Abstract
Homophthalic anhydride reacts with different aromatic amines to produce N-substituted homophthalimides. Bromination of the latter produces 4,4-dibromo-homophthalimide derivatives that can be used as precursors for spiro-derivatives. The dibromo derivatives react with different binucleophilic reagents to produce several spiro-isoquinoline derivatives. Reaction of the dibromo derivatives with malononitrile produces dicyanomethylene derivatives which react with different binucleophiles to produce new spiro-derivatives. Structures of the newly synthesized compounds are proved using spectroscopic methods such as IR, 1H-NMR and 13C-NMR. The newly synthesized compounds were tested for their antimicrobial and antioxidant activities, showing weak or no antimicrobial activity. On the other hand select compounds showed promising antioxidant activities.
Keywords: microwave, spiro-compounds, heterocycles, spiro-benzoxazoleisoquinoline, spiro-benzimidazoleisoquinoline, spiro-isoquinolinetriazole, spiro-isoquinolinepyrazole, spiro-isoquinolinepyrimidine, antimicrobial activity, antioxidant activity
1. Introduction
Spiro-compounds form a group of generally less investigated compounds. However, recently growing efforts have been made to synthesize and characterize these compounds. Many spiro-compounds possess very promising biological activities as anticancer agents [1,2], antibacterial agents [3,4], anticonvulsant agents [5,6,7], anti-tuberculosis agents [8], anti-Alzheimer’s agents [9], pain-relief agents [10,11], anti-dermatitis agents [12] and antimicrobial agents [13,14]. In addition to their medical uses, some spiro-compounds have found other uses in the agricultural and industrial fields. For example, they are used as antifungal agents [15], pesticides [16], laser dyes [17] and electroluminescent devices [18]. Spiro compounds have also been recently used as antioxidants [19,20]. We were prompted by these findings to try to synthesize new spiro-compounds with potential antimicrobial or antioxidant activities.
The microwave technique has several advantages over traditional methods of synthesis. Reduced reaction times [21,22,23,24], less effects on the environment and better reaction yields are some of the common advantages of using microwaves. In the present research project, we used both the microwave technique as well as conventional methods to prepare some spiro-compounds with expected biological activity.
2. Results and Discussion
2.1. Chemistry
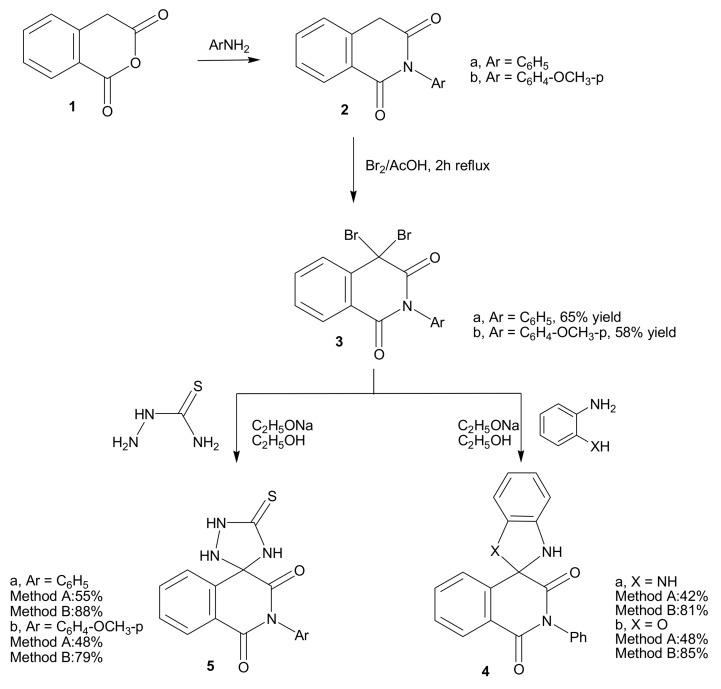
Homophthalic anhydride (1) was reacted with aromatic amines, namely aniline and p-anisidine, to afford N-arylhomophthalimide derivatives 2a,b, respectively, which were used as precursors for preparing new spiro-isoquinolines (Scheme 1). Compounds 2a,b, having an active methylene group, reacted with two equivalents of bromine in acetic acid to produce 2-aryl-4,4-dibromoisoquinoline-1,3-(2H,4H)dione derivatives 3a,b. The mass spectrum of compound 3a displayed the expected molecular ion peaks at m/z 394 (3.2%), 395 (6.7%), 396 (3.0%). Compound 3b gave molecular ion peaks at 424 (7.3%), 425 (15.1%), 426 (6.8%).
Scheme 1.
Reactions of dibromohomophthalimides with binucleophilic reagents; synthesis of 4 and 5.
Compound 3a underwent direct cyclocondensation when treated with each of o-phenylenediamine or o-aminophenol to produce 2'-phenyl-1,3-dihydro-1'H-spiro-[benzimidazole-2,4'-isoquinoline]-1',3' (2'H)-dione (4a) and 2'-phenyl-1'H,3H-spiro[1,3-benzoxazole-2,4'-isoquinoline]-1',3'(2'H)-dione (4b), respectively. Compounds 4a and 4b are new ring systems. Synthesis of compounds 4a,b was carried out under two different reaction conditions, namely by conventional heating and using microwave irradiation conditions. Thus, when the reaction was carried out in a refluxing ethanolic sodium ethoxide solution for 5 h under TLC monitoring, the product 4a was obtained in 42% yield, while 4b was obtained in 48% yield. However, when the same reaction was carried out by heating at 140 °C in a microwave oven for 15 min, the yields of 4a and 4b were 81% and 85%, respectively. It was then concluded that using microwave as a source of heat not only improves the reaction yield, but also significantly reduces the reaction time. The IR and 1H-NMR spectra of compounds 4a and 4b agreed with the proposed structures. Their mass spectra showed the molecular ion peaks at m/z 341 (12.3%) and 342 (8.6%), respectively.
