Abstract
A series of 1-acyl-3-(2'-aminophenyl) thiourea derivatives were designed and synthesized. The structures of all the newly synthesized compounds were identified by IR, elemental analysis, 1H-NMR and 13C-NMR. Their anti-intestinal nematode activities against Nippostrongylus brazilliensis were evaluated in rats by an oral route. Among these compounds, at concentrations of 10 mg/kg of rat, compound (1-(2'-furanyl)acyl-3- (2'-aminophenyl) thiourea) (5h) produced the highest activity with 89.4% deparasitization. The present work suggests that 1-acyl-3-(2'-aminophenyl) thiourea derivatives may become useful lead compounds for anti-intestinal nematode treatment.
Keywords: acyl, thiourea, deparasitization, Nippostrongylus brazilliensis
1. Introduction
Mebendazole and albendazole have been used against human and animal helminth parasites for more than two decades [1,2,3,4,5]. They are derived from benzimidazole which has a broad spectrum of activity and is used to treat nematode and trematode infections in domestic animals. The limited solubility of benzimidazoles may have a major influence on their absorption and clinical efficacy [6,7]. Furthermore, when used in lengthy therapies, they can produce side-effects, such as severe headaches, fever, fatigue, hair loss, and liver degeneration [8] and hence are not recommended for patients with hepatic problems. A way to overcome these problems is to use prodrugs [9,10,11], such as 4-amino-3-(3'-methoxycarbonyl-2'-thioureido)benzophenone [12]. It is a soluble prodrug, which is enzymatically cyclized to mebendazole in vitro. On the other hand, thiourea derivatives also exhibit potent antiviral, antibacterial and cytotoxic activities [13,14]. Several works demonstrate their activity against parasites such as Plasmodium falciparum, Trichomonas vaginalis and Trypanosoma. cruzi [15,16]. Based on these reports, we report herein the synthesis, characterization, and in vitro evaluation of anti-intestinal nematode activity of eight different novel thiourea derivatives bearing the o-aminobenzene moiety.
2. Results and Discussion
2.1. Synthesis and Characterization of 1-Acyl-3-(2-aminophenyl) thiourea Derivatives 5a-5h
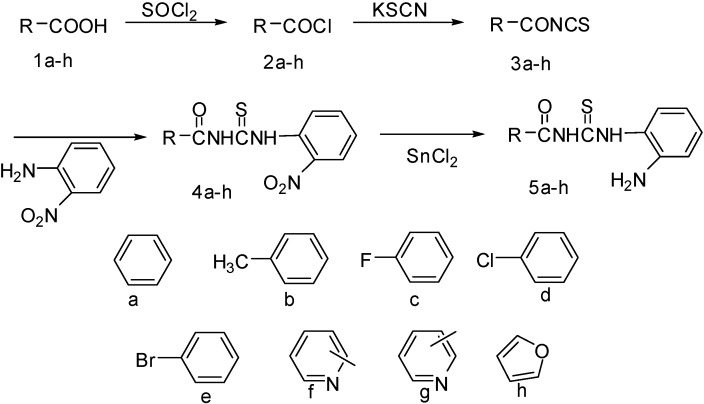
The synthetic route to the target compounds 5a-5h is shown in Scheme 1. Firstly, acids 1a-1h were acylated by SOCl2 followed by isothiocyanation and coupling reactions with 2-nitrobenzenamine to give 1-acyl-3-(2'-nitrophenyl) thioureas 4a-4h in moderate yield. Then the title compounds 5a-5h were successfully obtained in 50-60% overall yield using SnCl2 as reducing agent. Compounds 5a-5h were characterized by 1H-NMR, 13C-NMR and elemental analysis. All results are in full agreement with the proposed structures. For example, the 1H-NMR spectrum of compound 5b showed a singlet at 2.40 ppm (CH3), singlets at 12.9 and 11.6 ppm (NHCSNH) and a multiplet from δ = 7.91 to δ = 6.75 for aromatic hydrogens. Moreover, the 13C-NMR spectrum showed δ 29.6 (CH3), 154.19, 141.62, 133.95, 130.41, 125.70, 124.63, 122.27, 119.28, 115.78, 114.44, 113.15, 109.34 (benzene C), 165.1 (C=O), 180.2 (C=S), all consistent with its proposed structure. The elemental analyses results were in good agreement with those calculated for the suggested formulae. The melting points are sharp, indicating the purity of these compounds.
