Abstract
Hexamethylene-1,6-bis-(N,N-dimethyl-N-dodecylammonium bromide) (1), pentamethylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2), tetramethylene-1,4-bis(N,N-dimethyl-N-dodecylammonium bromide) (3), trimethylene-1,3-bis(N,N-dimethyl-N-dodecylammonium bromide) (4) and ethylene-1,2-bis(N,N-dimethyl-N-dodecylammonium bromide) (5) have been obtained and characterized by FTIR and NMR spectroscopy. DFT calculations have also been carried out. The optimized bond lengths, bond angles and torsion angles calculated by Hartree-Fock/3-21G(d,p) approach have been presented. MIC values for A. niger, P. chrysogenum, C. albicans have been determined and the relationship between MIC and spacer length has been discussed.
Keywords: polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides); FTIR and NMR spectra; DFT calculations; antifungal activity
1. Introduction
Quaternary ammonium salts were introduced as antimicrobial agents by Domagk over seventy years ago [1]. The first generation of quaternary ammonium compounds (QAC) were standard benzalkonium chlorides, i.e., alkylbenzyldimethyl-ammonium chloride, with specific alkyl distributions, i.e., C12, 40%; C14, 50% and C16, 10% [2]. The second generation of QAC was obtained by substitution of aromatic rings in alkylbenzyldimethylammonium chlorides by chlorine or alkyl groups to get the products like alkyldimethylethylbenzylammonium chloride with alkyl distributions of C12, 50%; C14, 30%; C16, 17% and C18, 3%. The dual quaternary ammonium salts are the third generation of QAC. These products are a mixture of equal proportion of alkyldimethylbenzylammonium chloride with alkyl distribution C12, 68%; C14, 32% and alkyldimethylethylbenzylammonium chloride with alkyl distribution C12, 50%; C14, 30%; C16, 17% and C18, 3%. The twin chain quaternary ammonium salts, like didecyldimethylammonium chloride are the fourth generation of QAC. The concept of the synergistic combination in the dual QACs has been applied to twin chain quaternary ammonium salts. The mixture of dialkyldimethylammonium chloride (dioctyl, 25%; didecyl, 25%, octyldecyl, 50%) with benzalkonium chloride (C12, 40%; C14, 50%; C16, 10%) is the newest blend of quaternary ammonium salts which represents the fifth generation of QACs [2]. Because of the increasing resistance of microorganisms to commonly used disinfectants, the synthesis of new types of microbiocides is a very important topic. One of the new groups with good antimicrobial activity are cyclic quaternary ammonium salts [3]. Some cyclic quaternary ammonium salts have been obtained previously by intramolecular cyclisation of amine derivatives [4,5,6,7,8,9]. Another way, i.e., reaction of alkyl halides with cyclic amines can lead to chiral cyclic quaternary ammonium salts [10].
In recent years the number of applications of quaternary ammonium salts has increased considerably. They are used as biocides [11,12,13,14,15], and phase-transfer catalysts, especially in enantioselective reactions [16,17,18,19,20,21]. Pyrrolidinium salts are analogues of oxotremorine and are used as muscarinic agonist [5]. Some of quaternary ammonium salts exist as ionic liquids, which can be used as ”green solvents” [22,23,24,25,26] and electrolytes for liquid batteries [27,28].
In this work we report the synthesis, FTIR and NMR spectroscopy, DFT calculations and antimicrobial properties of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-5. These compounds belong to a new class of quaternary ammonium salts, the so called gemini surfactants, where two tertiary amines are connected at the nitrogen atoms by a spacer. For symmetric gemini surfactants the notation [m-s-m] is applicable, where m is the number of carbon atoms in alkyl chain and s is a number of methylene groups in a spacer. Antimicrobial activity of [12-s-12] against Staphylococcus aureus depends on the length of spacer, and for s = 4 MIC is four times lower than for s = 2 [29]. Gemini alkylammonium surfactants are very promising microbiocides.
2. Results and Discusion
2.1. Synthesis
Polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-4 have been obtained by reaction of N,N-dimethyl-N-dodecylamine with 1,6-dibromohexane, 1,5-dibromopentane, 1,4-dibromo-butane and 1,3-dibromopropane, respectively. Ethylene-1,2-bis(N,N-dimethyl-N-dodecyloammonium bromide) (5) has been obtained by reaction of N,N,N’,N’-tetramethylethylenediamine with 1-bromo-decane. The reaction times were significantly shorter in comparison to procedures described in the literature, which is due to the more polar solvent that we used. The reaction yields were very high and varied from 83 to 92%. The analysis of melting points of gemini surfactants 1-5 shows the relationship between m.p. and the number of carbon atoms in the spacer (Figure 1).
Figure 1.
The relationship between melting points of 1-5 and number of carbon atoms in the spacer.
A higher number of carbon atoms in the spacer corresponded to a higher melting point. This clearly suggests stronger hydrophobic interactions between the hydrocarbon chains with elongation of spacer and better packaging in the crystal. Solubility in water of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) also depends on the spacer length. Ethylene-1,2-bis(N,N-dimethyl-N-dodecyloammonium bromide) (5) is soluble in water below 0.1% wt./wt. while hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) is readily soluble in water.
2.2. DFT calculations
The structure and numbering for 1-5 are given in Figure 2. The structures optimized at the HartreeFock/3-21G(d,p) level of theory are shown in Figure 3. The geometry parameters, energy and dipole moments computed using the Hartree-Fock/3-21G(d,p) method are given in Table 1. The calculated energy depends on the number of carbon atoms in the spacer. The relative stabilizing energy ΔE (a.u.) is a difference between calculated energy for 5, i.e., compound with ethylene group as a spacer and for 1, i.e., a compound with a hexamethylene spacer (Table 1). According to this assumption the most stable is hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1, Figure 4).