Similarly, compounds 3a,b reacted with thiosemicarbazide under both conventional and microwave reaction conditions and produced 2-phenyl-5'-thioxo-1H-spiro[isoquinoline-4,3'-[1,2,4]triazolidine]-1,3(2H)-dione (5a) and 2-(4-methoxyphenyl)-5'-thioxo-1H-spiro-[isoquinoline-4,3'-[1,2,4]-triazol-idine]-1,3(2H)-dione (5b), respectively. Compounds 5 also have a new ring system. Again, comparing the reaction times and overall yields for both traditional and microwave methods showed that traditional methods took 4 h, to give 55% and 48% yields of 5a and 5b, respectively, while the microwave method needed only 15 min to give 88% and 79% yields of 5a and 5b, respectively. The comparison again proves the advantages of using the microwave technique. Analytical and spectral data of 5a and 5b were in agreement with the proposed structures.
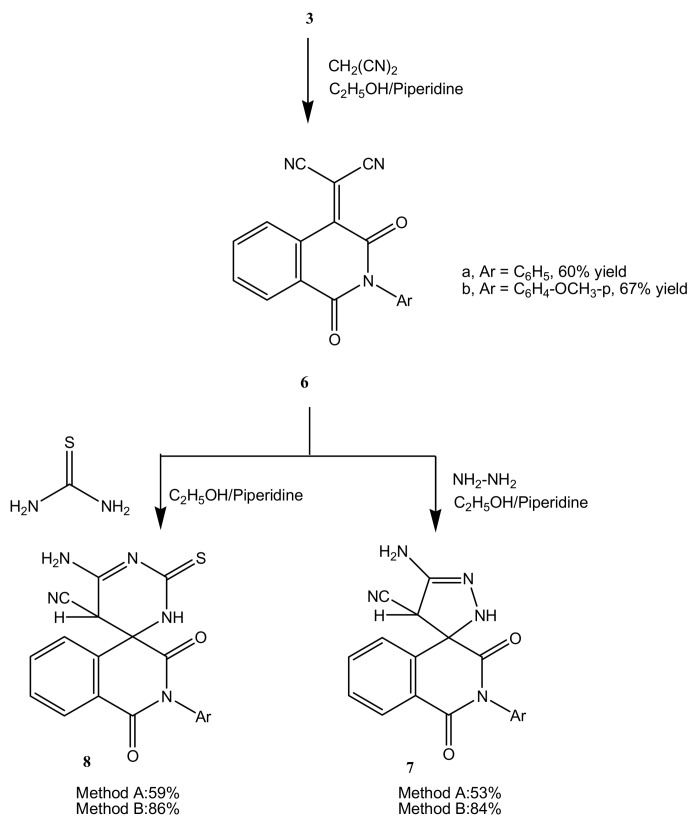
On the other hand, compounds 3a,b reacted with malononitrile, as an activated methylene compound, by refluxing under TLC monitoring in absolute ethanol containing a catalytic amount of piperidine for 3 h, to produce (1,3-dioxo-2-phenyl-2,3-dihydroisoquinolin-4(1H)-ylidene)-malononitrile (6a) and [2-(4-methoxyphenyl)-1,3-dioxo-2,3-dihydroisoquinolin-4(1H)-ylidene]-malononitrile (6b), respectively (Scheme 2). Attempts to carry out the same reaction under microwave conditions failed however, due to the formation of a complex mixture of products that could not be separated. Compounds 6a and 6b gave acceptable analytical and spectral data.
Scheme 2.
Reactions of isoquinolinylidenemalononitriles with hydrazine hydrate and thiourea; synthesis of 7 and 8.
Compound 6a could be used as a precursor for some spiro-isoquinolines containing five and six-membered heterocycles. Thus, compound 6a reacted with each of hydrazine hydrate and thiourea to produce 5'-amino-1,3-dioxo-2-phenyl-2,2',3,4'-tetrahydro-1H-spiro[isoquinoline-4,3'-pyrazole]-4'-carbonitrile (7) and 6'-amino-1,3-dioxo-2-phenyl-2'-thioxo-2,2',3,5'-tetrahydro-1H,3'H-spiro-[isoquinoline-4,4'-pyrimidine]-5'-carbonitrile (8), respectively. Compound 8 has a new ring system. Synthesis of compounds 7 and 8 was performed under TLC monitoring in refluxing ethanol solution containing catalytic amounts of piperidine for 4 h and the yields were 53% and 59%, respectively. Carrying out the same reaction under microwave assisted conditions reduced the reaction time to 15 min and increased the yields to 84% and 86%, respectively, for 7 and 8. Acceptable analytical data were obtained for the new compounds 7 and 8.
2.2. Biological Evaluation
2.2.1. Antimicrobial evaluation
The newly synthesized heterocyclic compounds listed in Table 1 were tested for their antimicrobial activity against the following microorganisms: Escherichia coli, Pseudomonas putida, Bacillus subtilis, Streptococcus lactis, Aspergillus niger, Penicillium sp. and Candida albicans. The preliminary screening of the investigated compounds was performed using the filter paper disc-diffusion method. The most active compounds were 4a, 5a, 5b and 8, which were slightly inhibitory to the microorganisms. The rest of compounds showed no sensitivity at all to the tested organisms, and the results are summarized in Table 1.
Table 1.
