Abstract
Four phenolics, salviaplebeiaside (1), γ-tocopherol (2), chrysosplenol-D (4), and isovitexin (5), along with α-tocoquinone (3) and β-sitosterol (6) were isolated from the aerial parts of Vitex negundo var. cannabifolia. The isolation was performed using bio-assay tracking experiments. The structures of compounds 1-5 were established by spectroscopic means. The antibacterial activities of the compounds were assessed against Escherichia coli, Bacillus subtilis, Micrococcus tetragenus and Pseudomonas fluorescens. Chrysosplenol-D (4) exhibited activities against all the four spoilage microorganisms.
Keywords: Vitex negundo var. cannabifolia, Verbenaceae, phenolics, antibacterial activities, spoilage microorganisms
1. Introduction
Vitex negundo var. cannabifolia (Verbenaceae) is a shrub growing mainly in Yangzi River basin of China [1]. The plant was used as herbal medicine for the treatment of many diseases, such as colds, malaria, inflammation, sores and beriberi [2]. Some iridoids, lignans and other components in the species were reported in previous phytochemical studies [3,4,5]. The juice extracted from aerial parts of the plant has been used as a folk antiseptic in Anhui province of China for preventing meat from rotting. In this study, four phenolics (compounds 1, 2, 4 and 5), along with two other compounds (3 and 6), were isolated and identified. Their antibacterial activities were examined and compound 4 was found to exhibit inhibitory activity against the four tested bacterial spp..
2. Results and Discussion
2.1. Antibacterial Activities of the Crude Extracts
The EtOH extract of the powdered dry aerial parts of V. negundo var. cannabifolia was successively fractionated with petroleum ether (PE), CHCl3 and n-BuOH. The n-BuOH fraction was further fractionated by Diaion HP-20 column chromatography (CC) using H2O, 50% EtOH and 95% EtOH in a sequential elution process to yield three fractions A-C.
The antibacterial activities of the EtOH extract, as well as the PE, CHCl3, B, C fractions against Escherichia coli, Bacillus subtilis, Micrococcus tetragenus, and Pseudomonas fluorescens were evaluated by the hole plate diffusion method (see Experimental section 3.10 for more details) [6]. The EtOH extract and the four other fractions were individually dissolved and diluted with DMSO to obtain serial concentrations of 100, 50 and 25 mg·mL-1. The inhibition activities were evaluated by diameters of the inhibition zones (Figure 1).
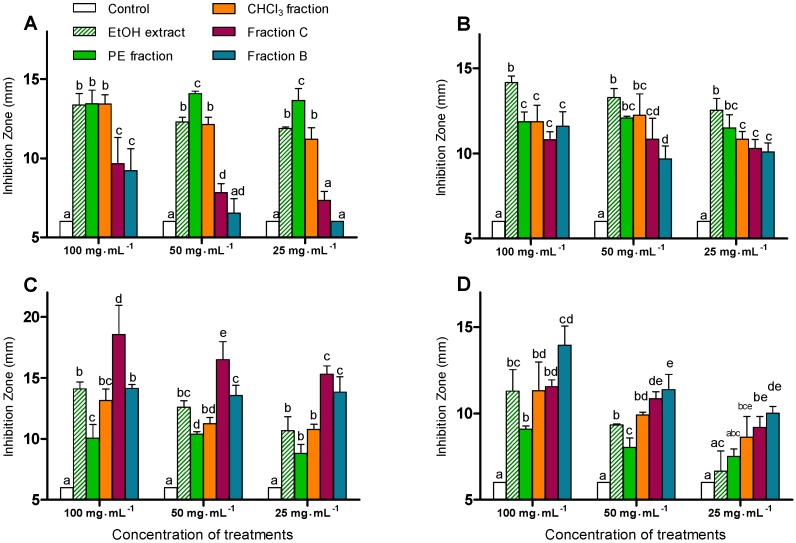
Figure 1.
Antibacterial activities of the EtOH extract as well as PE, CHCl3, B and C fractions. Data are means ± SD, n = 3. Within each concentration treatment, columns containing the same letter are not significantly different according to Duncan’s new multiple range test at P<0.05. [(A) E. coli, (B) B. subtilis, (C) M. tetragenus, (D) P. fluorescens].