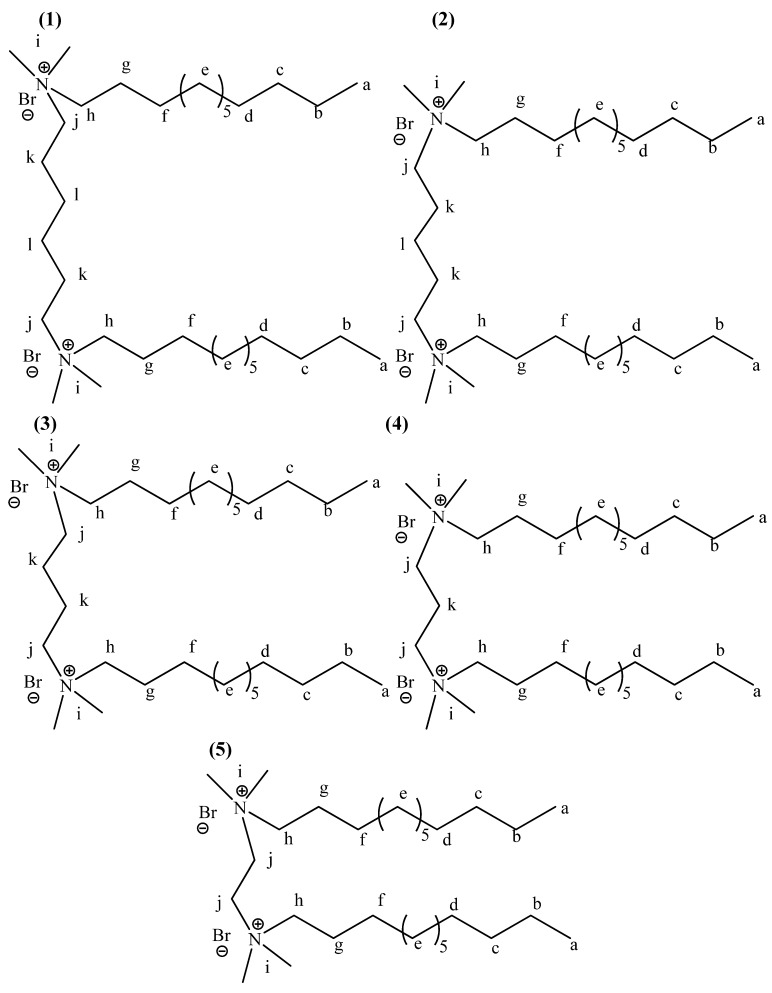
Figure 2.
Structures and numbering of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecyl-ammonium bromide) (1), pentamethylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2), tetramethylene-1,4-bis(N,N-dimethyl-N-dodecylammonium bromide) (3), trimethylene-1,3-bis-N,N-dimethyl-N-dodecylammonium bromide) (4) and ethylene-1,2-bis(N,N-dimethyl-N-dodecylammonium bromide) (5).
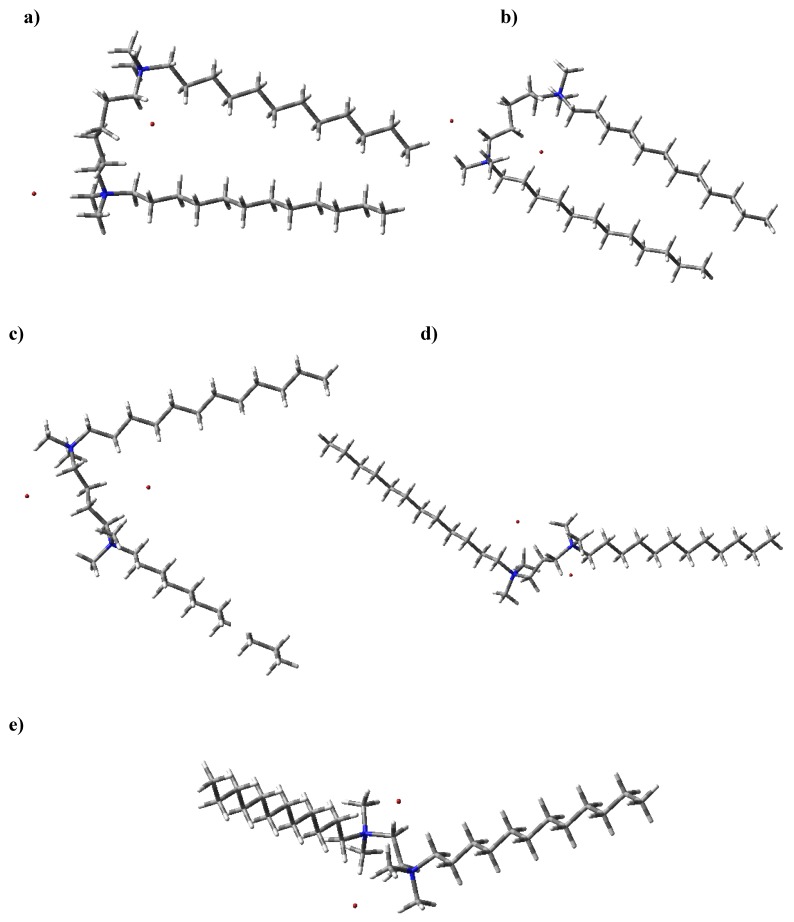
Figure 3.
Structures of 1-5 optimized by the Hartree-Fock/3-21G(d,p) method; (a) hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1), (b) penta-methylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2), (c) tetramethylene-1,4-bis(N,N-dimethyl-N-dodecylammonium bromide) (3), (d) trimethylene-1,3-bis(N,N-dimethyl-N-dodecylammonium bromide) (4) and (e) ethylene-1,2-bis(N,N-dimethyl-N-dodecylammonium bromide) (5).
Table 1.
Selected parameters of investigated compounds 1-5 calculated by the Hartree- Fock/3-21G(d,p) method.
| Parameters | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Energy (a.u) | -6551.697749 | -6412.885113 | -6474.064183 | -6396.071637 | -6357.506938 |
| Dipole moment (Debye) | 20.4558 | 17.1183 | 4.7632 | 7.1234 | 6.5095 |
| Bond length (Å) | |||||
| N+…Br- | 3.895 4.235 |
3.932 3.902 |
3.862 3.954 |
4.116 4.116 |
3.638 3.768 |
| C(i)-H…Br- | 3.681 | 3.727 | 3.674 | 3.297 | 3.383 |
| C(j)-H…Br- | 3.859 | 3.551 | 3.477 | 3.213 | 3.111 |
| C(l)-H…Br- | 3.851 | 1.550 | |||
| C(h)-H…Br- | 3.661 | 4.003 | |||
| N-C(h) | 1.537 | 1.536 | 1.534 | 1.550 | |
| N-C(i) | 1.506 | 1.501 | 1.503 | 1.536 | 1.529 |
| N-C(j) | 1.520 | 1,540 | 1.539 | 1.541 | 1.550 |
| C(j)-C(k) | 1.540 | 1.538 | 1.528 | ||
| C(h)-C(g) | 1.533 | 1.535 | 1.533 | 1.541 | 1.544 |
| Bond angle (o) | |||||
| N-C(h)-C(g) | 115.7 | 116.4 | 116.2 | 114.5 | 114.5 |
| N-C(j)-C(k) | 115.0 | 116.7 | 115.7 | 110.2 | - |
| C(j)-N-C(h) | 110.0 | 113.0 | 111.6 | 108.2 | 110.8 |
| C(i)-N-C(h) | 107.1 | 108.3 | 108.1 | 110.6 | 113.4 |
| Dihedral angle (o) | |||||
| N-C(h)-C(g)-C(f) | 167.4 | 177.8 | 179.9 | -179.4 | -174.0 |
| N-C(j)-C(k)-C(l) | 160.0 | -158.9 | -179.9 | - | - |
| C(i)-N-C(h)-C(g) | 168.7 | 56.6 | 175.4 | -69.7 | -49.1 |
| C(i)-N-C(j)-C(k) | 43.7 | -176.4 | 148.5 | 161.4 | - |
| C(a)-C(b)-C(c)-C(d) | 179.7 | -179.6 | 180.0 | 180.0 | 180.0 |
| C(j)-N-C(h)-C(g) | 47 | 172.1 | 58.6 | 172.9 | -166.2 |
Figure 4.
