forming conjugated metabolites using hydrophilic molecules such as GSH and glucuronic acid. Phase 2 enzymes can be classified into four different categories: i) classical conjugating enzymes: glutathione S-transferases (GSTs) and UDP-glucuronosyl transferases (UGTs); ii) enzymes contributing to biosynthesis/recycling of thiols: GCL, GSH reductase, and Txn reductase; iii) enzymes involved in the reduction of reactive intermediates: NAD(P)H: quinine oxidoreductases (NQOs) and epoxide hydrolase (EH); iv) stress-response proteins: heme oxygenase-1 (HO-1) and ferritin [16,17]. Due to their role in maintaining redox balance, thiol homeostasis, and excretion of reactive metabolites (e.g., peroxides, epoxides, aldehydes, quinones), phase 2 detoxifying enzymes are now often classified as antioxidant proteins [16]. Further, because electrophiles can evoke GSH depletion and macromolecular damage, many environmental chemicals are regarded as oxidative stressors. One such example is a tobacco-related chemical benzo[a]pyrene (B[a]P): its reactive metabolic intermediate B[a]P-7,8 dihydrodiol-9,10 epoxide is a potent carcinogenic electrophile that depletes GSH and causes DNA damage [18].
GSTs are ubiquitous, multifunctional enzymes that detoxify endogenous and exogenous electrophiles, including epoxides, aldehydes, and peroxides. There are seven distinct classes of GSTs based on amino-acid sequence similarities, physical structure of the genes, and immunological cross-reactivity: alpha (α), mu (μ), omega (ω), pi (π), sigma (σ), theta (θ), and zeta (ζ) [19]. Animal knockout studies have revealed the functional role of GSTs in the susceptibility to environmental electrophiles. In Gstp1/p2 null mice, the number of skin papillomas was significantly increased after exposure to the carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and 12-O-tetradodecanoyl-13-acetate (TPA) [20]. Lung cancer incidence after exposure to B[a]P was increased in Gstp1-/- mice [21]. In humans, cytosolic GSTs display polymorphisms which likely contribute to inter-individual differences in the response to xenobiotics and susceptibility to cancer [19]. The Gstm1 null genotype is associated with an almost 2-fold higher risk for nasopharyngeal carcinoma in humans or animals [22].
UGTs catalyze the glucuronic acid conjugation reaction that mediates the major excretory pathway for pollutants and drugs, as well as endogenous compounds such as bilirubin, steroids, and hormones [23]. A number of studies have confirmed the protective role of UGTs against environmental chemicals and carcinogens: i) susceptibility to B[a]P carcinogenesis was enhanced in ugt-deficient cultured rat skin fibroblast [24]; ii) elevated UGT1A1 in breast cancer cells reduced DMBA-DNA adduct formation [25].
NQO1 (previous names include DT-diaphorase or quinone reductase type 1), a key enzyme belonging to the family of homodimeric flavoproteins, facilitates quinone excretion by catalyzing the reduction of quinones to hydroquinones through a single-step two-electron reduction reaction [26]. Since the alternative one-electron reduction of quinone can form semihydroquinone, which is capable of generating ROS through redox-cycling, NQO1 functions to prevent oxidative DNA damage by environmental stressors. Further, NQO1 plays an important role in preserving endogenous antioxidants by maintaining ubiquinone and α-tocopherol quinone in their reduced forms [27,28,29]. Therefore, mice with targeted disruption of nqo1 were highly susceptible to B[a]P-induced mouse skin carcinogenesis [30]. NQO1*2, the C609T substituted polymorphic form of NQO1, has low NQO1 enzymatic activity compared with wild-type [31] and has been associated with greater risk of cancer incidence including pediatric leukemia [32], colorectal cancer [33], and gastric cardiac carcinoma [34].
HO-1 catalyzes the catabolic metabolism of heme to produce carbon monoxide and bilirubin [35,36]. Due to its role in removing the potent pro-oxidant heme and generating endogenous antioxidants carbon monoxide and bilirubin, HO-1 exhibits strong antioxidant capacity [36]. Ferritin, an iron storage protein, exhibits its antioxidative function by sequestering free iron that is potentially toxic when reacted with hydrogen peroxide [37,38].
3. Regulation of Antioxidant Genes by Nrf2
3.1. Antioxidant Response Element (ARE)
It has long been known that many phase 2 genes, including GSTs and NQO, are regulated through a cis-acting element, the antioxidant response element (ARE), located in their promoters. ARE was first identified in the 5′-flanking region of the rat Gsta2 subunit gene (TAATGGTGACAAAGCA) [39] and this enhancer is essential for the inducible expression of Gsta2 in response not only to phenolic antioxidants and metabolizable planar aromatic compounds, but also hydrogen peroxide and ROS [40]. Similarly, ARE (TCACAGTGACTTGGCA) of the rat nqo1 gene is necessary for the inducible expression of this gene [41,42]; subsequently it was found that the rat nqo1 ARE was highly conserved in the human nqo1 gene (TCACAGTGACTCAGCA) [43]. In addition, GCLC and GCLM, which are genes encoding GSH biosynthetic enzyme subunits, contain multiple functional AREs; the human GCLM promoter retains tandem AREs, which are in opposite orientations [44,45]. In the promoter of the human GCLC gene, a reverse ARE (TCCCCGTGACTCAGCG) located at -3118 bp was identified as a functional ARE, responsible for induction in response to β-naphthoflavone and putative chemopreventive agents [46]. In an early study by Wasserman and Fahl et al. [47], the core sequence of ARE was proposed to be 5′-A/GTGAC/GNNNGCa/c-3′. However, as more AREs are identified in a wide array of phase 2 genes, great variability in the core sequence of ARE was found, indicating that consensus ARE sequences may be dependent on the specific interrogated gene.
3.2. Nrf2 Signaling for the Regulation of ARE-Driven Genes
Based on the critical role of the ARE in antioxidant gene expression, the identification of transcription factors interacting with the ARE have long been a topic of interest. Extensive studies during the past decade have proven the notion that the transcription factor NF-E2-related factor 2 (Nrf2) is an essential element for regulation of the ARE [48,49,50,51]. Since the first demonstration that induction of nqo1 and gst gene expression was abrogated in t-butylhydroxyanisole (BHA)-treated nrf2/- mouse [48], numerous studies have confirmed the crucial role of Nrf2 in the regulation of ARE-bearing genes in response to oxidants and electrophiles. Hepatic and gastric activities of GSTs and Nqo1 were reduced in nrf2-deficient mice compared with wild-type mice, and the induction of these enzymes by a chemopreventive agent, oltipraz, was almost blunt in nrf2-/- mouse [52]. When the dominant mutant Nrf2 was overexpressed, hemin-inducible expression of HO-1 was largely inhibited [53].
Many chemicals can induce the translocation of Nrf2 from the cytoplasm to the nucleus, where it can transactivate AREs with other bZIP transcription factor partners, including small Maf proteins (Maf F, Maf G, and Maf K) and ATF4 [51,54,55,56,57]. As Nrf2 activators, several phytochemicals, typical cancer preventive agents, GSH-depleting agents, electrophiles, and heavy metals are known to induce the expression of ARE-driven genes. In an attempt to elucidate common gene clusters regulated by Nrf2, several comparative analyses of global gene expression have been performed in nrf2-/- mice following treatment with enzyme-inducing chemicals. In the initial demonstration by Kwak et al., the expression of 300 genes was increased by 3H-1,2,-dithiole-3-thione (D3T) treatment in wild-type mouse liver, whereas 77% of these inducible genes were not altered in nrf2-/- mice [58]. These Nrf2-dependent genes include clusters of phase 2 metabolizing enzymes such as GSTs and Nqo1, antioxidants, several cytochrome P450 enzymes, general enzymes, stress-response proteins, and the molecular chaperone-proteasome. Many of these Nrf2-dependent gene clusters were confirmed in other comparative gene analyses following treatment with isothiocyanates such as sulforaphane, phenolic antioxidants BHA and t-butyl hydroxyquinone (tBHQ), and synthetic oleanane triterpenoids [59,60,61,62,63,64,65,66], and representative antioxidant genes under the control of Nrf2 are summarized in Table 1. These comprehensive experimental approaches together with animal studies showing the loss of cytoprotective effect of enzyme inducers in nrf2-/- mice support the concept that Nrf2-target genes are primarily responsible for the advantageous action of phase 2 enzyme inducers.
