Abstract
A series of Schiff’s bases (E)-N-2-aryliden-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazides 2a-l and N-(2-(substituted phenyl)-4-oxo-thiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamides 3a-l were synthesized and evaluated for their antioxidant activity by the phosphomolybdenum method. Most of the Schiff’s bases and thiazolidine-4-ones bearing two hydroxyl groups on the phenyl ring showed excellent antioxidant activity in comparison with ascorbic acid. Preliminary investigation on cytotoxic and antifungal activity was done on some representative samples.
Keywords: coumarin, hydrazides, aromatic Schiff’s bases, N-(2-aryl-4-oxo-thiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamide, antioxidant activity, biological activity
1. Introduction
The structural and therapeutic diversity of small heterocyclic molecules coupled with their commercial viability has long fascinated organic and medicinal chemists. Heterocycles containing the coumarin ring system include some novel pharmacologically active compounds such as dicumarol, warfarin, mercumatilin and novobiocin. Natural coumarins affect the formation and scavenging of ROS and influence free radical-mediated oxidative damage [1].
Azomethine group (-C=N-)-containing compounds, typically known as Schiff’s bases, have been synthesized via condensation of primary amines with active carbonyls. It is well established that the biological activity of hydrazone compounds is associated with the presence of the active (-CO-NH-N=C-) pharmacophore and these compounds form a significant category of compounds in medicinal and pharmaceutical chemistry with several biological applications that include antitumoral [2,3], antifungal [4,5,6,7,8,9], antibacterial [4,5,6,7,8,9,10,11], antimicrobial [12] and anthelmintic uses [13]. Schiff’s base complexes play an important role in designing metal complexes related to synthetic and natural oxygen carriers [14].
In recent years, 4-thiazolidinones and 2,4-thiazolidinediones have been among the most extensively investigated classes of organic compounds. Thiazolidine derivatives are reported to show a variety of biological activities. The presence of a thiazolidine ring in penicillin and related derivatives was the first recognition of its occurrence in nature [15]. Thiazolidine-4-one represents a prevalent scaffold in drug discovery [16]. Literature surveys show