The relationship between number of carbon atoms in the spacer and relative energy ΔE (a.u.) of 1-5.
The highest stabilizing energy of 1 is a consequence of intramolecular hydrophobic interactions between two alkyl chains (Figure 3a). In compound 5 with an ethylene spacer, the distance between two nitrogen atoms is 3.8 Ǻ, whereas the thickness of the dodecyl group is over 5.1 Ǻ. These distances were calculated using the data from Table 1. Because of the N···N distance and geometry conditions, the structure of 5 with an ethylene group in the spacer is much more open and two dodecane chains cannot interact each other. Thus the stabilizing energy of 5 is low. In general as the spacer becomes longer the alkyl chains get closer together and compounds are more stabilized. In case of pentamethylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2) the stability of the structure is slightly decreased by conformational strain (Figure 4). Additionally, bromide anions in 1-4 are engaged in three non-linear weak intramolecular interactions with carbon atoms (Figure 3, Table 1).
In 5 the bromide atom forms only two non-linear weak intramolecular C-H···Br interactions. Bromide anions interact also via Coulombic attractions with positively charged nitrogen atoms. The N+···Br- distances are given in Table 1. The conclusions concerning stability of 1-5 in gas phase are in accordance with the stability of 1-5 in the solid state expressed by an increasing melting points for compounds with longer spacer.
2.3. FTIR spectra study
The FTIR spectra of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-5 were measured in KBr pellets at 20 °C.
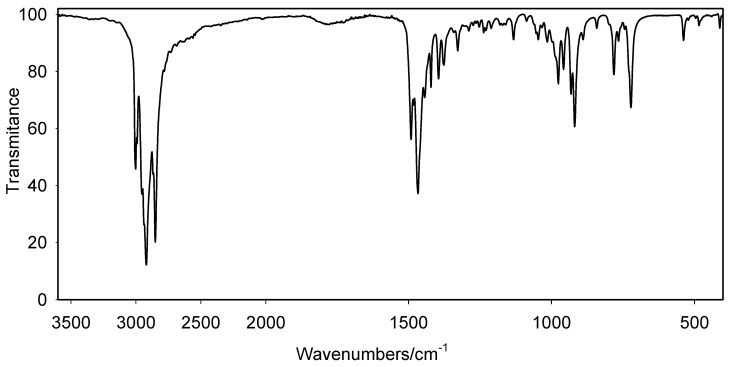
The spectra show typical bands of stretching asymmetric (νas) and symmetric (νa) vibrations as well as bands of deformation vibrations (δ), at 2850-2980 cm-1 and 1360-1490 cm-1, respectively (Figure 5). No significant changes were observed between the FTIR spectra of 1 with the longest spacer and 5 with the shortest spacer.
Figure 5.
FTIR spectrum of ethylene-1,2-bis(N,N-dimethyl-N-dodecylammonium bromide) (5).
2.4. 1H-NMR and 13C-NMR spectra
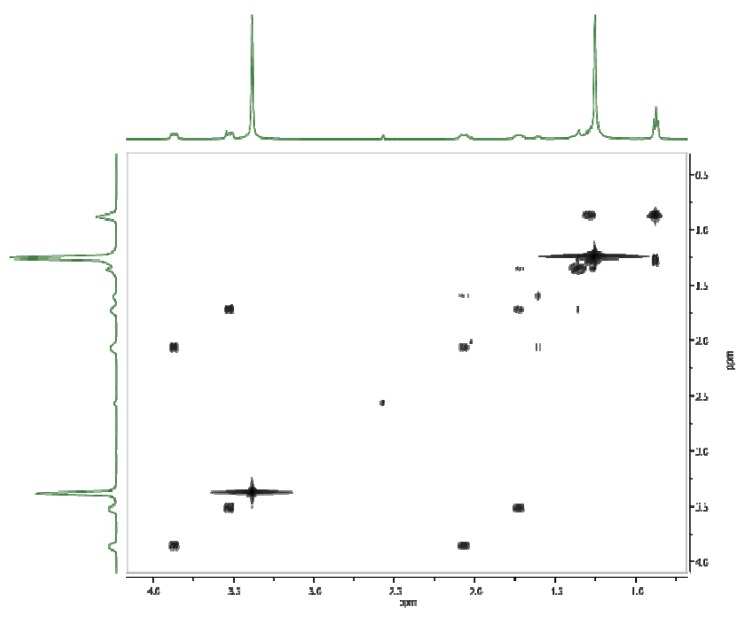
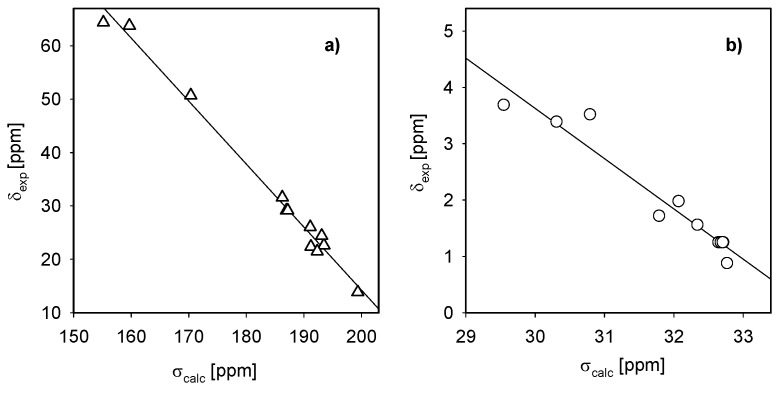
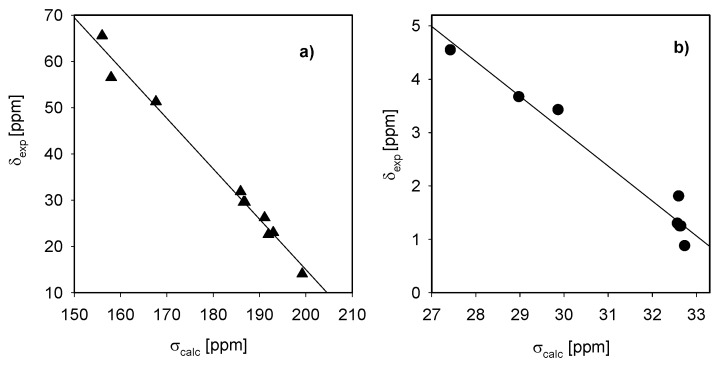
The proton chemical shift assignments (Table 2) of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-5 are based on 2D COSY experiments, in which the proton-proton connectivity is observed through the off-diagonal peaks in the counter plot (Figure 6). The relations between the experimental 1H and 13C chemical shifts (δexp) and the GIAO (Gauge-Independent Atomic Orbitals) isotropic magnetic shielding tensors (σcalc) are shown in Figure 5 and Figure 6. Both correlations are linear, described by the relationship: δexp = a + b·σcalc The a and b parameters are given in Table 2. It has been reported in the literature [20] that the correlation between the experimental chemical shifts and calculated isotropic screening constants are usually better for carbon-13 atoms than for protons. The protons are