Scheme 1.
Synthesis of compounds 5a-5h.
2.2. Anti-intestinal Nematode Activity
From Table 1, we can see that some of compounds showed significant anti-intestinal nematode activity in a two-day in vivo test in rats. At concentrations of 10 mg/kg of rat, compound 5h produced the highest activity against Nippostrongylus brazilliensis with 89.4 % deparasitization. For anti-intestinal nematode activity, it appears that a variety of substituents can be introduced on the phenyl ring without significantly altering the activity relative to the unsubstituted phenyl analogue 5a. For example, the substituted F, CH3, Cl, and Br acyl derivatives all have the almost same activity as 5a. Moreover, the 3'-pyridylacyl derivative 5g is slightly more active than the corresponding 2'-isomer 5f. On the other hand, the structural variation between compounds 5b and 5f results in different activity. Compound 5a, 5b, 5c, 5d and 5e contains benzene moieties, while 5f and 5g have a pyridyl group moiety. This pyridyl group appears to be particularly responsible for anti-intestinal nematode activity. Compounds 5f and 5g, which contain a pyridyl moiety, and 5h that contains a furanyl moiety all seemed to be much more effective in terms of anti-intestinal nematode activity. Because compound 5h displayed anti-intestinal nematode potency that is comparable to albendazole (10 mg/kg), further anti-intestinal nematode activity assay was carried out for compound 5h. It was found that at concentrations of 18 mg/kg of rat, 5h produced the highest activity against Nippostrongylus brazilliensis with 100%, effectiveness, which implies further possibilities for lead compound development.
Table 1.
Results of the chemotherapeutic trials of thiourea derivatives bearing O-aminobenzene moieties in rats.
| Number of worms recovered from rats (including worms in rats at post mortem) | ||||||
|---|---|---|---|---|---|---|
| Dose(mg/kg) | 1 | 2 | 3 | Average | Deparasitization(%) | |
| Control | 0 | 200 | 198 | 199 | 199 | |
| Albendazole | 10 | 0 | 0 | 0 | 0 | 100.00 |
| 5a | 10 | 180 | 170 | 176 | 175 | 13.7 |
| 5b | 10 | 178 | 180 | 178 | 178 | 10.5 |
| 5c | 10 | 183 | 177 | 174 | 178 | 10.5 |
| 5d | 10 | 188 | 183 | 184 | 185 | 7.0 |
| 5e | 10 | 177 | 170 | 170 | 172 | 13.5 |
| 5f | 10 | 109 | 110 | 108 | 109 | 45.2 |
| 5g | 10 | 108 | 107 | 96 | 103 | 48.2 |
| 5h | 10 | 22 | 23 | 19 | 21 | 89.4 |
3. Conclusions
In summary, various types of 1-acyl-3-(2-aminophenyl) thioureas were synthesized and their varying biological activities towards the N. brazilliensis was demonstrated. Among these compounds, 5h produced the highest activity against N. brazilliensis with 89.4% deparasitization. The present work suggest that 5h may be a useful lead compound for anti-intestinal nematode medicine development. Further studies of the structure-biology activity relationships around the designed compounds are underway.
4. Experimental
4.1. General
All the reagents and solvents were of the commercial quality and were used without purification. Elemental analysis was performed on a PE-2400 elemental analyzer, the C, H and N analysis were repeated twice. 1H-NMR and 13C-NMR spectra were obtained in DMSO-d6 with TMS as internal standard on a Bruker AM-400 spectrometer. Chemical shifts are reported as ppm. Melting points were determined by an X-6 micro-melting point apparatus and are uncorrected.
4.2. General Procedure for the Preparation of 1-Acyl-3-(2-aminophenyl)thioureas 5a-5h
According to our reported procedure [17] different acids 1a-1h were treated with SOCl2 and KSCN, respectively, affording moderate yields of around 75% of the intermediates 3a-3h, which were used directly without further purification. The subsequent nucleophilic reactions of 3a-3h with 2-nitro-benzenamine led to the key intermediates 4a-4h, respectively. Then reduction of 4a-4h with SnCl2 in CH3COOH afforded the target compounds 5a-5h, which were recrystallized twice from DMF/H2O.
