Abstract
Thirteen novel triterpenoid saponins, designed as amide derivatives of the natural cytotoxic saponin β-hederin, were synthesized by a stepwise glycosylation strategy. The in vitro cytotoxic activity of these compounds was evaluated against five different tumor cell lines. Most of the evaluated compounds showed effective inhibitory activity against at least one tumor cell line at micromolar concentrations. The preliminary structure-activity relationships (SAR) indicate that mide derivatization at C-28 resulted in highly cytotoxic derivatives on specific tumor cell lines, and also resulted in an increase in the antitumor selectivity of β-hederin.
Keywords: triterpenoid saponins, β-hederin, tumor cytotoxicity, synthesis
1. Introduction
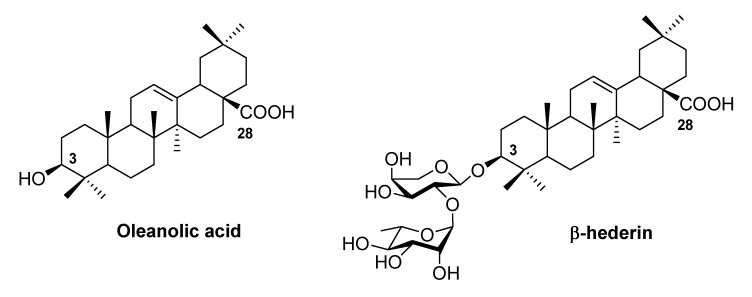
Oleanolic acid (OA, see Figure 1) is a pentacyclic triterpenoid widely distributed in Nature and possessing various important bioactivities, such as antitumor, anti-HIV, hepatoprotection, and anti-inflammatory properties[1,2,3]. OA also serves as an aglycon of many natural saponins, which display significantly higher levels of activity than OA itself. For instance, β-hederin (oleanolic acid 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside, see Figure 1) [4], an OA glycoside that bears a unique disaccharide at C3-OH, shows excellent inhibitory activity against many tumor cells [5,6]. In our previous studies, the chemical synthesis of β-hederin and its glycosylated derivatives was completed, and their antitumor activity was evaluated [7,8].
Figure 1.
Structures of OA and β-hederin.
Recently, some researchers have explored the structure-activity relationships of other pentacyclic triterpenic compounds, such as ursolic acid [9,10] and betulonic acid [11]. Those studies led to similar conclusions that the derivatives with a substitution of amino groups at C-28 often showed stronger cytotoxic antitumor activity. Therefore, we were inspired to investigate the possibility of similar properties in the medicinal chemistry of triterpenoid saponins. The present work describes the initial study of the synthesis of 13 novel amide derivatives of β-hederin and the evaluation of their cytotoxic activity against tumors.
2. Results and Discussion
2.1. Synthesis
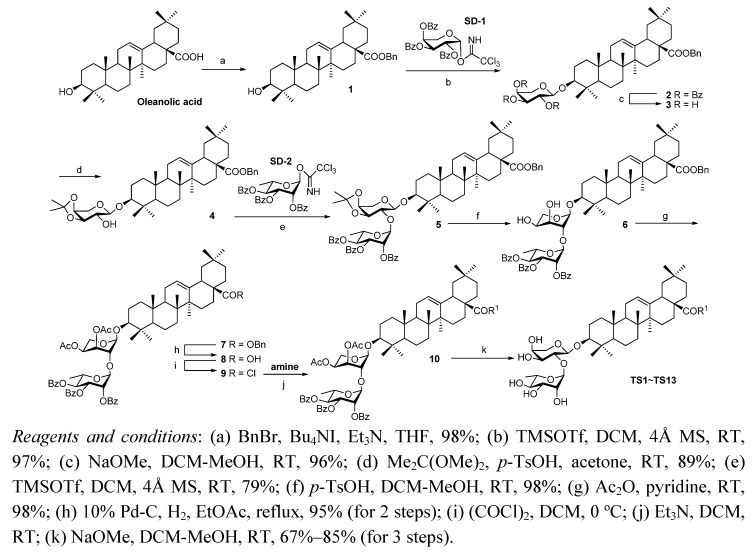
As far as β-hederin is concerned, the hydroxyl group at C3 is connected with a disaccharide moiety to form the so-called OA 3-glycosides. Therefore, only if the sugar substructure was well protected, can the free carboxyl group at C-28 be modified by various acylations. Based on our previous work [7,8,12], we developed a facile method to prepare the amide derivatives of β-hederin using OA, L-arabinose, L-rhamnose and some commercially available amines as the starting materials.
The compounds were synthesized as depicted in Scheme 1. To protect the carboxyl group of OA, benzyl ester 1 was prepared by the combination of OA, BnBr and K2CO3 in THF-H2O with Bu4NI as a phase transfer catalyst. Compound 1 was glycosylated with perbenzoylated arabinosyl trichloroacetimidate SD-1 [13] under the promotion of TMSOTf to produce an excellent yield of compound 2. Debenzoylation of compound 2 in the MeOH solution of NaOMe yielded saponin 3 without affecting the benzyl ester at C-28. Selective protection of 3-OH and 4-OH of the arabinose residue was successfully accomplished using 2,2-dimethoxypropane to furnish compound 4, which was combined with perbenzoylated rhamnosyl trichloroacetimidate SD-2 [14] under the same glycosylation conditions to generate compound 5 with a 79% yield. When the isopropylidene moiety was removed to prepare the intermediate 6, the 1C4 conformation of the arabinose residue resulted produced via conformational inversion. Based on the 1H-NMR spectrum of compound 6, the J1'-2' value of the arabinose residue was changed to 1.3, much smaller than the normal value of the α-arabinosyl conformation (usually not less than 5.0). Moreover, the chemical shift (δ) of anomic C atom (109.3) is larger than that of the natural product (104.8). From the point of view of the chemical structure, the aglycone and perbenzoylated rhamnose connected with 1-OH and 2-OH of the arabinose residue, respectively, increased steric hindrance to such an extent that a chair inversion of arabinose resulted. Next, the two free hydroxyl groups were shielded by acetylation in pyridine solution to furnish compound 7, whose benzyl group was easily removed through catalytic hydrogenation to produce compound 8 without affecting the double bond between C-12 and C-13 of OA. Compound 8 was treated with oxalyl chloride to yield compound 9, which then reacted with the appropriate amino compounds in the presence of Et3N to generate corresponding compounds 10. Last, rapid and complete removal of all acyl groups in basic solution yielded the final products TS1-TS13. Fortunately, the arabinose ring returned to the normal 4C1 conformation after the final deprotection, which was confirmed by the 1H-NMR data.
Scheme 1.
Synthesis of amide derivatives of β-hederin.
