Abstract
Despite gaps in our knowledge of how phytochemicals interfere with cellular functions, several natural plant products are utilized to prevent or treat a wide range of diseases. Identification of an agent with therapeutic potential requires multiple steps involving in vitro studies, efficacy and toxicity studies in animal models, and then human clinical trials. This review provides a brief introduction on natural products that may help to treat and/or prevent bronchial asthma and describes our current understanding of their molecular mechanisms based on various in vitro, in vivo, and clinical studies. We focus on the anti-inflammatory and anti-vascular actions of the plant products and other roles beyond the anti-oxidative effects.
Keywords: antioxidants, bronchial asthma, plant
Introduction
Phytotherapy, regarded as an “alternative medicine”, is one of the complementary approaches using extracts from natural origin as medicines or health-promoting agents. In recent years, natural products have received great attention for disease prevention due to their various health benefits and noticeable lack of toxicity and side effects [1]. Many dietary plant products such as grains, nuts, cereals, soy, spices, flaxseed oil, fruits, vegetables, medicinal plants, and herbs contain various phytochemical constituents, such as phenolics, carotenoids, alkaloids, nitrogen and organosulfur compounds, and vitamins.
Recent in vivo and in vitro studies have shown a potential anti-inflammatory role for some of the known natural compounds with antioxidant activity. Combinations of natural antioxidants offer a variety of mechanisms for reducing oxygen metabolites in tissues, altering signaling pathways, and modulating transcription factors, and they might play key roles in reducing reactive oxygen species (ROS)-dependent damage. Numerous bioactive plant compounds, including several polyphenols, have been recently tested for their antiangiogenic potential.
Asthma and chronic obstructive pulmonary disorder (COPD) are chronic inflammatory diseases of the airways characterized by airway hyperresponsiveness and airflow limitation with acute bronchoconstriction, swelling of the airway walls, chronic mucus plug formation, and airway wall remodeling. Increasing evidence has indicated that oxidative stress, one of the causative mechanisms of asthma, is thought to be a central event in inflammatory responses through activation of transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), resulting in gene expression of proinflammatory mediators [2]. Antioxidants with good bioavailability may thus protect against the direct injurious effects of oxidants but also may fundamentally alter the inflammatory events in the pathogenesis of various airway diseases.
This review will provide recent objective findings on the anti-oxidative, anti-inflammatory, and anti-vascular actions of plant products in asthma and the future perspectives in this field.
Oxidative Stress in Asthma
The lung is continuously exposed to oxidants, either generated endogenously by metabolic reactions (e.g. from mitochondrial electron transport during respiration or released from phagocytes) or derived from exogenous sources (e.g. air pollutants and cigarette smoke) [3,4,5,6].
There is now substantial evidence that oxidative stress plays a critical role in the pathogenesis of various lung disorders including asthma, COPD, acute lung injury, pulmonary fibrosis, and lung cancer [7,8,9,10,11]. Asthma is a chronic inflammatory disease of the airway characterized by airway eosinophilia, goblet cell hyperplasia with mucus hypersecretion, and hyperresponsiveness to inhaled allergens and nonspecific stimuli [12,13]. In addition, increased oxidative stress is related to severity of asthma, propagation of inflammatory response, and reduction of responsiveness to corticosteroids [14].
Allergen-activated and recruited inflammatory cells such as eosinophils, macrophages, monocytes and neutrophils from asthmatic patients produce more ROS than do those from normal subjects [15,16]. The constitutive airway cells such as epithelial cells are also a potential source of ROS [17]. In addition to cellular sources of ROS, several asthma mediators including lipid mediators, chemokines, adhesion molecules, and eosinophil granule proteins are potential stimuli of ROS production [18,19,20,21,22]. Besides, some environmental factors such as ozone and diesel exhaust particles may cause an extreme increase of ROS generation in the airway [23]. ROS can perturb airway cells thereby exacerbating many of the pathophysiologic features associated with asthma. ROS directly stimulate histamine release from mast cells and mucus secretion from airway epithelial cells [24,25,26]. The increased release of ROS can also directly damage to epithelial cells and cause cell shedding [15]. Studies have demonstrated that ROS lead to endothelial barrier dysfunction with subsequent increase of cell permeability to fluid, macromolecules, and inflammatory cells [7]. Overproduction of ROS or depression of the protective system in cells results in bronchial hyperreactivity, which is characteristic of asthma [27,28,29].
ROS induce contraction of airway smooth muscle, and this effect is enhanced when the epithelium is injured or removed [27]. In fact, H2O2 has been shown to cause contraction of airway smooth muscle and airway hyperresponsiveness in animal models [30]. Studies with animal models have indicated that ROS contribute to airway hyperresponsiveness by increasing vagal tone due to damage of oxidant-sensitive β-adrenergic receptors as well as decreasing mucociliary clearance [31,32]. Moreover, ROS can decrease numbers and function of epithelial cilia, increase mucus production, alter release of inflammatory mediators, and cause influx of inflammatory cells [7].
Under pathologic conditions, ROS exert a multitude of actions through various signaling pathways involving mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/Akt, and protein kinase C [PKC]), thereby activating pro-inflammatory gene transcription factors such as NF-κB, AP-1, and hypoxia-inducible factor (HIF)-1α [30,33,34,35,36,37,38,39,40,41]. ROS are also involved in production of a number of inflammatory mediators, most notably eicosanoids, by activating phospholipase A2 (PLA2) [42,43]. ROS even play a role in responses to innate immune stimuli including lipopolysaccharide and viruses as well as to acquire immune responses such as antigen interactions with IgE or IgG antibodies [44,45,46].
Antioxidant System in the Lung – Balancing Intracellular Oxidation
The lung has several natural antioxidant mechanisms to neutralize overproduced oxidants (ROS, reactive nitrogen species, and lipid peroxides), which include enzymatic as well as non-enzymatic antioxidants. These antioxidant defense systems form a tightly regulated network to resist any change in the redox environment of intra- and extracellular space [47]. Enzymatic antioxidants include catalase, glutathione peroxidase (GPX) and superoxide dismutase (SOD), and non-enzymatic antioxidants are vitamin C, vitamin E, albumin, uric acid, ceruloplasmin, and glutathione (GSH) [48,49,50]. Changes in these enzymatic and non-enzymatic antioxidants can disrupt homeostasis of ROS in bronchial cells.
Deficiency of endogenous antioxidant defenses has been reported in asthma [51]. Devereux and colleagues have proposed that individuals in westernized societies have progressively reduced their consumption of fruit and vegetables and as a result, decreased pulmonary antioxidant defenses, making them more susceptible to inhaled irritants and allergens [52]. As many antioxidants are supplied from the diet, attention has been paid to intake of the micronutrient antioxidants (vitamins A, C, and E, polyphenols and carotenoids) and to know how it may help to protect individuals from an oxidizing environment and/or inflammatory airway disease.
Plants have two primary strategies to detoxify harmful oxidant radicals; one is direct enzymatic breakdown of oxidant radicals using SOD, catalse, ascorbate peroxidase, peroxidase, glutathione reductase and monodehydroascorbate reductase [53]. These enzyme systems convert various oxidant radicals to reduced products. The second strategy is synthesis of antioxidant molecules such as vitamin C and vitamin E. These antioxidant compounds possess a hydroxyl group (-OH) on the ring structure, with an associated electron deficiency, which are highly reactive towards ROS [54,55].
Antioxidant Molecules in Plants and the Their Role in Bronchial Asthma
In the next paragraphs, we described the antioxidant and anti-inflammatory properties of some important natural bioactive compounds, which exert favorable effects on bronchial asthma (Table 1). These agents would be useful not only for reducing oxidative stress in asthma but also for the control of inflammation. Most of their actions are related to their ability to direct enzymatic breakdown via endogenouse anti-oxidative enzymes, synthesis of various anti-oxidants and quenchers, thereby inhibiting cytokine, chemokine or adhesion molecule synthesis and/or action. However, only a few plant-derived compounds have been submitted to clinical trials to test their potential as antioxidative and anti-inflammatory agents.
Table 1.
Plant antioxidants and their postulated mechanism of effect.