1-Phenylacyl-3-(2'-aminophenyl) thiourea (5a): Yield 60%, mp 154~156oC. IR (KBr) : 3165, 1628, 1255 cm−1; 1H-NMR δ: 12.52 (s, 1H, NH), 11.44 (s, 1H, NH), 8.03-6.80 (m, 9H, Ar-H), 5.30 (s, 2H, Ar-NH); 13C-NMR δ: 180.34, 165.48, 154.39, 133.62, 132.95, 130.41, 130.10, 128.63, 128.27, 127.28, 125.78, 124.05, 119.32, 118.44; Anal. Calcd. for C14H13N3OS (271.2): C 61.97, H 4.83, N 15.49; found C 62.00, H 4.83, N 15.60.
1-(4'-Methylphenyl)acyl-3-(2'-aminophenyl) thiourea (5b): Yield 58%, mp 164~165oC. IR (KBr) : 3208, 1645, 1240 cm−1; 1H-NMR δ: 12.91 (s, 1H, NH), 11.64 (s, 1H, NH), 7.91-6.75 (m, 8H, Ar-H), 5.32 (s, 2H, Ar-NH), 2.40 (s, 3H, CH3); 13C-NMR δ: 180.24, 165.18, 154.19, 141.62, 133.95, 130.41, 125.70, 124.63, 122.27, 119.28, 115.78, 114.44, 113.15, 109.34, 29.64; Anal. calcd. for C15H15N3OS (285.1): C 63.13, H 5.30, N 14.73; found C 63.56, H 5.41, N 14.73.
1-(4'-Fluorophenyl)acyl-3-(2'-aminophenyl) thiourea (5c): Yield 53%, mp 173~174oC. IR (KBr): 3235, 1650, 1235 cm−1; 1H-NMR δ: 12.93 (s, 1H, NH), 11.67 (s, 1H, NH), 8.12-6.80 (m, 8H, Ar-H), 5.35 (s, 2H, Ar-NH); 13C-NMR δ: 180.27, 166.18, 165.27, 155.29, 140.18, 139.19, 134.62, 133.95, 126.41, 124.70, 123.68, 122.58, 121.20, 118.54; Anal. calcd. for C14H12FN3OS (289.2): C 58.12, H 4.18, N 14.52; found C 58.56, H 4.14, N 14.68.
1-(4'-Chlorophenyl)acyl-3-(2'-aminophenyl) thiourea (5d): Yield 58%, mp 182~184oC. IR (KBr): 3225, 1630, 1240 cm−1; 1H-NMR δ: 12.90 (s, 1H, NH), 11.34 (s, 1H, NH), 7.97-6.80 (m, 8H, Ar-H), 5.23(s, 2H, Ar-NH); 13C-NMR δ: 180.23, 165.19, 154.67, 141.13, 139.37, 136.89, 135.05, 130.78, 128.95, 126.73, 125.47, 121.40, 120.85, 119.44; Anal. calcd. for C14H12ClN3OS (305.7): C 54.99, H 3.96, N 13.74; found C 56.00, H 3.94, N 13.80.
1-(4'-Bromophenyl)acyl-3-(2'-aminophenyl) thiourea(5e): Yield 50%, mp 156~158oC. IR (KBr): 3230, 1680, 1245 cm−1; 1H-NMR δ: 12.70 (s, 1H, NH), 11.44(s, 1H, NH), 7.92-6.75 (m, 8H, Ar-H), 5.19(S, 2H, Ar-NH); 13C-NMR δ: 180.30, 165.21, 154.89, 141.19, 139.17, 136.69, 135.75, 130.80, 128.99, 126.80, 125.87, 122.67, 121.78, 120.56; Anal. calcd. for C14H12BrN3OS (350.2): C 48.01, H 3.45, N 12.00; found C 48.06, H 3.54, N 12.08.