Antimicrobial activities of the newly synthesized compounds.
| Comp.No. | Inhibition zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Gram-negative | Gram-positive | Fungi | Yeast | ||||
| E. coli | P. putida | B. subtilis | S. lactis | A. niger | P. sp. | C. albicans | |
| 4a | 8 | 4 | 4 | 6 | 3 | 2 | 0 |
| 4b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5a | 5 | 3 | 5 | 5 | 4 | 3 | 0 |
| 5b | 10 | 9 | 10 | 8 | 6 | 5 | 0 |
| 6a | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 10 | 8 | 8 | 7 | 8 | 5 | 0 |
| Chloram-phenicol® | 22 | 21 | 18 | 19 | 20 | 12 | 0 |
| Ampicilin® | 24 | 20 | 19 | 22 | 24 | 14 | 14 |
E. coli = Escherichia coli; P. putida = Pseudomonas putida; B. subtilis = Bacillus subtilis; S. lactis = Streptococcus lactis; A. niger = Aspergillus niger; P. sp. = Penicillium sp; C. albicans = Candida albicans. The sensitivity of microorganisms to the tested compounds is identified in the following manner *: Highly sensitive = Inhibition zone 15-20 mm; Moderately sensitive = Inhibition zone: 10-15 mm; Slightly sensitive = Inhibition zone: 5-10 mm; Not sensitive = Inhibition zone: 0 mm;* Each result represents the average of triplicate readings.
2.2.2. Anti-oxidant activity screening
The newly synthesized compounds were tested for anti-oxidant activity as reflected in the ability to inhibit lipid peroxidation in rat brain and kidney homogenates and rat erythrocyte hemolysis. The pro-oxidant activities of the aforementioned compounds were assessed by their effects on bleomycin-induced DNA damage. Table 2 shows the anti-oxidant assays by erythrocyte hemolysis, which reveals that compounds 7 and 8 manifested potent anti-oxidative activity in the lipid peroxidation assay and considerable inhibitory activity in the hemolysis assay. Table 3 shows the anti-oxidant assays by the ABTS method. Again, compounds 7 and 8 showed interesting anti-oxidant activity. Table 2 and Table 3 also show that compounds 4a and 4b have moderate anti-oxidant properties. All compounds have been tested on bleomycin-dependent DNA damage. The results indicate that some compounds, namely 4a, 4b, 7 and 8, may have some protective activity towards DNA from the damage induced by bleomycin (Table 4).
Table 2.
Anti-oxidant assays by erythrocyte hemolysis (A/B × 100).
| Compounds | Absorbance of samples (A) | Hemolysis (%) |
|---|---|---|
| Complete hemolysis with distilled water (B) | 0.660 | – |
| Ascorbic acid | 0.026 | 3.93 |
| 4a | 0.048 | 7.27 |
| 4b | 0.052 | 7.87 |
| 5a | 0.213 | 32.37 |
| 5b | 0.187 | 28.33 |
| 6a | 0.062 | 9.39 |
| 6b | 0.068 | 10.30 |
| 7 | 0.030 | 4.54 |
| 8 | 0.033 | 5.00 |
Table 3.
Anti-oxidant assays by ABTS method [Abs. (control) − Abs. (test)/Abs. (control) × 100].
| Compounds | Absorbance of samples | Inhibition (%) |
|---|---|---|
| ABTS control | 0.54 | 0 |
| Ascorbic acid | 0.06 | 88.9 |
| 4a | 0.18 | 66.7 |
| 4b | 0.20 | 63.0 |
| 5a | 0.40 | 25.9 |
| 5b | 0.42 | 22.2 |
| 6a | 0.30 | 44.4 |
| 6b | 0.32 | 40.7 |
| 7 | 0.11 | 79.6 |
| 8 | 0.10 | 81.5 |
Table 4.
Assays for bleomycin-dependent DNA damage.
| Compound | Absorbance of samples |
|---|---|
| Ascorbic acid | 0.020 |
| 4a | 0.038 |
| 4b | 0.040 |
| 7 | 0.024 |
| 8 | 0.026 |
3. Experimental
3.1. General
Melting points were determined in open glass capillaries on a Gallenkamp melting point apparatus and are uncorrected. IR spectra (KBr discs) were recorded on a Shimadzu FTIR-8201PC spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded on a Varian Mercury 300 MHz, and a Varian Gemini 200 MHz. spectrometers using TMS as an internal standard and DMSO-d6 as solvent. Chemical shifts were expressed as δ (ppm) units. Mass spectra were recorded at 70 eV on a Shimadzu GCMS-QP1000EX using an inlet type injector. All reactions were followed by TLC (silica gel, aluminum sheets 60 F254, Merck). The Microanalytical Center of Cairo University performed the microanalyses. Microwave reactions were performed with a Millstone Organic Synthesis Unit (MicroSYNTH with touch control terminal) with a continuous focused microwave power delivery system in a pressure glass vessel (10 mL) sealed with a septum under magnetic stirring. The temperature of the reaction mixture was monitored using a calibrated infrared temperature control under the reaction vessel, and control of the pressure was performed with a pressure sensor connected to the septum of the vessel.
3.1.1. 2-aryl-4,4-dibromoisoquinoline-1,3-(2H,4H)dione derivatives 3a,b
A solution of either of 2a (2.37 g, 0.01 mol) or 2b (2.76 g, 0.01 mol) in glacial acetic acid (20 mL) was heated under reflux with bromine (1.1 mL, 3.0 g, 0.02 mole) for 2 h. After cooling, the reaction mixture was poured onto ice-water and the solid that precipitated was filtered off, dried and crystallized from the proper solvent.
4,4-Dibromo-2-phenylisoquinoline-1,3-(2H,4H)dione (3a): crystallized from ethanol in 65% yield as white crystals, m.p. 236-237 °C; 1H-NMR: 7.10-8.30 (m, 9H, Ar-H); 13C-NMR: 70.2 (sp3 C-4), 120.5, 123.2, 124.3, 126.1, 127.4, 130.0, 131.5, 134.6, 135.2, 136.4 (aromatic C), 158.4, 167.6 (2 CO); IRν: 3066 cm−1 (aromatic CH), 1645 (broad, 2C=O), 1605, 1500 (aromatic C=C); MS: M+ m/z 394 (3.2%), 395 (6.7%), 396 (3.0%); Anal. Calcd. for C15H9Br2NO2 (395.04): C (45.60%), H (2.30%), Br (40.45%), N (3.55%); Found: C (45.3%), H (2.4%), Br (45.5%), N (3.7%).