located on the periphery of the molecule, thus are more sensitive to solute-solvent interactions than carbon atoms which are more hidden. For this reason the correlation between the experimental and calculated data for protons is worse than that for carbon atoms. The differences between calculated and experimental shifts for protons and carbons are due to different phases. The calculated shifts describe single molecules in the gas phase, while experimental shifts include all interactions in the condensed phase.
Table 2.
Chemical shifts (δ, ppm) in CD3Cl calculating GIAO nuclear magnetic shielding tensors (σcalc) for hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) and ethylene-1,2-bis(N,N-dimethyl-N-dodecylammonium bromide) (5). The predicted GIAO chemical shifts were computed from the linear equation δexp. = a + b·σcalc with a and b determined from the fit the experimental data ( r is the correlation coefficient).
| δexp. | δcalc | σcalc | δexp. | δcalc | σcalc | ||
| Hexamethylene-1,6-bis(N,N-dimetyl-N-dodecyldodecylammonium bromide) (1) | |||||||
| Carbon-13 | Proton | ||||||
| C (a) | 13.84 | 14.98 | 199.37 | H (a) | 0.88 | 1.16 | 32.767 |
| C (b) | 22.63 | 21.93 | 193.49 | H (b) | 1.25 | 1.27 | 32.644 |
| C (c) | 31.60 | 30.49 | 186.25 | H(c) | 1.25 | 1.21 | 32.711 |
| C (d) | 29.17 | 29.62 | 186.98 | H (d) | 1.25 | 1.22 | 32.699 |
| C (e) | 29.17 | 29.35 | 191.11 | H (e) | 1.25 | 1.24 | 32.676 |
| C (f) | 26.06 | 24.74 | 191.23 | H (f) | 1.25 | 1.22 | 32.704 |
| C(g) C(h) C(i) C(j) C(k) C(l) a |
22.38 64.38 50.73 63.81 21.51 24.42 |
24.60 67.19 49.27 61.86 23.33 22.38 250.5599 |
155.19 170.35 159.70 192.31 193.11 |
H(g) H(h) H(i) H(j) H(k) H(l) |
1.72 3.52 3.39 3.69 1.98 1.56 |
2.03 2.92 3.35 4.03 1.78 1.54 30.40303 |
31.787 30.790 30.308 29.54 732.06 832.339 |
| b | -1.1816 | -0.892456 | |||||
| r2 | 0.98999 | 0.93883 | |||||
| Ethylene-1,2-bis-(N,N-dimethyl-N-dodecylammonium bromide) (5) | |||||||
| Carbon-13 | Proton | ||||||
| C (a) | 14.3 | 15.84 | 199.21 | H (a) | 0.88 | 1.24 | 32.732 |
| C (b) | 22.99 | 22.64 | 192.97 | H (b) | 1.30 | 1.35 | 32.568 |
| C (c) | 31.84 | 30.32 | 185.92 | H(c) | 1.25 | 1.29 | 32.648 |
| C (d) | 29.53 | 29.62 | 186.56 | H (d) | 1.25 | 1.30 | 32.637 |
| C (e) | 29.53 | 29.31 | 186.85 | H (e) | 1.25 | 1.31 | 32.620 |
| C (f) | 26.20 | 24.67 | 191.10 | H (f) | 1.25 | 1.31 | 32.623 |
| C(g) C(h) C(i) C(j) a |
22.60 65.54 51.29 56.51 |
23.77 62.89 50.24 60.78 232.9141 |
191.93 156.04 167.64 157.57 |
H(g) H(h) H(i) H(j) |
1.81 3.67 3.43 4.55 |
1.33 3.69 3.11 4.71 22.6482 |
32.598 28.976 29.866 27.423 |
| b | -1.0897 | -0.6541 | |||||
| r2 | 0.98592 | 0.96714 | |||||
Figure 6.
COSY NMR spectrum for hexamethylene-1,6-bis(N,N-dimethyl-N-dodecyldodecylammonium bromide) (1).
2.5. Antimicrobial activity
Minimal inhibitory concentration (MIC) values for all polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium) bromides against A. niger ATCC 16404 and A. niger LOCK 0439 are higher than those against P. chrysogenum LOCK 0531 and C. albicans ATCC 10231 (Table 3).
Table 3.