| Bioactive compounds | Activity and Potential mechanisms of effect | |
|---|---|---|
| Polyphenols | ||
| Flavonoids | Flavans | Donation of hydrogen atom to radicals |
| Flavanones | Chelation of redox-active metals | |
| Isoflavanones | Inhibition of lipid peroxidation | |
| Flavones | Regulation of the enzyme activities | |
| Isoflavones | Inhibition of mast cell/basophil activation | |
| Inhibition of eosinophlic degranulation | ||
| Anthocyanidins | Switching allergic immune response to Th1 profile | |
| Chalcones | Regulation of various transcription factors and mediators involving angiogenesis: HIF-1, VEGF, MMPs, EGFR and inhibit NF-κB, PI3K/Akt, and ERK1/2 signaling pathways | |
| Flavonolignans | ||
| Curcumin | Prevention of lipid peroxydation | |
| Radical scavenger/neutralizer | ||
| Decrease the levels of iNOS | ||
| Regulation of cytokines such as IL-2, IL-5, and GM-CSF through maintaining HDAC2 activity | ||
| Inhibition of mast cell activation | ||
| Inhibition of neutrophil function | ||
| Resveratrol | Inducing and stabilizing antioxidant enzymes | |
| Inhibition of prostaglandin production | ||
| Decrease the phosphorylation of ERK1/2, cyclooxygenase-2 activity, and activity of various transcription factors including NF-κB, STAT3, HIF-1α, and β-catenin | ||
| Inhibit protein kinases (src, PI3K, JNK and Akt) | ||
| Inhibit production of inflammatory mediators (IFN-γ, TNF, COX-2, iNOS, CRP and various interleukins) | ||
| Sirtuin1 activation | ||
| Modulate innate immune response | ||
| Inhibition of angiogenetic pathway that is mediated through expression of MMPs, VEGF, cathepsin D, ICAM-1 and E-selectin | ||
| Antioxidant vitamins | ||
| Carotenoids | Lycopene | Quenches singlet oxygen without degradation |
| Lutein | Regulation of various transcription factors (AP-1, NF-κB) | |
| β-cryptoxanthin | Supression production of inflammatory cytokines | |
| α-carotene | Reduce induction of IGF-1 | |
| β-carotene | ||
| Vitamin C | Ascorbic acid | Donation of hydrogen atom to radicals |
| Dehydro-ascorbic acid | Inhibiting the JNK/AP-1 signaling pathways | |
| prostaglandin inhibition | ||
| Vitamin E | Tocopherols | Hydroperoxide scavenger |
| (α, β, γ, and δ) | Modulation of the functional activity of T-lymphocytes and enhance the phagocytic activity of peripheral granulocytes | |
| Tocotrienols | ||
| (α, β, γ, and δ) | ||
| Inhibit monocyte response to LPS and LPS-induced degrdation of IκB and JNK activation | ||
| Regulation of endothelial cell signals | ||
| Membrane stabilization | ||
| Inhibition of IgE production | ||
| Organosulfur compounds | ||
| α-lipoic acid | Quenches reactive oxygen species | |
| Regenerates/recycles endogenous and exogenous antioxidants | ||
| Chelates redox metals | ||
| Modulate the activity of transcription factors | ||
| Volatile compounds | ||
| Phytoncides | Insecticide | |
| Antibacterial/antifungal activity | ||
| Radical scavenging activity | ||
| Enhance the activity of NK cells | ||
| Restoring antioxidants | ||
| Modulate the activity of transcription factor, NF-κB | ||
| Attenuate allergic inflammation | ||
AP-1, activator protein-1; COX, cyclooxygenase; CRP, C-reactive protein; EGFR, epidermal growth factor receptor; GM-CSF, granulocyte macrophage colony-stimulating factor; HDAC, histone deacetylase ; HIF, hypoxia inducible factor; IκB, inhibitory kappaB; IFN, interferon; iNOS, inducible nitric oxide synthase; JNK, Jun N-terminal kinase; LPS, lipopolysaccharide; MMPs, matrix metalloproteinases; NF-κB, nuclear factor-kappaB; NK, natural killer; PI3K, phosphoinositide 3-kinase; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
Flavonoids
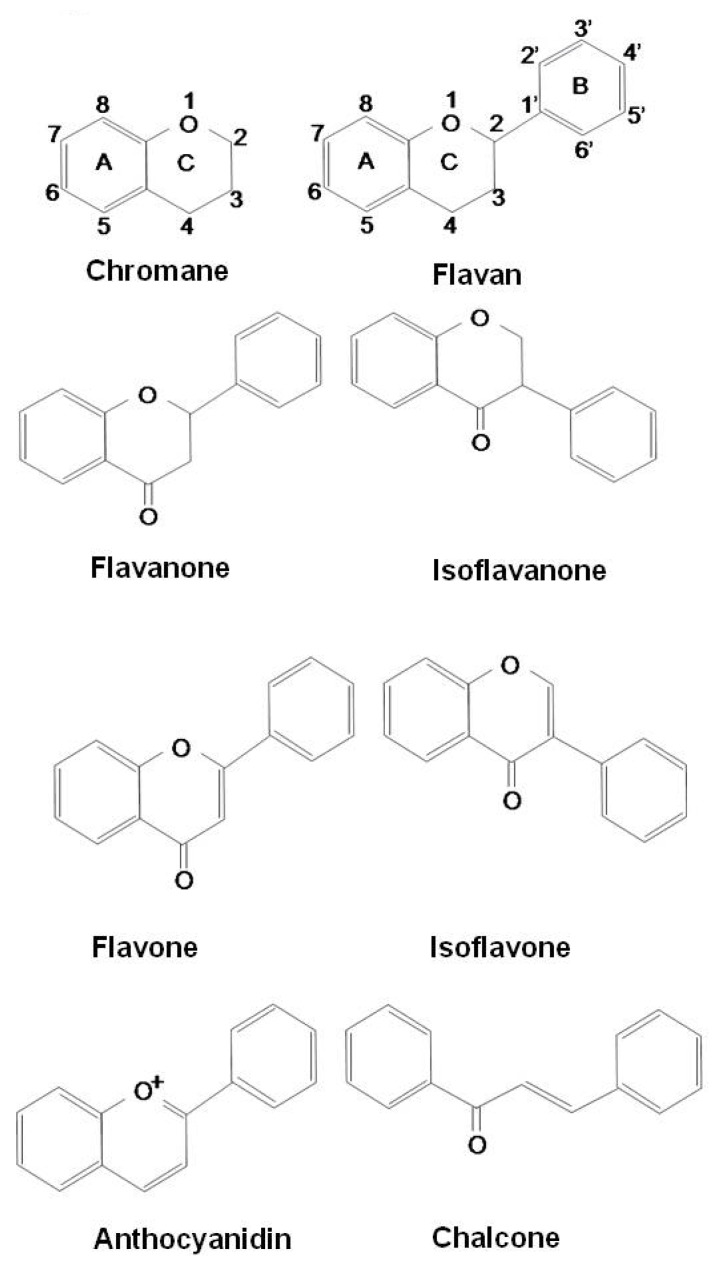
Flavonoids constitute the most important single group of polyphenols of low molecular weight polyphenolic secondary plant metabolites, with more than 8,000 compounds described [56,57,58]. They are found in fruits, vegetables, nuts, seeds, stems, flowers, roots, tea, wine, and coffee and are common substances in our daily diet [59,60]. Their structure is a heterocyclic hydrocarbon, chromane, and substitution of its ring C in position 2 or 3 with a phenyl group (B-ring) results in flavans or isoflavans. An oxo-group in position 4 leads to flavanones and isoflavanones. The presence of a double bond between C2 and C3 provides flavones and isoflavones. An additional double bond in-between C1 and C2 makes these compounds colorful anthocyanidins. Based on their structure, flavonoids are categorized into eight groups: flavans, flavanones, isoflavanones, flavones, isoflavones, anthocyanidins, chalcones, and flavonolignans (Figure 1).
Figure 1.
Structures of flavonoids.
Flavonoids interfere with oxidation of lipids and other molecules by rapid donation of hydrogen atoms to ROO• radicals. This hydrogen (electron) donating ability of a flavonoid molecule that acts to scavenge reactive radical species is primarily associated with the presence of a B-ring catechol group (dihydroxylated B-ring) [61]. One important structural feature, which is partially responsible for the antioxidant properties of flavonoids, is the presence of 2,3-unsaturation in conjugation with a 4-oxo group in the C-ring. In addition, the presence of functional groups involving both hydroxyl groups of B-ring and the 5-hydroxy group of A-ring is an important contributor in the ability of flavonoids to chelate redox-active metals and thus prevents catalytic breakdown of H2O2 [61]. The phenoxy radical intermediates are relatively stable, so they do not initiate further radical reaction and act as terminators of the reaction chains by interacting with other free radicals. Flavonoids are ideal scavengers of peroxyl radicals due to their favourable reduction potentials relative to alkyl peroxyl radicals, and thus they are effective inhibitors of lipid peroxidation [61]. Therefore, this strong anti-oxidative property of flavonoids has made them protective against airway diseases linked to oxidative stress [62,63]. In fact, several epidemiologic data suggest beneficial effects of flavonoids on asthma. A population-based case-control study has suggested that apple consumption or red wine intake were negatively associated with asthma prevalence or severity, respectively, perhaps due to a protective effect of flavonoids [64]. Moreover, a 30-year longitudinal epidemiological study reported that the incidence of asthma is lower in populations with higher intake of flavonoids [65].
Beyond anti-oxidative effects, flavonoids inhibit release of histamine and other preformed granule-associated mediators by inhibiting the activation of basophils and mast cells [66]. Flavonoids also inhibit synthesis of IL-4, IL-13, and CD40 ligand but initiate generation of new phospholipid-derived mediators. One of the well characterized flavonoids, quercetin inhibits eosinophilic secretion of Charcot-Leyden crystal protein and eosinophil cationic protein in a concentration-dependent manner [67]. Moreover, the inhibitory action of quercetin on other inflammatory cells appears to surpass any other clinically available compounds [57]. Very recently, Li et al. have also demonstrated that apigenin exhibits an anti-inflammatory activity in a murine asthma model and can switch the immune response to allergens toward the T-helper type 1 cell (Th1) profile [68]. These findings suggest that flavonoids are anti-allergenic and anti-inflammatory agents effective in treating/preventing of asthma.
Vascular changes are one of the major components for asthmatic pathogenesis [69]. The changes include an increase in vascular permeability, vascular dilation/engorgement, and vasculogenesis/angiogenesis [69]. Flavonoids and their related compounds have been shown to modulate expression of HIF-1, VEGF, matrix metalloproteinases (MMPs), and epidermal growth factor receptor but also inhibit NF-κB, PI3K/Akt, and ERK1/2 signaling pathways [70,71,72,73,74,75,76,77]. These observations have suggested that flavonoids as well as their related compounds inhibit certain steps of angiogenesis that are cell migration, microcapillary tube formation, and MMP expression [77,78].
Curcumin (diferuloylmethane)
The active component of turmeric is curcumin, a polyphenolic phytochemical with anti-inflammatory, antiamyloid, antiseptic, antitumor, antiallergic, and anti-oxidative properties [79]. In addition to its culinary uses, curcumin has been used as a traditional medicine for liver disease (particularly jaundice), indigestion, urinary tract diseases, blood purification, inflamed joints (rheumatoid arthritis), insect bites, dermatological disorders, and atherosclerosis [79]. Curcumin has been shown to be eight times more powerful than vitamin E in preventing lipid peroxidation [79,80]. It has also been suggested that curcumin plays a role in reducing oxidative stress by downregulating nitric oxide (NO) formation, scavenging or neutralizing free radicals, especially superoxide anion, and breaking the oxidative chain reaction caused by free radicals [80,81,82].
Curcumin has been implicated as an anti-inflammatory agent, but the precise mechanisms of its action are largely unknown. However, studies have demonstrated that curcumin decreases the level of inducible NO synthase induced by IFN-γ in lung tissue and expression of cytokines such as IL-2, IL-5, and GM-CSF through acting as a HDAC activator or inhibits histamine release from mast cells [79,80,83,84,85]. Such regulations by curcumin attenuate asthma phenotypes, reducing asthmatic symptoms, recruitment of eosinophils to the airway, and airway hyper-responsiveness. These findings indicate that curcumin may be useful as an adjuvant therapy for asthma.
