Abstract
Dichloromethane root extract of Rennellia elliptica Korth. showed strong inhibition of Plasmodium falciparum growth in vitro with an IC50 value of 4.04 µg/mL. A phytochemical study of the dichloromethane root extract has led to the isolation and characterization of a new anthraquinone, 1,2-dimethoxy-6-methyl-9,10-anthraquinone (1), and ten known anthraquinones: 1-hydroxy-2-methoxy-6-methyl-9,10-anthraquinone (2), nordamnacanthal (3), 2-formyl-3-hydroxy-9,10-anthraquinone (4), damnacanthal (5), lucidin-ω-methyl ether (6), 3-hydroxy-2-methyl-9,10-anthraquinone (7), rubiadin (8), 3-hydroxy-2-methoxy-6-methyl-9,10-anthraquinone (9), rubiadin-1-methyl ether (10) and 3-hydroxy-2-hydroxymethyl-9,10-anthraquinone (11). Structural elucidation of all compounds was accomplished by modern spectroscopic methods, notably 1D and 2D NMR, IR, UV and HREIMS. The new anthraquinone 1, 2-formyl-3-hydroxy-9,10-anthraquinone (4) and 3-hydroxy-2-methyl-9,10-anthraquinone (7) possess strong antiplasmodial activity, with IC50 values of 1.10, 0.63 and 0.34 µM, respectively.
Keywords: anthraquinone, Rennellia elliptica, antiplasmodial, Rubiaceae
1. Introduction
Rennellia elliptica Korth. (Rubiaceae) is a tropical shrub locally known in Malaysia as Segemuk or Mengkudu Rimba. This plant is widely distributed, along riverbanks or lowland forest, in Peninsular Malaysia, Sumatra and Borneo [1]. Decoctions of the root are taken by the locals for various purposes, including as an aphrodisiac, for body aches and as a post natal tonic [2]. Not much has been reported on the chemical constituents or biological properties of this plant. A preliminary study by Yusoff et al. on the roots of the plant reported one anthraquinone compound [3]. Our screening of some Malaysian plant extracts for antiplasmodial activity showed that the dichloromethane roots extract of R. elliptica is a potential source of antiplasmodial compounds (IC50 = 4.04 µg/mL). This paper reports the isolation, structure elucidation and antiplasmodial activity of a series of anthraquinone compounds, including a new one, from the root of R. elliptica.
2. Results and Discussion
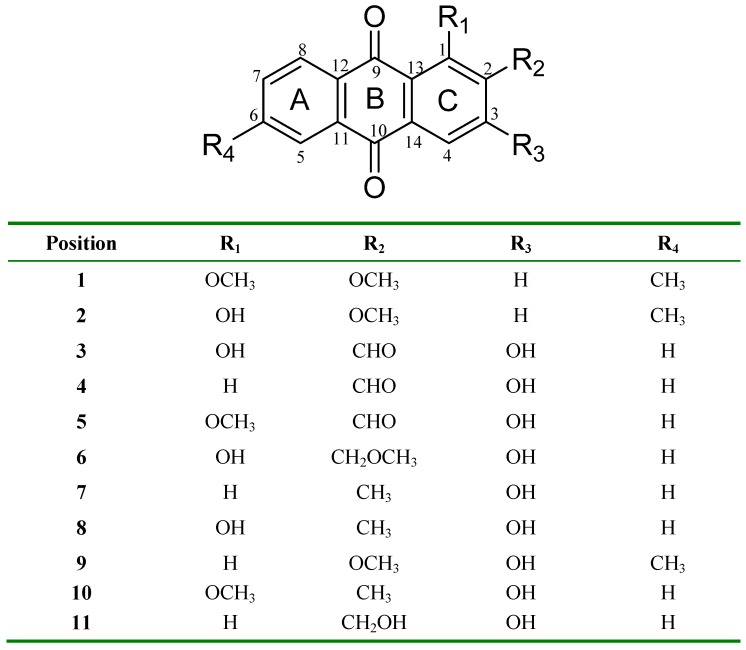
Eleven anthraquinones (Figure 1) were isolated from roots of R. elliptica after extensive chromatographic separation and were characterized by analysis of spectroscopic data and by comparison with literature values [4,5,6,7,8,9,10,11,12,13]. The structures of compounds 4 and 6 were also confirmed using x-ray crystallography [14,15]. The compound 1-hydroxy-2-methoxy-6-methyl-9,10-anthraquinone (2) was described by Mittie and Biswas in 1928 [16]. However, since then there have been no further reports on the natural occurrence of this compound and there is no spectroscopic data available in the literature for comparison. Hence, we elucidated the structure by careful analysis of MS, IR, UV and NMR data, followed by confirmation through x-ray crystallography [17]. In this paper we include the full spectroscopic data for this compound.