1-(2'-Pyridyl)acyl-3-(2'-aminophenyl) thiourea (5f): Yield 78%, mp 180~181oC. IR (KBr): 3190, 1675, 1250 cm−1; 1H-NMR δ: 12.90 (s, 1H, NH), 11.68 (s, 1H, NH), 8.02-7.83 (m, 4H, Py-H), 7.03-6.75 (m, 4H, Ar-H), 5.20 (s, 2H, Ar-NH); 13C-NMR δ: 180.36, 166.90, 154.48, 151.23, 147.67, 137.50, 130.48, 126.76, 125.51, 124.56, 124.00, 119.96,114.90; Anal. calcd for C13H12N4OS (272.3): C 57.34, H 4.44, N 20.57; found C 57.36, H 4.44, N 20.68.
1-(3'-Pyridyl)acyl-3-(2'-aminophenyl) thiourea (5g): Yield 49%, mp 180~181oC. IR (KBr): 3215, 1665, 1242 cm−1; 1H-NMR δ: 12.91 (s, 1H, NH), 11.64 (s, 1H, NH), 8.02-7.83 (m, 4H, Py-H), 7.03-6.75 (m, 4H, Ar-H), 5.21 (s, 2H, Ar-NH); 13C-NMR δ: 180.36, 166.92, 154.48, 151.23, 147.57, 137.51, 130.48, 126.76, 125.55, 124.56, 124.02, 119.99, 114.90; Anal. calcd. for C13H12N4OS (272.3): C 57.34, H 4.44, N 20.57; found C 57.37, H 4.44, N 20.66.
1-(2'-Furanyl)acyl-3-(2'-aminophenyl) thiourea (5h): Yield 48 %, mp 121~122oC. IR (KBr): 3220, 1640, 1250 cm−1; 1H-NMR δ: 12.91 (s, 1H, NH), 11.64 (s, 1H, NH), 8.09-6.83 (m, 3H, furyl-H), 7.03-6.75 (m, 4H, Ar-H), 5.20 (s, 2H, Ar-NH); 13C-NMR δ: 180.30, 166.78, 154.45, 147.23, 143.65, 130.48, 125.53, 124.56, 119.63, 115.36, 109.71; Anal. calcd. for C12H11N3O2S (261.3): C 55.16, H 4.24, N 16.08; found C 55.20, H 4.24, N 16.09.
4.3. Biological Assays
All analogues were tested against N. brazilliensis to evaluate their anti-intestinal nematode activities using the screening method described by Cavier [18].. The compounds were dissolved in dimethyl formamide (DMF) and serially diluted with water containing Triton X-80 (0.1 mg/L) to get the required test concentrations. Each rat in the respective group received 10 mg/kg body weight using oral candle. These compounds were tested on ten groups of rats, each containing three rats. Evaluations were based on a percentage scale of 0-100, in which 100 was total kill and 0 was no activity. All results are shown in Table 1. The reference compound was albendazole, and water containing DMF (0.5 mg/L) and Triton X-80 (0.1 mg/L) was used as a negative control. The trials commenced on the 10th day after infecting each of 30 rats with 250 N. brazilliensis larvae. The percentage deparasitization was calculated using the following formula:
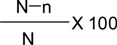 |
where N = average number of worms found in the control animals and n = average number of worms found in the groups of treated animals (including worms in rats at post mortem).
Acknowledgements
The authors wish to acknowledge that this project is supported by National Institute for Diseases (2010A102) and Shanghai Health Bureau (2009Y109).
Footnotes
Sample Availability: Samples of the compounds 5a-5h are available from the authors.