Resveratrol
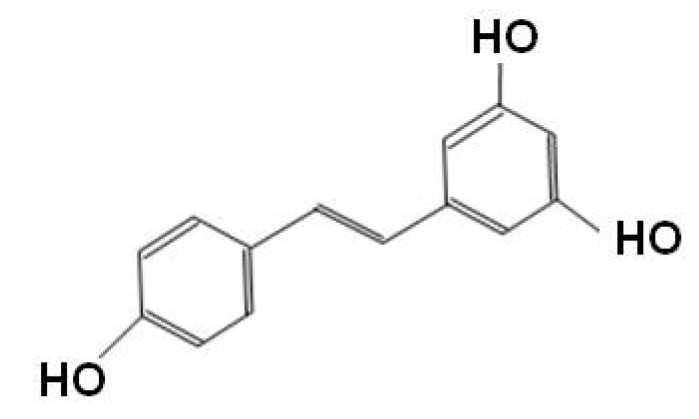
Resveratrol (3,4΄,5-trihydroxystilbene), more famous for being a constituent of red wine, is a phytoalexin found in various plants including grapes, berries, and peanuts [86]. Resveratrol is one of the polyphenolic compounds consisting of two phenol rings connected by a 2-carbon methylene bridge with several benefits on cardiovascular protection, anticancer effect, and a positive regulator of several aspects of metabolism (Figure 2) [87,88,89].
Figure 2.
Sturucture of resveratrol.
Resveratrol is capable of scavenging intracellular ROS by inducing and stabilizing antioxidant enzymes such as catalase, SOD, and glutathione peroxidase hemoxygenase [90,91]. In addition to its reducing properties, resveratrol has been shown to attenuate inflammation via inhibition of prostaglandin production and to decrease the phosphorylation of ERK1/2, cyclooxygenase-2 activity, and activity of various transcription factors including NF-κB, STAT3, HIF-1α, and β-catenin [92,93,94,95,96,97]. Resveratrol has also been known to inhibit protein kinases (e.g. src, PI3K, JNK, and Akt) and production of inflammatory mediators (e.g. IFN-γ, TNF, COX-2, iNOS, CRP and various interleukins) [98,99,100,101,102,103]. Moreover, recent studies have reported that resveratrol activates sirtuin1 (SIRT1) which is linked with apoptosis and longevity [104,105]. SIRT1 modulates poly (ADP-ribose) polymerase-1 (PARP-1) activity upon DNA damage. Activation of SIRT1 by resveratrol leads to a decrease in PARP-1 activity and promotes cell survival, which can attenuate the inflammatory reaction.
Resveratrol is also able to modulate innate immune response by inhibiting expression of costimulatory molecules (CD80 and CD86) and major histocompatibility complex classes I and II in bone marrow-derived dendritic cells [106] and to inhibit angiogenesis pathway that is mediated through expression of MMPs, VEGF, cathepsin D, ICAM-1, and E-selectin. These findings suggest that resveratrol can be a very attractive compound for preventing/treating asthma since this compound displays multiple therapeutic effects, showing antioxidative, anti-inflammatory, immume modulating, and vascular protective property.
Antioxidant Vitamins
Carotenoids
Total vitamin A consists of preformed vitamin A (retinol) and provitamin A compounds, defined as carotenoids, the most important being beta-carotene. Carotenoids are pigments found in plants and microorganisms [107]. Various studies have indicated that carotenoids may prevent or inhibit certain types of cancer, atherosclerosis, immunological disorders, asthma, and other diseases [56,108,109,110,111,112]. The antioxidant activity of carotenoids arises primarily as a consequence of the ability of the conjugated double-bonded structure to delocalize unpaired electrons [113]. Among them, β-carotene quenches singlet oxygen without degradation and reacts with free radicals such as the peroxyl, hydroxyl, and superoxide radicals. This process prevents chain reactions that could result in lipid peroxidation or DNA damage, both of which have been postulated as being precursors of disease processes [56,114,115].
To date, our understanding of the role of carotenoids in asthma is limited. Based on the results from the literature search, carotenoids, in particular, β-carotene seem to exhibit bimodal behavior depending on some factors; they show antioxidant behavior at low oxygen partial pressures, usually below 150 Torr, but at high pressures of oxygen, they may lose antioxidant property, or even become pro-oxidants [116]. Concentration of carotenoids also influences their anti/pro-oxidant properties in a similar manner; at high carotenoid concentrations, there is a propensity for pro-oxidant behavior [116,117]. In asthmatics, reduced levels of total carotenoids (lycopene, lutein, β-cryptoxanthin, α-carotene and β-carotene) in the whole blood were observed, as compared to healthy controls [118]. Additionally, hypovitaminosis A induces respiratory epithelial changes, such as metaplasia, and may predispose to respiratory infections, which may exacerbate acute asthmatic attacks in children [119,120,121,122].
Carotenoids may regulate activation of a variety of transcription factors [123]. Treatment of cells exposed to oxidative stress with β-carotene suppresses oxidative stress-induced activation of NF-κB and production of IL-6, TNF-α, and inflammatory cytokines. Carotenoids may influence the process of apoptosis in healthy cells. While the pro-apoptotic protein Bax is down-regulated after induction of external stimuli, β-carotene is able to increase expression of the anti-apoptotic protein Bcl-2 in normal cells [124]. In addition, β-carotene exhibits a pro-apoptotic effect in colon and leukemic cancer cells, and this effect occurs by a redox-dependent mechanism linked with NF-κB activity [125]. Lycopene has also been shown to regulate transcription factors. Mammary cancer cells treated with lycopene have shown to inhibit AP-1 binding and reduce induction of insulin-like growth factor-I. These dual roles of vitamin A including carotenoids on apoptosis provide the capability of carotenoids as an effective anti-inflammatory agent in various diseases.
Vitamin C
Vitamin C (ascorbic acid) is a very important and powerful antioxidant, which works in aqueous environment of the body. It presents in two biologically active forms, ascorbic acid and its oxidized derivative, dehydro-ascorbic acid, which are interconvertible [126]. Vitamin C acts as a hydrogen donor to reverse oxidation and protects membranes against oxidation (i.e., reducing agent). However, its effects, at very high doses, have been a subject of intense debate for many years [127,128]. Vitamin C prevents acid-catalyzed generation of N-nitroso compounds and competes with the secondary amines and amides for nitrosating species [129]. The intake of high doses of vitamin C (up to 2,000 mg/day) has been tried but its beneficial action has never been really established [130,131]. Some clinical studies have demonstrated that low level of vitamin C is associated with asthma risk in children, and asthmatic subjects had significantly decreased ascorbic acid and conversely supplementation of vitamin C benefits asthmatic adults who smoke, reducing cough and wheeze [132,133].
Vitamin C is also able to regulate factors that may influence gene expression, apoptosis, and other cellular functions. In fact, it protects against cell death triggered by various stimuli, and major proportion of this protection is associated with its antioxidant ability [129]. Vitamin C regulates the AP-1 complex including Fos and Jun superfamilies. Treatment of cells exposed to UV-B irradiation with vitamin C results in a 50% decrease in JNK phosphorylation, which activates AP-1, therewith inhibiting the JNK/AP-1 signaling pathways [134]. At present, however, evidence from randomized-controlled trials is insufficient to recommend a specific role for vitamin C in the treatment of asthma due to variable study design and generally poor reporting system [135].
Vitamin E
Vitamin E is a fat-soluble vitamin that exists in eight different forms. It is consists of a group of substances belonging to two closely related families, the tocopherols and tocotrienols, with each existing in a number of isomeric forms (α, β, γ, and δ) [136]. Alpha-tocopherol is the most active form of vitamin E in humans and is considered the major membrane-bound antioxidant employed by cells [137,138,139,140]. Its main antioxidant function is protection against lipid peroxidation [141]. Alpha-tocopherol is converted to α-tocopherol radical by donation of labile hydrogen to a lipid or lipid peroxyl radical, thus preventing the increase of oxidative radicals being more produced. In the case of damage to fatty acids (e.g. polyunsaturated fatty acids in cell membranes), lipid peroxidation alters the function of the cell membrane and possibly cause irreversible damage to metabolic pathways [114,115,142,143]. There is an interaction between vitamin E and other nutrients, particularly selenium and vitamin C in the antioxidant role. The α-tocopherol radical can thus be reduced to the original α-tocopherol form by ascorbic acid [144].
Normal plasma level of tocopherol may enhance lipoxygenation of arachidonic acid, whereas high tocopherol level exerts a suppressive effect as a hydroperoxide scavenger. Receptor-mediated activation of neutrophils in individuals with asthma results in synthesis of leukotrienes, which are known to play important roles in asthma pathophysiology [126]. Vitamin E may induce immunological effects via modulation of the functional activity of T-lymphocytes and enhance the phagocytic activity of peripheral granulocytes [145].
Several epidemiological trials have reported the efficacy of vitamin E supplements in bronchial asthma [146,147,148,149]. Vitamin E intake was generally unrelated to asthma status but was significantly lower in severe asthma than in mild asthma [133]. Currently, any randomized controlled trial and case-control study do not confirm the positive effect of an eventual supplementation of vitamin E. However, there are opposing regulatory functions of vitamin E isoforms thus we have to be cautious on interpretation of vitamin E studies. γ-Tocopherol also appears to be a more potent anti-inflammatory agent than α-tocopherol [150]. It decreases systemic oxidative stress and inhibits monocyte response to lipopolysaccharide (LPS) and LPS-induced degradation of IκB and JNK activation. There is a contradictory study demonstrating that γ-tocopherol elevates inflammation and ablates the anti-inflammatory benefit of the α-tocopherol by regulation of endothelial cell signals during leukocyte recruitment in experimental asthma [151]. Dietary tocopherols are taken up from the intestine and transported via the lymph to the blood and then to the liver. In the liver, α-tocopherol is transferred to plasma lipoproteins, resulting in retention of γ-tocopherol in tissues at 10% that of α-tocopherol [152]. On interpretating these two contradictory results, we should consider their serum levels with caution since low plasma level of γ-tocopherol (1.2–7.0 μM) may act as pro-oxidant, while higher level of γ-tocopherol (19.5 μM at 8 days) exerts anti-oxidative and anti-inflammatory effects [150,151].