Figure 1.
Anthraquinones 1-11, isolated from roots of R. elliptica Korth.
The new compound, 1,2-dimethoxy-6-methyl-9,10-anthraquinone (1) was obtained as bright yellow amorphous solid. The HREIMS displayed a [M + H]+ peak at 283.0968 [calc 283.3067] suggesting a molecular formula of C17H14O4. The absorption maxima in the UV spectrum were observed at 373, 341 and 257 nm, indicative of an anthraquinone moiety [18]. The IR spectrum did not show presence of chelated carbonyl and hydroxyl groups. The sp2 C-H stretch for the aromatic ring was observed at 3,081 cm-1. With the exception of the sharp singlet in the downfield region for the hydrogen-bonded hydroxyl group, the 1H-NMR spectrum resembles that of compound 2, suggesting a similar substitution pattern. Splitting pattern of the five aromatic proton signals suggested substitutions on both rings. Two overlapping doublets centered at δH 8.17 are due to H-8 (d, J = 7.8 Hz) and H-4 (d, J = 8.7 Hz), the peri-hydrogens. A doublet at δH 7.28 (J = 8.7 Hz) is due to H-3, meanwhile H-7 gave another doublet of doublet at δH 7.58 (Jo = 7.8 Hz, Jm = 1.7 Hz). These assignments were confirmed by their respective correlations in the COSY spectrum. H-5 resonated as a singlet at 8.06 ppm. In addition, two sharp singlets at δH 2.53 (3H, s) and 4.02 (6H, s) due to a methyl and two methoxy groups, respectively, were also observed.
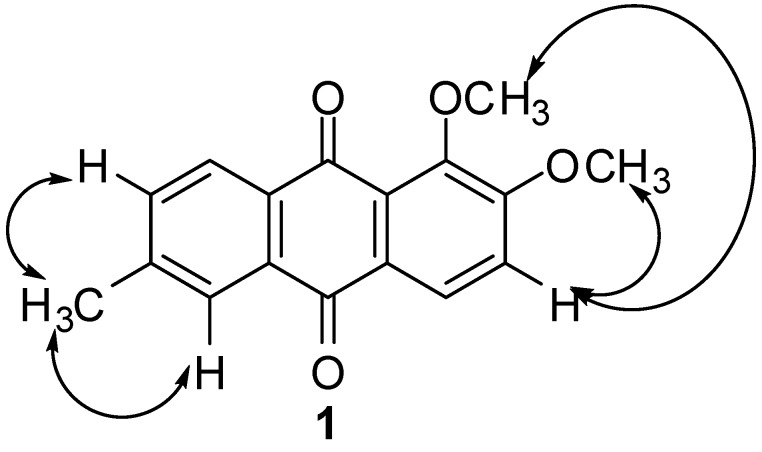
The location of the methoxy groups were established at C-1 and C-2 of ring C based on its NOE correlation with H-3. Thus, the only possible location for the methyl substituent is at C-6. This assignment was confirmed through NOE correlations of the methyl group with H-5 and H-7. These NOE correlations are illustrated in Figure 2. The placement of methyl group at C-6 was further confirmed by HMBC experiment which showed a 3J correlation with H-7. The methine carbons (C-3, C-4, C-5, C-7 and C-8) were assigned through HMQC correlations while the quaternary carbons (C-1, C-2, C-6, C-11, C-12, C-13 and C-14) were assigned based on careful analysis of HMBC spectrum. Both carbonyl carbons in this compound resonated very closely to each other with only 0.01 ppm difference at δC 182.70 and 182.71, which further confirmed the unchelated nature of the carbonyls.