2.2. Biological evaluation
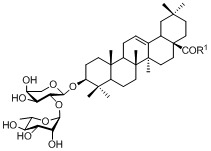
The preliminary in vitro biological evaluation was performed by a standard MTT assay to investigate the cytotoxicity of these saponins against five tumor cell lines: HeLa (cervical), MCF-7 (breast), HL-60 (leukemia), HT1080 (fibrosarcoma), and HepG2 (liver). The synthetic β-hederin was used as a reference compound. As shown in Table 1, although most of the evaluated compounds were found to be less active than β-hederin, they showed effective inhibitory activity against at least one tumor cell line at micromolar concentrations. It is worth emphasizing that compounds with a substitution of piperazine or methylpiperazine (TS8 and TS9) displayed more potent activity than β-hederin. However, none of the evaluated compounds showed any toxicity towards the HT1080 or HepG2 cell lines within the investigated concentration range. The SAR indicated that the conversion of β-hederin into an amide yielded a moderately active derivative. The modified saponins with the aliphatic amine substructure seemed to display stronger activity than those bearing aromatic amines. Generally, the amide derivatization at C-28 resulted in highly cytotoxic derivatives on specific tumor cell lines, which means the antitumor selectivity of β-hederin was increased.
Table 1.
Structures and tumor cytotoxicity of amide derivatives of β-hederin.
 | ||||||
|---|---|---|---|---|---|---|
| Compd. | R1 | IC50 (μM) | ||||
| HeLa | MCF-7 | HL-60 | HT1080 | HepG2 | ||
| TS1 |  |
> 100 | > 100 | 14.46 | > 50 | > 50 |
| TS2 | 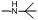 |
> 100 | > 100 | 12.78 | > 200 | > 100 |
| TS3 | 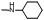 |
> 100 | > 100 | 11.53 | > 100 | > 100 |
| TS4 | 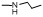 |
24.14 | > 100 | > 100 | > 200 | > 100 |
| TS5 | 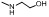 |
> 100 | 19.72 | > 100 | > 100 | > 100 |
| TS6 | 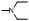 |
> 100 | 13.80 | > 100 | > 50 | > 50 |
| TS7 | 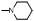 |
> 100 | > 100 | 15.58 | > 50 | > 50 |
| TS8 | 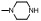 |
8.80 | 9.89 | > 100 | > 50 | > 50 |
| TS9 | 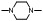 |
4.74 | 17.13 | > 100 | > 100 | > 100 |
| TS10 | 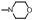 |
> 100 | > 100 | 18.20 | > 100 | > 100 |
| TS11 | 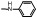 |
> 200 | > 100 | > 100 | > 100 | > 100 |
| TS12 | 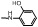 |
> 100 | > 100 | 16.29 | > 100 | > 100 |
| TS13 | 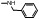 |
18.99 | 14.83 | > 100 | > 50 | > 50 |
| β-hederin | 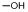 |
12.47 | 9.53 | 8.26 | > 50 | 19.72 |
3. Experimental
3.1. General
Commercial reagents were used without further purification unless otherwise stated. Solvents were dried and redistilled prior to use in the usual way. Analytical TLC was performed on silica gel HF254 plates. Preparative column chromatography (CC) was performed with silica gel H. Melting points were measured with a Büchi B-540 Melting Point apparatus. Optical rotations were measured at the sodium D-line at room temperature (RT) with a Perkin–Elmer 241MC polarimeter. 1H- and 13C-NMR spectra were recorded on a Bruker Avance AV600 MHz spectrometer using Me4Si as the internal standard. HRMS spectra were recorded on a high resolution ESI-FTICR mass spectrometer.
3.2. Benzyl oleanolate 3-O-3,4-O-isopropylidene-α-L-arabinopyranoside (4)
A suspension of OA (1.00 g, 2.2 mmol), BnBr (0.42 mL, 3.5 mmol), K2CO3 (0.60 g, 4.4 mmol) and Bu4NI (0.08 g, 0.22 mmol) in 40:1 THF-H2O (41 mL) was stirred overnight at RT. The mixture was then filtered, and the filtrate was concentrated under vacuum and purified by silica gel column chromatography (8:1 petroleum ether–EtOAc) to give oleanolic acid benzyl ester 1 (1.17 g, 98%) as a white amorphous solid. Compound 1 (1.00 g, 1.83 mmol), trichloroacetimidate SD-1 (1.27 g, 2.10 mmol) and powdered 4Å molecular sieves (MS, 500 mg) were mixed in dry DCM (25 mL) and stirred at RT for 20 min. A dry DCM solution (2.0 mL) of TMSOTf (0.02 mL, 0.01 mmol) was then added dropwise and the mixture was stirred for approximately 1.5 h until the reagents were completely consumed. The mixture was neutralized with Et3N (0.20 mL) and filtered. The filtrate was concentrated and purified by CC (8:1 petroleum ether–EtOAc) to generate compound 2 (1.87 g, 97%) as a white foam. A fresh solution of NaOMe in MeOH (1.0 mol/L, 1.70 mL) was added to a solution of 2 (1.50 g, 1.50 mmol) in 1:2 DCM–MeOH (40 mL). The mixture was stirred at RT for 2 h, neutralized with Dowex H+ resin, filtered, the filtrate was concentrated, and the residue subjected to CC (EtOAc) to yield saponin 3 (991 mg, 96%) as a white amorphous solid. Me2C(OMe)2 (0.31 mL, 2.50 mmol) and p-TsOH (17.2 mg) was added to a solution of 3 (679 mg, 1.00 mmol) in dry acetone (10 mL). The mixture was stirred for 4 h before Et3N (0.20 mL) was added. The solution was concentrated and purified by CC (6:1 petroleum ether–EtOAc) to generate afford compound 4 (634 mg, 89%) as a white foam. [α]25D +45.0 (c 1.60, CHCl3); 1H-NMR (CDCl3): δ 7.34 (m, 5H, Ar-H), 5.28 (t, J = 3.0 Hz, 1H, H-12), 5.07 (dd, J = 18.7, 12.6 Hz, 2H, PhCH2), 4.22–4.17 (m, 3H, H-1', H-4', H-5'-1), 4.06 (dd, J = 7.8, 6.1 Hz, 1H, H-3'), 3.75 (dd, J = 13.9, 3.5 Hz, 1H, H-5'-2), 3.63 (dd, J = 7.8, 7.8 Hz, 1H, H-2'), 3.12 (dd, J = 11.5, 4.6 Hz, 1H, H-3), 2.91 (dd, J = 13.8, 3.3 Hz, 1H, H-18), 2.30 (br s, 1H, OH), 1.54, 1.36 (s each, 3H each, O-(CH3)2C-O), 1.11, 0.98, 0.92, 0.89, 0.88, 0.82, 0.60 (s each, 3H each, 7×Me); HRMS: calcd for C38H59O7 (M-Bn): 627.4261; found: m/z 627.4255.