Table 1.
Antioxidant genes regulated by Nrf2.
| Function | Gene | Species (organs) | Reference |
|---|---|---|---|
| GSH biosynthesis | GCLC | Mouse (liver, lung) Human (HaCaT;keratinocyte) |
[58,67] [68] |
| GCLM | Mouse (liver, lung) Human (IMR-32;neuroblastoma cell) (HaCaT) |
[58,67] [68,69] |
|
| GSR | Mouse (liver, lung) Human (IMR-32, HaCaT) |
[70,71] [68,69] |
|
| Glutathione peroxidases | GPx1 | Mouse (cardiovascular, lung) | [72] |
| GPx2 | Mouse (liver, lung) Rat (liver) Human (Caco-2;colon Cell) Rat (liver) |
[73,74] [75] [76] |
|
| Thioredoxin reductase | TXNRD | Mouse (liver, lung) Human (IMR-32) |
[65,67] [69] |
| Thioredoxin | TXN | Mouse (liver) | [58] |
| Peroxiredoxin | PRDX1 | Mouse (liver,lung) | [67,74] |
| PRDX6 | Human (A549; lung derived cell line) | [77] | |
| Superoxide dismutase | SOD1 | Mouse (liver) | [71] |
| SOD2 | Mouse (liver) | [71] | |
| SOD3 | Mouse (lung) | [67] | |
| Catalase | Mouse (liver, lung) | [71,73] | |
| Glutathione S-transferases | GSTA1 | Mouse (liver, lung, small intestine) | [64,67,71] |
| GSTA2 | Mouse (liver, lung, small intestine) | [58,64,67] | |
| GSTA3 | Mouse (liver, lung, small intestine) | [64,67,71] | |
| GSTA4 | Mouse (liver) | [65] | |
| GSTM1 | Mouse (liver, small intestine) | [58,64,65] | |
| GSTM2 | Mouse (liver, small intestine) | [58,64,65] | |
| GSTM3 | Mouse (liver, small intestine) Human (IMR-32) |
[58,64,65] [69] |
|
| GSTM4 | Mouse (liver) | [65] | |
| GSTM5 | Mouse (liver) | [59] | |
| GSTM6 | Mouse (liver) | [65] | |
| MGST2 | Mouse (small intestine) | [64] | |
| MGST3 | Mouse (liver, small intestine) | [58,64] | |
| UDP-glucuronosyl transferase | UGT1A6 | Mouse (liver) | [74] |
| UGT2B1 | Mouse (liver) | [71] | |
| UGT2B5 | Mouse (liver, small intestine) | [58,64] | |
| Reduction | NQO1 AKR1A AKR1B8 |
Mouse (liver, lung, small intestine) Human (IMR-32) Mouse (liver, small intestine) Mouse (liver, small intestine) |
[58,64,67] [69] [58,64] [64,67] |
| Heme oxygenase-1 | HO-1 | Mouse (liver) Rat (liver) Human (IMR-32, HaCaT) |
[59,76] [68,69] |
| Hydrolysis | EPHX | Mouse (liver, small intestine) | [58,64] |
| Iron transport | Ferritin H | Mouse (lung) Human (HaCaT) |
[73] [68] |
| Ferritin L | Mouse (liver) Human (HaCaT) |
[65] [68] |
|
| Detoxication of heavy metals Transport |
MT І | Mouse (embryonic fibroblasts) Human (HepG2 cell;hepatoma) |
[56] [78] |
| MT ІІ | Mouse (embryonic fibroblasts) Human (HepG2 Cell) |
[56] [78] |
|
| MRP2 | Mouse (liver) | [71] | |
| MRP3 | Mouse (liver) Human (NSCLC, HBE1) |
[71] [79] |
|
| 26S Proteasome | Psma1,4,5,6,7 Psmb1,2,3,4,5,6 |
Mouse (liver) Mouse (liver) |
[58] [58] |
| Psmc1,3 Psmd1,5,7,11,12,13 |
Mouse (liver) Mouse (liver) |
[58] [58] |
|
3.3. Keap1 as a Protein Inhibitor of Nrf2
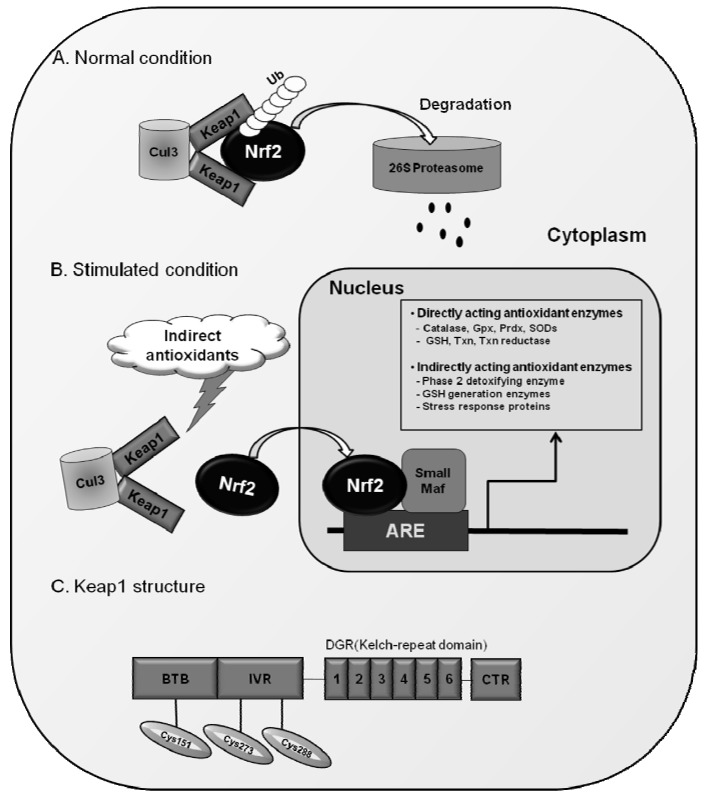
Nrf2 has six highly conserved homologous regions named Neh1 to Neh6 [80]. The Neh1 domain has a bZIP region interacting with partner proteins for heterodimerization [81]. The Neh3 domain interacts with chromodomain helicase DNA-binding protein 6 (CHD6) [82], and two acidic transactivation domains, Neh4 and Neh5, cooperatively bind with CBP (cAMP response element-binding protein-binding protein) [81]. The Neh2 domain located in the N-terminus of Nrf2 is known to be a regulatory domain responding to oxidative stress: Neh2 interacts with cytosolic protein, Kelch-like ECH-associated protein 1 (Keap1) and negatively controls Nrf2 function [83]. Keap1, which was originally isolated as an Nrf2-binding protein, is an actin-binding protein and has been thought to inhibit the function of Nrf2 by simply sequestering the protein in the cytoplasm [83,84]. However, as described in Figure 1, recent advances in Nrf2-Keap biology revealed that Keap1 functions as an adaptor protein between Nrf2 and Cul3, a component of the E3 ligase complex, and this binding promotes the continuous degradation of Nrf2 by the proteasome under normal conditions [85,86,87].
Figure 1.
Nrf2 and its regulation by Keap1. (A) Under normal conditions, Nrf2 is degraded by Keap1-Cul3-dependent pathway; (B) In the presence of indirect antioxidants, Keap1-Nrf2 binding is disturbed and Nrf2 transactivates ARE-driven genes in the nucleus; (C) Protein structure of Keap1 and reactive cysteine residues for the action of Nrf2.