Figure 2.
NOESY Correlations of 1.
Close inspection of all spectroscopic data confirmed that compound 1 is 1,2-dimethoxy-6-methyl-9,10-anthraquinone, the 1-methyl ether of compound 2. Complete 1H and 13C data of compounds 1 and 2 are presented in Table 1.
Table 1.
1H- (300 MHz) and 13C- (75.5 MHz) NMR Data of Compounds 1 and 2 in CDCl3.
| Position | Compound 1 | Compound 2 | ||||
|---|---|---|---|---|---|---|
| δH | δC | HMBC | δH | δC | HMBC | |
| 1 | - | 159.1 | - | - | 154.0 | - |
| 2 | - | 149.6 | - | - | 152.7 | - |
| 3 | 7.28, 1H, d, J = 8.7 Hz | 115.9 | C-2, C14 | 7.19, 1H, d, J = 8.4 Hz | 115.6 | C-2, C-14 |
| 4 | 8.17, 1H, d, J = 8.7 Hz | 125.2 | C-3, C-10,C-13, C-14 | 7.89, 1H, d, J = 8.4 Hz | 121.0 | C-3, C-10, C-13, C-14 |
| 5 | 8.06, 1H, s | 126.9 | C-7, C-10, C-11 | 8.12, 1H, s | 127.8 | 6-CH3, C-10, C-11 |
| 6 | - | 144.6 | - | - | 146.2 | - |
| 7 | 7.58, 1H, dd, Jo = 7.8 Hz, Jm = 1.7 Hz | 134.7 | 6-CH3, C-8, C-12 | 7.61, 1H, d, J = 8.1 Hz | 134.6 | C-6,C-8 |
| 8 | 8.17, 1H, d, J = 7.8 Hz | 127.0 | C-6, C-9 | 8.23, 1H, d, J = 8.1 Hz | 127.1 | C-7, C-9, C-12 |
| 9 | - | 182.7 | - | - | 189.1 | - |
| 10 | - | 182.7 | - | - | 181.8 | - |
| 11 | - | 132.9 | - | - | 134.0 | - |
| 12 | - | 132.9 | - | - | 131.1 | - |
| 13 | - | 127.4 | - | - | 116.1 | - |
| 14 | - | 127.5 | - | - | 125.5 | - |
| 1-OH | - | - | - | 13.20,1H,s | - | |
| 1-OCH3 | 4.02, 3H, s | 61.3 | C-1 | - | - | - |
| 2-OCH3 | 4.02, 3H, s | 56.3 | C-1,C-2,C-3 | 4.04, 3H, s | 56.4 | C-1,C-3 |
| 6-CH3 | 2.53, 3H, s | 21.8 | C-5, C-6, C-7 | 2.56, 3H, s | 22.0 | C-5, C-6, C-7 |
Anthraquinones isolated from the root of R. elliptica exhibited the typical substitution pattern of Rubia type anthraquinones, with most of them substituted only on ring C [19]. The anthraquinones from Morinda and Prismatomeris spp. in the same family also exhibited a similar substitution pattern [4,5,6,7,8,9,12,13,20,21,22]. Genus Rennellia is classified in the same tribe as Morinda and Prismatomeris [23], so the anthraquinones may be produced through similar biosynthetic pathways, which explains the similarity in the substitution patterns [19]. However, anthraquinones 1, 2 and 9 which are substituted on both rings have a methyl substitution at C-6, differing from the anthraquinones of Prismatomeris and Morinda which are typically hydroxyl or methoxy substituted at C-6.