4,4-Dibromo-2-(4-methoxyphenyl)isoquinoline-1,3-(2H,4H)dione (3b): crystallized from dilute dioxane in 58% yield as white crystals, m.p. 255-256 °C; 1H-NMR: 3.60 (s, 3H, OCH3), δ 6.80-7.90 ppm (m, 8H, Ar-H); 13C-NMR: 57.1 (OCH3), 71.0 (sp3 C-4), 115.3, 118.2, 124.2, 126.0, 127.2, 130.3, 131.6, 134.4, 135.1, 152.2 (aromatic C), 155.4, 163.6 (2 CO); IRν: 3060 cm−1 (aromatic CH), 1640 (broad, 2C=O), 1605, 1500 (aromatic C=C); MS: M+ m/z 424 (7.3%), 425 (15.1%), 426 (6.8%); Anal. Calcd. for C16H11Br2NO3 (425.07): C (45.21%), H (2.61%), Br (37.60%), N (3.30%); Found: C (45.1%), H (2.5%), Br (45.0%), N (3.6%).
3.1.2. Cyclocondensation of 3a with o-phenylenediamine and o-aminophenol; formation of 4a,b.
Method A: Compound 3a (3.95 g, 0.01 mole) was heated under reflux with either of o-phenylenediamine (1.08 g, 0.01 mol) or o-aminophenol (1.09 g, 0.01 mol) in ethanolic sodium ethoxide solution [prepared by dissolving metallic sodium (0.27 g, 0.012 mol) in absolute ethanol (25 mL)] for 5 h, under TLC monitoring. The reaction mixture was then cooled, acidified with few drops of conc. hydrochloric acid and the solid that precipitated was filtered at the pump and crystallized from the appropriate solvent.
Method B: The same reactants of method A were heated in microwave at 500 W and 140 °C for 15 min. The reaction mixture was treated in a similar manner to method A to obtain compounds 4a,b.
2'-Phenyl-1,3-dihydro-1'H-spiro[benzimidazole-2,4'-isoquinoline]-1',3'(2'H)-dione (4a): Crystallized from dimethylformamide as grey crystals, m.p. 218-219 °C, in 42% yield (Method A) or 81% yield (Method B); 1H-NMR: 3.80 (s, 2H, 2NH, D2O exchangeable), 6.40-7.70 (m, 13H, Ar-H); 13C-NMR: 83.4 (sp3 spiro C), 113.0, 115.9, 120.2, 122.9, 127.3, 127.8, 128.3, 128.9, 131.2, 133.7, 135.1, 135.9, 136.8 (aromatic C), 155.4, 163.6 (2 CO); IRν: 3180 cm−1 (broad, NH), 3065 (aromatic CH), 1645,1655 (2C=O), 1605, 1500 (aromatic C=C); MS: M+ m/z 341 (12.3%); Anal. Calcd. for C21H15N3O2 (341.36): C (73.89%), H (4.43%), N (12.31%); Found: C (74.1%), H (4.4%), N (12.1%).
2'-Phenyl-1'H,3H-spiro[1,3-benzoxazole-2,4'-isoquinoline]-1',3'(2'H)-dione (4b): Crystallized from dioxane as white crystals, m.p. 237-238 °C, in 48% yield (Method A) and 85% yield (Method B); 1H-NMR: 4.30 (s, 1H, NH, D2O exchangeable), 6.60-7.80 (m, 13H, Ar-H); 13C-NMR: 83.4 (sp3 spiro C), 116.0, 117.9, 118.2, 121.2, 124.9, 125.8, 127.5, 128.1, 128.5, 129.9, 132.1, 134.5, 135.4, 136.3, 138.8, 144.9 (aromatic C), 160.0, 168.9 (2 CO); IRν: 3210 cm−1 (NH), 3065 (aromatic CH), 1655,1670 (2C=O), 1605, 1500 (aromatic C=C); MS: M+ m/z 342 (8.6%); Anal. Calcd. for C21H14N2O3 (342.35): C (73.68%), H (4.12%), N (8.18%); Found: C (73.3%), H (4.2%), N (7.9%).
3.1.3. Cyclocondensation of 3a,b with thiosemicarbazide; formation of 5a,b
Method A: Each of compounds 3a (3.95 g, 0.01 mol) and 3b (4.25 g, 0.01 mol) was heated under reflux with thiosemicarbazide (0.91 g, 0.01 mol) in ethanolic sodium ethoxide solution [prepared by dissolving metallic sodium (0.27 g, 0.012 mol), in absolute ethanol (25 mL)] for 4 h, under TLC monitoring. The reaction mixture was then cooled, acidified with few drops of conc. hydrochloric acid and the solid that precipitated was filtered at the pump and crystallized from the appropriate solvent.
Method B: The same reactants of method A were heated in microwave at 500 W and 140 °C for 15 min. The reaction mixture was treated in a similar manner to method A to give compounds 5a,b.