Since the dissociation of Nrf2 from Keap1 is the primary mechanism for Nrf2 activation, it will be an intriguing problem to determine how these two molecules interact with each other in different cellular environments. Recently, Yamamoto and colleagues have proposed the “hinge and latch” model to explain the response of Nrf2-Keap1 complex to stimuli [88,89]. Nrf2 contains 2 Keap1 binding sites within the Neh2 domain: DLG motif and ETGE motif, which enable the formation of a complex of one molecule of Nrf2 and two molecules of Keap1 [90,91]. Of note, these two sites have different binding affinities: the affinity of DLG motif to Keap1 is much weaker than that of ETGE. This led to the hypothetical “latch” binding of DLG-Keap1, which can be easily disturbed by Keap1 conformational changes of [88,89,90,91]. Since DLG binding involves the subsequent Cul3-proteasomal degradation of Nrf2, alterations in the DLG-Keap1 binding can result in the rescue of Nrf2 from degradation and accumulation of this protein within the nucleus. In recognition that Keap1 is a cysteine-rich protein (human and murine Keap1 contain 27 and 25 cysteines, respectively), modifications in sulfhydryl residues of Keap1 protein were initially speculated to result in protein conformational changes [83]. In fact, oxidative stress conditions and many exogenous chemicals alter the reducing status of cysteine residues of Keap1 and lead to Nrf2 translocation. As reactive cysteine residues mediating Nrf2 activation, an initial study by Dinkova-Kostova et al [92], has identified Cys257, Cys273, Cys288, and Cys297 to be dexamethasone-modified cysteine residues using mass spectrometry analysis. Subsequent independent studies confirmed that Cys273 and Cys288 are essential for Nrf2 activation in response to phase 2 enzyme inducers such as dithiolethiones and sulforaphane [91]. In addition to these, Cys151 was demonstrated to be required for the effect of sulforaphane and tBHQ [93,94,95]. Taken together, these results indicate that Keap1 is a sensor protein responding to oxidative and environmental stresses through dynamic changes in cysteine reducing status (Figure 1).
4. Functional Role of the Nrf2 System: From Comparative Animal Studies
Since the first finding of Nrf2 as a master regulator of indirect antioxidant genes, various comparative animal studies using nrf2-/- mice have been performed to investigate the role of Nrf2 in the mammalian defense system. It is now widely accepted that cells and animals with a nrf2 null genotype are much more sensitive to environmental or oxidative stress conditions, leading to accelerated macromolecular damage, mutations, and apoptosis. The initial study by Chan et al. [96], demonstrated that nrf2-/- mice are highly susceptible to butylated hydroxytoluene (BHT)-induced lung damage and lethality in comparison to wild-type mice. A following independent study showed that Nrf2 is a critical factor for determining susceptibility to hyperoxic concentration of oxygen-induced lung injury in mouse [97,98]. As a proof of its role in the CNS, astrocytes from nrf2-/- mice showed higher rates of cell death in response to hydrogen peroxide treatment [62]. Murine embryonic fibroblasts (MEFs) isolated from nrf2-/- mice showed higher levels of cell death in response to treatment with the redox-cycling ROS generator menadione and GSH-depleting anticancer agent cisplatin [99,100]. Incubation with diquat dibromide, another redox cycling bipyridylium herbicide, MEFs displayed markedly decreased cell viability, increased lipid peroxidation and GSH oxidation in comparison to wild-type cells [101].
Many electrophiles can cause oxidative stress leading to DNA mutations and carcinogenesis. As Nrf2 is a prime regulator for the expression of electrophile-detoxifying enzymes, the Nrf2 system has been recognized as a susceptibility determinant in response to chemical carcinogens. The first demonstration by Ramos-Gomez et al. showed that the incidence of gastric tumors was significantly increased in nrf2-/- mice following B[a]P treatment and B[a]P-DNA adduct levels were concomitantly increased in these mutant mice [52,102]. Aoki et al. demonstrated that exposure of nrf2-/- mice to diesel exhaust particles, which are postulated as a probable causal factor in lung cancer, resulted in higher levels of oxidative DNA damage with concomitant increases in lung injury [103]. The incidence of urinary bladder carcinoma by BBN was significantly higher in nrf2-/- mice than in wild-type mice [104]. When treated with arsenic, nrf2-/- mice showed more severe pathological changes in the liver and bladder, and arsenic-induced DNA hypomethylation was significantly elevated in the absence of nrf2 [105].
Recent reports support the role of Nrf2 in the inhibition of inflammatory injuries. Following treatment with lipopolysaccharide (LPS), peritoneal neutrophils from nrf2-/- mice exhibited increased NADPH oxidase-dependent ROS generation and levels of TNF-alpha, IL-6 and chemokines (Mip2 and Mcp-1) compared to wild-type neutrophils [106]. Nrf2-/- macrophages were more susceptible to damage induced by reactive oxygen/nitrogen species, as well as acrolein and cadmium, macrophage toxins [107]. In addition to its role in inflammation, Nrf2 plays an inhibitory role in the fibrogenic process: bleomycin-induced pulmonary fibrosis and cyclosporine-mediated renal fibrosis were aggravated in nrf2-/- mice [108,109]. All together these reports confirmed the critical role of direct and indirect antioxidant proteins, which are under the control of Nrf2, for cytoprotection against divergent arrays of oxidative damage.
5. Indirect Antioxidants Activating the Nrf2 System
Given that Nrf2 primarily governs the expression of antioxidant genes, activation of Nrf2 signaling by specific chemicals can be conceded as one of effective means for prevention of oxidative injuries. In particular, naturally occurring chemicals from vegetables and fruits have been recognized to exhibit antioxidant, ROS-eliminating properties. β-Carotene is an example of direct antioxidants, while various phytochemicals exert their antioxidant activity through Nrf2 signaling. As recently reviewed by Surh et al. [110], phytochemicals activating Nrf2 signaling include isothiocyanates, dithiolethiones, resveratrol, curcumin, CAPE (caffeic acid phenethyl ester; from honeybee hives), epigallicatechin gallate (from green tea), allyl sulfides (from garlic), xanthohumols (from hop), and cinnamaldehyde. The beneficial effects of these phytochemicals have been demonstrated by numerous studies, and in particular, cancer preventive efficacy has been highlighted in many animal and human studies (Table 2).
Table 2.
Indirect antioxidants and their effects on chemical toxicity.
| Indirect antioxidants | Effect on target organ toxicity |
|---|---|
Sulforaphane 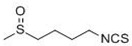
|
Protection against tumor formation induced by many carcinogens: mammary, colon, lung, pancreatic, gastric, intestine, skin, and bladder (mouse, rat, hamster) [16,111,112,113,114,115] |
D3T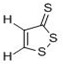
|
Inhibition of aflatoxin B1 induced hepatic tumorigenesis (rat) [116,117]. |
Oltipraz
|
Inhibition of carcinogenesis induced by various carcinogens in bladder, colon, kidney, liver, lung, pancreas, and stomach (mouse, rat) [116,118,119]. |
Resveratrol
|
Inhibition of growth of variety tumors: skin, breast, gastric, colon, small intestine, lung, esophageal, prostate, liver, and pancreatic cancers (mice, rat) [120,121,122]. Human clinical trials in breast cancer patients [123]. |
Curcumin 
|
Inhibition of tumor development in skin, liver, oral, esophageal, stomach, intestinal, colon, and bladder (mouse, rat) [124]. Human clinical trials in patients with advanced pancreatic cancer and other disease [125,126]. |
CAPE
|
Anti-proliferation property in cancer cells [127]. |
The isothiocyanate sulforaphane (1-isothiocyanato-(4R)-(methylsulfinyl)butane) was isolated from broccoli by a bioassay screen of plant extracts for the identification of NQO1 inducers [128]. Accumulating lines of evidence show that sulforaphane is one of the most potent phase 2 enzyme inducers, and its indirect antioxidant properties have been demonstrated in various chemical-carcinogenesis animal studies. In female Sprague Dawley rats, oral administration of sulforaphane with doses of 75, 100, or 150 μmol/day for four days strongly inhibited tumor incidence, multiplicity, and burden by DMBA treatment [115]. In male Fisher rats treated with azoxymethane, sulforaphane inhibited the formation of colonic aberrant crypt foci [111]. Sulforaphane also inhibited lung adenomas induced by tobacco carcinogens [129] and suppressed the incidence of pancreatic tumors induced by N-nitroso-bis(2-oxopropyl)amine. Further studies with nrf2-/- mice have revealed that Nrf2 is the molecular target of sulforaphane. Nrf2-deficient mice lost antioxidant gene inducibility following sulforaphane treatment, and did not show the protective effect of sulforaphane in a B[a]P-induced gastric tumor formation model [113].