Figures 1A-D show the significant inhibitory activities of the crude extracts at all three tested concentrations. The activity against E. coli was enhanced as the polarity of the fractions decreased (Figure 1A), and the PE fraction showed significantly higher activity against E. coli at the concentrations of 50 and 25 mg·mL-1 versus the other fractions. However, there was no significant difference in the inhibition activity against B. subtilis among the four fractions (Figure 1B). The activity of fraction C against M. tetragenus was significantly higher than that of any other fraction (Figure 1C). Furthermore, at concentrations 50 and 25 mg·mL-1, both fractions C and B showed significantly higher activities compared to the PE and CHCl3 fractions. In Figure 1D, the inhibitory activities of the fractions against P. fluorescens were enhanced with the increase of the fractions’ polarity. Both fractions C and B displayed higher activities than the PE or CHCl3 fraction at all three concentrations. Overall fraction B showed a stronger inhibition against P. fluorescens. These results suggest that the bio-active components against E. coli were present in the lower-polarity (PE) fraction, while those against M. tetragenus and P. fluorescens were located in higher-polarity fractions (C and B).
2.2. Isolation and Identification of the Compounds
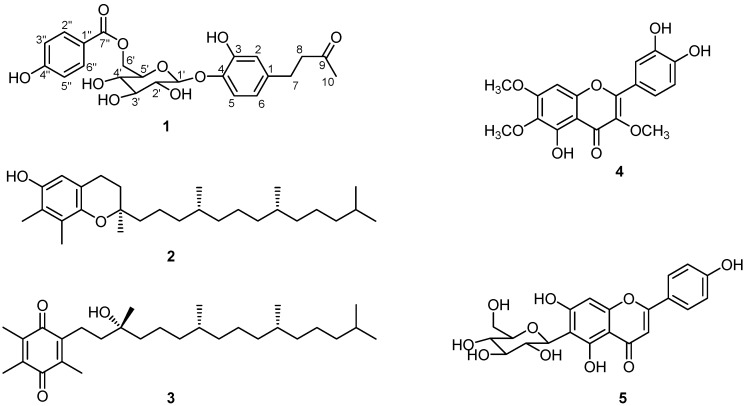
The PE, B and C fractions were presumed to contain bio-active compounds from the results of the above assays, and were therefore separated by a combination of silica gel, ODS-A, and Sephadex LH-20 CC to yield compounds 1-6 (Figure 2).
Figure 2.
Structures of Compounds 1-5.
Compound 1 was obtained as a pale yellow gum. Its molecular formula was determined as C23H26O10 by the negative HRESI-TOF-MS signal at m/z 461.1462. The 1H-, 13C-NMR and HSQC spectra showed the signals of a carbonyl group (δC 211.13), a tertiary methyl group [δH 2.023 (3H, s), δC 30.00], a 1,2,4-trisubstituted benzene [δH 6.933 (1H, d, J = 8.4 Hz), δH 6.574 (1H, br s), and δH 6.284 (1H, dd, J = 8.4, 1.6 Hz)], and a β-D-glucopyranosyl moiety [δH 4.625 (1H, d, J = 5.2 Hz), δC 104.30, 77.59, 75.83, 74.88, 72.07, 64.83]. The high field region of the 1H-NMR spectrum gave a 4H singlet (δH 2.617), which was correlated with two carbon signals at δC 45.81 and 30.20 in the HSQC spectrum. The above information was characteristic of a phenylbutanone glucoside, containing a glucopyranosyl moiety, and an aromatic ring (A-ring) with a butanone substituent [7]. The 4H singlet (CH2-7 and CH2-8) mentioned above was believed to be the results of the magnetic equivalence of those protons. The 1H- and 13C-NMR spectra of 1 were very similar to those of vitexfolin C, which hads a p-hydroxybenzoyl group (B-ring) at its 2′ position, and by careful comparison of the spectral data of 1 with those of vitexfolin C [7], the presence of a p-hydroxybenzoyl group [δH 7.753 (2H, d, J = 8.8 Hz), δH 6.648 (2H, d, J = 8.8 Hz), δC 168.14 (C-7″), 165.63 (C-4″), 133.03 (C-2″, 6″), 120.95 (C-1″), 116.82 (C-3″, 5″)] in 1 could indeed be readily deduced. The key differences between the NMR spectra of 1 and those of vitexfolin C were the chemical shifts of the 1H- and 13C-NMR signals of the 2′, 5′ and 6′ positions. In the NMR spectra of 1, the chemical shifts of H-2′, C-2′ and C-5′ were upshifted to δH 3.446, δC 74.88 and δC 75.83 ppm, respectively, while those of H-5′, H-6′a, H-6′b and C-6′ were downshifted to δH 3.641, δH 4.575, δH 4.275, and δH 64.83 ppm, respectively, suggesting that the β-D-glucopyranosyl moiety in 1 was 1′,6′-disubstituted instead of 1′,2′-disubstituted as in vitexfolin C. By analysis on the reported phenylbutanone glucosides in Vitex spp., 1 was assumed to be a 1′-(A-ring),6′-(B-ring)-disubstituted glucopyranose [7]. The hypothesis was confirmed by analysis of the HMBC spectrum, which showed cross peaks between H-6′ (δH 4.575 and δH 4.275) and C-7″ (δC 168.14), as well as between H-1′ (δH 4.625) and C-4 (δC 144.99). On the basis of this evidence, 1 was identified as 4-{4-O-[6-(4-hydroxybenzoyl)-O-β-D-glucopyranosyl]-3-hydroxyphenyl}-2-butanone. The structure was previously reported as salviaplebeiaside from Salvia plebeian [8].