2-Phenyl-5'-thioxo-1H-spiro[isoquinoline-4,3'-[1,2,4]triazolidine]-1,3(2H)-dione (5a): Crystallized from ethanol as white crystals, m.p. 188-189 °C, in 55% yield (Method A) and 88% yield (Method B); 1H-NMR: 2.10 (s,1H, NH, D2O exchangeable), 2.30 (s,1H, NH, D2O exchangeable), 2.40 (s, 1H, NH, D2O exchangeable), 7.10-8.30 (m, 9H, Ar-H); 13C-NMR: 77.7 (sp3 spiro C), 121.1, 123.4, 127.6, 128.4, 128.8, 129.8, 132.1, 134.0, 135.2, 136.8 (aromatic C), 154.9 (CS), 159.5, 166.9 (2 CO); IRν: 3220, 3185, 3150 cm−1 (NH), 3060 (aromatic CH), 1645,1670 (2C=O), 1600, 1490 (aromatic C=C); MS (70 eV): M+ m/z 324 (11.3%); Anal. Calcd. for C16H12N4O2S (324.36): C (59.25%), H (3.73%), N (17.27%), S (9.89%); Found: C (59.5%), H (3.9%), N (16.9%), S (10.0%).
2-(4-Methoxyphenyl)-5'-thioxo-1H-spiro[isoquinoline-4,3'-[1,2,4]-triazolidine]-1,3(2H)-dione (5b): Crystallized from dilute dioxane as white crystals, m.p. 202-203 °C, in 48% yield (Method A) and 79% yield (Method B); 1H-NMR: 2.10 (s, 1H, NH, D2O exchangeable), 2.30 (s, 1H, NH, D2O exchangeable), 2.40 (s, 1H, NH, D2O exchangeable), 3.70 (s, 3H, OCH3), 7.10-8.30 (m, 9H, Ar-H); IR ν: 3220, 3185, 3150 cm−1 (NH), 3060 (aromatic CH), 1640, 1660 (2C=O), 1600, 1490 (aromatic C=C); MS: M+ m/z 354 (13.8%). Anal. Calcd. for C17H14N4O3S (354.38): C (57.62%), H (3.98%), N (15.81%), S (9.05%); Found: C (57.3%), H (3.9%), N (16.1%), S (9.3%).
3.1.4. Reaction of 3a,b with malononitrile: formation of 6a,b
To a solution of each of compounds 3a (3.95 g, 0.01 mol) and 3b (4.25 g, 0.01 mol) in absolute ethanol (30 mL) containing a catalytic amount of piperidine was added malononitrile (0.66 g, 0.01 mol). The reaction mixture was heated under reflux for 3 h, under TLC monitoring, then cooled and poured onto ice-cold water. The solid product that separated was filtered off, dried and crystallized from ethanol.
(1,3-Dioxo-2-phenyl-2,3-dihydroisoquinolin-4(1H)-ylidene)-malononitrile (6a): Obtained in 60% yield as pale yellow crystals, m.p. 224-225 °C; 1H-NMR: 7.00-8.10 (m, 9H, Ar-H); 13C-NMR: 83.1 (ethylenic C), 112.0 (CN), 122.4, 124.7, 127.1, 127.8, 128.2, 128.6, 129.9, 131.0, 133.1, 133.9 (aromatic C), 147.3 (C3), 158.1, 163.0 (2 CO); IRν: 3060 cm−1 (aromatic CH), 2185 (CN), 1710, 1660 (2C=O), 1600, 1490 (aromatic C=C); MS: M+ m/z 299 (28.2%); Anal. Calcd. for C18H9N3O2 (299.28): C (72.24%), H (3.03%), N (14.04%); Found: C (72.0%), H (3.3%), N (14.3%).
[2-(4-Methoxyphenyl)-1,3-dioxo-2,3-dihydroisoquinolin-4(1H)-ylidene]malononitrile (6b): Obtained as yellow crystals in 67% yield, m.p. 241-242 °C; 1H-NMR: 3.70 (s, 3H, OCH3), 6.70-8.00 (m, 8H, Ar-H); 13C-NMR: 57.3 (OCH3), 83.0 (ethylenic C), 113.1 (CN), 115.2, 124.1, 126.4, 127.2, 127.9, 128.3, 129.5, 131.0, 133.1, 144.2 (aromatic C), 146.9 (C3), 158.4, 163.2 (2 CO; IRν: 3060 cm−1 (aromatic CH), 2195 (CN), 1710, 1670 (2C=O), 1600, 1490 (aromatic C=C); MS: M+ m/z 329 (24.8%); Anal. Calcd. for C19H11N3O3 (329.31): C (69.30%), H (3.37%), N (12.76%); Found: C (69.5%), H (3.3%), N (12.4%).
3.1.5. 5'-Amino-1,3-dioxo-2-phenyl-2,2',3,4'-tetrahydro-1H-spiro-[isoquinoline-4,3'-pyrazole]-4'-carbonitrile (7).
Method A: A solution of 6a (2.99 g, 0.01 mol) in absolute ethanol (30 mL) containing a catalytic amount of piperidine was heated under reflux with hydrazine hydrate (1.1 mL, 0.02 mol) for 4 h under TLC monitoring. The reaction mixture was then cooled, poured onto ice-cold water. The solid that separated was filtered off, dried and crystallized from dilute dimethylformamide.
Method B: The same reactants of method A were heated in microwave at 500 W and 140 °C for 15 min. The reaction mixture was treated in a similar manner to method A to obtain compound 7.
Compound 7 was obtained as white crystals, m.p. 254-255 °C, in 53% yield (Method A) and 84% yield (Method B); 1H-NMR: 2.10 (s, 2H, NH2, D2O exchangeable), 3.50 (s,1H, pyrazole H4), 6.10 (s, 1H, NH, D2O exchangeable) 6.90-8.10 (m, 9H, Ar-H); 13C-NMR: 44.1 (pyrazole C4), 55.3 (sp3 spiro C), 116.1 (CN), 120.2, 123.1, 126.9, 128.2, 128.9, 129.1, 129.7, 132.0, 135.1, 144.4 (aromatic C), 150.9 (pyrazole C3), 155.3, 169.2 (2 CO); IR ν: 3340, 3315, 3260 cm−1 (NH2 and NH), 3080 (aromatic CH), 2180 (CN), 1670,1640 (2C=O), 1600, 1490 (aromatic C=C); MS: M+ m/z 331 (7.8%); Anal. Calcd. for C18H13N5O2 (331.33): C (65.25%), H (3.95%), N (21.14%); Found: C (65.5%), H (4.1%), N (21.5%).