References and Notes
- 1.Ceballos L., Elissondo M., Bruni S.S., Denegri G., Alvarez L., Lanusse C. Flubendazole in cystic echinococcosis therapy: pharmaco-parasitological evaluation in mice. Parasitol. Int. 2009;58:354–358. doi: 10.1016/j.parint.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Cumino A.C., Elissondo M.C., Denegri G.M. Flubendazole interferes with a wide spectrum of cell homeostatic mechanisms in Echinococcus granulosus protoscoleces. Parasitol. Int. 2009;58:270–277. doi: 10.1016/j.parint.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi I., Kajisa M., Farid A. S., Yamanaka A., Horii Y. Paralytic ileus and subsequent death caused by enteric parasite, strongyloides papillosus, in mongolian gerbils. Vet. Parasitol. 2009;162:100–105. doi: 10.1016/j.vetpar.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Vanparijs O. Chemotherapy of experimental echinococcus multilocularis in jirds. Parasitol.Res. 1990;76:238–240. doi: 10.1007/BF00930820. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y. H., Wang X. G., Chen Y. T. Continuous long-term albendazole therapy in intraabdominal cystic echinococcosis. Chin. Med. J. 1991;104:930–933. [PubMed] [Google Scholar]
- 6.Geary T. G., Conder G. A., Bishop B. The changing landscape of antiparasitic drug discovery for veterinary medicine. Trends. Parasitol. 2004;20:449–455. doi: 10.1016/j.pt.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Mc Kellar Q. A., Scott E. W. The benzimidazole anthelmintic agents. J. Vet. Pharmacol. Ther. 1990;13:223–247. doi: 10.1111/j.1365-2885.1990.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 8.Coles G. C., Jackson F., Pomroy W. E., Prichard R. K. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006;136:167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Luis F., Hernandez-Campos A., Yepez-Mulia L., Cedillob R., Castilloa R. Synthesis and hydrolytic stability studies of albendazole carrier prodrugs. Bioorg. Med. Chem. Lett. 2001;11:1359–1362. doi: 10.1016/s0960-894x(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen L. S., Slok F., Bundgaard H. N-Alkoxycarbonyl prodrugs of mebendazole with increased water solubility. Int. J. Pharm. 1994;102:231–239. doi: 10.1016/0378-5173(94)90060-4. [DOI] [Google Scholar]
- 11.Nielsen L. S., Bundgaard H., Falch E. Prodrugs of thiabendazole with increased water-solubility. Acta Pharm. Nord. 1992;4:43–49. [PubMed] [Google Scholar]
- 12.Dawson M., Watson T. R. 4-Amino-3-(3′-methoxycarbonyl-2′-thioureido)benzophenone, a prodrug of mebendazole. Eur. J. Drug. Metab. Pharmacokinet. 1983;8:329–334. doi: 10.1007/BF03188765. [DOI] [PubMed] [Google Scholar]
- 13.Pervze H., Iqbal H. P., Tahir M.Y., Nasim F.H., Choudhary M.I., Khan K.M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4-substituted isatin-3-thiosemicarbazones. J. Enzym. Inhib. Med. Chem. 2008;23:848–854. doi: 10.1080/14756360701746179. [DOI] [PubMed] [Google Scholar]
- 14.Küçükgüzel I., Güniz Küçükgüzela S., Rollasa S., Kirazb M. Some 3-thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials. Bioorg. Med. Chem. Lett. 2001;11:1703–1707. doi: 10.1016/S0960-894X(01)00283-9. [DOI] [PubMed] [Google Scholar]
- 15.Greenbaum D. C., Mackey Z., Hansell E., Doyle P., Gut J., Caffrey C. R., Lehrman J., Rosenthal P. J., Mckerrow J. H., Chibale K. Synthesis and structure-activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against plasmodium falciparum, trypanosoma brucei, and trypanosoma cruzi. J. Med. Chem. 2004;47:3212–3219. doi: 10.1021/jm030549j. [DOI] [PubMed] [Google Scholar]
- 16.Bharti N., Husain K., Garza M. T. G., Vega D. E. C., Garza J. C., Cardenas B. D. M., Naqvi F. Synthesis and in vitro antiprotozoal activity of 5-nitrothiophene-2-carboxaldehyde thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2002;12:3475–3478. doi: 10.1016/S0960-894X(02)00703-5. [DOI] [PubMed] [Google Scholar]
- 17.Xue S., Duan L., Ke S., Zhu J. Synthesis and herbicidal activities of pentylchrysanthemacyl thiourea pyrimidine derivatives and related fuse ring compounds. Chin. J. Org. Chem. 2004;24:686–690. [Google Scholar]
- 18.Cavier R. Chemotherapy of Helminthiasis. Vol. 1. Pergamon Press; Oxford, UK: 1973. p. 215. [Google Scholar]