The minimal inhibitory concentration (MIC) (μM/mL) of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) (1-5) for conidia and vegetative cells.
| Compound | Strains | |||
|---|---|---|---|---|
|
A.niger ATCC 16404 |
P.chrysogenum LOCK 0531 |
A.niger LOCK 0439 |
C.albicans ATCC 10231 |
|
| (1) | 0.12 | 0.06 | 0.12 | 0.015 |
| (2) | 0.12 | 0.06 | 0.24 | 0.015 |
| (3) | 0.15 | 0.095 | 0.375 | 0.037 |
| (4) | 0.3 | 0.15 | 0.4 | 0.075 |
| (5) | 0.3 | 0.15 | 0.4 | 0.075 |
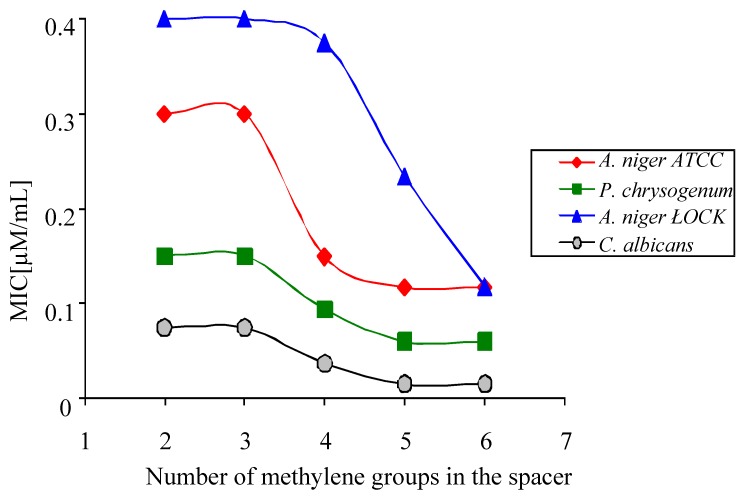
Aspergillus niger is a fungus, which are very resistant to chemical disinfectants, what make them very difficult to removed from the surface and air. The highest antifungal activity was shown by hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) and pentamethylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2). In general for all fungal strains a longer spacer corresponds to a lower MIC (Figure 9). This phenomenon may result from the fact that polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) with long spacers are more flexible and connect more easily with the conidial surface. The MIC value of dodecyltrimethylammonium chloride, which is a monomer analog of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides), against A. niger is 20 times higher than the MIC value for hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) [2]. This means that the same biocidal effect can be obtained using at least ten times less dimeric surfactant instead of a monomeric surfactant. These are fundamental results for the application of gemini surfactants like polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides), as chemical microbiocides.
Figure 9.
The relationship between number of methylene groups in the spacer of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-5 and MIC of conidia.
The minimal concentrations which inhibit 48-hour mycelium development for hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) and pentamethylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2) are significantly higher than those for conidia. (Table 4). The higher MIC values for mycelium in comparison to conidia result from the character of mycelium growth. Fragments of hypha growing by apical elongation are sensitive to disinfectants, while portions of the hyphae away from the tips are more resistant [30]. This resistance is due to a thicker cellular wall that hinders penetration of microbiocide molecules. Another reason of this resistance is wall porosity, which decreases with age [31].
Table 4.
(MIC, μM/mL) of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) and pentamethylene-1,5-bis(N,N-dimethyl-N-dodecylammonium bromide) (2) for mycelium and pseudomycelium.
| Compound | Strains | |||
|---|---|---|---|---|
|
A.niger ATCC 16404 |
P.chrysogenum LOCK 0531 |
A.niger LOCK 0439 |
C.albicans ATCC 10231 |
|
| 1 | 0.31 | 0.31 | 0.31 | 0.31 |
| 2 | 0.31 | 0.76 | 0.76 | 0.45 |
3. Experimental
3.1. General
The NMR spectra were measured with a Varian Gemini 300VT spectrometer, operating at 300.07 and 75.4614 MHz for 1H and 13C, respectively. Typical conditions for the proton spectra were: pulse width 32°, acquisition time 5s, FT size 32 K and digital resolution 0.3 Hz per point, and for the carbon spectra pulse width 60°, FT size 60 K and digital resolution 0.6 Hz per point, the number of scans varied from 1,200 to 10,000 per spectrum. The 13C and 1H chemical shifts were measured in CDCl3 relative to an internal standard of TMS. All proton and carbon-13 resonances were assigned by 1H (COSY) and 13C (HETCOR). All 2D NMR spectra were recorded at 298 K on a Bruker Avance DRX 600 spectrometer operating at the frequencies 600.315 MHz (1H) and 150.963 MHz (13C), and equipped with a 5 mm triple-resonance inverse probe head [1H/31P/BB] with a self-shielded z gradient coil (90° 1H pulse width 9.0 μs and 13C pulse width 13.3 μs). Infrared spectra were recorded in the KBr pellets using a FT-IR Bruker IFS 66 spectrometer. The ESI (electron spray ionization) mass spectra were recorded on a Waters/Micromass (Manchester, UK) ZQ mass spectrometer equipped with a Harvard Apparatus syringe pump. The sample solutions were prepared in methanol at a concentration of approximately 10-5 M. The standard ESI – MS mass spectra were recorded at a 30 V cone voltage.
3.2. Computational details
The calculations were performed using the Gaussian 03 program package [32] at the Hartree-Fock [33,34] levels of theory with the 3-21 basis set [33]. The NMR isotopic shielding constants were calculated using the standard GIAO (Gauge-Independent Atomic Orbital) approach [32,33,34,35] of GAUSSIAN 03 program package [36].
3.3. Antimicrobial study
Fungal strains. The antifungal activity of the gemini surfactants was evaluated against Aspergillus brasiliensis (previously A. niger) ATCC 16404, Aspergillus niger LOCK 0439, Penicillium chrysogenum LOCK 0531 and Candida albicans ATCC 10231.
Antifungal activity. Minimal inhibitory concentrations (MIC values) against conidia of moulds were measured by a tube standard 2-fold dilution method. Malt Extract Broth (Merck) was used for the antifungal tests. Moulds were preincubated on MEA slant for 5 days at 28 ºC, yeast – for 1 day at 37 ºC. Conidia suspensions of each strain were prepared by adding sterile water containing 0.1% (w/w) Tween 80 to the slant. The yeast cell suspension of Candida albicans was prepared by similar procedure but without Tween 80. The conidia and yeast cells were adjusted to 1-2 × 106 cells/ml by counting them in Thoma chamber. One mL of conidia suspension was mixed with 1 mL of media containing the tested compounds and incubated at 28 ºC for 72 h – moulds, 37 ºC for 48 h - yeast. The MICs were defined as the lowest concentration of the compounds at which there was no visible growth. Minimal inhibitory concentrations (MIC values) against mycelium of moulds were measured by the suspension method. Conidia and yeast cells suspensions were prepared in the same way as for the conidia test. Next, 1.5 mL of inoculum was mixed with 12 mL of MEB medium and incubated for 48 h - moulds at 28 ºC, yeast- 37 ºC. After this time, 1.5 mL of tested gemini surfactants in different concentrations were added and all cultures were incubated another 48 h. The control sample was culture of mycelium without gemini surfactants. In this case, the MICs were defined as the lowest concentration of the compounds at which the development of mycelium in comparison to the control sample was inhibited. Each experiment was repeated three times and the mean values were used to compute the MICs.