We screened the anthraquinones isolated from the roots of R. elliptica for antiplasmodial activity based on the promising screening results of dichloromethane crude extract (IC50 = 4.04 µg/mL). The in vitro antiplasmodial activity of anthraquinones isolated from R. elliptica against a chloroquine sensitive strain of P. falciparum (3D7) is shown in Table 2. Compound 7 displayed the strongest inhibition activity, with an IC50 value of 0.34 µM, followed by compound 4 with an IC50 value of 0.63 µM. Sittie et al. established that an aldehyde group at C-2 and a phenolic hydroxy group at C-3 on the anthraquinone skeleton enhance the activity of anthraquinones against the growth of P. falciparum [24]. Our results show that methyl group at C-2 together with phenolic hydroxy group at C-3 as in compound 7 also gave significant activity. It should also be noted that both compounds 4 and 7 do not possess hydroxyl substituents at the peri positions. The new anthraquinone 1 also exhibited strong inhibition, with an IC50 value of 1.1 µM. Interestingly, anthraquinone 2, which structurally differs only at C-1 (hydroxyl substituent instead of methoxy substituent) did not show any significant activity. The position of substituents on anthraquinone skeleton clearly influences the antiplasmodial activity, which warrants further investigation. We are currently synthesizing anthraquinone derivatives with various substitution patterns for the purpose of establishing structure-activity relationships.
Table 2.
Antimalarial Activities of Anthraquinones from R. elliptica Korth.
| Sample | IC50 (µM) |
|---|---|
| 1 | 1.10 |
| 2 | na† |
| 3 | 72.46 |
| 4 | 0.63 |
| 5 | 51.28 |
| 6 | 2.10 |
| 7 | 0.34 |
| 8 | na† |
| 9 | nt‡ |
| 10 | na† |
| 11 | nt‡ |
| Chloroquine diphosphate | 6.30* |
Each sample was tested in duplicate; The IC50 values were obtained from average values of percent inhibition within a series of concentration; Notes: na† –no activity; nt‡ – not tested; * unit in nM.
3. Experimental
3.1. Instrumentation
1H- and 13C-NMR spectra were run on a Bruker 300 Ultrashield NMR (Switzerland) at 300 and 75.5 MHz, respectively, using CDCl3 or acetone-d6 (Merck) as solvent. Chemical shifts are reported in ppm and δ scale with the coupling constants given in Hz. Melting points were determined using a Hinotek X-4 (China) melting point apparatus equipped with a microscope and are uncorrected. HREIMS spectra were obtained on a Thermo Finnigan Automass Multi (Shimadzu, Japan). IR spectra were obtained using Perkin-Elmer 1600 series FTIR spectrometer (USA) using KBr pellets. UV spectra were recorded on Shimadzu UV-160A spectrometer (Japan) in absolute ethanol (Scharlau) and alkaline ethanol.
3.2. Chemicals and Reagents
Column chromatography was carried out using silica gel (silica gel 60, 230-400 mesh, Merck, Germany). n-Hexane, dichloromethane and methanol for column chromatography were freshly distilled from industrial grade solvents. The fractions collected were monitored using analytical TLC (Merck, Germany), pre-coated with silica gel 60 F254 of 0.25 mm thickness and visualized under UV light at 245 nm and 356 nm. PTLC was carried out using pre-coated plate with PSC-Fertigplatten Kieselgel 60 F254 (1.0 mm thickness, 20 × 20 cm) purchased from Merck. Plasmodium falciparum (3D7) strain was used for in vitro antiplamodial tests. All chemicals used for determination of antiplasmodial activity were purchased from Sigma-Aldrich, unless otherwise stated.
3.3. Plant Material
The roots of R. elliptica were collected from Kuala Keniam, National Park, Pahang, Malaysia at altitude 165 m above sea level and were identified by Dr Shamsul Khamis, Universiti Putra Malaysia. The voucher specimens (SK1512/08) were deposited at Herbarium of Institute of Bioscience, Universiti Putra Malaysia and Universiti Teknologi MARA.