By comparison their spectroscopic data (see the Experimental section) with those reported in the references, compounds 2-5 were identified as γ-tocopherol, α-tocoquinone, chrysosplenol-D and isovitexin, respectively [9,10,11,12]. Compound 6 were identified as β-sitosterol by comparing its Rf value with that of an authentic sample in a TLC experiment.
2.3. Antibacterial Activities of Compounds 1-5
The antibacterial activities of 1-5 against E. coli, B. subtilis, M. tetragenus, and P. fluorescens were also evaluated by the hole plate diffusion method. Compound 4 exhibited weak activities against E. coli, B. subtilis and M. tetragenus, with minimal inhibitory concentration (MIC) values of 500, 500 and 250 μg·mL-1, respectively. At the same concentrations, ampicillin sodium displayed much stronger inhibition against the corresponding microorganisms. Nevertheless, the inhibitory activity of 4 against P. fluorescens was comparable with that of ampicillin sodium (MIC, 500 μg·mL-1, see Table 2).
Table 2.
Antibacterial Activities of Compounds 1-5.
| Compd. | MIC (μg·mL-1) a | ||||
|---|---|---|---|---|---|
| E. coli | B. subtilis | M. tetragenus | P. fluorescens | ||
| 1 | >1000 | >1000 | >1000 | >1000 | |
| 2 | >1000 | >1000 | >1000 | >1000 | |
| 3 | >1000 | >1000 | >1000 | >1000 | |
| 4 | 500 | 500 | 250 | 500 | |
| 5 | >1000 | >1000 | >1000 | >1000 | |
| AMP b | 0.122 | 0.061 | 0.244 | 250 | |
a: The results were the average of three readings; b: ampicillin sodium.
3. Experimental
3.1. General
IR spectra were obtained on a Nicolet 8700 FT-IR spectrophotometer (Thermo, USA). The 1H-NMR (400 MHz), 13C-NMR (100 MHz) and 2D-NMR spectra such as HSQC and HMBC were recorded on a Bruker Avance-400 (Bruker, Switzerland). Chemical shifts are expressed in ppm (δ). The ESI-MS data were obtained with a Finnigan LTQ LC/MS system (Thermo, USA) by direct injection. The HRESI-TOF-MS was recorded on an Agilent 6210 instrument (Agilent Technologies, Inc., USA) by direct injection. Silica gel 60 (200-300 mesh, Qingdao Marine Chemical Co. Ltd., Qingdao, PR China) and YMC GEL ODS-A (50 μm, YMC Co. Ltd., Japan) were used for CC. MeOH was used as the eluant in Sephadex LH-20 CC. TLC was performed using precoated silica gel plates (GF254, Liangchen Chemical Co. Ltd., Huoshan, Anhui province, PR China), or precoated RP-18 silica gel plates (F254, Merck, Germany), with detection by spraying with 10% H2SO4/ethanol reagent followed by heating.
3.2. Plant Material
Aerial parts of V. negundo var. cannabifolia were collected in the rural area of Xuan-Cheng, Anhui Province, China, in summer of 2008, and identified by Prof. Sheng-Ni Tian, Anhui Agricultural University, and Prof. Yue-Hong Yan, Hunan University of Science and Technology. A voucher specimen was deposited at the Laboratory of Botany, School of Life Sciences, Anhui Agricultural University.