3.3. Benzyl oleanolate 3-O-2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl-(1→2)-3,4-O-isopropylidene-α-L-arabinopyranoside (5)
A mixture of compound 4 (560 mg, 0.78 mmol), trichloroacetimidate SD-2 (630 mg, 1.00 mmol) and powdered 4Å MS (300 mg) in dry DCM (10 mL) was stirred at RT for 20 min. A dry DCM solution of TMSOTf (0.005 mmol) was added dropwise, and the mixture was stirred for 2 h, followed by the addition of Et3N (0.20 mL) and filtration. The filtrate was concentrated and subjected to CC (8:1 petroleum ether–EtOAc) to furnish disaccharide saponin 5 (730 mg, 79%) as a white foam. [α]25D +96.7 (c 2.58, CHCl3); 1H-NMR (CDCl3): δ 8.12–7.21 (m, 20H, Ar-H), 5.87 (dd, J = 10.2, 3.3 Hz, 1H, H-3"), 5.76 (s, 1H, H-1"), 5.65 (m, 2H, H-2", H-4"), 5.30 (t, J = 3.0 Hz, 1H, H-12), 5.07 (dd, J = 22.4, 12.6 Hz, 2H, PhCH2), 4.53 (m, 1H, H-5"), 4.47 (d, J = 3.0 Hz, 1H, H-1'), 4.25 (m, 2H, H-3', H-4'), 4.17 (m, 1H, H-5'-1), 3.90 (dd, J = 3.0, 3.0 Hz, 1H, H-2'), 3.79 (m, 1H, H-5'-2), 3.17 (dd, J = 11.3, 4.1 Hz, 1H, H-3), 2.92 (m, 1H, H-18), 1.55, 1.35 (s each, 3H each, O-(CH3)2C-O), 1.34 (d, J = 6.1 Hz, 3H, H-6"), 1.14, 0.95, 0.93, 0.92, 0.90, 0.89, 0.64 (s each, 3H each, 7×Me); HRMS: calcd for C65H81O14 (M-Bn): 1085.5626; found: m/z 1085.5619.
3.4. Benzyl oleanolate 3-O-2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (6)
Compound 5 (705 mg, 0.60 mmol) was dissolved in DCM–MeOH (1:2, 40 mL), and then p-TsOH (78 mg) was added. The solution was stirred at RT for 3 h, after which Et3N (0.40 mL) was added, and the mixture was concentrated and purified by CC (2:1 petroleum ether–EtOAc) to yield compound 6 (666 mg, 98%) as a white amorphous solid. [α]25D +77.3 (c 2.27, CHCl3); 1H-NMR (CDCl3): δ 8.10–7.23 (m, 20H, Ar-H), 5.84 (dd, J = 10.2, 3.1 Hz, 1H, H-3"), 5.65 (m, 2H, H-2", H-4"), 5.36 (s, 1H, H-1"), 5.29 (br s, 1H, H-12), 5.07 (dd, J = 18.7, 12.6 Hz, 2H, PhCH2), 4.81 (d, J = 1.3 Hz, 1H, H-1'), 4.34 (m, 1H, H-5"), 4.11–3.98 (m, 3H, H-2', H-4', OH), 3.82 (m, 1H, H-5'-1), 3.67 (m, 1H, H-5'-2), 3.45 (d, J = 7.9 Hz, 1H, H-3'), 3.18 (dd, J = 11.0, 3.2 Hz, 1H, H-3), 2.91 (m, 1H, H-18), 2.52 (br s, 1H, OH), 1.34 (d, J = 6.0 Hz, 3H, H-6"), 1.12, 1.05, 0.92, 0.89, 0.88, 0.84, 0.61 (s each, 3H each, 7×Me); HRMS: calcd for C62H77O14 (M-Bn): 1045.5313; found: m/z 1045.5307.
3.5. Oleanolic acid 3-O-2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-acetyl-α-L-arabinopyranoside (8)
A solution of compound 6 (600 mg, 0.53 mmol) and Ac2O (0.25 mL, 2.65 mmol) in dry pyridine (5 mL) was stirred at RT overnight. The solvent was evaporated in a vacuum, and the resulting residue was dissolved in DCM (20 mL), washed with water (15 mL × 3), and dried over MgSO4. The mixture was filtered, and the filtrate was concentrated to produce crude compound 7 as a white amorphous solid, which was then dissolved in dry EtOAc (20 mL). After adding in 100 mg of 10% Pd–C, the solution was refluxed and bubbled up with H2 (25 mL/min) for 5 h. Pd–C was removed through filtration, and the filtrate was concentrated to dryness, which was purified by CC (3:1 petroleum ether–EtOAc) to generate compound 8 (569 mg, 95%, for the 2 steps) as a white amorphous solid. [α]25D +57.2 (c 1.68, CHCl3); 1H-NMR (CDCl3): δ 8.17–7.27 (m, 15H, Ar-H), 5.85 (dd, J = 10.2, 3.3 Hz, 1H, H-3"), 5.57 (s, 1H, H-1"), 5.65 (m, 2H, H-2", H-4"), 5.28 (t, J = 3.0 Hz, 1H, H-12), 4.77 (d, J = 3.0 Hz, 1H, H-1'), 4.53 (m, 1H, H-5"), 4.25–4.21 (m, 2H, H-3', H-4'), 4.18 (m, 1H, H-5'-1), 3.93 (m, 1H, H-2'), 3.71 (m, 1H, H-5'-2), 3.16 (dd, J = 11.3, 4.1 Hz, 1H, H-3), 2.94 (m, 1H, H-18), 1.94 (s, 6H, 2×CH3CO), 1.37 (d, J = 6.0 Hz, 3H, H-6"), 1.19, 1.15, 1.06, 0.90, 0.89, 0.83, 0.78 (s each, 3H each, 7×Me); HRMS: calcd for C62H77O14 (M+Na): 1155.5603; found: m/z 1155.5597.
3.6. General procedure for the synthesis of oleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranosides
Oxalyl chloride (0.37 mL, 4.40 mmol) was added dropwise to a solution of compound 8 (500 mg, 0.44 mmol) in redistilled DCM (10 mL). The mixture was stirred at RT for 6 h. Then, the solvents were co-evaporated with toluene for complete removal of the excess oxalyl chloride to yield compound 9 as a yellow foam. A solution of compound 9 in redistilled DCM (10 mL) was treated with the corresponding amine (0.80 mmol) and 5 drops of Et3N and stirred for 4 h under nitrogen. The mixture was washed with satd aq NaCl (10 mL × 3), dried over MgSO4, and concentrated in vacuo to yield the crude product 10 which was dissolved in dry 1:2 DCM–MeOH (12 mL) and treated with a fresh solution of NaOMe in MeOH (1.0 mol/L, 1.00 mL). The solution was stirred at RT for 2 h, neutralized with Dowex H+ resin to pH 7, and filtered. The filtrate was concentrated and subjected to CC (20:10:1 CHCl3–MeOH–H2O) to give the target saponins in yields of 67%–85% (for the 3 steps).