3.4. Extraction and Isolation of Anthraquinones
The powdered air-dried roots of R. elliptica (1 kg) were successively extracted with hexane, dichloromethane and methanol (5 L each) at room temperature for 72 hours. The filtrate was concentrated in vacuo to give 27 g of a brown coloured residue. The dichloromethane crude extract (9 g) was pre-mixed with silica (1:1) and introduced onto a packed column (5 cm × 60 cm) of acid-washed silica gel (previously shaken with 2% oxalic acid for 2 hours, filtered and dried at 90 ºC). The column was eluted gradiently using compositions of solvents of increasing polarity (n-Hex-CH2Cl2, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9 and 100 CH2Cl2 v/v; CH2Cl2-MeOH, 99:1, 95:5, 9:1 v/v). Fractions of 200 mL each were collected and combined based on TLC pattern into eight major fractions (A to H) for further separation procedures.
Compound 3 (92 mg) was obtained upon recrystallization of fraction A from CH2Cl2. The golden yellow precipitate from fraction C was recrystallized from CH2Cl2 to yield crystals of 4 (42 mg). The remaining liquid from fraction C was dried and subjected to column chromatography (15 mm × 330 mm) eluted isocratically with CH2Cl2 to give 2 (7 mg) and 5 (20 mg). Fraction D was purified using PTLC (100% CH2Cl2) to furnish 6 (23 mg). Compound 7 (20 mg) was purified from fraction E after recrystallization of the dark yellow precipitate from CH2Cl2. The remaining portion of fraction E was rechromatographed using a small column (15 mm × 330 mm) eluted with a gradient of CH2Cl2-MeOH (100, 99:1, 98:2, v/v) to give 50 fractions. Repeated PTLC (CH2Cl2-MeOH, 99:1, v/v) of these fractions afforded compounds 1 (5 mg), 8 (32 mg) and 9 (7 mg). The pale yellow precipitate from fraction F was redissolved in CH2Cl2 and MeOH. Purification of the MeOH soluble portion using repeated PTLC (CH2Cl2-MeOH, 97:3, v/v) yielded 10 (23 mg). Compound 11 (5 mg) was obtained upon recrystallization of light yellow precipitate from CH2Cl2 of fraction F. Spectral data for compounds 3-9 are in good agreement with published data [4,5,6,7,8,9,10,11,12,13].
1,2-Dimethoxy-6-methyl-9,10-anthraquinone (1). Bright yellow amorphous solid (CH2Cl2); m.p. 193-196 ºC. UV λmax (EtOH): 373, 341, 257 nm; IR νmax (KBr): 3,081, 2,945, 1,666, 1,601 cm-1; HREIMS 283.0968 [M + H]+ (calc for C17H14O4 282.3067); For 1H-NMR (CDCl3) and 13C-NMR (CDCl3) data, see Table 1.
1-Hydroxy-2-methoxy-6-methyl-9,10-anthraquinone (2). Red needles (CH2Cl2); m.p. 220-221 ºC. UV λmax (EtOH): 421, 278, 262 nm; IR νmax (KBr): 3,467, 1,653, 1,637 cm-1; HREIMS 269.0867 [M + H]+ (calc for C16H12O4 268.2796); For 1H-NMR (CDCl3) and 13C-NMR (CDCl3) data, see Table 1.
3.5. Determination of Antiplasmodial Activity
The antiplasmodial activity of dichloromethane extract and the isolated compounds were determined by methods as previously described [25]. The samples were dissolved in DMSO and kept at -20 °C until used. The malarial parasite P. falciparum (3D7) clone was propagated in a 24-well culture plate in the presence of 10, 1, 0.1, 0.01 and 0.001 µg/mL range of concentrations of each compound. Chloroquine diphosphate was used as positive control. The growth of the parasite was monitored by making a blood smear fixed with MeOH and stained with Geimsa (Merck). The antiplasmodial activity of each compound was expressed as an IC50 value, defined as the concentration of the compound causing 50% inhibition of parasite growth relative to an untreated control.
4. Conclusions
A phytochemical study on dried roots of R. elliptica afforded a new anthraquinone, 1,2-dimethoxy-6-methyl-9,10-anthraquinone (1), together with ten known anthraquinones 2-11. Several anthraquinones strongly inhibited in vitro growth of a chloroquine sensitive strain of Plasmodium falciparum (3D7), with the strongest inhibition shown by compound 7. The antiplasmodial data suggested that the roots extract of R. elliptica is a potential source of new antiplasmodial agents.