Alpha-lipoic acid
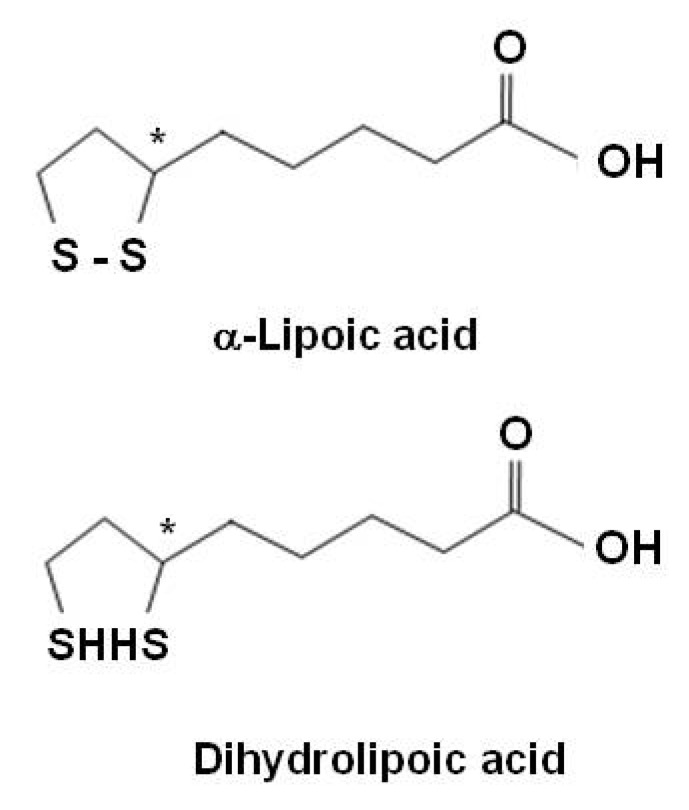
α-Lipoic acid (LA), an organosulfur compound derived from octanoic acid, is a natural compound also referred as thiothic acid. LA is readily absorbed from the diet and is converted rapidly to its reduced dithiol form, dihydrolipoic acid (DHLA) (Figure 3). Both LA and DHLA are powerful antioxidants [153]. Most LA in food is derived from lipoamide-containing enzymes and is bound to the amino acid, lysine (lipollysine). Plant sources that are rich in lipoyllysine include spinach, broccoli, and tomatoes. LA is a ''non-vitamin" nutrient that is essential to life. It is not classified as a vitamin because it is produced in the body. It is generally involved in oxidative decarboxylations of keto acids and is presented as a growth factor for some organisms. Although LA is involved in cellular energy production, its chief role as a dietary supplement may be as a powerful antioxidant. Unlike other antioxidants, LA is both fat and water-soluble and is easily absorbed and transported across cell membranes. LA directly quenches reactive oxygen species, regenerates/recycles endogenous and exogenous antioxidants such as vitamins C and E and GSH, chelates redox metals including Cu(II) and Fe(II), repairs of oxidised proteins, and modulates the activity of transcription factors such as NF-κB [56,153]. LA has the ability to regenerate other antioxidants like vitamin C, vitamin E, and GSH for further use after they have eradicated free radicals [56].
Figure 3.
Structures of α-lipoic acid and dihydrolipoic acid.
LA has been used clinically for treating oxidant-induced diseases, such as ischemia-reperfusion injury, cardiovascular disease including atherosclerosis, HIV infection, and diabetic neuropathy [154,155,156,157]. LA also possesses radio-protective properties and furthermore minimizes the pathological consequences of cigarette smoke [158]. In 1986, when Chernobyl nuclear reactor disaster was occurred, LA was found to markedly reduce the ill effects of radiation on liver and kidney function. In addition, LA is very effective in the treatment of heavy metal poisoning and Aminata mushroom poisoning [154].
In allergic airway inflammation, LA has been shown to reduce significantly serum IgE concentration, attenuate Th2 cytokines, IL-4, IL-5, IL-13, and IL-18, and reduce NF-κB- activation by decreasing intracellular ROS levels in a murine model of asthma [159]. In addition, LA also suppresses HIF-1α activation linked to VEGF expression that is a critical player in asthma pathogenesis [160]. These reports indicate that LA acts as a multi-functional molecule in airway inflammatory disorders; i.e., a potent anti-oxidant and a modulator of gene expression leading to evoke inflammatory cascades and/or plasma exudation. Until now, there is no clinical study with LA on asthmatic patients, thus, based on these positive data of LA on protective effects for the allergic airway diseases in animal models, we expect well-designed and large scaled clinical trials with LA for asthma to be performed in near future.
Selenium
Since Schwarz and Foltz established selenium as an essential trace element in the diet for prevention of diseases in 1957, it is well known as a potent nutritional antioxidant [161]. Selenium is derived from both vegetable and animal products, particularly seafood, liver, and cereals. As a member of the sulfur family of elements, selenium shares several chemical properties with sulfur, including valence states and the ability to form covalent bonds with carbon [161]. Selenium is unique among antioxidants in that it exerts its biological effects through direct incorporation into proteins (selenoproteins) as the amino acid selenocysteine. Some selenoproteins that have been characterized as important antioxidant enzymes include GPX-1, GPX-4, thioredoxin reductase-1 and thioredoxin reductase-2, and selenoprotein P [162,163,164]. The selenium-dependent enzyme, GPX recycles glutathione, reducing lipid peroxidation by catalyzing the reduction of peroxides, including hydrogen peroxide [165]. Selenium is also involved in a process of innate and adaptive immune responses [166,167].
Moreover, selenium stabilizes activated platelets which play an important physiological role in allergic processes and immunological mechanisms i.e., selenium blocks the allergic inflammation [168]. In asthma, platelets participate by acting as inflammatory cells, by releasing mediators, spasmogens and/or by interacting with other inflammatory cell types [168]. Selenium affects the expression of endothelial cell adhesion molecules, E-selectin, P-selectin, ICAM-1, VCAM-1, and ELAM-1, which are crucial in the inflammatory process for recruitment of inflammatory cells into the target tissue [169,170,171].
Despite of these positive data on selenium use in asthmatics, there are still some conflicting findings of selenium supplementation in animal and human studies for asthma prevalence or severity of asthma, and thus the issue regarding selenium is not conclusive [172,173,174,175].
Antioxidative Diet
Diet and nutrition may affect the onset and course of chronic inflammatory airway diseases. Serum lycopene and vitamin A concentrations have been found to be significantly lower in asthmatics than in those without asthma [132,133]. In contrast, vitamin E intake is generally unrelated to asthma status but the level of vitamin E in serum is significantly lower in severe asthmatics than in mild asthmatics [133]. Among children, consumption of fresh fruits, particularly fruits high in vitamin C, has been related to a lower prevalence of asthma symptoms and improved lung functions [132,133]. Dietary supplementation or adequate intake of lycopene, vitamin A, flavonoids, and vitamin C rich foods may be beneficial in asthmatic subjects. As for the prevention of the airway disease, many foods may affect development of COPD [132]. Food rich in vitamin C and E may play an especially important role in the prevention and treatment of bronchial asthma.
In fact, studies on lung function decrement and bronchial asthma in adults suggest that daily intake of vitamin C at levels exceeding the current recommended dietary allowance (60 mg/day among nonsmokers and 100 mg/day among smokers) may have a protective effect [132]. In one study, an increase of 40 mg/day in vitamin C intake led to an approximate 20 mL increase in FEV1 [132]. An epidemiological study on the impact of dietary selenium and carotenoids on major causes of mortality and morbidity (including asthma) in elderly women showed a lower risk of mortality in subjects with higher serum selenium and serum total carotenoids levels [175].
Volatile Compounds
Phytoncides
Phytoncides are natural volatile compounds emitted by trees and plants as a protective mechanism against the harmful insects and animals or microorganism. The major ingredients of phytoncide are highly volatile terpenoids such as α-pinene, carene, and myrcene [176]. Terpenoids are frequently found in essential oils from plants. Monoterpenoids have been isolated from the oils of many higher plants and are valuable in the perfumery and flavor industries. They are also found in nature as insect pheromones and defense secretions and in many marine organisms, where they are usually halogenated. There are four different types of phytoncide solutions introduced by Japanese researchers; chemical components released from trees (A-type), highly bactericidal plants (AB-type), flowering grass (D-type), and non-allergic plants (G-type) [176]. Inhalation of phytoncides is reported as forest bathing and aromatherapy (relaxing effects) [177,178] as well as antibacterial effects [179,180]. Chemical and pharmacological studies have shown that some species produce active principles that exert anti-gastropathic, anti-inflammatory, anti-oxidant, and radical scavenging activity [181,182,183]. It has been reported that physiological effects of phytoncides contribute to the improvement of various disorders including accelerated aging, allergies, multiple sclerosis, and Parkinson disease [176].
In addition, phytoncide is reported to enhance the activity of human NK cells [184]. It has also been introduced that fragrance from trees has a regulatory effect on immune function in humans and a restorative effect on the stress-induced immune suppression in mice [185,186].
There is no report about the roles of phytoncide in asthma. However, we have found that administration of phytoncide solution reduces asthmatic phenotypes of an animal model of asthma, restoring GSH level (unpublished data). To define the exact mechanism of phytoncide in asthma, further study is required. In future, we expect that volatile compounds like phytoncide are applicable to the patients with asthma as an additional therapeutic approach.
Conclusions and Closing Remarks
Asthma is characterized by ongoing inflammation and accompanied by increased oxidative stress and subsequent lung injury. ROS generation through endogenous or exogenous pathway is critical to asthmatic inflammatory responses. Though endogenous antioxidant mechanisms are present to counteract the ROS-mediated inflammatory responses, two opposing mechanisms lose their balance in an inflammatory state. Modulation of these events by enhancing antioxidant levels offers unique opportunities for therapeutic strategies for disease prevention, blocking inflammation or inhibition of airway remodeling. Clearly, the concentrations used in in vitro studies (1–100 μM) are at physiologically achievable in vivo. Though many animal studies have been revealed possible therapeutic effect of natural bioactive compounds, the available literature regarding dietary manipulation as asthma therapy is largely unconvincing currently. However, there have been remarkable advances continuously in cellular and molecular mechanisms affected by these compounds. Beyond its anti-oxidative activities, plant antioxidants have been demonstrated their anti-inflammatory effects through regulation of various inflammatory cells and mediators, vascular protective effects, and controlling roles in a wide range of signaling pathways. Natural biological compounds could be used alone or in association with other available anti-inflammatory drugs, allowing a reduction in costs and/or side effects. The question remains of whether these data are relevant enough for human disease outcomes, where exposure to natural biological compounds is chronic and at relatively low concentrations, depending on bioavailability and metabolism. The complexity of each reaction and the vast differences in physiologic influences make the clinical research difficult in regard to clinical studies using antioxidant and biologic therapies. Therefore, future studies must include various compounding factors including sufficient dose adjusted, and search for more effective, powerful natural biological compounds should be continued. Based on those studies, the long-term supplementation, large population-based randomized controlled trials with placebo are needed in order to clarify the role and effects of antioxidants in the clinical settings.