3.6.1. N-isopropyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS1)
White powder, m.p. 176.4–178.3 ºC; [α]25D +11.5 (c 0.13, CH3OH); 1H-NMR (pyridine-d5): δ 6.92 (d, 1H, J = 7.2 Hz, NH), 6.12 (s, 1H, H-1''), 5.43 (br s, 1H, H-12), 4.90 (d, 1H, J = 5.4 Hz, H-1'), 4.72 (m, 1H, H-2''), 4.62–4.54 (m, 3H, H-2', H-3'', H-5''), 4.34–4.27 (m, 5H, H-3', H-4', H-5'-1, H-4'', NCH(CH3)2), 3.82 (m, 1H, H-5'-2), 3.25 (dd, 1H, J = 11.3, 4.1 Hz, H-3), 3.01 (br d, 1H, J = 13.8 Hz, H-18), 1.63 (d, 3H, J = 6.5 Hz, H-6''), 1.26, 1.20, 1.75, 1.16, 1.08, 0.96, 0.92, 0.91, 0.86 (s each, 3H each, 9×Me); 13C-NMR(pyridine-d5): δ 176.5 (C-28), 144.9 (C-13), 122.6 (C-12), 104.9 (C-1'), 101.7 (C-1''), 88.7 (C-3), 75.8 (C-2'), 74.1, 74.1 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.9 (C-4'), 64.9 (C-5'), 55.8 (C-5), 47.9 (C-9), 46.7 (C-17), 46.1 (C-19), 42.2 (C-14), 41.8 (C-18), 41.4 (C-NH), 39.8 (C-4), 39.5 (C-8), 38.9 (C-1), 37.0 (C-10), 34.4 (C-21), 33.8 (C-29), 33.2 (C-7), 33.1 (C-22), 30.8 (C-20), 28.0, 27.8 (C-15, C-23), 26.5 (C-2), 26.0 (C-27), 23.7, 23.7, 23.5 (C-30, C-11, C-16), 22.9, 22.3 (CH(CH3)2), 18.6, 18.6 (C-6, C-6''), 17.3 (C-26), 17.0 (C-24), 15.7 (C-25); HRMS: calcd for C44H73NO10 (M+Na): 798.5232; found: m/z 798.5227.
3.6.2. N-tert-butyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS2)
White powder, m.p. 187.5–189.3 ºC; [α]25D +7.5 (c 0.24, CH3OH); 1H-NMR (pyridine-d5): δ 7.15 (d, 1H, J = 7.0 Hz, NH), 6.12 (s, 1H, H-1''), 5.43 (br s, 1H, H-12), 4.89 (d, 1H, J = 5.3 Hz, H-1'), 4.73 (m, 1H, H-2''), 4.64–4.54 (m, 3H, H-2', H-3'', H-5''), 4.33–4.27 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.81 (m, 1H, H-5'-2), 3.24 (dd, 1H, J = 11.5 Hz, 4.0, H-3), 2.87 (br d, 1H, J = 13.8 Hz, H-18), 1.62 (d, 3H, J = 6.1 Hz, H-6''), 1.44 (s, 9H, t-Bu), 1.25, 1.17, 1.10, 0.98, 0.91, 0.89, 0.88 (s each, 3H each, 7×Me); 13C- NMR (pyridine-d5): δ 176.8 (C-28), 145.0 (C-13), 122.7 (C-12), 104.9 (C-1'), 101.7 (C-1''), 88.7 (C-3), 75.9 (C-2'), 74.1, 73.9 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.7 (C-4'), 64.8 (C-5'), 55.9 (C-5), 50.7 (C-NH), 47.9 (C-9), 47.0 (C-17), 46.8 (C-19), 42.4 (C-14), 42.3 (C-18), 39.8 (C-4), 39.5 (C-8), 39.0 (C-1), 37.0 (C-10), 34.5 (C-21), 33.5 (C-29), 33.2 (C-7), 33.0 (C-22), 30.8 (C-20), 28.8 (C(CH3)3), 28.1, 27.8 (C-15, C-23), 26.6 (C-2), 25.8 (C-27), 23.9, 23.9, 23.7 (C-30, C-11, C-16), 18.6, 18.6 (C-6'', C-6), 18.0 (C-26), 17.0 (C-24), 15.7 (C-25); HRMS: calcd for C45H75NO10 (M+Na): 812.5289; found: m/z 812.5285.
3.6.3. N-cyclohexyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS3)
White powder, m.p. 201.5–203.7 ºC; [α]25D +27.1 (c 0.30, CH3OH); 1H-NMR (pyridine-d5): δ 6.71 (d, 1H, J = 7.5 Hz, NH), 6.14 (s, 1H, H-1''), 5.46 (br s, 1H, H-12), 4.90 (d, 1H, J = 5.3 Hz, H-1'), 4.74 (m, 1H, H-2''), 4.65–4.55 (m, 3H, H-2', H-3'', H-5''), 4.33–4.28 (m, 4H, H-3', H-4', H-5'-1, H-4''), 4.06 (m, 1H, NCH), 3.82 (m, 1H, H-5'-2), 3.25 (dd, 1H, J = 11.3, 3.8 Hz, H-3), 3.09 (br d, 1H, J = 9.8 Hz, H-18), 2.12–1.78 (m, 10H, hexanyl), 1.62 (d, 3H, J = 6.1 Hz, H-6''), 1.27, 1.18, 1.09, 0.98, 0.95, 0.93, 0.89 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 176.5 (C-28), 145.0 (C-13), 122.7 (C-12), 104.9 (C-1'), 101.7 (C-1''), 88.8 (C-3), 75.9 (C-2'), 74.1, 73.9 (C-3', C-4''), 72.5, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.8 (C-4'), 64.8 (C-5'), 55.9 (C-5), 48.6 (C-NH), 48.0 (C-9), 46.8 (C-17), 46.3 (C-19), 42.3 (C-14), 41.9 (C-18), 39.8 (C-4), 39.5 (C-8), 38.9 (C-1), 37.0 (C-10), 34.5 (C-21), 33.6 (C-29), 33.2 (C-7), 33.0 (C-22), 30.9 (C-20), 28.1, 27.9 (C-15, C-23), 26.5 (C-2), 26.0 (C-27), 25.9, 25.6, 25.5 (hexanyl), 23.8, 23.8, 23.7 (C-30, C-11, C-16), 18.6, 18.6 (C-6'', C-6), 17.8 (C-26), 17.0 (C-24), 15.6 (C-25); HRMS: calcd for C47H77NO10 (M+Na): 838.5547; found: m/z 838.5544.