3.3. Extraction
Dry stems and leaves of V. negundo var. cannabifolia (3.0 kg) were ground to a fine powder, which were extracted with 95% EtOH three times (24, 48 and 48 h with 10 L each time) by percolation at room temperature. The EtOH percolate was concentrated in vacuo to a syrup (EtOH extract, 420 g). This syrup was suspended in H2O and the aqueous suspension (1000 mL) was successively extracted with PE (500 mL, 3 times), CHCl3 (500 mL, 3 times), and n-BuOH (500 mL, 3 times) at room temperature. The PE and the CHCl3 extracts, on concentration, yielded a dark green syrup (PE fraction, 60 g) and a brown syrup (CHCl3 fraction, 40 g) respectively. The n-BuOH extraction, upon concentration under reduced pressure, afforded 90 g of a brown syrup. This syrup was further fractionated by Diaion HP-20 CC using H2O, 50% EtOH and 95% EtOH in a sequential elution process, yielding three fractions A-C.
3.4. Isolation of the Compounds
The PE fraction was subjected to CC on silica gel, eluted with PE-EtOAc mixtures of increasing polarity (from 15:1 to 8:1), to give three fractions (PE1-PE3). Fraction PE1 was further subjected sequentially to silica gel CC using 99:1 PE-EtOAc as eluant, and to ODS-A CC using methanol as eluant, to afford 2 (13 mg), 3 (21 mg) and 6 (60 mg). Fraction B (53 g) was subjected sequentially to silica gel CC eluted with CH2Cl2-CH3OH mixtures of increasing polarities (from 10:1 to 5:1), to ODS-A CC using CH3OH-H2O (2:3) as eluant, and to Sephadex LH-20 CC to afford 1 (27 mg) and 5 (10 mg). Fraction C (4 g) was subjected to silica gel CC eluted with CH2Cl2-MeOH (10:1) and repeated Sephadex LH-20 CC to yield 4 (24 mg).
3.5. Salviaplebeiaside (1)
Pale yellow gum. IR (KBr) νmax (cm–1): 3,420, 2,923, 1,702, 1,609, 1,594, 1,511, 1,448, 1,281, 1,167, 1,073, 882, 852, 800, 772, 729 and 698. HRESI-TOF-MS: m/z 461.1462 [M – H]– (calcd. for C23H25O10–, 461.1453) in negative mode. 1H- and 13C-NMR data, see Table 1.
Table 1.
1H- and 13C-NMR data of 1 (in methanol-d4).
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC | |
|---|---|---|---|---|---|---|
| 1 | ─ | 138.13 | 3′ | 3.446 b | 77.59 | |
| 2 | 6.574 br s | 117.13 | 4′ | 3.357 m | 72.07 | |
| 3 | ─ | 148.38 | 5′ | 3.641 m | 75.83 | |
| 4 | ─ | 144.99 | 6′a | 4.575 br d (11.6) | 64.83 | |
| 5 | 6.933 d (8.4) | 118.89 | 6′b | 4.275 dd (11.6, 7.6) | ||
| 6 | 6.284 dd (8.4, 1.6) | 120.34 | 1″ | ─ | 120.95 | |
| 7 | 2.617 br s a | 30.20 | 2″ | 7.753 d (8.8) | 133.03 | |
| 8 | 2.617 br s a | 45.81 | 3″ | 6.648 d (8.8) | 116.82 | |
| 9 | ─ | 211.13 | 4″ | ─ | 165.63 | |
| 10 | 2.023 s | 30.00 | 5″ | 6.648 d (8.8) | 116.82 | |
| 1′ | 4.625 d (5.2) | 104.30 | 6″ | 7.753 d (8.8) | 133.03 | |
| 2′ | 3.446 b | 74.88 | 7″ | ─ | 168.14 |
a, b signals were overlapped.
3.6. γ-Tocopherol (2)
Yellow oil; ESI-MS m/z 417 [M + H]+, 415 [M – H]–; 1H-NMR (CDCl3) δ: 6.372 (1H, s, H-5), 2.669 (2H, m, H-4), 2.136, 2.113 (each 3H, s, H-7a, 8a), 1.243 (3H, s, H-2a), 0.872-0.846 (12H, m, H-4′a, 8′a, 12′a, 13′); 13C-NMR (CDCl3) δ: 146.3 (C-6), 145.9 (C-8a), 125.9 (C-8), 121.7 (C-7), 118.4 (C-4a), 112.3 (C-5), 75.6 (C-2), 40.2 (C-1'), 39.5 (C-11'), 37.5 (C-3', 5', 7', 9'), 32.9 (C-4', 8'), 31.6 (C-3), 28.0 (C-12'), 24.9 (C-10'), 24.5 (C-6'), 24.2 (C-2a), 22.8 and 22.7 (C-12'a and C-13'), 22.4 (C-4), 21.1 (C-2'), 19.8 (C-4'a, 8'a), 11.9 (C-7a, 8a).