Acknowledgments
We thank Professor Mie-Jae Im for critical readings of the manuscript. This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084144).
Footnotes
Sample Availability: Not Available.
References
- 1.Manson M.M., Farmer P.B., Gescher A., Steward W.P. Innovative agents in cancer prevention. Recent Results Cancer Res. 2005;166:257–275. doi: 10.1007/3-540-26980-0_17. [DOI] [PubMed] [Google Scholar]
- 2.Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 3.Nohl H., Kozlov A.V., Gille L., Staniek K. Cell respiration and formation of reactive oxygen species: facts and artefacts. Biochem. Soc. Trans. 2003;31:1308–1311. doi: 10.1042/bst0311308. [DOI] [PubMed] [Google Scholar]
- 4.Liu P.L., Chen Y.L., Chen Y.H., Lin S.J., Kou Y.R. Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: Role of AIF and EndoG. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L739–L749. doi: 10.1152/ajplung.00099.2005. [DOI] [PubMed] [Google Scholar]
- 5.Vayssier-Taussat M., Camilli T., Aron Y., Meplan C., Hainaut P., Polla B.B., Weksler B. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1293–H1300. doi: 10.1152/ajpheart.2001.280.3.H1293. [DOI] [PubMed] [Google Scholar]
- 6.Wilson M., Lightbody J.H., Donaldson K., Sales J., Stone V. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol. Appl. Pharmacol. 2002;84:172–179. doi: 10.1006/taap.2002.9501. [DOI] [PubMed] [Google Scholar]
- 7.Henricks P.A., Nijkamp F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B., Gutteridge J.M., Cross C.E. Free radicals, antioxidants, and human disease: Where are we now? J. Lab. Clin. Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 9.MacNee W. Oxidative stress and lung inflammation in airway disease. Eur. J. Pharmacol. 2001;429:195–207. doi: 10.1016/S0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 10.Repine J.E., Bast A., Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am. J. Respir. Crit. Care Med. 1997;156:341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 11.Dworski R. Oxidant stress in asthma. Thorax. 2000;55:S51–S53. doi: 10.1136/thorax.55.suppl_2.S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay A.B. Asthma and inflammation. J. Allergy Clin. Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-G. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet J., Jeffery P.K., Busse W.W., Johnson M., Vignola A.M. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 14.Barnes P.J. Reactive oxygen species in asthma. Eur. Respir. Rev. 2000;10:240–243. [Google Scholar]
- 15.Schauer U., Leinhaas C., Jäger R., Rieger C.H. Enhanced superoxide generation by eosinophils from asthmatic children. Int. Arch. Allergy Appl. Immunol. 1991;96:317–321. doi: 10.1159/000235515. [DOI] [PubMed] [Google Scholar]
- 16.Teramoto S., Shu C.Y., Ouchi Y., Fukuchi Y. Increased spontaneous production and generation of superoxide anion by blood neutrophils in patients with asthma. J. Asthma. 1996;33:149–155. doi: 10.3109/02770909609054546. [DOI] [PubMed] [Google Scholar]
- 17.Rochelle L.G., Fischer B.M., Adler K.B. Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radic. Biol. Med. 1998;24:863–868. doi: 10.1016/S0891-5849(97)00375-4. [DOI] [PubMed] [Google Scholar]
- 18.Bruijnzeel P.L., Koenderman L., Kok P.T., Hameling M.L., Verhagen J. Platelet activating factor (PAF-acether) induced leukotriene C4 formation and luminal dependent chemiluminescence of human eosinophils. Pharm. Res. Comm. 1986;18:61–69. doi: 10.1016/0031-6989(86)90039-1. [DOI] [PubMed] [Google Scholar]
- 19.Chihara J., Hayashi N., Kakazu T., Yamamoto T., Kurachi D., Nakajima S. RANTES augments radical oxygen products from eosinophils. Int. Arch. Allergy Immunol. 1994;104:52–53. doi: 10.1159/000236752. [DOI] [PubMed] [Google Scholar]
- 20.Tenscher K., Metzner B., Schopf E., Norgauer J., Czech W. Recombinant human eotaxin induces oxygen radical production, upregulation in human eosinophils via a pertussis toxin-sensitive heterotrimeric guanine nucleotide-binding protein. Blood. 1996;88:3195–3199. [PubMed] [Google Scholar]
- 21.Nagata M., Sedgwick J.B., Vrtis R., Busse W.W. Endothelial cells upregulate eosinophil superoxide generation via VCAM-1 expression. Clin. Exp. Allergy. 1998;29:550–561. doi: 10.1046/j.1365-2222.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- 22.Rankin J.A., Harris P., Ackerman S.J. The effect of eosinophil granule major basic protein on lung-macrophage superoxide anion generation. J. Allergy Clin. Immunol. 1992;89:746–752. doi: 10.1016/0091-6749(92)90383-D. [DOI] [PubMed] [Google Scholar]
- 23.Health effects of outdoor air pollution. Committee of the environmental and occupational health assembly of the American Thoracic Society. Am. J. Respir. Crit. Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 24.Rahman I., MacNee W. Reactive oxygen species. In: Barnes P.J., Drazen J., Rennard S., Thomson N., editors. Asthma and COPD. Academic Press; London, UK: 2002. pp. 243–254. [Google Scholar]
- 25.Barnes P.J., Chung K.F., Page C.P. Inflammatory mediators of asthma: An update. Pharmacol. Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- 26.Barnes P.J. Cytokines as mediators of chronic asthma. Am. J. Respir. Crit. Care Med. 1994;150:42–49. doi: 10.1164/ajrccm/150.5_Pt_2.S42. [DOI] [PubMed] [Google Scholar]
- 27.Hulsmann A.R., Raatgeep H.R., den Hollander J.C., Stijnen T., Saxena P.R., Kerrebijn K.F., de Jongste J.C. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. Am. J. Respir. Crit. Care Med. 1994;149:519–525. doi: 10.1164/ajrccm.149.2.8306055. [DOI] [PubMed] [Google Scholar]
- 28.Cortijo J., Marti-Cabrera M., de la Asuncion J.G., Pallardó F.V., Esterase A., Bruseghini L., Viña J., Morcillo E.J. Contraction of human airways by oxidative stress protection by N-acetylcysteine. Free Radic. Biol. Med. 1999;27:392–400. doi: 10.1016/S0891-5849(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi-Hashjin G., Folkerts G., Henricks P.A., Verheyen A.K., van der Linde H.J., van Ark I., Coene A., Nijkamp F.P. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am. J. Respir. Crit. Care Med. 1996;153:1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- 30.Lee K.S., Kim S.R., Park S.J., Park H.S., Min K.H., Lee M.H., Jin S.M., Jin G.Y., Yoo W.H., Lee Y.C. Hydrogen peroxide induces vascular permeability via regulation of vascular endothelial growth factor. Am. J. Respir. Cell Mol. Biol. 2006;35:190–197. doi: 10.1165/rcmb.2005-0482OC. [DOI] [PubMed] [Google Scholar]
- 31.Adam L., Bouvier M., Jones T.L. Nitric oxide modulates beta(2)-adrenergic receptor palmitoylation and signaling. J. Biol. Chem. 1999;274:26337–26343. doi: 10.1074/jbc.274.37.26337. [DOI] [PubMed] [Google Scholar]
- 32.Owen S., Pearson D., Suarez-Mendez V., O’Driscoll R., Woodcock A. Evidence of free-radical activity in asthma. N. Engl. J. Med. 1991;325:586–587. doi: 10.1056/NEJM199108223250816. [DOI] [PubMed] [Google Scholar]
- 33.Cho Y.J., Seo M.S., Kim J.K., Lim Y., Chae G., Ha K.S., Lee K.H. Silica-induced generation of reactive oxygen species in Rat2 fibroblasts: Role in activation of mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 1999;262:708–712. doi: 10.1006/bbrc.1999.1274. [DOI] [PubMed] [Google Scholar]
- 34.Ding M., Shi X., Dong Z., Chen F., Lu Y., Castranova V., Vallyathan V. Freshly fractured crystalline silica induces activator protein-1 activation through ERKs and p38 MAPK. J. Biol. Chem. 1999;274:30611–30616. doi: 10.1074/jbc.274.43.30611. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Ilasaca M., Crespo P., Pellici P.G., Gutkind J.S., Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.C., Lee K.S., Park S.J., Park H.S., Lim J.S., Park K.H., Im M.J., Choi I.W., Lee H.K., Kim U.H. Blockade of airway hyperresponsiveness and inflammation in a murine model of asthma by a prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acid. FASEB J. 2004;18:1917–1919. doi: 10.1096/fj.04-2212fje. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.S., Park H.S., Park S.J., Kim S.R., Min K.H., Jin S.M., Li L., Lee Y.C. An antioxidant modulates expression of receptor activator of NF-κB in asthma. Exp. Mol. Med. 2006;38:217–229. doi: 10.1038/emm.2006.27. [DOI] [PubMed] [Google Scholar]
- 38.Schenk H., Klein M., Erdbrugger W., Dröge W., Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-κB and AP-1. Proc. Natl. Acad. Sci. USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper R., Wu K., Chang M.M., Yoneda K., Pan R., Reddy S.P., Wu R. Activation of nuclear factor-kappa b transcriptional activity in airway epithelial cells by thioredoxin but not by N-acetyl-cysteine and glutathione. Am. J. Respir. Cell Mol. Biol. 2001;25:178–185. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- 40.Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 41.Tikoo K., Lau S.S., Monks T.J. Histone H2 phosphorylation is coupled to poly(ADP-ribosylation) during reactive oxygen species-induced cell death in renal proximal tubular epithelial cells. Mol. Pharmacol. 2001;60:394–402. doi: 10.1124/mol.60.2.394. [DOI] [PubMed] [Google Scholar]
- 42.Zor U., Ferber E., Gergely P., Szücs K, Dombrádi V., Goldman R. Reactive oxygen species mediate phorbol ester-regulated tyrosine phosphorylation and phospholipase A2 activation: potentiation by vanadate. Biochem. J. 1993;295:879–888. doi: 10.1042/bj2950879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman R., Ferber E., Zort U. Reactive oxygen species are involved in the activation of cellular phospholipase A2. FEBS Lett. 1992;309:190–192. doi: 10.1016/0014-5793(92)81092-Z. [DOI] [PubMed] [Google Scholar]