3.6.4. N-propyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS4)
White powder, m.p. 178.6–181.0 ºC; [α]25D +17.7 (c 0.27, CH3OH); 1H-NMR (pyridine-d5): δ 7.31 (m, 1H, NH), 6.15 (s, 1H, H-1''), 5.39 (br s, 1H, H-12), 4.88 (d, 1H, J = 5.4 Hz, H-1'), 4.73 (m, 1H, H-2''), 4.63–4.55 (m, 3H, H-2', H-3'', H-5''), 4.34–4.27 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.80 (m, 1H, H-5'-2), 3.48–3.42 (m, 1H, NCH2CH2CH3), 3.27–3.21 (m, 2H, H-3, NCH2CH2CH3), 3.07 (br d, 1H, J = 13.0 Hz, H-18), 1.61 (d, 3H, J = 5.4 Hz, H-6''), 1.58–1.54 (m, 2H, NCH2CH2CH3), 1.24, 1.15, 1.07, 0.91, 0.90, 0.89, 0.86 (s each, 3H each, 7×Me), 0.84–0.81 (t, 3H, NCH2CH2CH3); 13C-NMR (pyridine-d5): δ 177.4 (C-28), 144.9 (C-13), 122.7 (C-12), 104.9 (C-1'), 101.7 (C-1''), 88.7 (C-3), 75.8 (C-2'), 74.1, 74.0 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.8 (C-4'), 64.8 (C-5'), 55.8 (C-5), 47.9 (C-9), 46.8 (C-17), 46.4 (C-19), 42.2, 41.9, 41.6 (NCH2CH2CH3, C-14, C-18), 39.7 (C-4), 39.5 (C-8), 38.8 (C-1), 37.0 (C-10), 34.4 (C-21), 33.8 (C-29), 33.2 (C-7), 33.0 (C-22), 30.9 (C-20), 28.0, 27.8 (C-15, C-23), 26.5 (C-2), 26.1 (C-27), 23.8 (C-30), 23.7 (C-11), 23.7 (C-16), 23.4 (NCH2CH2CH3), 18.6, 18.5 (C-6, C-6''), 17.5 (C-26), 17.0 (C-24), 15.6 (C-25), 11.8 (NCH2CH2CH3); HRMS: calcd for C44H73NO10 (M+Na): 798.5234; found: m/z 798.5230.
3.6.5. N-(2-hydroxyethyl)oleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS5)
White powder, m.p. 184.6–187.3ºC; [α]25D +0.3 (c 0.30, CH3OH); 1H-NMR (pyridine-d5): δ 7.51 (m, 1H, NH), 6.13 (s, 1H, H-1''), 5.42 (br s, 1H, H-12), 4.90 (d, 1H, J = 5.4 Hz, H-1'), 4.74 (m, 1H, H-2''), 4.62–4.54 (m, 3H, H-2', H-3'', H-5''), 4.32–4.28 (m, 4H, H-3', H-4', H-5'-1, H-4''), 4.02–4.00 (m, 2H, NCH2CH2OH), 3.89–3.87 (m, 1H, NCH2CH2OH), 3.86–3.82 (m, 1H, H-5'-2), 3.65–3.59 (m, 1H, NCH2CH2OH), 3.26 (dd, 1H, J = 11.1, 4.1 Hz, H-3), 3.06 (br d, 1H, J = 12.8 Hz, H-18), 1.62 (d, 3H, J = 6.6 Hz, H-6''), 1.26, 1.22, 1.07, 0.96, 0.91, 0.90, 0.86 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 178.1 (C-28), 144.7 (C-13), 122.9 (C-12), 104.9 (C-1'), 101.7 (C-1''), 88.7 (C-3), 75.8 (C-2'), 74.0, 74.0 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.8 (C-5''), 68.8 (C-4'), 64.9 (C-5'), 61.4 (NCH2CH2OH), 55.8(C-5), 47.9 (C-9), 46.7 (C-17), 46.4 (C-19), 43.0 (NCH2CH2OH), 42.1, 41.9 (C-14, C-18), 39.7 (C-4), 39.5 (C-8), 38.8 (C-1), 36.9 (C-10), 34.3 (C-21), 33.6 (C-29), 33.1 (C-7), 32.8 (C-22), 30.9 (C-20), 28.0, 27.8 (C-15, C-23), 26.5, 26.1 (C-2, C-27), 23.8, 23.7, 23.7 (C-30, C-11, C-16), 18.6, 18.5 (C-6, C-6''), 17.3, 17.0 (C-26, C-24), 15.5(C-25); HRMS: calcd for C43H71NO11 (M+Na): 800.5027; found: m/z 800.5022.
3.6.6. N,N-diethyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS6)
White powder, m.p. 159.4–161.2 ºC; [α]25D -20.0 (c 0.14, CH3OH); 1H-NMR (pyridine-d5): δ 6.17 (s, 1H, H-1''), 5.43 (br s, 1H, H-12), 4.90 (d, 1H, J = 5.4 Hz, H-1'), 4.75 (m, 1H, H-2''), 4.64–4.56 (m, 3H, H-2', H-3'', H-5''), 4.32–4.27 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.83 (m, 1H, H-5'-2), 3.41–3.26 (m, 6H, H-3, H-18, N(CH2CH3)2), 1.63 (d, 3H, J = 6.6 Hz, H-6''), 1.27, 1.18, 1.14, 1.14, 1.09, 0.96, 0.93, 0.93, 0.88 (s each, 3H each, 9×Me); 13C-NMR (pyridine-d5): δ 175.0 (C-28), 145.6 (C-13), 121.8 (C-12), 105.0 (C-1'), 101.8 (C-1''), 88.9 (C-3), 76.0 (C-2'), 74.2, 74.0 (C-3', C-4''), 72.7, 72.5 (C-2'', C-3''), 70.0 (C-5''), 68.9 (C-4'), 64.9 (C-5'), 56.1 (C-5), 48.2 (C-9), 47.8 (C-17), 47.2 (C-19), 44.2 (NCH2CH3), 42.3, 42.3 (C-14, C-18), 39.8 (C-4), 39.6 (C-8), 38.9 (C-1), 37.2 (C-10), 34.5 (C-21), 33.6 (C-29), 33.3, 33.3 (C-7, C-22), 30.6 (C-20), 30.4 (NCH2CH3), 28.6, 28.2 (C-15, C-23), 26.7 (C-2), 26.0 (C-27), 24.3, 23.9, 22.9 (C-30, C-11, C-16), 18.6 (C-6, C-6''), 17.7 (C-26), 17.2 (C-24), 15.8 (C-25), 13.7, 13.7 (N(CH2CH3)2); HRMS: calcd for C45H75NO10 (M+Na): 812.5289; found: m/z 812.5281.