3.1.6. 6'-Amino-1,3-dioxo-2-phenyl-2'-thioxo-2,2',3,5'-tetrahydro-1H,3'H-spiro[isoquinoline-4,4'-pyrimidine]-5'-carbonitrile (8)
Method A: A solution of 6a (2.99 g, 0.01 mol) in absolute ethanol (30 mL) containing a catalytic amount of piperidine was heated under reflux with thiourea (0.76 g, 0.01 mol) for 4 h under TLC monitoring. The reaction mixture was then cooled, poured onto ice-cold water. The solid that separated was filtered off, dried and crystallized from dilute dimethylformamide.
Method B: The same reactants of method A were heated in microwave at 500 W and 140 °C for 15 min. The reaction mixture was treated in a similar manner to method A to obtain compound 8.
Compound 8 was obtained as white crystals, m.p. 243-244°C, in 59% yield (Method A) and 86% yield (Method B); 1H-NMR: 2.10 (s, 2H, NH2, D2O exchangeable), 2.70 (s, 1H, NH, D2O exchangeable), 3.30 (s, 1H, pyrimidine H5), 6.90-7.90 (m, 9H, Ar-H); 13C-NMR: 39.3 (pyrimidine C5), 54.9 (sp3 spiro C), 118.4 (CN), 121.1, 124.6, 126.3, 128.8, 129.4, 129.8, 130.76, 132.7, 135.9, 143.6 (aromatic C), 150.9 (pyrimidine C4), 158.1 (CS), 161.3, 169.2 (2 CO); IR ν: 3300, 3285, 3180 cm−1 (NH2 and NH), 3070 (aromatic CH), 2150 (CN), 1670,1645 (2C=O), 1600, 1490 (aromatic C=C); MS: M+ m/z 375 (5.8%); Anal. Calcd. for C19H13N5O2S (375.40): C (60.79%), H (3.49%), N (18.66%), S (8.54%); Found: C (61.0%), H (3.6%), N (18.5%), S (8.6%).
3.2. Antimicrobial Screening
The newly synthesized heterocyclic compounds were tested for their antimicrobial activity against the following microorganisms: (a) Gram-negative: Escherichia coli and Pseudomonas putide; (b) Gram-positive: Bacillus subtilis and Streptococcus lactis; (c) Fungi: Aspergillus niger and Penicillium sp.; (d) Yeast: Candida albicans. Media: Three types of specific media were used in this study:
Medium 1: For bacteria (Nutrient Medium), consisting of (g/L distilled water): peptone, 5 and meat extract, 3. pH was adjusted to 7.0.
Medium 2: For fungi (Potato Dextrose Medium), consisting of (g/L distilled water): Infusion from potatoes, 4 and D(+)glucose, 20. pH was adjusted to 5.5.
Medium 3: For yeast (Universal Medium), consisting of (g/L distilled water): yeast extract, 3; malt extract, 3; peptone, 5 and glucose, 10. pH was adjusted to 5.5.
For solid media, 2% agar was added. All media were sterilized at 121 °C for 20 min.
3.2.1. Procedure (Filter paper diffusion method) [25]
Proper concentrations of microbial suspensions were prepared from 1 (for bacteria) to 3 (for yeast and fungi)-day-old liquid stock cultures incubated on a rotary shaker (100 rpm). In the case of fungi, five sterile glass beads were added to each culture flask. The mycelia were then subdivided by mechanical stirring at speed No. 1 for 30 min. Turbidity of microorganisms was adjusted with a spectrophotometer at 350 nm to give an optical density of 1.0. Appropriate agar plates were aseptically surface inoculated uniformly by a standard volume (ca. 1 mL) of the microbial broth culture of the tested microorganism, namely E. coli, P. putida, B. subtilis, S. lactis, A. niger, Penicillium sp. and C. albicans.
Whatman No. 3 filter paper discs of 10 mm diameter were sterilized by autoclaving for 15 min at 121 °C. Test compounds were dissolved in 80% ethyl alcohol to give final concentration of 5 μg/mL. The sterile discs were impregnated with the test compounds (5 μg/disc). After the impregnated discs have been air dried, they were placed on the agar surface previously seeded with the organism to be tested. Discs were gently pressed with forceps to insure thorough contact with the media. Three discs were arranged per dish, suitably spaced apart, i.e. the discs should be separated by a distance that is equal to or slightly greater than the sum of the diameters of inhibition produced by each disc alone. Each test compound was conducted in triplicate. Plates were kept in the refrigerator at 5 °C for 1 h to permit good diffusion before transferring them to an incubator at 37 °C for 24 h for bacteria and at 30 °C for 72 h for yeast and fungi.
3.3. Anti-Oxidant Screening
3.3.1 Assay for erythrocyte hemolysis
Blood was obtained from rats by cardiac puncture and collected in heparinized tubes. Erythrocytes were separated from plasma and the buffy coat and washed three times with 10 volumes of 0.15 M NaCl. During the last washing, the erythrocytes were centrifuged at 2,500 rpm for 10 min to obtain a constantly packed cell preparation. Erythrocyte hemolysis was mediated by peroxyl radicals in this assay system [26]. A 10% suspension of erythrocytes in pH 7.4 phosphate-buffered saline (PBS) was added to the same volume of 200 mM 2,2'-azobis(2-amidinopropane)dihydrochloride (AAPH) solution (in PBS) containing samples to be tested at different concentrations. The reaction mixture was shaken gently while being incubated at 37 °C for ~h. The reaction mixture was then removed, diluted with eight volumes of PBS and centrifuged at 2,500 rpm for 10 min. The absorbance A of the supernatant was read at 540 nm. Similarly, the reaction mixture was treated with eight volumes of distilled water to achieve complete hemolysis, and the absorbance B of the supernatant obtained after centrifugation was measured at 540 nm. The percentage hemolysis was calculated by equation (1 − A/B) × 100%. The data were expressed as mean standard deviation. L-Ascorbic was used as a positive control.