Acknowledgements
The authors would like to thank Ministry of Higher Education and Universiti Teknologi MARA for financial support (FRGS grant 600-IRDC/ST/FRGS/TN.5/3/2) and Shamsul Khamis from Universiti Putra Malaysia for plant identification. Special thanks to Aty Widyawaruyanti from Department of Phytochemistry and Pharmacognosy, Faculty of Pharmacy, University of Airlangga, Indonesia for determination of antiplasmodial activities. Che Puteh Osman thanks Universiti Teknologi MARA for scholarship awarded to her.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Suratman The Indonesian Species of Rennellia Korth. (Rubiaceae) Biodiversitas. 2008;9:259–263. [Google Scholar]
- 2.Mat Salleh K., Latiff A. Tumbuhan Ubatan Malaysia. Universiti Kebangsaan Malaysia & Kem, Sains; Bangi, Malayisa: 2002. p. 560. [Google Scholar]
- 3.Yusoff N.I., Latip J., Liew H.L., Latiff A. Preliminary Phytochemical Study of Plants in the Endau Rompin State Park, Pahang: Anthraquinone from Roots of Rennellia elliptica Korth. (Rubiaceae) In: Ismail S.M.; Isa, M.M.; Ahmad W.Y.; Ramli, M.R.; Latiff A., editors. Endau Rompin Park: Physical and Biological Environmental Management. Forestry Department Peninsular Malaysia; Kuala Lumpur, Malaysia: 2004. [Google Scholar]
- 4.Chang P., Chen C. Isolation and Characterization of Antitumor Anthraquinones from Morinda umbellata. Chin. Pharm. J. (Taipei) 1995;47:347–353. [Google Scholar]
- 5.Chang P., Lee K.-H. Cytotoxic antileukemic anthraquinones from Morinda parvifolia. Phytochemistry. 1984;23:1733–1736. doi: 10.1016/S0031-9422(00)83480-9. [DOI] [Google Scholar]
- 6.Ee G.C.L., Wen Y.P., Sukari M.A., Go R., Lee H.L. A new anthraquinone from Morinda citrifolia roots. Nat. Prod. Res. (Formerly Nat. Prod. Lett.) 2009;23:1322–1329. doi: 10.1080/14786410902753138. [DOI] [PubMed] [Google Scholar]
- 7.Ismail N.H., Ali A.M., Aimi N., Kitajima M., Takayama H., Lajis N.H. Anthraquinones from Morinda elliptica. Phytochemistry. 1997;45:1723–1725. doi: 10.1016/S0031-9422(97)00252-5. [DOI] [Google Scholar]
- 8.Rath G., Ndonzao M., Hostettmann K. Antifungal Anthraquinones from Morinda lucida. Int. J. Pharmacogn. 1995;33:107–114. doi: 10.3109/13880209509055208. [DOI] [Google Scholar]
- 9.Xiang W., Song Q.-S., Zhang H.-J., Guo S.-P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia. 2008;79:501–504. doi: 10.1016/j.fitote.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.El-Hady S., Bukuru J., Kesteleyn B., Van Puyvelde L., Van T.N., De Kimpe N. New Pyranonaphthoquinone and Pyranonaphthohydroquinone from the Roots of Pentas longiflora. J. Nat. Prod. 2002;65:1377–1379. doi: 10.1021/np020110e. [DOI] [PubMed] [Google Scholar]
- 11.Feng Z.-M., Jiang J.-S., Wang Y.-H., Zhang P.-C. Anthraquinones from the Roots of Prismatomeris tetrandra. Chem. Pharm. Bull. 2005;53:1330–1332. doi: 10.1248/cpb.53.1330. [DOI] [PubMed] [Google Scholar]
- 12.Kanokmedhakul K., Kanokmedhakul S., Phatchana R. Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J. Ethnopharmacol. 2005;100:284–288. doi: 10.1016/j.jep.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Tuntiwachwuttikul P., Butsuri Y., Sukkoet P., Prawat U., Taylor W.C. Anthraquinones from the Roots of Prismatomeris malayana. Nat. Prod. Res. 2008;22:962–968. doi: 10.1080/14786410701650261. [DOI] [PubMed] [Google Scholar]