3.4. Synthesis
Hexamethylene-1,6-bis(N,N-dimethyl-N-dodecyldodecylammonium bromide) (1). N,N-Dimethyl-N-dodecylamine (18.2 g, 0,09 M) was mixed with 1,6-dibromohexane (10,7 g, 0.04 M) in acetonitrile (80 mL). The reaction mixture was heated under reflux for 5 h. The solvent was evaporated under reduced pressure and the residue was dried over P4O10 and then recrystallized from acetonitrile, yield 84%, m.p. 231-232 °C; Elemental analysis: found (calc) %C 60.54 (60.88); %H 11.75 (11.12); %N 4.03 (4.18); ES+MS m/z 255 (C34H74N2/2); 1H-NMR: δ 0.88 [6H, H(a)], 1,25 [4H, H(b)], 1.25 [4H, H(c)], 1.25 [4H, H(c)], 1.25 [4H, H(d)], 1.25 [20H, H(e)], 1.25 [4H, H(f)], 1.72 [4H, H(g)], 3.52 [4H, H(h)], 3.39 [12H, H(i)], 3.69 [4H, H(j)], 1.98 [4H, H(k)], 1.56 [4H, H(l)]; 13C-NMR: δ 13.84 C(a), 22.63 C(b), 31.60 C(c), 29.17 C(d), 29.17 C(e), 26.06 C(f), 22.38 C(g), 64.38 C(h), 50.73 C(i), 63.81 C(j), 21.51 C(k), 24.42 C(l)
Pentamethylene-1,5-bis(N,N-dimethyl-N-dodecyldodecylammonium bromide) (2). N,N-Dimethyl-dodecylamine (9.3 g, 0.04 M) was mixing with 1,5-dibromopentane (5.0 g, 0.02 M) in acetonitrile (80 mL). The reaction mixture was heated under reflux for 4 h. The solvent was evaporated under reduced pressure and the residue was dried over P4O10 and then recrystallized from acetonitrile, yields 86%, m.p. 226-227 °C; Elemental analysis found (calc) %C 59.99 (60.35); %H 11.58 (11.05); %N 4.02 (4.27); ES+MS m/z 248 (C33H72N2/2); 1H-NMR: δ 0.88 [6H, H(a)], 1,36 [4H, H(b)], 1.25 [4H, H(c)], 1.25 [4H, H(c)], 1.25 [4H, H(d)], 1.25 [20H, H(e)], 1.25 [4H, H(f)], 1.73 [4H, H(g)], 3.52 [4H, H(h)], 3.38 [12H, H(i)], 3.86 [4H, H(j)], 2.08 [4H, H(k)], 1.62 [2H, H(l)]; 13C-NMR: δ 13.98 C(a), 22.79 C(b), 31.75 C(c), 29.28 C(d), 29.28 C(e), 26.20 C(f), 22.46 C(g), 64.45 C(h), 50.69C(i), 63.76 C(j), 21.73 C(k), 22 53 C(l).
Tetraethylene-1,4-bis(N,N-dimethyl-N-dodecyldodecylammonium bromide) (3). N,N-Dimethyl-dodecylamine (9.7 g, 0.05 M) was mixing with 1,4-dibromobutane (4.9 g 0.03 M) in acetonitrile (80 mL). The reaction mixture was heated under reflux for 5 h. The solvent was evaporated under reduced pressure and the residue was dried over P4O10 and then recrystallized from mixture acetonitrile:aceton, yields 92%, m.p. 225-226 °C; Elemental analysis found (calc) %C 58.74 (59.80); %H 11.47 (10.98); %N 4.18 (4.36); ES+MS m/z 241(C32H70N2/2); 1H-NMR: δ 0.88 [6H, H(a)], 1,26 [4H, H(b)], 1.26 [4H, H(c)], 1.26 [4H, H(c)], 1.26 [4H, H(d)], 1.26 [20H, H(e)], 1.26 [4H, H(f)], 1.76 [4H, H(g)], 3.46 [4H, H(h)], 3.33 [12H, H(i)], 3.85 [4H, H(j)], 2.08 [4H, H(k)]; 13C-NMR: δ 13.81 C(a), 22.60 C(b), 31.59 C(c), 29.19 C(d), 29.19 C(e), 26.08 C(f), 22.37 C(g), 64.75 C(h), 50.67 C(i), 63.17C(j), 19.57 C(k).
Threemethylene-1,3-bis(N,N-dimethyl-N-dodecyldodecylammonium bromide) (4). N,N-dimethyl-dodecylamine (9.7 g, 0.05 M) was mixing with 1,3-dibromopentane (4.6 g, 0.03 M) in acetonitrile (80 mL). The reaction mixture was heated under reflux for 6 h. The solvent was evaporated under reduced pressure and the residue was dried over P4O10 and then recrystallized from mixture acetonitrile:aceton, yields 86%, m.p. 199-200 °C; Elemental analysis found (calc) %C 58.81 (59.22); %H 10.56 (10.90); %N 4.17 (4.46); ES+MS m/z 234 (C31H68N2/2); 1H-NMR: δ 0.88 [6H, H(a)], 1,35 [4H, H(b)], 1.26 [4H, H(c)], 1.26 [4H, H(d)], 1.26 [20H, H(e)], 1.26 [4H, H(f)], 1.79 [4H, H(g)], 3.55 [4H, H(h)], 3.42 [12H, H(i)], 3.82 [4H, H(j)], 2.77 [4H, H(k)]; 13C-NMR: δ 13.87 C(a), 22.73 C(b), 31.66 C(c), 29.27 C(d), 29.27 C(e), 26.13 C(f), 22.42 C(g), 66.00 C(h), 50.99, C(i), 60.64 C(j), 18.55 C(k).