3.3.2. Anti-oxidant activity screening assay-ABTS method
For each of the investigated compounds, 2 mL of ABTS [2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)] solution (60 mM) was added to MnO2 suspension, all prepared in phosphate buffer (pH 7, 0.1 M). The mixture was shaken, centrifuged, filtered to remove excess MnO2, and the absorbance (Acontrol) of the resulting green-blue solution (ABTS radical solution) was adjusted at ca. 0.5 at λ 734 nm. Then, 50 μL of (2 mM) solution of the test compound in spectroscopic grade MeOH/ phosphate buffer (1:1) was added. The absorbance (Atest) was measured and the reduction in color intensity was expressed as % inhibition. The % inhibition for each compound is calculated from the following equation [27]:
Ascorbic acid (vitamin C) was used as standard anti-oxidant (positive control). Blank sample was run without ABTS and using MeOH/phosphate buffer (1:1) instead of sample. Negative control sample was run with MeOH/phosphate buffer (1:1) instead of tested compound.
3.3.3. Bleomycin-dependent DNA damage
The assay was done according to Aeschlach et al. [28] with minor modifications. The reaction mixture (0.5 mL) contained DNA (0.5 mg/mL), bleomycin sulfate (0.05 mg/mL), MgCl2 (5 mM), FeCl3 (50 μM) and samples to be tested at different concentrations. L-Ascorbic acid was used as a positive control. The mixture was incubated at 37 °C for 1 h. The reaction was terminated by addition of 0.05 mL EDTA (0.1 M). The color was developed by adding 0.5 mL thiobarbituric acid (TBA) (1%, w/v) and 0.5 mL HCl (25%, v/v) followed by heating at 80 °C for 10 min. After centrifugation, the extent of DNA damage was measured by increase in absorbance at 532 nm.
4. Conclusions
New spiro-compounds have been synthesized using both traditional methods and microwave-assisted conditions. The latter methods proved much more efficient in reducing reaction times as well as increasing the overall yield of the reactions. The newly synthesized compounds were tested for their antimicrobial and antioxidant activities. Some compounds showed moderate or weak antimicrobial activity, whereas compounds 7 and 8 showed promising antioxidant activity.
Acknowledgements
This research is financed by Al-Taif University, Al-Taif, Kingdom of Saudi Arabia, Project # 1-430-456.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Young-Won C., Angela S., Bao-Ning S., Qiuwen M., Hee-Byung C., Soedarsono R., Leonardus K., Agus R., Norman F., Steven S., Douglas K. Potential anticancer activity of naturally occurring and semisynthetic derivatives of aculeatins A and B from Amomum aculeatum. J. Nat. Prod. 2008;3:390–395. doi: 10.1021/np070584j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen-Liang W., Tian-Jiao Z., Hong-Wen T., Zhen-Yu L., Yu-Chun F., Qian-Qun G., Wei-Ming Z. Three novel, structurally unique spirocyclic alkaloids from the halotolerant B-17 fungal strain of Aspergillus variecolor. Chem. Biodivers. 2007;4:2913–2919. doi: 10.1002/cbdv.200790240. [DOI] [PubMed] [Google Scholar]
- 3.van der Sar S., Blunt J., Munro M. Spiro-Mamakone A: a unique relative of the spirobisnaphthalene class of compounds. Org. Lett. 2006;8:2059–2069. doi: 10.1021/ol060434k. [DOI] [PubMed] [Google Scholar]
- 4.Hyeong Beom P., Nam Hyun J., Joon Hee H., Jung Hoon C., Jung Hoon C., Jung-Hyuck C., Kyung Ho Y., Chang-Hyun O. Synthesis and in-vitro activity of novel 1beta-methylcarbapenems having spiro[2,4]heptane moieties. Arch. Pharm. 2007;340:530–537. doi: 10.1002/ardp.200700060. [DOI] [PubMed] [Google Scholar]
- 5.Jolanta O., Krzysztof K. Synthesis and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane, 2-azaspiro[4.5]decane-1,3-dione and 3-cyclohexylpyrrolidine-2,5-dione: Part IV. Acta Pol. Pharm. 2006;63:101–108. [PubMed] [Google Scholar]
- 6.Krzysztof K., Jolanta O., Malgorzata D. Synthesis, physicochemical and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane- and [4.5]decane-1,3-diones: part V. Eur. J. Med. Chem. 2008;43:53–61. doi: 10.1016/j.ejmech.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Jolanta O., Krzysztof K., Ewa T. Impact of aromatic substitution on the anticonvulsant activity of new N-(4-arylpiperazin-1-yl)-alkyl-2-aza-spiro[4,5]decane-1,3-dione derivatives. Pharmacol. Rep. 2006;58:207–214. [PubMed] [Google Scholar]
- 8.Chande M.S., Verma R.S., Barve P.A., Khanwelkar R.R., Vaidya R.B., Ajaikumar K.B. Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: a Michael addition approach. Eur. J. Med. Chem. 2005;40:1143–1148. doi: 10.1016/j.ejmech.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Masakazu F., Kenji H., Jiro K. Spiro compound, process for preparing the same and use thereof as drugs. WO/2001/066546 (App. PCT/JP2001/001793) Int. Pat. 2001