that thiazolinylhydrazones exhibit antitubercular and antimicrobial activities [15], and their pronounced antioxidant [17] and antifungal [18] activity has also been reported. Thiazolidine-4-ones have many interesting activity profiles, namely COX-1 inhibitors [19], inhibitors of the bacterial enzyme MurB, which was a precursor acting during the biosynthesis of peptidoglycan [20], non-nucleoside inhibitors of HIV-RT [21] and anti-histaminic agents [22]. Depending on the substituents, 4-oxothiazolidine ring can induce different pharmacological properties such as antibacterial [23], antimycobacterial [24], anticonvulsant [25] or anti-inflammatory activity [26] and it has been reported that the introduction of arylidene moieties at different positions of the thiazolidinone ring enhanced biological activity [27,28,29]. Some authors examined the ability of this ligand structure to form complexes with some radionuclides for potential use in nuclear medicine [30]. Thus, coumarins containing a Schiff’s base and a thiazolidinone moiety are expected to have enhanced biological activities.
2. Results and Discussion
2.1. Synthesis
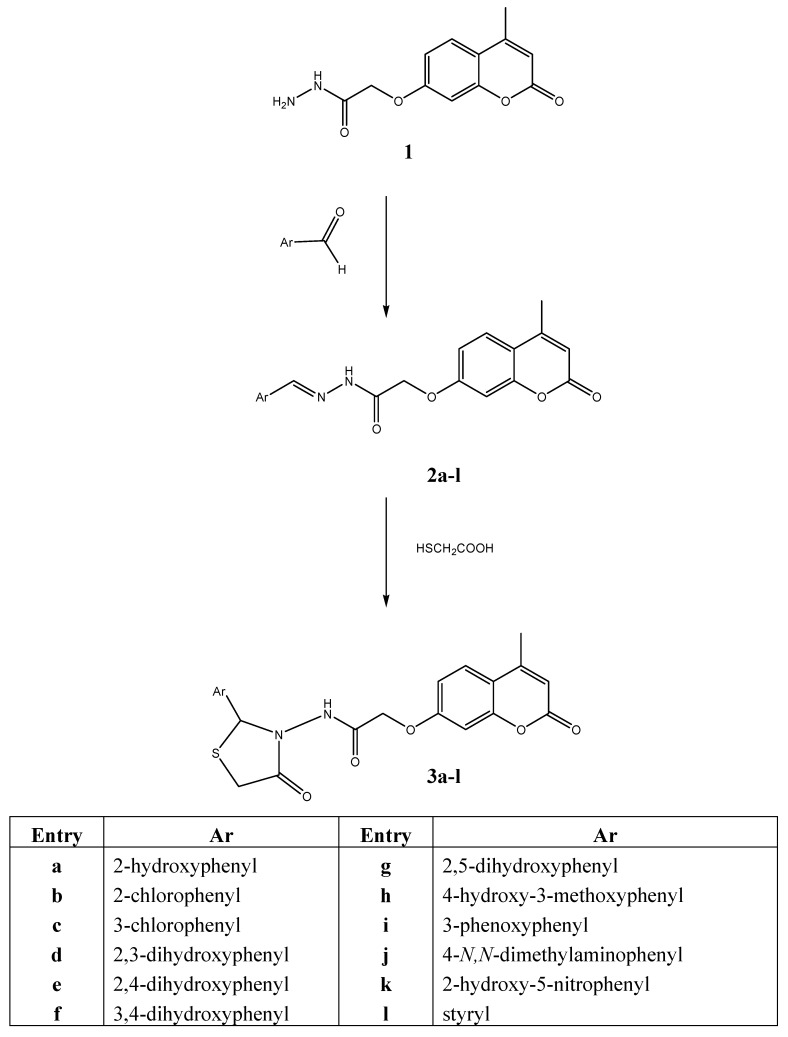
In our ongoing research to synthesize potentially biologically active thiazolidinone derivatives we have now described a series of (E)-N-2-aryliden-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)aceto-hydrazides 2a-l and N-(2-aryl-4-oxo-thiazolidine-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-acetamides 3a-l (Scheme 1). The series of Schiff’s bases 2a-l was prepared similarly to those previously described [5,6,12,13,31], by refluxing solutions of different suitable aromatic aldehydes and hydrazide 1 in absolute ethanol for 2 to 4 hours, in a presence of catalytic amount of glacial acetic acid. The structures of the products 2a-l were inferred from their analytical and spectral data.
Scheme 1.
Synthesis of 2,3-disubstituted-1,3-thiazolidine-4-ones 3a-l.
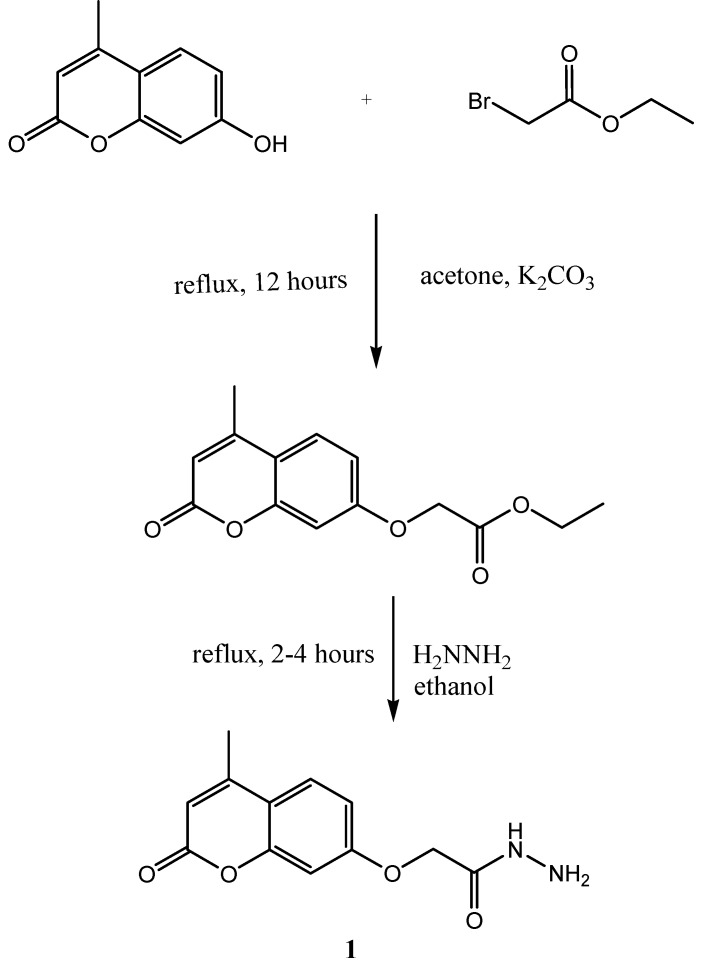
Starting material 1 was prepared as indicated in Scheme 2. Its IR spectrum showed absorption bands in the 3,317 cm-1 (hydrazide NH-NH2), 3,269 cm-1 (aromatic C-H), 1,711 cm-1(-C=O carbonyl stretching) and 1,621–1,640 cm-1 (-CO-NH-NH2 groups) regions, respectively. The 1H-NMR spectrum exhibited a singlet due to the –CO-NH-NH2, NH proton at δ 9.32 ppm. Methylene protons (-OCH2-) resonated as singlets at 4.85 ppm.