3.7. α-Tocoquinone (3)
Yellow oil; ESI-MS m/z 447 [M + H]+, 445 [M – H]–; 1H-NMR (CDCl3) δ: 2.539 (2H, m, H-1'), 2.032 (3H, s, H-3a), 2.003 (6H, s, H-2a, 5a), 1.228 (3H, s, H-3'a), 0.867-0.830 (12H, m, H-4′a, 8′a, 12′a, 13′); 13C-NMR (CDCl3) δ: 187.8 (C-4), 187.3 (C-1), 144.5 (C-6), 140.6 (C-5), 140.5 (C-3), 140.3 (C-2), 72.8 (C-3'), 42.4 (C-4'), 40.3 (C-2'), 39.4 (C-14'), 37.7 (C-6'), 37.5 (C-10'), 37.4 (C-12'), 32.7 (C-7', 11'), 29.7 (C-8'), 27.9 (C-15'), 26.7 (Me-3'), 24.9 (C-13'), 24.6 (C-9'), 22.8 (Me-15'), 22.7 (Me-15'), 21.5 (C-1'), 21.4 (C-5'), 19.8 (Me-7',11'), 12.5 (Me-2), 12.4 (Me-2), 11.9 (Me-5).
3.8. Chrysosplenol-D (4)
Yellow amorphous powder; ESI-MS m/z 361 [M + H]+, 383 [M + Na]+, 359 [M – H]–; 1H-NMR (DMSO-d6) δ: 7.56 (1H, d, J = 2.1 Hz, H-2'), 7.45 (1H, dd, J = 8.4, 2.1 Hz, H-6'), 6.91 (1H, d, J = 8.4 Hz, H-5'), 6.80 (1H, s, H-8), 3.93 (3H, s, 7-OCH3), 3.81 (3H, s, 3-OCH3), 3.76 (3H, s, 6-OCH3); 13C-NMR (DMSO-d6) δ: 178.1 (C-4), 158.5 (C-7), 156.0 (C-9), 151.7 (C-5), 151.5 (C-2), 149.3 (C-4'), 145.4 (C-3'), 137.6 (C-3), 131.5 (C-6), 120.6 (C-1'), 120.3 (C-6'), 115.7 (C-2'), 115.3 (C-5'), 105.5 (C-10), 91.2 (C-8), 60.0, 59.6.3 (6, 3-OCH3), 56.4 (7-OCH3).
3.9. Isovitexin (5)
Yellow amorphous powder; ESI-MS m/z 455 [M + Na]+, 431 [M – H]–; 1H-NMR (CD3OD) δ: 7.71 (2H, d, J = 8.8 Hz, H-2', 6'), 6.81 (2H, d, J = 8.8 Hz, H-3', 5'), 6.47 (1H, s, H-8), 6.38 (1H, s, H-3), 4.81 (1H, d, J = 10 Hz, H-1"), 4.07 (1H, dd, J = 9.6, 9.2 Hz, H-2"), 3.78 (1H, dd, J = 12.0, 2.0 Hz, H-6"a), 3.65 (1H, dd, J = 12.0, 5.2 Hz, H-6"b), 3.40-3.29 (3H, m, H-3", 4", 5"); 13C-NMR (CD3OD) δ: 184.0 (C-4), 166.2 (C-2), 165.0 (C-7), 162.8 (C-4'), 162.0 (C-5), 158.7 (C-9), 129.4 (C-2', 6'), 123.1 (C-1'), 117.0 (C-3', 5'), 109.2 (C-6), 105.2 (C-10), 103.9 (C-3), 95.3 (C-8), 82.6 (C-5"), 80.2 (C-3"), 75.3 (C-1"), 72.6 (C-2"), 71.8 (C-4"), 62.9 (C-6").