Ethylene-1,2--bis (N,N-dimethyl-N-dodecyldodecylammonium bromide) (5). N,N,N’,N’-Tetra-methylethylamine (2 g, 0.02 M) was mixing with bromododecane (4.6 g, 0.03 M) in acetonitrile (30 mL). The reaction mixture was heated under reflux for 4 h. The solvent was evaporated under reduced pressure and the residue was dried over P4O10 and then recrystallized from mixture ethanol:ethyl acetate, yields 83%, m.p. 186-187 °C; Elemental analysis found (calc) %C 58.49 (58.62); %H 10.83 (10.82); %N 4.57 (4.56); ES+MS m/z 227 (C30H66N2/2); 1H-NMR): δ 0.88 [6H, H(a)], 1,30 [4H, H(b)], 1.25 [4H, H(c)], 1.25 [4H, H(d)], 1.25 [20H, H(e)], 1.25 [4H, H(f)], 1.81 [4H, H(g)], 3.67 [4H, H(h)], 3.43 [12H, H(i)], 4.55 [4H, H(j)]; 13C-NMR (CDCl3): δ 14.03 C(a), 22.99 C(b), 31.84 C(c), 29.53 C(d), 29.53 C(e), 26.20 C(f), 22.60 C(g), 65.54 C(h), 51.29, C(i), 56.51 C(j).
4. Conclusions
Polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-4 have been obtained with good yield after short reaction times by reaction of N,N-dimethyl-N-dodecylamine with 1,6-dibromohexane, 1,5-dibromopentane, 1,4-dibromobutane and 1,3-dibromopropane. Ethylene-1,2-bis-(N,N-dimethyl-N-dodecyloammonium bromide) (5) has been prepared in good yield by reaction of N,N,N’,N’-tetramethylethylenediamine with 1-bromodecane. The structures of the title compounds have been analyzed by FTIR and NMR spectroscopy, as well as by DFT calculations. Properties of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides) 1-5, including energy, melting points, solubility and antifungal activity, depend strongly on the length of the spacer. The longer the spacer the better the water solubility, stability and antifungal activity. Hexamethylene-1,6-bis(N,N-dimethyl-N-dodecyloammonium bromide) shows the best antifungal activity and can be used as an efficient microbiocide.
Figure 7.
Experimental chemical shifts (δexp, CD3Cl) in hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide) (1) vs. the isotropic magnetic shielding (σcalc) from the GIAO/HF/3-21G(d,p) calculations for molecules δexp = a + b·σcalc : (a) 13C and (b) 1H.
Figure 8.
Experimental chemical shifts (δexp, CD3Cl) in ethylene-1,2-bis(N,N-dimethyl-N-dodecylammonium bromide) (5) vs. the isotropic magnetic shielding (σcalc) from the GIAO/HF/3-21G(d,p) calculations for molecules δexp = a + b σcalc : (a) 13C and (b) 1H.
Acknowledgments
Authors thank to Zofia Żakowska from Institute of Fermentation Technology and Microbiology, Technical University of Lodz for helpful comments and advice which helped to develop ideas put forward. This work was supported by the funds from Adam Mickiewicz University, Faculty of Chemistry. The microbiological study were supported by KBN grant “Fungicidal properties of new gemini surfactants” N N401 027736.
Footnotes
Sample Availability: Samples of the compounds (1-5) are available from the authors.
References and Notes
- 1.Domagk G. A new class of disinfectants. Dtsch. Med. Wochenscher. 1935;61:829–832. doi: 10.1055/s-0028-1129654. [DOI] [Google Scholar]
- 2.Block S.S. Disinfection, Sterilization, and Preservation. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2001. [Google Scholar]
- 3.Dega-Szafran Z., Dulewicz E., Brycki B. Synthesis and characterization of 1-carbalkoxymethyl-4-hydroxy-1-methylpiperidinium chlorides. ARKIVOC. 2007;vi:90–102. [Google Scholar]
- 4.Erickson J.G., Keps J.S. Reactions of Long-chain Amines. IV. Preparation of N-Alkylpyrrolidines, N,N-Dialkylpyrrolidinium Chlorides and N,N-Dialkylpiperidinium Chlorides. J. Am. Chem. Soc. 1955;77:485–456. doi: 10.1021/ja01607a082. [DOI] [Google Scholar]
- 5.Ringdahl B., Roch M., Jenden D.J. Tertiary 3- and 4-Haloalkylamine Analogues of Oxotremorine as Prodrugs of Potent Muscarinic Agonists. J. Med. Chem. 1988;31:160–164. doi: 10.1021/jm00396a024. [DOI] [PubMed] [Google Scholar]
- 6.Chukhadzhyan É.O., Chukhadzhyan Él.O., Shakhatuni K.G., Gevorkyan N.T. Synthesis of Chlorine-Substituted Naphto- and Benzisoindolinium Salts by Base-catalyzed Intramolecular Cyclization of Ammonium Salts. Chem. Heterocycl. Compound. 1998;34:912–915. doi: 10.1007/BF02311325. [DOI] [Google Scholar]
- 7.Schmitt K.D. Surfactant-Mediated Phase Transfer as an Alternative to Propanesultone Alkylation. Formation of a New Class of Zwitterionic Surfactants. J. Org. Chem. 1995;60:5474–5479. doi: 10.1021/jo00122a028. [DOI] [Google Scholar]
- 8.Venkataramu S.D., Macdonell G.D., Purdum W.R., Dilbeck G.A., Berlin K.D. Polyphosphoric Acid Catalyzed Cyclization of Aralkenyl-Substituted Quaternary Ammonium Salts. J. Org. Chem. 1977;42:2195–2200. doi: 10.1021/jo00433a001. [DOI] [Google Scholar]
- 9.Walker M.A. An Unusual Tandem Cyclization-Stevens Rearrangement Mediated by Ph3P/DEAD or Bu3P/ADDP. Tetrahedron Lett. 1996;37:8133–8136. doi: 10.1016/0040-4039(96)01891-6. [DOI] [Google Scholar]
- 10.Glaeske K.W., West F.G. Chirality Transfer from Carbon to Nitrogen to Carbon via Cyclic Ammonium Ylides. Org. Lett. 1999;1:31–33. doi: 10.1021/ol9905349. [DOI] [Google Scholar]
- 11.Manivannan G. Disinfection and Decontamination: Principles, Applications and Related Issues. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2008. [Google Scholar]
- 12.Menger F.M., Keiper J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000;39:1906–1920. doi: 10.1002/1521-3773(20000602)39:11<1906::AID-ANIE1906>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Paulus W. Directory of Microbiocides for the Protection of Materials. Springer; Dordrecht, The Netherlands: 2005. [Google Scholar]