Scheme 2.
Synthesis of (4-methyl-2-oxo-2H-chromen-7-yloxy)acetic acid hydrazide 1.
The IR spectra of compounds 2a-l showed characteristic bands at 3,448–3,278 cm-1 (OH; NH), 1,709 cm-1 and 1,672 cm-1(C=O, lactone) and 1,620 cm-1 (C=O, amide, HC=N azomethine). The 1H- NMR spectra did not only show the absence of NH2 protons at 3.38, but also the presence of N=CH proton at 8.30 ppm.
N-(2-aryl-4-oxo-thiazolidine-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamides 3a-l were obtained by the reaction described in [29], which was performed by refluxing the solutions of Schiff’s bases 2a-l and thioglycolic acid in 1,4-dioxane in the presence of anhydrous ZnCl2 for 6-8 hours. Formation of 2,3-disubstituted 4-thiazolidinones 3a-l was confirmed by IR spectroscopy, which showed the ring C=O stretching characteristic of 1,3-thiazolidine-4-ones ring in the range of νmax 1,690–1,730 cm-1.
1H-NMR spectra for 3a-l showed methylene CH2 (COCH2S) protons of the 4-thiazolidinone ring between δ 3.34–3.38 ppm as the singlet signal and δ5.26–5.29 ppm for CH (SCHN); proton of the 4-thiazolidinone ring as a singlet signal.
2.2. Antioxidant activity
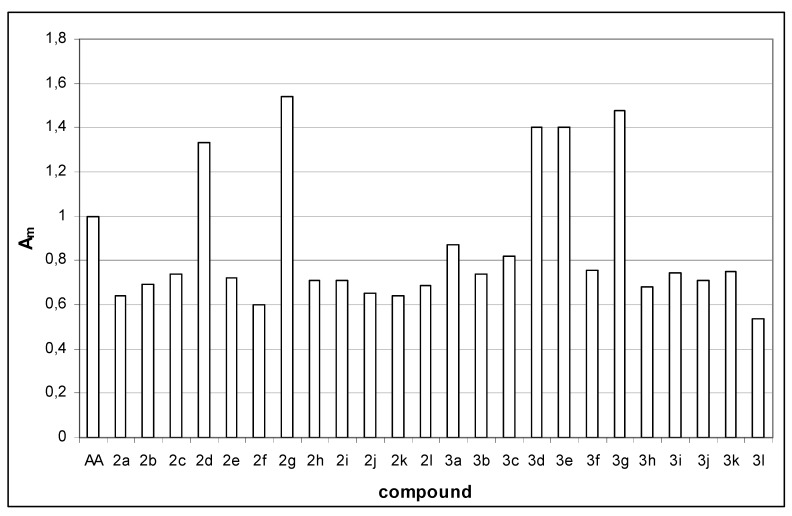
Data in Figure 1. show that substituents on the phenyl ring have a great influence on antioxidant activity. In descending order the effects of the various substituents on the phenyl ring of the Schiff’s bases were found to be: 2,5(OH)2 (2g) > 2,3-(OH)2 (2d) > 3-Cl (2c) > 2,4-(OH)2 (2e) > 3 - phenoxy (2i) > 3-OCH3-4-OH (2h) > 2-Cl (2b) > styryl (2l) > 4-N(CH3)2 (2j) > 2-OH-5-NO2 (2k)> 2-OH (2a) > 3,4-(OH)2 (2f). Among the Schiff’s compounds 2g and 2d have better antioxidant activities than ascorbic acid (1.54 and 1.34 times better, respectively). Both of these compounds have two electron donating OH groups on phenyl ring, one of them being in ortho position in both cases. They also posses another electron donating group, the presence of which obviously contributes to increased antioxidant activity, as the compound 2a with only one OH group in the ortho position did not show relevant antioxidant activity.
Figure 1.
Antioxidant activities of novel coumarin derivatives relative to ascorbic acid (Am – activity relative to ascorbic acid (AA) on a molar basis).
The effects of various substituents on phenyl ring of 1,3-thiazolidine-4-ones in descending order were found to be: 2,5-(OH)2 (3g) > 2,3-(OH)2 (3d) > 2,4-(OH)2 (3e) > 2-OH (3a) > 3-Cl (3c) > 3,4-(OH)2 (3f) > 2-OH-5-NO2 (3k) > 3-phenoxy (3i) > 2-Cl (3b) > 4-N(CH3)2 (3j) > 3-OCH3-4-OH (3h) > styryl (3l). Among the series of 1,3-thiazolidine-4-ones, compounds 3g, 3d and 3e have better antioxidant activity than ascorbic acid (1.48, 1.41 and 1.40 times better, respectively). All of these compounds also have two electron donating OH groups on the phenyl ring, one of them being in an ortho position. Presence of another OH group, no matter the position on the phenyl ring, obviously contributes to increased antioxidant activity, like in the series of Schiff’s bases.