3.10. Anti-bacterial Assays of the Crude Extracts
Anti-bacterial activities were evaluated by the hole plate diffusion method [6]. The test microorganisms were E. coli, B. subtilis, M. tetragenus, and P. fluorescens, which were obtained from the School of Basic Medical Sciences, Anhui Medical University, Hefei, P.R. China. The EtOH extract, PE and CHCl3 fractions, as well as fractions B and C were individually dissolved and diluted with DMSO to obtain serial concentrations of 100, 50 and 25 mg·mL-1. Six mm wide holes were bored with a sterilized steel borer into the Nutrient Agar Media (beef extract 3 g, peptone 10 g, agar 17 g, NaCl 5 g, H2O 1,000 mL, pH 7.2) in the Petri dish inoculated with the test microorganism. The solution of the compound (60 μL) at a specific concentration was added into each of the holes. DMSO was used as the negative control. The plates were then incubated at 37 °C for 24 hours. The diameters of the inhibition zones were measured and recorded. The assays were performed three times in order to guarantee reproducibility of results (see Figure 1).
3.11. Anti-bacterial Assays of the Compounds
Compounds 1-5 and ampicillin sodium (positive control) were individually dissolved and diluted with DMSO to obtain serial concentrations of 1000, 500, 250, 125 and 62.5 μg·mL-1 (for ampicillin sodium, the solutions were serially diluted from 1000 to 0.03 μg·mL-1). The anti-bacterial assays were also performed by the hole plate diffusion method as described above. The inhibition zones around the holes were measured and the MIC, which was defined as the lowest concentration being able to inhibit any visible bacterial growth, was recorded. The assays were performed three times for statistical analysis (see Table 2).
3.12. Statistical Analysis
The data in Figure 1 are presented as means ± SD. The values were evaluated by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Differences were considered significant at P < 0.05.
4. Conclusions
This is the first report on the isolation of salviaplebeiaside, γ-tocopherol and α-tocoquinone from the genus Vitex. Compounds 1-5 were reported from investigated species for the first time. The apoptosis-inducing and antimalarial activities, as well as vascular relaxation effects of 4 had been reported [13,14,15,16]. With respect to antibacterial activities, the inhibition effects of 4 on Staphylococcus aureus, Cladosporium cucumerinum and Bacillus cereus were documented [11,17]. Our study indicated the inhibition activities of 4 on four spoilage microorganisms for the first time. It is known that P. fluorescens plays an important role in rotting of meat. The results of the present assays suggest chrysosplenol-D could be used as a potential antiseptic food additive. Furthermore, this compound might also be one of the key components to account for the medicinal usage of the plant.
In our previous work, the compositions of the essential oil from the aerial parts of V.negundo var. cannabifolia and their antiseptic activities were analyzed [18]. However, neither the essential oil, nor the compounds isolated from the PE fraction displayed any significant inhibitory activity against P. fluorescens.
A characteristic of phenolic-rich high-polar fraction in the fruits of the investigated species was revealed by previous phytochemical studies [5]. Our work also revealed that phenolics were the predominant components in the high-polar fraction of the aerial parts of the plant. As the high-polar fraction was confirmed as the significant antiseptic fraction by our bio-assays, the phenolics could be the key antiseptic constituents of V.negundo var. cannabifolia, even though more chemical examinations have to be done for this plant.
Acknowledgements
We are grateful to Guang-Hong Liu, Hua-Shi Furnishing Inc., Xuan-Cheng, Anhui Province, for collecting the plant material, and for partial financial support. We also thank Shu Wei, Anhui Agricultural University, for providing advice for the paper, and Yu-Sang Wang, University of Science and Technology of China, Chinese Academy of Sciences, for NMR measurements, and Hua-Rong Tan, Biotechnology Center of Anhui Agricultural University, for ESI-MS measurements, as well as Feng-Lin Hu, School of Forestry & Landscape Architecture, Anhui Agricultural University, for HRESI-TOF-MS measurements. This work was supported by Outstanding Youth Foundation of Anhui Province (No. 08040106804), and Starting Foundation for Young Talents of Anhui Agricultural University (2005).
Footnotes
Sample Availability: Samples of the compounds 1-6 are available from the authors.