- 44.Liu T., Castro S., Brasier A.R., Jamaluddin M., Garofalo R.P., Casola A. Reactive oxygen species mediate virus-induced STAT activation: Role of tyrosine phosphatase. J. Biol. Chem. 2004;279:2461–2469. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 45.Hsu H.Y., Wen M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 46.Seifried H.E., Anderson D.E., Fisher E.I., Milner J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Chaudiere J., Ferrari-Iliou R. Intracellular antioxidants from chemical to biochemical mechanisms. Food Chem. Toxicol. 1999;37:949–962. doi: 10.1016/S0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 48.Heffner J.A., Repine J.E. State of the art: Pulmonary strategies of antioxidant defense. Am. Rev. Respir. Dis. 1989;140:531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- 49.Toth K.M., Clifford D.P., Berger E.M., White C.W., Repine J.E. Intact human erythrocyte prevent hydrogen peroxide mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J. Clin. Invest. 1984;74:292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Asbeck B.S., Hoidal J., Vercelloti G.M., Schwartz B.A., Moldow C.F., Jacob H.S. Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science. 1985;227:756–759. doi: 10.1126/science.2982213. [DOI] [PubMed] [Google Scholar]
- 51.Wood L.G, Gibson P.G., Garg M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003;21:177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 52.Devereux G., Seaton A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 53.Atkinson C.J., Nestby R., Fod Y.Y., Dodds P.A. Enhancing beneficial antioxidants in fruits: A plant physiological perspective. BioFactors. 2005;23:229–234. doi: 10.1002/biof.5520230408. [DOI] [PubMed] [Google Scholar]
- 54.Klein B.P., Kurilich A.C. Processing effects on dietary antioxidants from plant foods. HortScience. 2000;35:580–584. [Google Scholar]
- 55.Prior R.L., Cao G. Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. HortScience. 2000;35:588–592. [Google Scholar]
- 56.Balsano C., Alisi A. Antioxidant effects of natural bioactive compounds. Curr. Pharmaceu. Des. 2009;15:3063–3073. doi: 10.2174/138161209789058084. [DOI] [PubMed] [Google Scholar]
- 57.Middleton E.J., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 58.Williams C.A., Grayer R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004;21:539–573. doi: 10.1039/b311404j. [DOI] [PubMed] [Google Scholar]
- 59.Hollman P.C., Katan M.B. Health effects and bioavailability of dietary flavonols. Free Rad. Res. 1999;31:S75–S80. doi: 10.1080/10715769900301351. [DOI] [PubMed] [Google Scholar]
- 60.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 61.Schroeter H., Boyd C., Spencer J.P., Williams R.J., Cadenas E., Rice-Evans C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging. 2002;23:861–880. doi: 10.1016/S0197-4580(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 62.Rice-Evans C. Flavonoid antioxidants. Curr. Med. Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 63.Polovka M., Brezova V., Stasko A. Antioxidant properties of tea investigated by EPR spectroscopy. Biophys. Chem. 2003;106:39–56. doi: 10.1016/S0301-4622(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 64.Shaheen S.O., Sterne J.A., Thompson R.L., Songhurst C.E., Margetts B.M., Burney P.G. Dietary antioxidants and asthma in adults: population-based case-control study. Am. J. Respir. Crit. Care Med. 2001;164:1823–1828. doi: 10.1164/ajrccm.164.10.2104061. [DOI] [PubMed] [Google Scholar]
- 65.Knekt P., Kumpulainen J., Jarvinen R., Rissanen H., Hellövaara M., Reunanen A., Hhakulinen T., Aromaa A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 66.Middleton E. The role of hydrogen peroxide in basophil histamine release and the effect of selected flavonoids. J. Allergy Clin. Immunol. 1986;78:321–328. doi: 10.1016/S0091-6749(86)80083-5. [DOI] [PubMed] [Google Scholar]
- 67.Sloan R., Boran-Ragotzy R., Ackerman S.J., Drzewiecki G., Middleton E. The effect of plant flavonoids on eosinophil degranulation. J. Allergy Clin. Immunol. 1991;282 abstract. [Google Scholar]
- 68.Li R.R., Pang L.L., Du Q., Shi Y., Dai W.J., Yin K.S. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol. Immunotoxicology. 2010 doi: 10.3109/08923970903420566. in press. [DOI] [PubMed] [Google Scholar]
- 69.Park H.S., Kim S.Y., Kim S.R., Lee Y.C. Targeting abnomrla airway vasculatiry as a therapeutical strategy in asthma. Respirology. 2010;15:459–471. doi: 10.1111/j.1440-1843.2010.01724.x. [DOI] [PubMed] [Google Scholar]
- 70.Oak M.H., El Bedoui J., Schini-Kerth V.B. Antiangiogenic properties of naturalpolyphenols from red wine and green tea. J. Nutr. Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Cao Y., Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 72.Fang J., Zhou Q., Liu L.Z., Xia C., Hu X., Shi X., Jiang B.H. Angiogenin inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 73.Liu L.Z., Fang J., Zhou Q., Hu X., Shi X., Jiang B.H. Apigenin inhibits expressionof vascular endothelial growth factor and angiogenesis in human lung cancercells: Implication of chemoprevention of lung cancer. Mol. Pharmacol. 2005;68:635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- 74.Fang J., Xia C., Cao Z., Zheng J.Z., Reed E., Jiang B.H. Apigenin inhibits VEGF andHIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 75.Mojžišová G., Mojžiš J., Pilátová M., Vohárová V., Varinská L., Perjesi P., Sarišský M., Mirossay L. Antiproliferative and antiangiogenic effects of selected chalcones. Acta Pharmacol. Sin. 2006;27:338. [Google Scholar]
- 76.Mojžiš J., Varinská L., Perjesi P.G., Mojžišová G. Antiangiogenic effect of newlysynthesized chalcones. Eur. J. Cancer. 2007;5:87. [Google Scholar]
- 77.Mojzis J., Varinska L., Mojzisova G., Kostova I., Mirossay L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008;57:259–265. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Oak M.H., El Bedoui J., Schini-Kerth V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Kurup V.P., Barrios C.S. Immunomodulatory effects of curcumin in allergy. Mol. Nutr. Food Res. 2008;52:1031–1039. doi: 10.1002/mnfr.200700293. [DOI] [PubMed] [Google Scholar]
- 80.Biswas S., Rahman I. Modulation of steroid activity in chronic inflammation: A novel anti-inflammatory role for curcumin. Mol. Nutr. Food Res. 2008;52:987–994. doi: 10.1002/mnfr.200700259. [DOI] [PubMed] [Google Scholar]
- 81.Mortellini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28:1301–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 82.Brouet I., Ohshima H., Curcumin An anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthetase in activated macrophages. Biochem. Biophys. Res. Commun. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- 83.Barnes P.J. Histone deacetylase-2 and airway disease. Ther. Adv. Respir. Dis. 2009;3:235–243. doi: 10.1177/1753465809348648. [DOI] [PubMed] [Google Scholar]
- 84.Meja K.K., Rajendrasozhan S., Adenuga D., Biswas S.K., Sundar I.K., Spooner G., Marwick J.A., Chakravarty P., Fletcher D., Whittaker P., et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 2008;39:312–323. doi: 10.1165/rcmb.2008-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bremner P., Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J. Pharm. Pharmacol. 2002;54:453–472. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- 86.de la Lastra C.A., Villegas I. Resveratrol as an antioxidant and prooxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 87.Das S., Das D.K. Resveratrol: A therapeutic promise for cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2007;2:133–138. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 88.Shankar S., Singh G., Srivastava R.K. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front. Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 89.Pirola L., Fröjdö S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 90.Leonard S., Xia C., Jiang B.H., Stinefelt B., Klandorf H., Harris G.K., Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 91.Das D.K., Maulik N. Resveratrol in cardioprotection: A therapeutic promise of alternative medicine. Mol. Interven. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 92.Youn J., Lee J.S., Na H.K., Kundu J.K., Surh Y.J. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer. 2009;61:847–854. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 93.Li T., Wang W., Chen H., Li T., Ye L. Evaluation of anti-leukemia effect of resveratrol by modulating STAT3 signaling. Int. Immunopharmacol. 2010;10:18–25. doi: 10.1016/j.intimp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Tang X., Zhang Q., Nishitani J., Brown J., Shi S, Le A.D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin. Cancer Res. 2007;13:2568–2576. doi: 10.1158/1078-0432.CCR-06-2704. [DOI] [PubMed] [Google Scholar]