- 14.Zana R., Xia J. Gemini Surfactants. Synthesis, Interfacial and Solution PhaseBehavior, and Applications. Marcel Dekker; New York, NY, USA: 2004. [Google Scholar]
- 15.Fraise A.P., Lambert P.A., Maillard J.-Y. Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization. 4th ed. Blackwell Publishing; Malden, MA, USA: 2004. [Google Scholar]
- 16.Park E.J., Kim M.H., Kim D.Y. Enantioselective Alkylation of â-Keto Esters by Phase-Transfer Catalysis Using Chiral Quaternary Ammonium Salts. J. Org. Chem. 2004;69:6897–6899. doi: 10.1021/jo0401772. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.Y., Huh S.Ch., Kim S.M. Enantioselective Michael reaction of malonates and chalcones by phase-transfer catalysis using chiral quaternary ammonium salt. Tetrahedron Lett. 2001;42:6299–6301. doi: 10.1016/S0040-4039(01)01237-0. [DOI] [Google Scholar]
- 18.Kim D.Y., Park E.J. Catalytic Enantioselective Fluorination of α-Keto Esters by Phase-Transfer Catalysis Using Chiral Quaternary Ammonium Salts. Org. Lett. 2002;4:545–547. doi: 10.1021/ol010281v. [DOI] [PubMed] [Google Scholar]
- 19.Niess B., Jorgensen K.A. The asymmetric vinylogous Mannich reaction of dicyanoalkylidenes with α-amido sulfones under phase-transfer conditions. Chem. Commun. 2007:1620–1622. doi: 10.1039/B702172K. [DOI] [PubMed] [Google Scholar]
- 20.Orglmeister E., Mallat T., Baiker A. Quaternary ammonium derivatives of cinchonidine as new chiral modifiers for platinum. J. Catal. 2005;233:333–341. doi: 10.1016/j.jcat.2005.04.034. [DOI] [Google Scholar]
- 21.Breistein P., Karlsson S., Hendenström E. Chiral pyrrolidinium salts as organocatalysts in the stereoselective 1,4-conjugate addition of N-methylpyrrole to cyclopent-1-ene carbaldehyde. Tetahedron: Asymetry. 2006;17:107–111. doi: 10.1016/j.tetasy.2005.11.020. [DOI] [Google Scholar]
- 22.Fuijmori T., Fujiii K., Kanzaki R., Chiba K., Yamamoto H., Umebayashi Y., Ishiguro S. Conformational structure of room temperature ionic liquid N-butyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl) imide — Raman spectroscopic study and DFT calculations. J. Mol. Liq. 2007;131-132:216–224. doi: 10.1016/j.molliq.2006.08.054. [DOI] [Google Scholar]
- 23.Yoshinawa-Fujita M., Johansson K., Newman P., MacFarlane D.R., Forsyth M. Novel Lewis-base ionic liquids replacing typical anions. Tetrahedron Lett. 2006;47:2755–2758. doi: 10.1016/j.tetlet.2006.02.073. [DOI] [Google Scholar]
- 24.Henderson W.A., Passerini S. Phase Behavior of Ionic Liquid-LiX Mixtures: Pyrrolidinium Cations and TFSI- Anions. Chem. Mater. 2004;16:2881–2758. doi: 10.1021/cm049942j. [DOI] [PubMed] [Google Scholar]
- 25.Sun J., MacFarlane D.R., Forsyth M. A new family of ionic liquids based on the 1-alkyl-2-methyl pyrrolinium cation. Electrochim. Acta. 2003;48:1707–1711. doi: 10.1016/S0013-4686(03)00141-5. [DOI] [Google Scholar]
- 26.MacFarlane D.R., Forsyth S.A., Golding J., Deacon G.B. Ionic liquids based on imidazolium, ammonium and pyrrolidinium salts of the dicyanamide anion. Green Chem. 2002;4:444–448. doi: 10.1039/b205641k. [DOI] [Google Scholar]
- 27.Sakaaebe H., Matsumoto H. N-Methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl) imide (PP13–TFSI) – novel electrolyte base for Li battery. Electrochem. Commun. 2003;5:594–548. doi: 10.1016/S1388-2481(03)00137-1. [DOI] [Google Scholar]
- 28.Tigelaar D.M., Meador M.A.B., Bennett W.R. Composite Electrolytes for Lithium Batteries: Ionic Liquids in APTES Cross-Linked Polymers. Macromolecules. 2007;40:4159–4164. doi: 10.1021/ma062804q. [DOI] [Google Scholar]
- 29.Laatiris A., El Achouri M., Infante M.R., Bensouda Y. Antibacterial activity, structure and CMC relationship of alkanediyl α,ω- bis(dimethylammonium bromide) surfactants. Microbiol. Res. 2008;163:645–650. doi: 10.1016/j.micres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Manavathu E.K., Cutright J., Chandrasekar P.H. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J. Clin. Microbiol. 1999;37:858–861. doi: 10.1128/jcm.37.3.858-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraise A.P., Lambert P.A., Maillard J.-Y. Russell, Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization. Blackwell Publishing; Malden, MA, USA: 2004. [Google Scholar]
- 32.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Montgomery J.A., Vreven T., Jr., Kudin K.N., Burant J.C., Millam J.M., Iyengar S.S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G.A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M.X.Li., Knox J.E., Hratchian H.P., Cross J.B., Adamo C., Jaramillo J., Gomperts R., Stratmann R.E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Ochterski J.W., Ayala P.Y., Morokuma K., Voth G.A., Salvador P., Dannenberg J.J., Zakrzewski V.G., Dapprich S., Daniels A.D., Strain M.C., Farkas O., Malick D.K., Rabuck A.D., Raghavachari K., Foresman J.B., Ortiz J.V., Cui Q., Baboul A.G., Clifford S., Cioslowski J., Stefanov B.B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R.L., Fox D.J., Keith T., Al-Laham M.A., Peng C.Y., Nanayakkara A., Challacombe M., Gill P.M.W., Johnson B., Chen W., Wong M.W., Gonzalez C., Pople J.A. Gaussian 03, Revision C.01. Gaussian, Inc.; Wallingford, CT, USA: 2004. [Google Scholar]
- 33.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 34.Becke A.D. Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J. Chem. Phys. 1997;107:8554–8560. doi: 10.1063/1.475007. [DOI] [Google Scholar]
- 35.Hehre W.J., Random L., Schleyer P.v.R., Pople J.A. Ab Initio Molecular Orbital Theory. Wiley; New York, NY, USA: 1986. [Google Scholar]
- 36.Wolinski K., Hilton J.F., Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990;112:8251–8260. doi: 10.1021/ja00179a005. [DOI] [Google Scholar]