References and Notes
- 1.Pei J., Chen S.L. Flora Reipublicae Popularis Sinicae. Science Press; Beijing, China: 1982. pp. 143–145. Tomus 65 (1) [Google Scholar]
- 2.Xiao P.G. Modern Chinese Materia Medica. 1st. Chemical Industry Press; Beijing, China: 2002. pp. 453–457. [Google Scholar]
- 3.Taguchi H. Studies on the constituents of Vitex cannabifolia. Chem. Pharm. Bull. 1976;24:1668–1670. doi: 10.1248/cpb.24.1668. [DOI] [Google Scholar]
- 4.Iwagawa T., Nakahara A., Nakatani M. Iridoids from Vitex cannabifolia. Phytochemistry. 1993;32:453–454. doi: 10.1016/S0031-9422(00)95014-3. [DOI] [Google Scholar]
- 5.Yamasaki T., Kawabata T., Masuoka C., Kinjo J., Ikeda T., Nohara T., Ono M. Two new lignan glucosides from the fruit of Vitex cannabifolia. J. Nat. Med. 2008;62:47–51. doi: 10.1007/s11418-007-0184-1. [DOI] [PubMed] [Google Scholar]
- 6.Feng N., Ye W.H., Wu P., Huang Y.C., Xie H.H., Wei X.Y. Two new antifungal alkaloids produced by Streptoverticillium morookaense. J. Antibiot. 2007;60:179–183. doi: 10.1038/ja.2007.19. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama E., Fujimori S., Yamazaki M., Deyama T. Pharmacologically active components of Viticis Fructus (Vitex rotundifolia). II. The components having analgesic effects. Chem. Pharm. Bull. 1998;46:655–662. doi: 10.1248/cpb.46.655. [DOI] [PubMed] [Google Scholar]
- 8.Jin Q.H., Han X.H., Hwang J.H., Hong S.S., Park M.O., Lee C., Lee C.H., Lee D.H., Lee M.K., Hwang B.Y. A new phenylbutanone glucoside from Salvia plebeian. Nat. Prod. Sci. 2009;15:106–109. [Google Scholar]
- 9.Matsuo M., Urano S. 13C NMR spectra of tocopherols and 2,2-dimethylchromanols. Tetrahedron. 1976;32:229–231. doi: 10.1016/0040-4020(76)87006-8. [DOI] [Google Scholar]
- 10.Du G.S., Cai X.H., Shang J.H., Luo X.D. Non-alkaline constituents from the leaf of Alstonia scholaris. Chin. J. Nat. Med. 2007;5:259–262. (In Chinese) [Google Scholar]
- 11.Wang Y., Hamburger M., Gueho J., Hostettmann K. Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochemistry. 1989;28:2323–2327. doi: 10.1016/S0031-9422(00)97976-7. [DOI] [Google Scholar]
- 12.Ramarathnam N., Osawa T., Namiki M., Kawakishi S. Chemical studies on novel rice hull antioxidants. 2. Identification of isovitexin, a C-glycosyl flavonoid. J. Agr. Food Chem. 1989;37:316–319. doi: 10.1021/jf00086a009. [DOI] [Google Scholar]
- 13.Arisawa M., Hayashi T., Shimizu M., Morita N., Bai H., Kuze S., Ito Y. Isolation and cytotoxicity of two new flavonoids from Chrysosplenium grayanum and related flavonols. J. Nat. Prod. 1991;54:898–901. doi: 10.1021/np50075a029. [DOI] [PubMed] [Google Scholar]
- 14.Li W.X., Cui C.B., Cai B., Wang H.Y., Yao X.S. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J. Asian Nat. Prod. Res. 2005;7:615–626. doi: 10.1080/10286020310001625085. [DOI] [PubMed] [Google Scholar]
- 15.Chen Liu K.C.S., Yang S.L., Roberts M.F., Elford B.C., Phillipson J.D. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992;11:637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- 16.Okuyama E., Suzumura K., Yamazaki M. Pharmacologically active components of Viticis Fructus (Vitex rotundifolia). I. The components having vascular relaxation effects. Nat. Med. (Tokyo) 1998;52:218–225. [Google Scholar]
- 17.Stermitz F.R., Scriven L.N., Tegos G., Lewis K. Two flavonols from Artemisia annua which potentiate the activity of Berberine and Norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002;68:1140–1141. doi: 10.1055/s-2002-36347. [DOI] [PubMed] [Google Scholar]
- 18.Ling W.W., Zhang Z.Z., Ling T.J., Zhang Y.G. Constituents in the essential Oil of Vitex negundo Linn. var. cannabifolia (Sieb.et Zucc.) Hand.-Mazz. and their antibacterial activities. Sci. Technol. Food Ind. 2010 in press. (In Chinese) [Google Scholar]