- 95.Wu H., Liang X., Fang Y., Qin X., Zhang Y., Liu J. Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1 alpha expression in colon carcinoma cells. Biomed. Pharmacother. 2008;62:613–621. doi: 10.1016/j.biopha.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 96.Park S.Y., Jeong K.J., Lee J., Yoon D.S., Choi W.S., Kim Y.K., Han J.W., Kim Y.M., Kim B.K., Lee H.Y. Hypoxia enhances LPA-induced HIF-1alpha and VEGF expression: Their inhibition by resveratrol. Cancer Lett. 2007;258:63–69. doi: 10.1016/j.canlet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 97.Bourguignon L.Y., Xia W., Wong G. Hyaluronan-mediated CD44 Interaction with p300 and SIRT1 Regulates β-Catenin Signaling and NFkappaB-specific Transcription Activity Leading to MDR1 and Bcl-xL Gene Expression and Chemoresistance in Breast Tumor Cells. J. Biol. Chem. 2009;284:2657–2671. doi: 10.1074/jbc.M806708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rachon D., Rimoldi G., Wuttke W. In vitro effects of genistein and resveratrol on the production of interferon-gamma (IFNgamma) and interleukin-10 (IL-10) by stimulated murine splenocytes. Phytomedicine. 2006;13:419–424. doi: 10.1016/j.phymed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 99.Tao H.Y., Wu C.F., Zhou Y., Gong W.H., Zhang X., Iribarren P., Zhao Y.Q., Le Y.Y., Wang J.M. The grape component resveratrol interferes with the function of chemoattractant receptors on phagocytic leukocytes. Cell Mol. Immunol. 2004;1:50–56. [PubMed] [Google Scholar]
- 100.Wirleitner B., Schroecksnadel K., Winkler C., Schennach H., Fuchs D. Resveratrol suppresses interferon-gamma-induced biochemical pathways in human peripheral blood mononuclear cells in vitro. Immunol. Lett. 2005;100:159–163. doi: 10.1016/j.imlet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 101.Gao X., Deeb D., Media J., Divine G., Jiang H., Chapman R.A., Gautam S.C. Immunomodulatory activity of resveratrol: discrepant in vitro and in vivo immunological effects. Biochem. Pharmacol. 2003;66:2427–2435. doi: 10.1016/j.bcp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 102.Gao X., Xu Y.X., Janakiraman N., Chapman R.A., Gautam S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001;62:1299–1308. doi: 10.1016/S0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 103.Harikumar K.B., Aggarwal B.B. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 104.Kolthur-Seetharam U., Dantzer F., McBurney M.W., de Murcia G., Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 105.Alcaín F.J., Villalba J.M. Sirtuin activators. Expert Opin. Ther. Pat. 2009;19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- 106.Kim G,Y., Cho H., Ahn S.C., Oh Y.H., Lee C.M., Park Y.M. Resveratrol inhibits phenotypic and functional maturation of murine bone marrow-derived dendritic cells. Int. Immunopharmacol. 2004;4:245–253. doi: 10.1016/j.intimp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Rao A.V., Rao L.G. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Gallicchio L., Boyd K., Matanoski G., Tao X.G., Chen L., Lam T.K., Shiels M., Hammond E., Robinson K.A., Caulfield L.E., et al. Carotenoids and the risk of developing lung cancer: A systematic review. Am. J. Clin. Nutr. 2008;88:372–383. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 109.Young C.Y., Yuan H.Q., He M.L., Zhang J.Y. Carotenoids and prostate cancer risk. Mini Rev. Med. Chem. 2008;8:529–537. doi: 10.2174/138955708784223495. [DOI] [PubMed] [Google Scholar]
- 110.Melendez G.V, Okani E.T., Kiertsman B., Roncada M.J. Vitamin A status in children with pneumonia. Eur. J. Clin. Nutr. 1995;49:379–384. [PubMed] [Google Scholar]
- 111.Kukukbay H., Yakinci C., Kukukbay F.Z., Tutgut M. Serum vitamin A and beta-carotene levels in children with recurrent acute respiratory infections and diarrhoea in Malarya. J. Trop. Pediatr. 1997;43:337–340. doi: 10.1093/tropej/43.6.337. [DOI] [PubMed] [Google Scholar]
- 112.Mayne S.T. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996;10:690–701. [PubMed] [Google Scholar]
- 113.Mortensen A., Skibsted L.H., Truscott T.G. The interaction of dietary carotenoids with radical species. Arch. Biochem. Biophys. 2001;385:13–19. doi: 10.1006/abbi.2000.2172. [DOI] [PubMed] [Google Scholar]
- 114.Schock B.C., Young I.S., Brown V., Fitch P.S., Shields M.D., Ennis M. Antioxidants and oxidative stress in BAL fluid of atopic asthmatic children. Pediatr. Res. 2003;53:375–381. doi: 10.1203/01.PDR.0000049625.51462.D1. [DOI] [PubMed] [Google Scholar]
- 115.Barasi ME. Human Nutrition. Arnold Publishers; London, UK: 1997. Water soluble vitamins; pp. 171–175. [Google Scholar]
- 116.Rice-Evans C.A., Sampson J., Bramley P.M., Holloway D.E. Why do we expect carotenoids to be antioxidants in vivo? Free Radic. Res. 1997;26:381–398. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- 117.Patrick L. Beta-carotene: the controversy continues. Altern. Med. Rev. 2000;5:530–545. [PubMed] [Google Scholar]
- 118.Wood L.G., Garg M.L., Blake R.J., Garcia-Caraballo S., Gibson P.G. Airway and circulating levels of carotenoids in asthma and healthy controls. J. Am. Coll. Nutr. 2005;24:448–455. doi: 10.1080/07315724.2005.10719490. [DOI] [PubMed] [Google Scholar]
- 119.Zachman R.D. Role of vitamin A in lung development. J. Nutr. 1994;125:1634S–1638S. doi: 10.1093/jn/125.suppl_6.1634S. [DOI] [PubMed] [Google Scholar]
- 120.Chandra R.K., Vyas D. Vitamin A, immunocompetence and infection. Fed. Nutr. Bull. 1989;11:12–19. [Google Scholar]
- 121.Bloem M.W., Wedel M., Egger R.J. Mild vitamin A deficiency and risk of respiratory tract diseases and diarrhea in preschool and school children in northeastern Thailand. Am. J. Epidemiol. 1990;131:332–339. doi: 10.1093/oxfordjournals.aje.a115502. [DOI] [PubMed] [Google Scholar]
- 122.Villamor E., Fawzi W.W. Vitamin A supplementation: Implications for morbidity and mortality in children. J. Infect. Dis. 2000;182:S122–S133. doi: 10.1086/315921. [DOI] [PubMed] [Google Scholar]
- 123.Niles R.M. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat. Res. 2004;555:81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 124.Sharoni Y., Danilenko M., Dubi N., Ben-Dor A., Levy J. Carotenoids and transcription. Arch. Biochem. Biophys. 2004;430:89–96. doi: 10.1016/j.abb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 125.Karas M., Amir H., Fishman D., Danilenko M., Segal S., Nahum A., Koifmann A., Giat Y., Levy J., Sharoni Y. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr. Cancer. 2000;36:101–111. doi: 10.1207/S15327914NC3601_14. [DOI] [PubMed] [Google Scholar]
- 126.Riccioni G., Barbara M., Bucciarelli T., de Ilio C., D’Orazio N. Antioxidant vitamin supplementation in asthma. Ann. Clin. Lab. Sci. 2007;37:96–101. [PubMed] [Google Scholar]
- 127.Retsky K.L., Chen K., Zeind J., Frei B. Inhibition of copperinduced LDL oxidation by Vitamin C is associated with decreased copperbinding to LDL and 2-oxo-histidine formation. Free Radic. Biol. Med. 1999;26:90–98. doi: 10.1016/S0891-5849(98)00151-8. [DOI] [PubMed] [Google Scholar]
- 128.Cameron E., Pauling L. Supplemental ascorbate in supportive treatment of cancer—prolongation of survival times in terminal human cancer. Part 1. Proc. Natl. Acad. Sci. USA. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.You W.C., Zhang L., Gail M.H., Chang Y.S., Liu W.D., Ma J.L., Li J.Y., Jin M.L., Hu Y.R., Yang C.S., et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J. Natl. Cancer Inst. 2000;92:1607–1612. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- 130.Knekt P., Jarvinen R., Seppanen R., Rissanen A., Aromaa A., Heinonen O.P., Albanes D., Heinonen M., Pukkala E., Teppo L. Dietary antioxidants and the risk of lung-cancer. Am. J. Epidemiol. 1991;134:471–479. doi: 10.1093/oxfordjournals.aje.a116118. [DOI] [PubMed] [Google Scholar]
- 131.Divisi D., Di Tommaso S., Salvemini S., Garramone M., Crisci R. Diet and cancer. Acta Biomed. 2006;77:118–123. [PubMed] [Google Scholar]
- 132.Romieu I., Trenga C. Diet and obstructive lung diseases. Epidemiol. Rev. 2001;23:268–287. doi: 10.1093/oxfordjournals.epirev.a000806. [DOI] [PubMed] [Google Scholar]
- 133.Allen S., Britton J.R., Leonardi-Bee J.A. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax. 2009;64:610–619. doi: 10.1136/thx.2008.101469. [DOI] [PubMed] [Google Scholar]
- 134.Catani M.V., Rossi A., Costanzo A., Sabatini S., Levrero M., Melino G., Avigliano L. Induction of gene expression via activator protein-1 in the ascorbate protection against UV-induced damage. Biochem. J. 2001;356:77–85. doi: 10.1042/0264-6021:3560077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaur B., Rowe B.H., Arnold E. Vitamin C supplementation for asthma. Cochrane Database Syst. Rev. 2009;1:CD000993. doi: 10.1002/14651858.CD000993.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Riccioni G., D’Orazio N. The role of selenium, zinc and antioxidant vitamin supplementation in the treatment of bronchial asthma: Adjuvant therapy or not? Expert Opin. Investig. Drugs. 2005;14:1145–1155. doi: 10.1517/13543784.14.9.1145. [DOI] [PubMed] [Google Scholar]
- 137.Burton G.W., Ingold K.U. Vitamin E as an in vitro and in vivo antioxidant. Ann. N.Y. Acad. Sci. 1989;570:7–22. doi: 10.1111/j.1749-6632.1989.tb14904.x. [DOI] [PubMed] [Google Scholar]
- 138.Atkinson J., Epand R.F., Epand R.M. Tocopherols and tocotrienols in membranes: A critical review. Free Radic. Biol. Med. 2008;44:739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 139.De Luca H.F., Zierold C. Mechanisms and functions of vitamin D. Nutr. Rev. 1998;56:4–10. doi: 10.1111/j.1753-4887.1998.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 140.Moriguchi S., Muraga M. Vitamin E and immunity. Vitam. Horm. 2000;59:305–336. doi: 10.1016/S0083-6729(00)59011-6. [DOI] [PubMed] [Google Scholar]