Dithiolethiones including D3T and oltipraz are organosulfur compounds [130]. Oltipraz, a congener of D3T, was initially developed for the treatment of schistosomiasis and has been extensively studied as a typical cancer chemopreventive agent. In various rodent organs, such as bladder, colon, kidney, liver, lung, pancreas, stomach, trachea, and blood, oltipraz pre-treatment inhibited carcinogenesis induced by divergent carcinogens including azoxymethane, aflatoxin B1, and B[a]P [116,117,118]. In addition, phase I and II clinical trials have demonstrated the cancer preventive efficacy of oltipraz in humans. A single dose of oltipraz (125~1,000 mg/m2) in humans increased enzymatic activity of NQO1, GCL, and GST in colonic mucosa [131]. NQO1 activity in peripheral mononuclear cells was also elevated in oltipraz-treated individuals without showing significant side effects. In a later randomized double-blind study in Qidong, People’s Republic of China, the intake of low-dose oltipraz increased the phase 2 conjugation reaction of aflatoxin B1, indicating the facilitated detoxification of this potent carcinogen [132]. Anti-carcinogenic effects of dithiolethiones were found to be Nrf2-dependent: the induction of antioxidant genes by dithiolethiones (D3T and oltipraz) was blunt and cancer preventive efficacy of oltipraz has been lost in nrf2-deficient mice [50,52,58,104]. Keap1 has been proposed as the molecular target of dithiolethiones, and Wakabayashi et al. demonstrated that D3T led to intermolecular disulfide bond formation between Cys273 and Cys288 of two Keap1 proteins [91].
Resveratrol (3,5,4’-trihydroxystilbene) is a nonflavonoid polyphenol found in peanuts, grapes, and red wines [133]. Numerous studies have suggested that resveratrol in red wine can reduce the incidence of coronary heart disease and cancer [122]. Resveratrol has an intrinsic antioxidant property, which depends on the redox properties of phenolic hydroxyl groups [134], and its strong antioxidant properties were evidenced by the inhibition of polyunsaturated fatty acid oxidation, which is a core pathologic marker of atherosclerosis [135]. Recent reports showed that the protective effects of resveratrol are mediated by the activation of Nrf2: pretreatment with resveratrol protected H2O2-induced PC12 cell death and this protection was accompanied by upregulation of HO-1 [136]. Resveratrol restored cigarette smoke-depleted GSH levels by upregulating GCL expression through Nrf2 in human lung epithelial cells, and protected cells against cigarette smoke-mediated oxidative stress [137]. Further, enzymatic activities of antioxidant enzymes such as NQO1, Gpx, GR, GST, and SOD were increased after pretreatment with resveratrol in cultured hepatocytes [138].
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a major yellow pigment naturally isolated from the rhizomes of Curcuma longa and used as a coloring and flavoring agent [139]. Curcumin has various beneficial properties including anti-inflammatory, antioxidant, and chemopreventive activities [122,126]. Curcumin elevated enzymatic activity and protein levels of HO-1 through Nrf2 signaling in porcine renal epithelial proximal tubule cells (LLC-PK1) [140]. Dietary administration of curcumin in mice increased nuclear Nrf2, ARE binding activity, and target gene expression in the liver and lungs. As a consequence, curcumin-treated mice showed significantly reduced DNA adduct formation, oxidative damage, and inflammation following B[a]P challenge [141].
6. Pleiotropic Effects of Small Molecule Nrf2 Activators
Health benefits of statins in reducing cardiovascular risk are not solely dependent on their cholesterol-lowering effects: basic and clinical studies have demonstrated that the inhibition of inflammation, maintenance of endothelial function, and modulation of platelet function cooperatively contribute to the protective effect of statins [142,143]. Based on this concept, it has been widely speculated that the use of statins can prevent/ameliorate stroke, Alzheimer’s and renal diseases, and cancers [143,144,145]. Recent advances in understanding the mechanism of action of statins suggest that these non-cholesterol beneficial effects can be explained by a single mechanism inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA reductase) and the consequent reduction in isoprenoids, which are essential for the posttranslational modification of several signaling proteins. Small molecule Nrf2 activators already gained attention for their cancer preventive efficacy. However, recent understanding of the gene clusters regulated by Nrf2 and its various pathophysiological implications provided in-depth characterization of Nrf2 signaling, indicating the pleiotropic effects of Nrf2 activators.
6.1. Anti-Inflammatory Function of Small Molecule Nrf2 Activators
Considerable evidence supports the notion that Nrf2 activation can suppress the inflammatory response although its mechanism has not been fully defined. As increased ROS generation is a core event of inflammation, enhanced cellular antioxidant capacity is believed to contribute to the repression of inflammatory injuries. In particular, potent anti-inflammatory function of HO-1 has been highlighted: increased carbon monoxide, an inhibitor of macrophage activity, is thought to participate in the anti-inflammatory role of HO-1 [35,146,147]. Consistent with these reports, a number of studies have shown that indirect antioxidant-mediated Nrf2 activation is strongly associated with the protection of animals from pro-inflammatory insults [148]. Indeed, a potent Nrf2 activator synthetic triterpenoid significantly reduced LPS-induced cytokine production and in turn suppressed mortality by LPS in wild-type mice; however these protective effects were not observed in nrf2-null mice [106]. In the context of chronic inflammation and its causal link to carcinogenesis, the anti-inflammatory role of small molecule Nrf2 activators appears to be one of mechanisms of cancer-preventive action.
6.2. Modulation of Proteasome Function by Nrf2 Activators: Implication in Protein Toxicity-Associated Diseases
A comparative global gene expression analysis in nrf2-/- mice led to the identification of multiple subunits of hepatic 26S proteasome as D3T-inducible, Nrf2-dependent genes [58]. In a subsequent study, it was shown that the expression of Psmb5, which is the catalytic core subunit of the 20S proteasome, can be up-regulated by proximal AREs [149]. In addition to increased expression of individual subunits, total enzymatic activity of the proteasome was significantly enhanced in multiple tissues from D3T-treated mice including liver, lung, kidney, and small intestines [150]. Since the proteasome, the 20S proteasome in particular, is known to be largely responsible for the degradation of oxidatively damaged proteins, under conditions with abnormal proteasome function, cells will be encountered by condition termed protein toxicity [151,152,153]. This protein toxicity has been highlighted in neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis. Therefore, the use of Nrf2 activators may prevent protein toxicity by maintaining proteasome function. In fact, sulforaphane treatment ameliorated hydrogen peroxide-induced protein oxidation and resulted cytotoxicity by modulating proteasome expression in murine neuroblastoma cells [154]. Degradation of SOD1 G93A protein, which has been postulated as an ALS-causing mutant protein, was facilitated in tissue homogenates from D3T-treated mice [150].
6.3. Lipid Metabolism and Nrf2 Activators.
Several recent findings indicated a potential role for Nrf2 in lipid metabolism. For instance, Shin et al., reported that nrf2-null MEFs showed markedly accelerated adipogenesis, which was triggered by rosiglitazone treatment, compared to wild-type MEFs [155]. In these cells, treatment with an Nrf2-activating triterpenoid effectively inhibited adipocyte differentiation; however nrf2-null cells were unaffected by triterpenoid. In a follow-up study, another synthetic triterpenoid 1-[2-cyano-3-,12-dioxo-oleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im) completely prevented the increase in weight gain of wild-type mice imparted by a high-fat diet, whereas nrf2-disrupted mice were not responsive to the preventive intervention of CDDO-Im [156]. Similar findings have been reported by Tanaka et al., and concentrations of free fatty acid and malondialdehyde equivalents were higher in nrf2-null mice compared with wild-type mice after four weeks on a high-fat diet [157].