3.6.7. 1-(piperidin-1-yl)ole-28-one 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS7)
White powder, m.p. 174.0–176.3ºC; [α]25D -12.5 (c 0.12, CH3OH); 1H-NMR (pyridine-d5): δ 6.17 (s, 1H, H-1''), 5.42 (br s, 1H, H-12), 4.89 (d, 1H, J = 5.4 Hz, H-1'), 4.75 (m, 1H, H-2''), 4.64–4.56 (m, 3H, H-2', H-3'', H-5''), 4.32–4.27 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.81 (d, 1H, J = 11.4 Hz, H-5'-2), 3.60–3.54 (m, 4H, H-piperidin-2,6), 3.40 (dd, 1H, J = 11.9, 4.2 Hz, H-3), 3.25 (br d, 1H, J = 13.0 Hz, H-18), 1.50 (m, 6H, H-piperidin-3,4,5), 1.62 (d, 3H, J = 6.6 Hz, H-6''), 1.27, 1.18, 1.09, 0.95, 0.94, 0.94, 0.88 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 174.5 (C-28), 145.5 (C-13), 121.8 (C-12), 105.0 (C-1'), 101.8 (C-1''), 88.8 (C-3), 75.9 (C-2'), 74.1, 73.9 (C-3', C-4''), 72.6, 72.5 (C-2'', C-3''), 69.9 (C-5''), 68.8 (C-4'), 64.8 (C-5'), 56.1 (C-5), 48.1, 47.6, 46.9, 46.7 (C-9, C-piperidine, C-17, C-19), 44.2 (C-piperidine), 42.3, 42.3 (C-14, C-18), 39.6 (C-4), 39.5 (C-8), 38.9 (C-1), 37.1 (C-10), 34.3 (C-21), 33.4 (C-29), 33.2, 33.2 (C-7, C-22), 30.6 (C-20), 30.1 (C-piperidine), 28.4, 28.1 (C-15, C-23), 26.5, 26.5 (C-2, C-27), 26.2, 25.1 (C-piperidine), 24.2, 23.8, 22.9 (C-30, C-11, C-16), 18.6 (C-6, C-6''), 17.3, 17.1 (C-26, C-24), 15.7 (C-25); HRMS: calcd for C46H75NO10 (M+Na): 824.5389; found: m/z 824.5386.
3.6.8. 1-(piperazin-1-yl)ole-28-one 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS8)
White powder, m.p. 230.3–231.1ºC; [α]25D -19.4 (c 0.32, CH3OH); 1H-NMR (pyridine-d5): δ 6.17 (s, 1H, H-1''), 5.41 (br s, 1H, H-12), 4.90 (d, 1H, J = 5.4 Hz, H-1), 4.75 (m, 1H, H-2''), 4.65–4.56 (m, 3H, H-2', H-3'', H-5''), 4.33–4.28 (m, 4H, H-3', H-4', H-5'-1, H-4''), 4.28–4.20 (m, 4H, H-piperizine-2,6), 3.82 (d, 1H, J = 11.4 Hz, H-5'-2), 3.43–3.77 (m, 5H, H-3, H-piperizine-3,5), 3.25 (br d, 1H, J = 13.3 Hz, H-18), 1.63 (d, 3H, J = 6.0 Hz, H-6''), 1.24, 1.19, 1.11, 0.94, 0.93, 0.88, 0.85 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 175.3 (C-28), 145.0 (C-13), 122.0 (C-12), 104.9 (C-1'), 101.7 (C-1''), 88.7 (C-3), 75.9 (C-2'), 74.1, 74.0 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.8 (C-4'), 64.9 (C-5'), 56.0 (C-5), 48.1 (C-9), 47.6, 46.7 (C-17, C-19), 44.4, 44.0, 44.0 (C-piperazine), 42.2, 42.2 (C-14, C-18), 39.5, 39.5, 38.9 (C-4, C-8, C-1), 37.1 (C-10), 34.1 (C-21), 33.1, 33.1, 33.1 (C-7, C-29, C-22), 30.5 (C-20), 30.2 (C-piperazine), 28.3, 28.1 (C-15, C-23), 26.5 (C-2), 26.1 (C-27), 24.1, 23.7, 22.7 (C-30, C-11, C-16), 18.6 (C-6, C-6''), 17.1, 17.0 (C-26, C-24), 15.6 (C-25); HRMS: calcd for C45H74N2O10 (M+Na): 825.5343; found: m/z 825.5336.
3.6.9. 1-(4-methylpiperazin-1-yl)ole-28-one 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS9)
White powder, m.p. 190.2–192.7ºC; [α]25D -17.5 (c 0.16, CH3OH); 1H-NMR (pyridine-d5): δ 6.20 (s, 1H, H-1''), 5.42 (br s, 1H, H-12), 4.89 (d, 1H, J = 5.4 Hz, H-1'), 4.77 (m, 1H, H-2''), 4.66–4.58 (m, 3H, H-2', H-3'', H-5''), 4.34–4.28 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.83–3.74 (m, 5H, H-5'-2, H-piperizine-2,6), 3.40 (dd, 1H, J = 11.7, 4.0 Hz, H-3), 3.25 (br d, 1H, J = 13.1 Hz, H-18), 2.33 (m, 4H, H-piperizine-3,5), 2.17 (s, 3H, NCH3), 1.63 (d, 3H, J = 6.6 Hz, H-6''), 1.26, 1.19, 1.10, 0.95, 0.94, 0.93, 0.88 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 174.8 (C-28), 145.4 (C-13), 121.8 (C-12), 104.9 (C-1'), 101.8(C-1''), 88.8 (C-3), 75.9 (C-2'), 74.1, 73.9 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.7(C-4'), 64.8 (C-5'), 56.0 (C-5), 55.6 (C-piperazine), 48.1 (C-9), 47.6, 46.7 (C-17, C-19), 46.0, 45.7, 44.1 (C-piperazine), 42.2, 42.2 (C-14, C-18), 39.6, 39.5, 38.9 (C-4, C-8, C-1), 37.1 (C-10), 34.2 (C-21), 33.4 (C-29), 33.2, 33.2 (C-7, C-22), 30.6 (C-20), 30.2 (NCH3), 28.3, 28.1 (C-15,C-23), 26.5 (C-2), 26.2 (C-27), 24.2, 23.8, 22.8 (C-30, C-11, C-16), 18.6 (C-6, C-6''), 17.3, 17.0 (C-26, C-24), 15.7 (C-25); HRMS: calcd for C46H76N2O10 (M+Na): 839.5500; found: m/z 839.5497.