- 141.Pryor W.A. Vitamin E and heart disease: Basic science to clinical intervention trials. Free Radic. Biol. Med. 2000;28:141–164. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 142.Devarah S., Harris A., Jialal I. Modulation of monocyte-macrophage function with alpha-tocopherol: implications for atherosclerosis. Nutr. Rev. 2002;60:8–14. doi: 10.1301/002966402760240381. [DOI] [PubMed] [Google Scholar]
- 143.Yoshikawa T., Yoshida N. Vitamin E and leukocyte-endothelial cell interactions. Antioxid. Redox Signal. 2000;2:821–825. doi: 10.1089/ars.2000.2.4-821. [DOI] [PubMed] [Google Scholar]
- 144.Kojo S., Vitamin C. basic metabolism and its function as an index of oxidative stress. Curr. Med. Chem. 2004;11:1041–1064. doi: 10.2174/0929867043455567. [DOI] [PubMed] [Google Scholar]
- 145.Pletsityi K.D., Vasipa S.B., Daydova T.V., Fomina V.G. Vitamin E: Immunocorrecting effect in bronchial asthma patients. Vopr. Med. Khim. 1995;41:33–36. [PubMed] [Google Scholar]
- 146.Picado C., Deulofeu R., Lleonart R., Agusti M., Mullol J., Quintó L., Torra M. Dietary. micronutrients/antioxidants and their relationship with bronchial asthma severity. Allergy. 2001;56:43–49. doi: 10.1034/j.1398-9995.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 147.Troisi R.J., Willett W.C., Weiss S.T., Trichopoulos D., Rosner B., Speizer F.E. A prospective study of diet and adult-onset asthma. Am. J. Respir. Crit. Care Med. 1995;151:1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 148.Trenga C., Koenig J.Q,, Williams P.V. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch. Environ. Health. 2001;56:242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 149.Pearson P.J., Lewis S.A., Britton J., Fogarty A. Vitamin E supplements in asthma: A parallel group randomized placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wiser J., Alexis N.E., Jiang Q., We W., Robinnette C., Roubey R., Peden D.B. In vivo gamma tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008;45:40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berdnikovs S., Abdala-Valencia H., McCary C., Somand M., Cole R., Garcia A., Bryce P., Cook-Mills J.M. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J. Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wolf G. How an increased intake of α-tocopherol can suppress the bioavailability of γ-tocopherol. Nutr. Rev. 2006;64:295–299. doi: 10.1111/j.1753-4887.2006.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 153.Packer L., Suzuki Y.J. Vitamin-E and alpha-lipoate — role in antioxidant recycling and activation of the NF-kappa B transcription factor. Mol. Aspects Med. 1993;14:229–239. doi: 10.1016/0098-2997(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 154.Bustamante J., Lodge J.K., Marcocci L., Tritschler H.J., Packer L., Rihn B.H. Alpha-lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998;24:1023–1039. doi: 10.1016/S0891-5849(97)00371-7. [DOI] [PubMed] [Google Scholar]
- 155.Cao X., Phillis J.W. The free radical scavenger, alpha-lipoic acid, protects against cerebral ischemia-reperfusion injury in gerbils. Free Radic. Res. 1995;23:365–370. doi: 10.3109/10715769509065257. [DOI] [PubMed] [Google Scholar]
- 156.Van Dam P.S. Oxidative stress and diabetic neuropathy: Pathophysiological mechanisms and treatment perspectives. Diabetes Metab. Res. Rev. 2002;18:176–184. doi: 10.1002/dmrr.287. [DOI] [PubMed] [Google Scholar]
- 157.Ametov A.S., Barinov A., Dyck P.J., Hermann R., Kozlova N., Litchy W.J., Low P.A., Nehrdich D., Novosadova M., O'Brien P.C., et al. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoid acid: The SYDNEY trial. Diabetes Care. 2003;26:770–776. doi: 10.2337/diacare.26.3.770. [DOI] [PubMed] [Google Scholar]
- 158.Ramakrishnan N., Wolfe W.W., Catravas G.N. Radioprotection of hematopoietic tissues in mice by lipoic acid. Radic. Res. 1992;130:360–365. doi: 10.2307/3578382. [DOI] [PubMed] [Google Scholar]
- 159.Cho Y.S., Lee J.C., Lee T.H., Lee E.Y., Lee K.U., Park J.Y., Moon H.B. α-Lipoic acid inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J. Allergy Clin. Immunol. 2004;114:429–435. doi: 10.1016/j.jaci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 160.Lee K.S., Park H.S., Park S.J., Kim S.R., Min K.H., Jin S.M., Park K.H., Kim U.H., Kim C.Y., Lee Y.C. A prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acid, regulates vascular permeability by reducing vascular endothelial growth factor expression in asthma. Mol. Pharmacol. 2005;68:1281–1290. doi: 10.1124/mol.105.016055. [DOI] [PubMed] [Google Scholar]
- 161.Schwarz S. Essentiality and metabolic functions of selenium. Med. Clin. North Am. 1976;60:745–758. doi: 10.1016/s0025-7125(16)31858-2. [DOI] [PubMed] [Google Scholar]
- 162.Saito Y., Sato N., Hirashima M., Takebe G., Nagasawa S., Takahashi K. Domain structure of bi-functional selenoprotein P. Biochem. J. 2004;381:841–846. doi: 10.1042/BJ20040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rayman M.P. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 164.Gromer S., Eubel J.K., Lee B.L., Jacob J. Human selenoproteins at a glance. Cell Mol. Life Sci. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Anonymous. Selenium-monograph. Altern. Med. Rev. 2003;8:63–71. [PubMed] [Google Scholar]
- 166.Bainbridge D.R. Use of (75Se)L-Selenomethionine as a label for lymphoid cells. Immunology. 1976;30:135–144. [PMC free article] [PubMed] [Google Scholar]
- 167.Gromer S., Eubel J.K., Lee B.L., Jacob J. Human selenoproteins at a glance. Cell Mol. Life Sci. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pretorius E., Oberholzer H.M., Vieira W.A., Smit E. Ultrastructure of platelets and fibrin networks of asthmatic mice exposed to selenium and Withania somnifera. Anat. Sci. Int. 2009;84:210–217. doi: 10.1007/s12565-008-0010-1. [DOI] [PubMed] [Google Scholar]
- 169.Jahnova E., Horvathova M., Gazdik F., Weissova S. Effects of selenium supplementation on expression of adhesion molecules in corticoid-dependent asthmatics. Bratisl. Lek. Listy. 2002;103:12–16. [PubMed] [Google Scholar]
- 170.Brown K.M., Arthur J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001;4:593–599. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- 171.Horváthová M., Jahnová E., Gazdik F. Effect of selenium supplementation in asthmatic subjects on the expression of endothelial cell adhesion molecules in culture. Biol. Trace Elem. Res. 1999;69:15–26. doi: 10.1007/BF02783912. [DOI] [PubMed] [Google Scholar]
- 172.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Allam M.F., Lucena R.A. Selenium supplementation for asthma. Cochrane DatabaseSyst. Rev. 2009;1:CD003538. doi: 10.1002/14651858.CD003538.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Riccioni G. Plasma lycopene and antioxidant vitamins in asthma: The PLAVA study. J. Asthma. 2007;44:429–432. doi: 10.1080/02770900701421880. [DOI] [PubMed] [Google Scholar]
- 175.Ray A.L., Semba R.D., Walston J., Ferrucci L., Cappola A.R., Ricks M.O., Xue Q.L., Fried L.P. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women’s health and aging studies. J. Nutr. 2006;136:172–176. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- 176.Hisama M., Matsuda S., Tanaka T., Shibayama H., Nomura M., Iwaki M. Suppression of mutagens-induced SOS response by phytoncide solution using salmonella typhimurium TA 1535/pSK1002 umu Test. J. Oleo. Sci. 2008;57:381–390. doi: 10.5650/jos.57.381. [DOI] [PubMed] [Google Scholar]
- 177.Aoshima H., Hamamoto K. Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncide. Biosci. Biotechnol. Biochem. 1999;63:743–748. doi: 10.1271/bbb.63.743. [DOI] [PubMed] [Google Scholar]
- 178.Hossain S.J., Aoshima H., Koda H., Kiso Y. Potentiation of the ionotropioc GABA receptor response by whiskey fragrance. J. Agric. Food Chem. 2002;50:6828–6834. doi: 10.1021/jf020448e. [DOI] [PubMed] [Google Scholar]
- 179.Li Q., Morimoto K., Inagaki H., Katsumata M., Hirata Y., Hirata K., Shimizu T., Li Y.J., Wakayama Y. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents. 2008;22:45–55. [PubMed] [Google Scholar]
- 180.Sadlon A.E., Lamson D.W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern. Med. Rev. 2010;15:33–47. [PubMed] [Google Scholar]
- 181.Nam J.H., Jung H.J., Choi J., Lee K.T., Park H.J. The anti-gastropathic and anti-rheumatic effect of nigaichigoside F1 and 23-hydroxytormentic acid isolated from the unripe fruits of Rubus Coreanus in a rat model. Biol. Pharm. Bull. 2006;29:967–970. doi: 10.1248/bpb.29.967. [DOI] [PubMed] [Google Scholar]
- 182.Oikawa S. Sequence-specific DNA damage by reactive oxygen species: Implications for carcinogenesis and aging. Environ. Health Prev. Med. 2005;10:65–71. doi: 10.1007/BF02897995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nose K. Role of reactive oxygen species in the regulation of physiological functions. Bio. Pharm. Bull. 2000;23:897–903. doi: 10.1248/bpb.23.897. [DOI] [PubMed] [Google Scholar]
- 184.Li Q., Nakadai A., Matsushima H., Miyazaki Y., Krensky A.M., Kawada T., Morimoto K. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol. Immunotoxicol. 2006;28:319–333. doi: 10.1080/08923970600809439. [DOI] [PubMed] [Google Scholar]
- 185.Komori T., Fujiwara R., Tanida M., Nomura J., Yokoyama M.M. Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation. 1995;2:174–180. doi: 10.1159/000096889. [DOI] [PubMed] [Google Scholar]
- 186.Shibata H., Fujiwara R., Iwamoto M., Matsuoka H., Yokoyama M.M. Immunological and behavioral effects of fragrance in mice. Int. J. Neurosci. 1991;57:151–159. doi: 10.3109/00207459109150355. [DOI] [PubMed] [Google Scholar]