6.4. Liver Regeneration and Nrf2
Another promising effect of Nrf2 activators is their potential role in liver regeneration after injury. A recent study by Beyer et al. demonstrated that liver regeneration after a partial hepatectomy is significantly delayed in nrf2-null mice compared to wild-type mice [158]. This finding has been explained by the observation that increased oxidative stress in the nrf2-null condition can lead to insulin/insulin-like growth factor resistance and further impairment of p38 MAPK and AKT signaling, which is necessary for liver cell proliferation [159]. A similar finding was reported by Wakabayashi et al.; however, this group highlighted the direct role of Nrf2 in the regulation of Notch1, suggesting that a loss of nrf2 impedes Notch function and thereby interferes with liver cell proliferation [160]. These results suggest that Nrf2 activators may have beneficial effects in recovery from liver damage, which can result from chronic alcohol consumption, chemical toxins, and drug/viral inflammation. In fact, this possibility has been supported by Kang et al.: oltipraz treatment of rats with established cirrhotic liver enhanced regeneration and reduced cirrhotic nodules with a concomitant improvement in survival [161,162]. Although the authors concluded that activation of CCAAT/enhancer binding protein β (C/EBPβ) and a consequent suppression of TGFβ expression is a primary mechanism of oltipraz-mediated regeneration, the potential involvement of Nrf2 signaling may be of interest for future investigations.
7. Conclusions
The utility of direct antioxidants has been confronted by several randomized clinical studies showing that vitamins C, E, and β-carotene do not reduce cancer incidence in humans. In addition, due to their short half-lives, direct antioxidants need to be administered frequently and relatively high dosages are required to sustain their physiological efficacy. Indirect antioxidants are defined as small molecules that can upregulate the expression of genes encoding antioxidant proteins through Nrf2. Eventually, this effect influences the physiological, biochemical, and/or cellular processes that inactivate free radicals or that prevent free radical-initiated chemical reactions. In contrast to the short half-life of direct antioxidants, indirect antioxidants act through gene regulation, so their physiological effects last longer than do those of direct antioxidants. Furthermore, indirect antioxidants are unlikely to evoke pro-oxidant effects, which have been a problem in the use of high dose vitamin E therapy. Of note, accumulating lines of evidence suggest that small molecule Nrf2 activators exert pleiotropic effects: prevention of cancer, amelioration of inflammatory injuries, protection against protein toxicity, promotion of liver regeneration after injuries, and maintenance of balanced lipid metabolism.
As for the development of indirect antioxidants, Keap1 is now accepted as a direct molecular target of antioxidant gene inducers. However, at this time, due to the limited understanding of the structural biology of Keap-Nrf2 proteins, the detailed mechanism of how specific chemicals react with specific sulfhydryl residues of the Keap1 protein remains to be uncovered. In general, inducers of antioxidant genes are diverse in their structures and chemical properties; however, one common feature is the high reactivity to sulfhydryl groups through the oxidation or alkylation reaction. Originally, Paul Talalay and colleagues defined 9 different chemical classes of sulfhydryl-reactive gene inducers, including isothiocyanates, dithiolethiones, a variety of Michael reaction acceptors, arsenicals and heavy metals, hydroperoxides, vicinal dimercaptans, oxidized diphenols, phenylene diamines, and quinones [163]. A recent study by Kobayashi et al., developed a zebrafish model of a Keap1 mutation, and classified several sulfhydryl reactive chemicals into distinct categories depending on the reactive cysteine residues required for their action [164]. In their classification, sulforaphane, D3T, and GSH depletor diethylmaleate are classified into the same class based on the requirement of Cys151 for Nrf2 activation, while Prostaglandin A2 and 15-deoxy-Δ12,14-prostaglandin J2, in a different class, require Cys273 for their action. On the other hand, an independent study reported that hydrogen peroxide modified multiple cysteine residues of Keap1, including Cys77, Cys297, Cys319, Cys369, and Cys434, indicating more nonspecific modifications in this case [165]. These suggest a clear notion that particular cysteine residues of the Keap1 protein respond to differential signals in a specific way. Therefore, an accurate understanding of the cysteine reactivity of the Keap1 protein will promote the development of more specific antioxidants for the activation of Nrf2. In conclusion, we propose that the development of specific small molecule Nrf2 activators might be a successful strategy to control or prevent a wide array human disease, which is associated with oxidative injuries.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant (2010-0013857 and 2010-0016808) funded by the Korean government.
References
- 1.Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 2.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliwell B., Gutteridge J. Free Radicals in Biology and Medicine. 3rd ed. Oxford University Press; New York, NY, USA: 2001. pp. 82–95. [Google Scholar]
- 4.Steinbrenner H., Sies H. Protection against reactive oxygen species by selenoproteins. Biochim. Biophys. Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B., Gutteridge J. Free Radicals in Biology and Medicine. 3rd ed. Oxford University Press; New York, NY, USA: 2001. pp. 262–316. [Google Scholar]
- 6.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 7.Fita I., Rossmann M.G. The active center of catalase. J. Mol. Biol. 1985;185:21–37. doi: 10.1016/0022-2836(85)90180-9. [DOI] [PubMed] [Google Scholar]
- 8.Schneider Y., Vincent F., Duranton B., Badolo L., Gosse F., Bergmann C., Seiler N., Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000;158:85–91. doi: 10.1016/S0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
- 9.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999;27:951–965. doi: 10.1016/S0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 10.Forgione M.A., Cap A., Liao R., Moldovan N.I., Eberhardt R.T., Lim C.C., Jones J., Goldschmidt-Clermont P.J., Loscalzo J. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.CIR.0000026820.87824.6A. [DOI] [PubMed] [Google Scholar]
- 11.Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 12.Rahman I., Biswas S.K., Jimenez L.A., Torres M., Forman H.J. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox. Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 13.Huang C.S., Chang L.S., Anderson M.E., Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J. Biol. Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- 14.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung. Cell Mol. Physiol. 2000;279:L1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 15.Conney A.H. Induction of drug-metabolizing enzymes: a path to the discovery of multiple cytochromes P450. Annu. Rev. Pharmacol. Toxicol. 2003;43:1–30. doi: 10.1146/annurev.pharmtox.43.100901.135754. [DOI] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova A.T., Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008;52(Suppl. 1):S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 17.Hayes J.D., McMahon M., Chowdhry S., Dinkova-Kostova A.T. Cancer Chemoprevention Mechanisms Mediated Through the Keap1-Nrf2 Pathway. Antioxid. Redox. Signal. 2010 doi: 10.1089/ars.2010.3221. in press. [DOI] [PubMed] [Google Scholar]
- 18.Jernstrom B., Dock L., Martinez M. Metabolic activation of benzoa.pyrene-7,8-dihydrodiol and benzoa.pyrene-7,8-dihydrodiol-9,10-epoxide to protein-binding products and the inhibitory effect of glutathione and cysteine. Carcinogenesis. 1984;5:199–204. doi: 10.1093/carcin/5.2.199. [DOI] [PubMed] [Google Scholar]