3.6.10. 1-morpholinoole-28-one 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS10)
White powder, m.p. 201.7–204.1ºC; [α]25D -10.1 (c 0.10, CH3OH); 1H-NMR (pyridine-d5): δ 6.16 (s, 1H, H-1''), 5.40 (br s, 1H, H-12), 4.88 (d, 1H, J = 4.9 Hz, H-1'), 4.73 (m, 1H, H-2''), 4.64–4.55 (m, 3H, H-2', H-3'', H-5''), 4.32–4.26 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.84–3.68 (m, 9H, H-5'-2, H-morpholine), 3.38 (dd, 1H, J = 11.5, 4.0 Hz, H-3), 3.24 (br d, 1H, J = 7.9 Hz, H-18), 1.62 (d, 3H, J = 5.7 Hz, H-6''), 1.25, 1.18, 1.09, 0.94, 0.93, 0.89, 0.88 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 175.0 (C-28), 145.2 (C-13), 122.0 (C-12), 105.0 (C-1'), 101.8 (C-1''), 88.8 (C-3), 75.9 (C-2'), 74.1, 74.0 (C-3', C-4''), 72.6, 72.5 (C-2'', C-3''), 69.9 (C-5''), 68.8 (C-4'), 67.2 (C-morpholine), 64.9 (C-5'), 56.0 (C-5), 48.1 (C-9), 47.6 (C-morpholine), 46.6, 46.4 (C-17, C-19), 44.1 (C-morpholine), 42.2 (C-14, C-18), 39.6, 39.5 (C-4, C-8), 38.9 (C-1), 37.1 (C-10), 34.2 (C-21), 33.3 (C-29), 33.2 (C-7, C-22), 30.6 (C-20), 30.0 (C-morpholine), 28.2, 28.1 (C-15, C-23), 26.6 (C-2), 26.2 (C-27), 24.2 (C-30), 23.8, 22.7 (C-11, C-16), 18.6 (C-6, C-6''), 17.2, 17.0 (C-26, C-24), 15.7 (C-25); HRMS: calcd for C45H73NO11 (M+Na): 826.5184; found: m/z 826.5180.
3.6.11. N-phenyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS11)
White powder, m.p. 156.7–158.3ºC; [α]25D -11.9 (c 0.15, CH3OH); 1H-NMR (pyridine-d5): δ 7.76–7.29 (m, 5H, Ar-H), 6.13 (s, 1H, H-1''), 5.55 (br s, 1H, H-12), 4.88 (d, 1H, J = 5.4 Hz, H-1'), 4.73 (m, 1H, H-2''), 4.60–4.52 (m, 3H, H-2', H-3'', H-5''), 4.32–4.28 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.80 (d, 1H, J = 11.1 Hz, H-5'-2), 3.22 (br d, 1H, J = 10.8 Hz, H-3), 3.02 (br d, 1H, J = 11.6 Hz, H-18), 1.60 (m, 3H, H-6''), 1.26, 1.14, 1.02, 0.96, 0.94, 0.77, 0.75 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 175.8 (C-28), 143.2 (C-13), 138.4, 127.4, 127.3, 127.0, 123.8, 123.7 (6×Ar-C), 122.5 (C-12), 104.2 (C-1'), 101.1 (C-1''), 88.1 (C-3), 75.2 (C-2'), 73.4, 73.2 (C-3', C-4''), 72.0, 71.8 (C-2'', C-3''), 69.3 (C-5''), 68.0 (C-4'), 64.0 (C-5'), 55.1 (C-5), 47.5, 47.2, 46.1 (C-9, C-17, C-19), 41.9 (C-14), 41.5 (C-18), 39.1 (C-4), 38.8 (C-8), 38.2 (C-1), 36.3 (C-21), 32.7 (C-29), 32.4, 32.1 (C-7, C-22), 30.2 (C-20), 27.4 (C-15), 27.1 (C-23), 25.8 (C-2), 25.5 (C-27), 23.6 (C-30), 23.2, 23.0 (C-11, C-16), 17.9, 17.8 (C-6, C-6''), 16.4, 16.3 (C-24, C-26), 14.9 (C-25); HRMS: calcd for C47H71NO10 (M+Na): 832.5078; found: m/z 832.5072.
3.6.12. N-(2-hydroxyphenyl)oleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS12)
White powder, m.p. 191.3–194.0ºC; [α]25D -28.6 (c 0.21, CH3OH); 1H-NMR (pyridine-d5): δ 12.27 (br s, 1H, Ph-OH), 9.07 (m, 1H, Ar-H), 8.98 (m, 1H, Ar-H), 7.13 (m, 1H, Ar-H), 7.00 (m, 1H, Ar-H), 6.14 (s, 1H, H-1''), 5.67 (br s, 1H, H-12), 4.87 (d, 1H, J = 5.3 Hz, H-1'), 4.72 (m, 1H, H-2''), 4.63–4.52 (m, 3H, H-2', H-3'', H-5''), 4.32–4.25 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.80 (br d, 1H, J = 9.6 Hz, H-5'-2,), 3.20 (dd, 1H, J = 11.6, 4.1 Hz, H-3), 3.07 (br d, 1H, J = 13.2 Hz, H-18), 1.60 (d, 3H, J = 6.1 Hz, H-6''), 1.26, 1.13, 1.02, 0.91, 0.89, 0.82, 0.70 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 175.7 (C-28), 147.2 (OH-Ph-C), 143.3 (C-13), 128.2 (Ar-C), 123.6, 123.4 (Ar-C, C-12), 119.9 (Ar-C), 119.3 (Ar-C), 114.8 (Ar-C), 104.2 (C-1'), 101.1 (C-1''), 88.1 (C-3), 75.2 (C-2'), 73.4, 73.2 (C-3', C-4''), 71.9, 71.8 (C-2'', C-3''), 69.2 (C-5''), 68.0 (C-4'), 64.1 (C-5'), 55.1 (C-5), 47.4, 47.3, 46.3 (C-9, C-17, C-19), 42.0 (C-14), 41.6 (C-18), 39.1 (C-4), 38.8 (C-8), 38.3 (C-1), 36.2 (C-10), 33.8 (C-21), 32.8, 32.4, 32.2 (C-7, C-22, C-29), 30.2 (C-20), 27.4 (C-15), 27.2 (C-23), 25.9 (C-2), 25.4 (C-27), 23.8, 23.3, 23.0 (C-11, C-16, C-30), 17.9, 17.8 (C-6, C-6''), 16.3, 16.2 (C-24, C-26), 14.9 (C-25); HRMS: calcd for C47H71NO11 (M+Na): 848.5027; found: m/z 848.5019.