- 14.Ismail N.H., Osman C.P., Ahmad R., Awang K., Ng S.W. 1,3-Dihydroxy-2-methoxymethyl-9,10-anthraquinone from Rennellia elliptica Korth. Acta Crystallogr. 2009;E65:o1433–o1434. doi: 10.1107/S1600536809017607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail N.H., Osman C.P., Awang K., Abdul Malek S.N., Ng S.W. 2-Formyl-3-hydroxy-9,10-anthroquinone. Acta Crystallogr. 2008;E64:o2164. doi: 10.1107/S1600536808032224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitter P.C., Biswas H. Inductive Method for the Study of Natural Products. I. Naturally occuring Anthraquinone Derivatives. J. Ind. Chem. Soc. 1928;5:769–778. [Google Scholar]
- 17.Ismail N.H., Osman C.P., Ahmad R., Awang K., Ng S.W. 1-Hydroxy-2-methoxy-6-methyl-9,10-anthraquinone from Rennellia elliptica Korth. Acta Crystallogr. 2009;E65:o1435. doi: 10.1107/S1600536809017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derksen G.C.H., Van Beek T.A., Atta ur R. Studies in Natural Products Chemistry. Volume 26, Part 7. Elsevier; Amsterdam, The Netherlands: 2002. Rubia tinctorum L; pp. 629–684. [Google Scholar]
- 19.Han Y.-S., der Heiden R.V., Verpoorte R. Biosynthesis of Anthraquinones in Cell Cultures of the Rubiaceae. Plant Cell Tissue Organ Cult. 2001;67:201–220. doi: 10.1023/A:1012758922713. [DOI] [Google Scholar]
- 20.Do Q.V., Pharm G.D., Mai N.T., Phan T.P.P., Nguyen H.N., Yea Y.Y., Ahn B.Z. Cytoxicity of Some Anthraquinones from the Stem of Morinda citrifolia Growing in Vietnam. Tap Chi Hoa Hoc. 1999;37:94–97. [Google Scholar]
- 21.Krohn K., Gehle D., Dey S.K., Nahar N., Mosihuzzaman M., Sultana N., Sohrab M.H., Stephens P.J., Pan J.-J., Sasse F. Prismatomerin, a New Iridoid from Prismatomeris tetrandra. Structure Elucidation, Determination of Absolute Configuration, and Cytotoxicity. J. Nat. Prod. 2007;70:1339–1343. doi: 10.1021/np070202+. [DOI] [PubMed] [Google Scholar]
- 22.Wongkrajang K., Jankam A., Kumpun S., Suksamrarn A., Yingyongnarongkul B.-e. Antifungal Anthraquinones from the Roots of Prismatomeris filamentosa; In Proceedings of 31st Congress on Science and Technology of Thailand (STT 31); Nakhon Ratchasima, Thailand. October 18-20; 2005. [Google Scholar]
- 23.Bremer B., Manen J.-F. Phylogeny and Classification of the Subfamily Rubioideae (Rubiaceae) Plant Syst. Evol. 2000;225:43–72. doi: 10.1007/BF00985458. [DOI] [Google Scholar]
- 24.Sittie A.A., Lemmich E., Olsen C.E., Hviid L., Kharazmi A., Nkrumah F.K., Christensen S.B. Structure-Activity Studies: In vitro Antileishmanial and Antimalarial Activities of Anthraquinones from Morinda lucida. Planta Med. 1999;65:259–261. doi: 10.1055/s-2006-960473. [DOI] [PubMed] [Google Scholar]
- 25.Widyawaruyanti A., Subehan, Kalauni S.K., Awale S., Nindatu M., Zaini N.C., Syafruddin D., Asih P.B.S., Tezuka Y., Kadota S. New prenylated flavones from Artocarpus champeden and their antimalarial activity in vitro. J. Nat. Med. 2007;61:410–413. doi: 10.1007/s11418-007-0153-8. [DOI] [Google Scholar]