- 19.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 20.Henderson C.J., Smith A.G., Ure J., Brown K., Bacon E.J., Wolf C.R. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc. Natl. Acad. Sci. USA. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie K.J., Henderson C.J., Wang X.J., Vassieva O., Carrie D., Farmer P.B., Gaskell M., Park K., Wolf C.R. Glutathione transferase pi plays a critical role in the development of lung carcinogenesis following exposure to tobacco-related carcinogens and urethane. Cancer Res. 2007;67:9248–9257. doi: 10.1158/0008-5472.CAN-07-1764. [DOI] [PubMed] [Google Scholar]
- 22.Nazar-Stewart V., Vaughan T.L., Burt R.D., Chen C., Berwick M., Swanson G.M. Glutathione S-transferase M1 and susceptibility to nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 1999;8:547–551. [PubMed] [Google Scholar]
- 23.Tukey R.H., Strassburg C.P. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 24.Vienneau D.S., DeBoni U., Wells P.G. Potential genoprotective role for UDP-glucuronosyltransferases in chemical carcinogenesis: initiation of micronuclei by benzo(a)pyrene and benzo(e)pyrene in UDP-glucuronosyltransferase-deficient cultured rat skin fibroblasts. Cancer Res. 1995;55:1045–1051. [PubMed] [Google Scholar]
- 25.Leung H.Y., Wang Y., Leung L.K. Differential effect of over-expressing UGT1A1 and CYP1A1 on xenobiotic assault in MCF-7 cells. Toxicology. 2007;242:153–159. doi: 10.1016/j.tox.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Nioi P., Hayes J.D. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat. Res. 2004;555:149–171. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Beyer R.E., Segura-Aguilar J., Di Bernardo S., Cavazzoni M., Fato R., Fiorentini D., Galli M.C., Setti M., Landi L., Lenaz G. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc. Natl. Acad. Sci. USA. 1996;93:2528–2532. doi: 10.1073/pnas.93.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landi L., Fiorentini D., Galli M.C., Segura-Aguilar J., Beyer R.E. DT-Diaphorase maintains the reduced state of ubiquinones in lipid vesicles thereby promoting their antioxidant function. Free Radic. Biol. Med. 1997;22:329–335. doi: 10.1016/S0891-5849(96)00294-8. [DOI] [PubMed] [Google Scholar]
- 29.Siegel D., Bolton E.M., Burr J.A., Liebler D.C., Ross D. The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol. Pharmacol. 1997;52:300–305. doi: 10.1124/mol.52.2.300. [DOI] [PubMed] [Google Scholar]
- 30.Long D.J., 2nd, Waikel R.L., Wang X.J., Perlaky L., Roop D.R., Jaiswal A.K. NAD(P)H:quinone oxidoreductase 1 deficiency increases susceptibility to benzo(a)pyrene-induced mouse skin carcinogenesis. Cancer Res. 2000;60:5913–5915. [PubMed] [Google Scholar]
- 31.Traver R.D., Siegel D., Beall H.D., Phillips R.M., Gibson N.W., Franklin W.A., Ross D. Characterization of a polymorphism in NAD(P)H: quinone oxidoreductase (DT-diaphorase) Br. J. Cancer. 1997;75:69–75. doi: 10.1038/bjc.1997.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiemels J.L., Pagnamenta A., Taylor G.M., Eden O.B., Alexander F.E., Greaves M.F. A lack of a functional NAD(P)H:quinone oxidoreductase allele is selectively associated with pediatric leukemias that have MLL fusions. United Kingdom Childhood Cancer Study Investigators. Cancer Res. 1999;59:4095–4099. [PubMed] [Google Scholar]
- 33.Lafuente M.J., Casterad X., Trias M., Ascaso C., Molina R., Ballesta A., Zheng S., Wiencke J.K., Lafuente A. NAD(P)H:quinone oxidoreductase-dependent risk for colorectal cancer and its association with the presence of K-ras mutations in tumors. Carcinogenesis. 2000;21:1813–1819. doi: 10.1093/carcin/21.10.1813. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J.H., Li Y., Wang R., Sarbia M., Guo W., Wen D.G., Wei L.Z., Chen Z.F., Kuang G., Zhang L.W., He M., Wu M.L., Wang S.J. The NAD(P)H: quinone oxidoreductase 1 C609T polymorphism and susceptibility to esophageal cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20:544–546. [PubMed] [Google Scholar]
- 35.Loboda A., Jazwa A., Grochot-Przeczek A., Rutkowski A.J., Cisowski J., Agarwal A., Jozkowicz A., Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 36.Prestera T., Talalay P., Alam J., Ahn Y.I., Lee P.J., Choi A.M. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE) Mol. Med. 1995;1:827–837. doi: 10.1007/BF03401897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison P.M., Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji Y., Ayaki H., Whitman S.P., Morrow C.S., Torti S.V., Torti F.M. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol. Cell Biol. 2000;20:5818–5827. doi: 10.1128/MCB.20.16.5818-5827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rushmore T.H., Pickett C.B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 40.Rushmore T.H., Morton M.R., Pickett C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 41.Favreau L.V., Pickett C.B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 42.Nioi P., McMahon M., Itoh K., Yamamoto M., Hayes J.D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem. J. 2003;374:337–348. doi: 10.1042/bj20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaiswal A.K. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991;30:10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- 44.Erickson A.M., Nevarea Z., Gipp J.J., Mulcahy R.T. Identification of a variant antioxidant response element in the promoter of the human glutamate-cysteine ligase modifier subunit gene. Revision of the ARE consensus sequence. J. Biol. Chem. 2002;277:30730–30737. doi: 10.1074/jbc.M205225200. [DOI] [PubMed] [Google Scholar]
- 45.Mulcahy R.T., Gipp J.J. Identification of a putative antioxidant response element in the 5’-flanking region of the human gamma-glutamylcysteine synthetase heavy subunit gene. Biochem. Biophys. Res. Commun. 1995;209:227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- 46.Mulcahy R.T., Wartman M.A., Bailey H.H., Gipp J.J. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J. Biol. Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 47.Wasserman W.W., Fahl W.E. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi M., Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 50.Kwak M.K., Egner P.A., Dolan P.M., Ramos-Gomez M., Groopman J.D., Itoh K., Yamamoto M., Kensler T.W. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat. Res. 2001;480-481:305–315. doi: 10.1016/S0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 51.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos-Gomez M., Kwak M.K., Dolan P.M., Itoh K., Yamamoto M., Talalay P., Kensler T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 54.He C.H., Gong P., Hu B., Stewart D., Choi M.E., Choi A.M., Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 55.Igarashi K., Kataoka K., Itoh K., Hayashi N., Nishizawa M., Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994;367:568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- 56.Katsuoka F., Motohashi H., Ishii T., Aburatani H., Engel J.D., Yamamoto M. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol. Cell Biol. 2005;25:8044–8051. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motohashi H., O’Connor T., Katsuoka F., Engel J.D., Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/S0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 58.Kwak M.K., Wakabayashi N., Itoh K., Motohashi H., Yamamoto M., Kensler T.W. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 59.Hu R., Xu C., Shen G., Jain M.R., Khor T.O., Gopalkrishnan A., Lin W., Reddy B., Chan J.Y., Kong A.N. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (-/-) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 60.Hu R., Xu C., Shen G., Jain M.R., Khor T.O., Gopalkrishnan A., Lin W., Reddy B., Chan J.Y., Kong A.N. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79:1944–1955. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Kwak M.K., Kensler T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J.M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 63.Nair S., Xu C., Shen G., Hebbar V., Gopalakrishnan A., Hu R., Jain M.R., Lin W., Keum Y.S., Liew C., Chan J.Y., Kong A.N. Pharmacogenomics of phenolic antioxidant butylated hydroxyanisole (BHA) in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Pharm. Res. 2006;23:2621–2637. doi: 10.1007/s11095-006-9099-x. [DOI] [PubMed] [Google Scholar]