3.6.13. N-benzyloleanolic amide 3-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (TS13)
White powder, m.p. 168.3–171.1ºC; [α]25D -9.5 (c 0.19, CH3OH); 1H-NMR (pyridine-d5): δ 8.02–8.00 (m, 1H, NH), 7.45 (m, 2H, Ar-H), 7.31 (m, 2H, Ar-H), 7.22 (m, 1H, Ar-H), 6.17 (s, 1H, H-1''), 5.37 (br s, 1H, H-12), 4.86 (d, 1H, J = 5.4 Hz, H-1'), 4.77 (m, 2H, H-2'', NHCH2Ph), 4.63–4.55 (m, 4H, H-2', H-3'', H-5'', NHCH2Ph), 4.31–4.25 (m, 4H, H-3', H-4', H-5'-1, H-4''), 3.80 (m, 1H, H-5'-2), 3.22 (dd, 1H, J = 11.5, 4.1 Hz, H-3), 3.14 (br d, 1H, J = 13.9 Hz, H-18), 1.61 (d, 3H, J = 6.6 Hz, H-6''), 1.23, 1.19, 1.07, 0.88, 0.88, 0.87, 0.85 (s each, 3H each, 7×Me); 13C-NMR (pyridine-d5): δ 177.5 (C-28), 144.8 (C-13), 140.9, 128.8, 128.8, 128.1, 128.1, 127.1 (Ar-C), 122.8 (C-12), 104.9 (C-1'), 101.8 (C-1''), 88.7 (C-3), 75.9 (C-2'), 74.1, 74.0 (C-3', C-4''), 72.6, 72.4 (C-2'', C-3''), 69.9 (C-5''), 68.8 (C-4'), 64.8 (C-5'), 55.8 (C-5), 47.9 (C-9), 46.9, 46.5 (C-17, C-19), 43.5 (CH2Ph), 42.1, 41.9 (C-14, C-18), 39.7 (C-4), 39.5 (C-8), 38.8 (C-1), 37.0 (C-10), 34.4 (C-21), 33.8 (C-7), 33.2 (C-29), 33.0 (C-22), 30.9 (C-20), 28.0, 27.9 (C-15, C-23), 26.5 (C-2), 26.1 (C-27), 23.7 (C-30), 23.7 (C-11), 23.7 (C-16), 18.6, 18.5 (C-6, C-6''), 17.4 (C-26), 17.0 (C-24), 15.6 (C-25); HRMS: calcd for C48H73NO10 (M+Na): 846.5234; found: m/z 846.5229.
4. Conclusions
In summary, 13 novel amide derivatives of β-hederin were synthesized and evaluated in vitro for tumor cytotoxicity. The results from the antitumor screening showed that compounds TS8 and TS9 possessed potent antitumor activity against HeLa and MCF-7 cell lines. The preliminary structure-activity relationships indicated that the conversion of the C-28 carboxylic acid group of β-hederin into an amide derivative often resulted in a loss of broad spectrum antitumor activity, but also resulted in an increase in the antitumor selectivity. To provide more clarity about the structure-activity relationship, further studies on additional systematic structural variations are underway.
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (No. 30772641) for the financial support.
Footnotes
Sample Availability: Samples are available from the authors.
References and Notes
- 1.Mahajan R.T., Chopda M.Z. Phyto-pharmacology of Ziziphus jujuba Mill–a plant review. Pharmacogn. Rev. 2009;3:320–329. [Google Scholar]
- 2.Ovesna Z., Vachalkova A., Horvathova K., Tothova D. Pentacyclic triterpenic acids: new chemoprotective compounds. Neoplasma. 2004;51:327–333. [PubMed] [Google Scholar]
- 3.Ma C., Nakamura N., Hattori M. Natural products and their derivatives, having anti-HIV-1 protease activity. Curr. Top. Med. Chem. 2003;3:77–99. [Google Scholar]
- 4.Nakanishi T., Tanaka K., Murata H., Somekawa M., Inada A. Phytochemical studies of seeds of medicinal plants. III. ursolic acid and oleanolic acid glycosides from seeds of Patrinia scabiosaefolia FISCHER. Chem. Pharm. Bull. 1993;41:183–186. doi: 10.1248/cpb.41.183. [DOI] [PubMed] [Google Scholar]
- 5.Barthomeuf C., Debiton E., Mshvidadze V., Kemertelidze E., Balansard G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med. 2002;68:672–675. doi: 10.1055/s-2002-33807. [DOI] [PubMed] [Google Scholar]
- 6.Jung H.J., Lee C.O., Lee K.T., Choi J., Park H.J. Structure-activity relationship of oleanane disaccharides isolated from Akebia quinata versus cytotoxicity against cancer cells and NO inhibition. Biol. Pharm. Bull. 2004;27:744–747. doi: 10.1248/bpb.27.744. [DOI] [PubMed] [Google Scholar]
- 7.Cheng M.S., Yan M.C., Liu Y., Zheng L.G., Liu J. Synthesis of β-hederin and Hederacolchiside A1: triterpenoid saponins bearing a unique cytotoxicity-inducing disaccharide moiety. Carbohydr. Res. 2006;341:60–67. doi: 10.1016/j.carres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Yan M.C., Liu Y., Lu W.X., Wang H., Sha Y., Cheng M.S. Facile synthesis and cytotoxicity of triterpenoid saponins bearing a unique disaccharide moiety: hederacolchiside A1 and its analogues. Carbohydr. Res. 2008;343:780–784. doi: 10.1016/j.carres.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Meng Y.Q., Song Y.L., Yan Z.K., Xia Y. Synthesis and in vitro cytotoxicity of novel ursolic acid derivatives. Molecules. 2010;15:4033–4040. doi: 10.3390/molecules15064033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Y.Q., Liu D., Cai L.L., Chen H., Cao B., Wang Y.Z. The synthesis of ursolic acid derivatives with cytotoxic activity and the investigation of their preliminary mechanism of action. Bioorg. Med. Chem. 2009;17:848–854. doi: 10.1016/j.bmc.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Kommera H., Kaluderovic G.N., Kalbitz J., Dräger B., Paschke R. Small structural changes of pentacyclic lupine type triterpenoid derivatives lead to significant differences in their anticancer properties. Eur. J. Med. Chem. 2010;46:3346–3353. doi: 10.1016/j.ejmech.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Sha Y., Yan M.C., Liu J., Liu Y., Cheng M.S. Facile synthesis of oleanolic acid monoglycosides and diglycosides. Molecules. 2008;13:1472–1486. doi: 10.3390/molecules13071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu B., Xie J., Deng S., Hui Y. First synthesis of a bidesmosidic triterpene saponin by a highly efficient procedure. J. Am. Chem. Soc. 1999;121:12196–12197. doi: 10.1021/ja9926818. [DOI] [Google Scholar]
- 14.Ziegler T., Bien F., Jurisch C. Chemoenzymatic synthesis of enantiomerically pure alkene 1,2-diols and glycosides thereof. Tetrahedron Asymmetry. 1998;9:765–780. doi: 10.1016/S0957-4166(98)00051-2. [DOI] [Google Scholar]