- 10.Frank R., Reich M., Jostock R., Bahrenberg G., Schick H., Henkel B., Sonnenschein H. Substituted spiro compounds and their use for producing pain-relief medicaments. 20080269271 (App. USPTO: 514278) US Pat. 2008
- 11.Hans S., Robert F., Reich M., Ruth O., Gregor B., Fritz T., Henkel B. Substituted spiro compounds and their use for producing pain-relief drugs. WO/2006/122769 (App. No.: PCT/EP2006/004651) Int. Pat. 2006
- 12.Nakao K., Ikeda K., Kurokawa T., Togashi Y., Umeuchi H., Honda T., Okano K., Mochizuki H. effect of trk-820, a selective kappa opioid receptor agonist, on scratching behavior in an animal model of atopic dermatitis. Nihon Shinkei Seishin Yakurigaku Zasshi. 2008;28:75–83. [PubMed] [Google Scholar]
- 13.Pawar M.J., Burungale A.B., Karale B.K. Synthesis and antimicrobial activity of spiro(chromeno[4,3-d][1,2,3]thiadiazole-4,1'-cyclohexanes), spiro(chromeno-[4,3-d][1,2,3]-selenadiazole-4,1'-cyclohexanes) and (spiro-chroman-2,1'-cyclohexan-4-one)-5-spiro-4-acetyl-2-(acetylamino)-∆2-1,3,4-thiadiazoline compounds. ARKIVOC. 2009;(XIII):97–107. [Google Scholar]
- 14.Thadhaney B., Sain D., Pernawat G., Talesara G.L. Synthesis and antimicrobial evaluation of ethoxyphthalimide derived from spiro[indole-3,5'-(1,3)thiazole(4,5-c)isoxazol]-2(1H)-ones via ring closure metathesis. Indian J. Chem. 2010;49B:368–373. [Google Scholar]
- 15.Hejiao H., Huijuan G., Erwei L., Xingzhong L., Yuguang Z., Yongsheng C. Decaspirones F-I, bioactive secondary metabolites from the saprophytic fungus Helicoma viridis. J. Nat. Prod. 2006;69:1672–1675. doi: 10.1021/np0603830. [DOI] [PubMed] [Google Scholar]
- 16.Lindell S., Sanft U., Thönessen M-Th. Heterocyclic spiro compounds as pesticides. WO/2001/011968 (App. PCT/EP2000/ 007851) Int. Pat. 2001
- 17.Kreuder W., Yu N., Salbeck J. Use of spiro compounds as LASER dyes. WO/1999/040655 (App. PCT/EP1999/000441) Int. Pat. 1999
- 18.Lupo D., Salbeck J., Schenk H., Stehlin T., Stern R., Wolf A. Spiro compounds and their use as electroluminescence materials. 5840217 (App. USPTO: 08/417390) US Pat. 2008
- 19.Sarma B.K., Manna D., Minoura M., Mugesh G. Structure, spirocyclization mechanism and Glutathione Peroxidase-like antioxidant activity of stable spirodiazaselenurane and spirodiazatellurane. J. Am. Chem. Soc. 2010;132:5364–5374. doi: 10.1021/ja908080u. [DOI] [PubMed] [Google Scholar]
- 20.Shimakawa S., Yoshida Y., Niki E. Antioxidant action of lipophilic nitroxyl radical, cyclohexane-1-spiro-2'-(4'-oxyimidazolidine-1'-oxyl)-5'-spiro-1''-cyclohexane against peroxidation under hypoxic conditions. Lipids. 2003;38:225–231. doi: 10.1007/s11745-003-1055-3. [DOI] [PubMed] [Google Scholar]
- 21.Sosnowski M., Skulski L. Microwave-accelerated iodination of some aromatic amines, using urea-hydrogen peroxide addition compound (UHP) as the oxidant. Molecules. 2002;7:867–870. doi: 10.3390/71200867. [DOI] [Google Scholar]
- 22.Gregg B., Golden K., Quinn J. Indium(III) trifluoromethanesulfonate as an efficient catalyst for the deprotection of acetals and ketals. J. Org. Chem. 2007;72:5890–5893. doi: 10.1021/jo0707075. [DOI] [PubMed] [Google Scholar]
- 23.Lerebours R., Wolf C. Palladium(II)-catalyzed conjugate addition of arylsiloxanes in water. Org. Lett. 2007;9:2737–2740. doi: 10.1021/ol071067v. [DOI] [PubMed] [Google Scholar]
- 24.Marion N., Gealageas R., Nolan S. [(NHC)AuI]-catalyzed rearrangement of allylic acetates. Org. Lett. 2007;9:2653–2656. doi: 10.1021/ol070843w. [DOI] [PubMed] [Google Scholar]
- 25.Coffen D.L., Korzan D.G. Synthetic quinine analogs. III. Frangomeric and anchimeric processes in the preparation and reactions of α,β-epoxy ketones. J. Org. Chem. 1971;36:390–395. doi: 10.1021/jo00802a006. [DOI] [Google Scholar]
- 26.Morimoto Y., Tanaka K., Iwakiri Y., Tokuhiro S., Fukushima S., Takeuchi Y. Protective effects of some neutral amino acids against hypotonic hemolysis. Biol. Pharm. Bull. 1995;18:1417–1422. doi: 10.1248/bpb.18.1417. [DOI] [PubMed] [Google Scholar]
- 27.Lissi E., Modak B., Torres R., Escobar J., Urzua A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of ABTS and DPPH methods. Free Radic. Res. 1999;30:471–477. doi: 10.1080/10715769900300511. [DOI] [PubMed] [Google Scholar]
- 28.Aeschlach R., Loliger J., Scott B.C., Murciao A., Butler J., Halliwell B., Aruoma O. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994;32:31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]