- 64.Thimmulappa R.K., Mai K.H., Srisuma S., Kensler T.W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 65.Yates M.S., Kwak M.K., Egner P.A., Groopman J.D., Bodreddigari S., Sutter T.R., Baumgartner K.J., Roebuck B.D., Liby K.T., Yore M.M., Honda T., Gribble G.W., Sporn M.B., Kensler T.W. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl.imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 66.Yates M.S., Tran Q.T., Dolan P.M., Osburn W.O., Shin S., McCulloch C.C., Silkworth J.B., Taguchi K., Yamamoto M., Williams C.R., Liby K.T., Sporn M.B., Sutter T.R., Kensler T.W. Genetic versus Chemoprotective Activation of Nrf2 Signaling: Overlapping yet Distinct Gene Expression Profiles between Keap1 Knockout and Triterpenoid Treated Mice. Carcinogenesis. 2009:2488–2494. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W., Yamamoto M., Petrache I., Tuder R.M., Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacLeod A.K., McMahon M., Plummer S.M., Higgins L.G., Penning T.M., Igarashi K., Hayes J.D. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis. 2009;30:1571–1580. doi: 10.1093/carcin/bgp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J., Lee J.M., Johnson J.A. Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells. J. Biol. Chem. 2002;277:388–394. doi: 10.1074/jbc.M109380200. [DOI] [PubMed] [Google Scholar]
- 70.Harvey C.J., Thimmulappa R.K., Singh A., Blake D.J., Ling G., Wakabayashi N., Fujii J., Myers A., Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reisman S.A., Yeager R.L., Yamamoto M., Klaassen C.D. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol. Sci. 2009;108:35–47. doi: 10.1093/toxsci/kfn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A., Rangasamy T., Thimmulappa R.K., Lee H., Osburn W.O., Brigelius-Flohe R., Kensler T.W., Yamamoto M., Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho H.Y., Reddy S.P., Debiase A., Yamamoto M., Kleeberger S.R. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic. Biol. Med. 2005;38:325–343. doi: 10.1016/j.freeradbiomed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 74.Okawa H., Motohashi H., Kobayashi A., Aburatani H., Kensler T.W., Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 75.Banning A., Deubel S., Kluth D., Zhou Z., Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol. Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Habeos I.G., Ziros P.G., Chartoumpekis D., Psyrogiannis A., Kyriazopoulou V., Papavassiliou A.G. Simvastatin activates Keap1/Nrf2 signaling in rat liver. J Mol. Med. 2008;86:1279–1285. doi: 10.1007/s00109-008-0393-4. [DOI] [PubMed] [Google Scholar]
- 77.Chowdhury I., Mo Y., Gao L., Kazi A., Fisher A.B., Feinstein S.I. Oxidant stress stimulates expression of the human peroxiredoxin 6 gene by a transcriptional mechanism involving an antioxidant response element. Free Radic. Biol. Med. 2009;46:146–153. doi: 10.1016/j.freeradbiomed.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeh C.T., Yen G.C. Effect of sulforaphane on metallothionein expression and induction of apoptosis in human hepatoma HepG2 cells. Carcinogenesis. 2005;26:2138–2148. doi: 10.1093/carcin/bgi185. [DOI] [PubMed] [Google Scholar]
- 79.Mahaffey C.M., Zhang H., Rinna A., Holland W., Mack P.C., Forman H.J. Multidrug-resistant protein-3 gene regulation by the transcription factor Nrf2 in human bronchial epithelial and non-small-cell lung carcinoma. Free Radic. Biol. Med. 2009;46:1650–1657. doi: 10.1016/j.freeradbiomed.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itoh K., Igarashi K., Hayashi N., Nishizawa M., Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell Biol. 1995;15:4184–4193. doi: 10.1128/MCB.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 82.Nioi P., Nguyen T., Sherratt P.J., Pickett C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xue F., Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a "tethering" mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 87.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tong K.I., Kobayashi A., Katsuoka F., Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 89.Tong K.I., Padmanabhan B., Kobayashi A., Shang C., Hirotsu Y., Yokoyama S., Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Padmanabhan B., Tong K.I., Ohta T., Nakamura Y., Scharlock M., Ohtsuji M., Kang M.I., Kobayashi A., Yokoyama S., Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 91.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.L., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eggler A.L., Liu G., Pezzuto J.M., van Breemen R.B., Mesecar A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eggler A.L., Luo Y., van Breemen R.B., Mesecar A.D. Identification of the highly reactive cysteine 151 in the chemopreventive agent-sensor Keap1 protein is method-dependent. Chem. Res. Toxicol. 2007;20:1878–1884. doi: 10.1021/tx700217c. [DOI] [PubMed] [Google Scholar]
- 95.Zhang D.D., Hannink M. Distinct cysteine residues in keap1 are required for keap1-dependent ubiquitination of nrf2 and for stabilization of nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan K., Kan Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho H.Y., Jedlicka A.E., Reddy S.P., Kensler T.W., Yamamoto M., Zhang L.Y., Kleeberger S.R. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 98.Cho H.Y., Jedlicka A.E., Reddy S.P., Zhang L.Y., Kensler T.W., Kleeberger S.R. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am. J. Respir. Cell Mol. Biol. 2002;26:42–51. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- 99.Cho J.M., Manandhar S., Lee H.R., Park H.M., Kwak M.K. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett. 2008;260:96–108. doi: 10.1016/j.canlet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 100.Kwak M.K., Ramos-Gomez M., Wakabayashi N., Kensler T.W. Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes. Methods Enzymol. 2004;382:414–423. doi: 10.1016/S0076-6879(04)82022-6. [DOI] [PubMed] [Google Scholar]
- 101.Osburn W.O., Wakabayashi N., Misra V., Nilles T., Biswal S., Trush M.A., Kensler T.W. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch. Biochem. Biophys. 2006;454:7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramos-Gomez M., Dolan P.M., Itoh K., Yamamoto M., Kensler T.W. Interactive effects of nrf2 genotype and oltipraz on benzoa.pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 103.Aoki Y., Sato H., Nishimura N., Takahashi S., Itoh K., Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 104.Iida K., Itoh K., Kumagai Y., Oyasu R., Hattori K., Kawai K., Shimazui T., Akaza H., Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 105.Jiang T., Huang Z., Chan J.Y., Zhang D.D. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicol. Appl. Pharmacol. 2009;240:8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thimmulappa R.K., Scollick C., Traore K., Yates M., Trush M.A., Liby K.T., Sporn M.B., Yamamoto M., Kensler T.W., Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu H., Jia Z., Zhang L., Yamamoto M., Misra H.P., Trush M.A., Li Y. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp. Biol. Med. (Maywood) 2008;233:463–474. doi: 10.3181/0711-RM-304. [DOI] [PubMed] [Google Scholar]
- 108.Cho H.Y., Reddy S.P., Yamamoto M., Kleeberger S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 109.Shin D.H., Park H.M., Jung K.A., Choi H.G., Kim J.A., Kim D.D., Kim S.G., Kang K.W., Ku S.K., Kensler T.W., Kwak M.K. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic. Biol. Med. 2010;48:1051–1063. doi: 10.1016/j.freeradbiomed.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Surh Y.J., Kundu J.K., Na H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 111.Chung F.L., Conaway C.C., Rao C.V., Reddy B.S. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 112.Clarke J.D., Dashwood R.H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fahey J.W., Haristoy X., Dolan P.M., Kensler T.W., Scholtus I., Stephenson K.K., Talalay P., Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzoa.pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Juge N., Mithen R.F., Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y., Kensler T.W., Cho C.G., Posner G.H., Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kensler T.W., Egner P.A., Dolan P.M., Groopman J.D., Roebuck B.D. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987;47:4271–4277. [PubMed] [Google Scholar]
- 117.Kensler T.W., Groopman J.D., Eaton D.L., Curphey T.J., Roebuck B.D. Potent inhibition of aflatoxin-induced hepatic tumorigenesis by the monofunctional enzyme inducer 1,2-dithiole-3-thione. Carcinogenesis. 1992;13:95–100. doi: 10.1093/carcin/13.1.95. [DOI] [PubMed] [Google Scholar]
- 118.Kensler T.W., Groopman J.D., Sutter T.R., Curphey T.J., Roebuck B.D. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 119.Roebuck B.D., Curphey T.J., Li Y., Baumgartner K.J., Bodreddigari S., Yan J., Gange S.J., Kensler T.W., Sutter T.R. Evaluation of the cancer chemopreventive potency of dithiolethione analogs of oltipraz. Carcinogenesis. 2003;24:1919–1928. doi: 10.1093/carcin/bgg173. [DOI] [PubMed] [Google Scholar]
- 120.Aziz M.H., Kumar R., Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int. J. Oncol. 2003;23:17–28. doi: 10.3892/ijo.23.1.17. [DOI] [PubMed] [Google Scholar]
- 121.Juan M.E., Vinardell M.P., Planas J.M. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- 122.Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 123.Athar M., Back J.H., Tang X., Kim K.H., Kopelovich L., Bickers D.R., Kim A.L. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh S., Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med. Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 125.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 126.Hatcher H., Planalp R., Cho J., Torti F.M., Torti S.V. Curcumin: from ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagaoka T., Banskota A.H., Tezuka Y., Saiki I., Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg. Med. Chem. 2002;10:3351–3359. doi: 10.1016/S0968-0896(02)00138-4. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y., Talalay P., Cho C.G., Posner G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Conaway C.C., Wang C.X., Pittman B., Yang Y.M., Schwartz J.E., Tian D., McIntee E.J., Hecht S.S., Chung F.L. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y., Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol. Cancer Ther. 2008;7:3470–3479. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Dwyer P.J., Szarka C.E., Yao K.S., Halbherr T.C., Pfeiffer G.R., Green F., Gallo J.M., Brennan J., Frucht H., Goosenberg E.B., Hamilton T.C., Litwin S., Balshem A.M., Engstrom P.F., Clapper M.L. Modulation of gene expression in subjects at risk for colorectal cancer by the chemopreventive dithiolethione oltipraz. J. Clin. Invest. 1996;98:1210–1217. doi: 10.1172/JCI118904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J.S., Shen X., He X., Zhu Y.R., Zhang B.C., Wang J.B., Qian G.S., Kuang S.Y., Zarba A., Egner P.A., Jacobson L.P., Munoz A., Helzlsouer K.J., Groopman J.D., Kensler T.W. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J. Natl. Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 133.Leiro J., Alvarez E., Arranz J.A., Laguna R., Uriarte E., Orallo F. Effects of cis-resveratrol on inflammatory murine macrophages: antioxidant activity and down-regulation of inflammatory genes. J. Leukoc. Biol. 2004;75:1156–1165. doi: 10.1189/jlb.1103561. [DOI] [PubMed] [Google Scholar]
- 134.Lopez-Velez M., Martinez-Martinez F., Del Valle-Ribes C. The study of phenolic compounds as natural antioxidants in wine. Crit. Rev. Food Sci. Nutr. 2003;43:233–244. doi: 10.1080/10408690390826509. [DOI] [PubMed] [Google Scholar]
- 135.Miller N.J., Rice-Evans C.A. Antioxidant activity of resveratrol in red wine. Clin. Chem. 1995;41:1789. [PubMed] [Google Scholar]
- 136.Chen C.Y., Jang J.H., Li M.H., Surh Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 137.Kode A., Rajendrasozhan S., Caito S., Yang S.R., Megson I.L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294:L478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 138.Rubiolo J.A., Mithieux G., Vega F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 139.Ammon H.P., Wahl M.A. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 140.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garg R., Gupta S., Maru G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzoa.pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 142.Calabro P., Yeh E.T. The pleiotropic effects of statins. Curr Opin Cardiol. 2005;20:541–546. doi: 10.1097/01.hco.0000181482.99067.bf. [DOI] [PubMed] [Google Scholar]
- 143.Shaw S.M., Fildes J.E., Yonan N., Williams S.G. Pleiotropic effects and cholesterol-lowering therapy. Cardiology. 2009;112:4–12. doi: 10.1159/000137692. [DOI] [PubMed] [Google Scholar]
- 144.D’Amico G. Statins and renal diseases: from primary prevention to renal replacement therapy. J. Am. Soc. Nephrol. 2006;17:S148–S152. doi: 10.1681/ASN.2005121341. [DOI] [PubMed] [Google Scholar]
- 145.Dulak J., Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr. Cancer Drug Targets. 2005;5:579–594. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Immenschuh S., Baumgart-Vogt E., Mueller S. Heme Oxygenase-1 and Iron in Liver Inflammation: a Complex Alliance. Curr. Drug Targets. 2010 doi: 10.2174/1389450111009011541. [DOI] [PubMed] [Google Scholar]
- 147.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010 doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 148.Khor T.O., Yu S., Kong A.N. Dietary cancer chemopreventive agents - targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 149.Kwak M.K., Wakabayashi N., Greenlaw J.L., Yamamoto M., Kensler T.W. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol. Cell Biol. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kwak M.K., Huang B., Chang H., Kim J.A., Kensler T.W. Tissue specific increase of the catalytic subunits of the 26S proteasome by indirect antioxidant dithiolethione in mice: enhanced activity for degradation of abnormal protein. Life Sci. 2007;80:2411–2420. doi: 10.1016/j.lfs.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 151.Halliwell B. Hypothesis: proteasomal dysfunction: a primary event in neurogeneration that leads to nitrative and oxidative stress and subsequent cell death. Ann. N Y Acad. Sci. 2002;962:182–194. doi: 10.1111/j.1749-6632.2002.tb04067.x. [DOI] [PubMed] [Google Scholar]
- 152.Poppek D., Grune T. Proteasomal defense of oxidative protein modifications. Antioxid. Redox Signal. 2006;8:173–184. doi: 10.1089/ars.2006.8.173. [DOI] [PubMed] [Google Scholar]
- 153.Stefani M., Dobson C.M. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003;27:27. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 154.Kwak M.K., Cho J.M., Huang B., Shin S., Kensler T.W. Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic. Biol. Med. 2007;43:809–817. doi: 10.1016/j.freeradbiomed.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 155.Shin S., Wakabayashi N., Misra V., Biswal S., Lee G.H., Agoston E.S., Yamamoto M., Kensler T.W. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shin S., Wakabayashi J., Yates M.S., Wakabayashi N., Dolan P.M., Aja S., Liby K.T., Sporn M.B., Yamamoto M., Kensler T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanaka Y., Aleksunes L.M., Yeager R.L., Gyamfi M.A., Esterly N., Guo G.L., Klaassen C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 158.Beyer T.A., Xu W., Teupser D., auf dem Keller U., Bugnon P., Hildt E., Thiery J., Kan Y.W., Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beyer T.A., Werner S. The cytoprotective Nrf2 transcription factor controls insulin receptor signaling in the regenerating liver. Cell Cycle. 2008;7:874–878. doi: 10.4161/cc.7.7.5617. [DOI] [PubMed] [Google Scholar]
- 160.Wakabayashi N., Shin S., Slocum S.L., Agoston E.S., Wakabayashi J., Kwak M.K., Misra V., Biswal S., Yamamoto M., Kensler T.W. Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci. Signal. 2010;3:ra52. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kang K.W., Choi S.H., Ha J.R., Kim C.W., Kim S.G. Inhibition of dimethylnitrosamine-induced liver fibrosis by 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione. (oltipraz) in rats: suppression of transforming growth factor-beta1 and tumor necrosis factor-alpha expression. Chem. Biol. Interact. 2002;139:61–77. doi: 10.1016/S0009-2797(01)00286-1. [DOI] [PubMed] [Google Scholar]
- 162.Kang K.W., Kim Y.G., Cho M.K., Bae S.K., Kim C.W., Lee M.G., Kim S.G. Oltipraz regenerates cirrhotic liver through CCAAT/enhancer binding protein-mediated stellate cell inactivation. FASEB J. 2002;16:1988–1990. doi: 10.1096/fj.02-0406fje. [DOI] [PubMed] [Google Scholar]
- 163.Talalay P., De Long M.J., Prochaska H.J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Holland R., Hawkins A.E., Eggler A.L., Mesecar A.D., Fabris D., Fishbein J.C. Prospective type 1 and type 2 disulfides of Keap1 protein. Chem. Res. Toxicol. 2008;21:2051–2060. doi: 10.1021/tx800226m. [DOI] [PMC free article] [PubMed] [Google Scholar]