Observing the overall data for antioxidant activity, it is clear that the presence of two hydroxyl groups has a great influence on radical scavenging activity. Schiff’s base 2g shows the greatest antioxidant activity of all investigated compounds, followed by the 1,3-thiazolidine-4-one 3g, both having 2,5-(OH)2 substituents on phenyl ring, which is in accordance with the results of Lin et al. [32] who reported correlation of radical-scavenging effects of coumarins with the number of hydroxyl groups.
3. Experimental
3.1. General
The melting points were taken on an Electrothermal capillary melting point apparatus and are uncorrected. Thin-layer chromatographies were performed using HF254 fluorescent silica gel plates (Merck), which were examined under UV 254 and 365 nm light. Silica gel (230–400 mesh) was used for flash chromatography separations. The elemental analysis for C, H and N were done on a Perkin-Elmer Analyzer 2440. Infrared spectra (ν/cm-1) were recorded on a Beckmann FT-IR 3303 instrument, using KBr disks. 1H- and 13C-NMR spectra were recorded on JEOL EX-270 MHz NMR Spectrometer at 293 K in DMSO-d6. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS. The absorbance was measured on a Helios γ UV visible spectrophotometer (Thermo Spectronic, Cambridge, UK).
3.2. General procedure for preparation of (E)-Ń’-arylidene-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-acetohydrazides 2a-l
A mixture of compound 1 (0.01 mole) and a suitable aromatic aldehyde (Ar/a-k; 0.01 mole) was refluxed in absolute ethanol (30 mL) in presence of a catalytic amount of glacial acetic acid for 2 to 4 hours. The reaction mixture was cooled and the precipitate was filtered and recrystallized from methanol to give compounds 2a-l.
(E)-N´’-(2-Hydroxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2a) [5]. M.p. 284–286 °C; yield (72%); FT-IR: νmax 3,440; 3,282; 3,101; 2,916; 2,853; 1,724; 1,685; 1,621; 1,537; 1,489; 1,393; 1,300 and 1,154 cm-1. 1H-NMR (δ, ppm): 2.41(s, 3H, CH3, C-4); 4.85 (s, 2H, CH2); 6.25 (s, 1H, C-3); 6.87 (s, 1H, H-8), 6.98 (d, 1H, H-6); 7.85 (d, 1H, H-5); 7.25-7.75 (m, 4H, arom.,) 8.33 (s, 1H, NH); 8.56 (s, 1H, HC=N-); 10.99 (s,1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2); 107.65 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 116.1 (C-3, Ar-); 118.4 (C-3, Ar-); 121.7 (C-5, Ar-); 127.8 (C-5); 130.5 (C-6, Ar-); 132.4 (C-1, Ar-); 161.2 (C-2, Ar-); 143.2 (C=N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-4); 172.42 (CO-NH). Anal. Calcd. for C19H16N2O5 (352.34): C, 64.77; H, 4.58; N, 8.95. Found: C, 64.76; H, 4.55; N, 7.92
(E)-N´-(2-Chlorobenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2b) [13]. M.p. 248–250 °C; yield (82%). FT-IR: νmax 3,289; 2,950; 2,864; 1,712; 1,700; 1,624; 1,560; 1,525; 1,398; 1,290 and 1,150 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.22 (s, 1H, H-3); 6.99 (s, 1H, H-8); 7.05 (d, 1H, H-6); 7.39-7.75 (m, 4H, arom.); 8.06 (d, 1H, H-5); 8.41(s, 1H, NH); 8.74 (s, 1H, HC=N-). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2); 107.65 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 127.1 (C-5, Ar-); 127.8 (C-5); 129.1 (C-3, Ar-); 130.4 (C-6, Ar‑); 132.5 (C-4, Ar-); 133.2 (C-1, Ar-); 134.2 (C-2, Ar-); 143.2 (C=N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. for C19H15ClN2O4 (370.79): C, 61.55; H, 4.08; N, 7.56. Found: C, 61.57; H, 4.08; N, 7.53.
(E)-N´-(3-Chlorobenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2c) [5]. M.p. 242–244 °C; yield (80%). FT-IR: νmax 3,448; 3,201; 3,102; 2,997; 1,713; 1,683; 1,617; 1,511; 1,434; 1,391; 1,274 and 1,136 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.82 (s, 2H, CH2); 6.23 (s, 1H, H-3); 6.98 (s, 1H, H-8); 7.02 (d, 1H, H-6); 7.20 (d, 1H, H-5); 7.41-7.74 (m, 4H, arom.); 8.00 (s, 1H, NH); 8.31 (s, 1H, HC=N-). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2); 107.65 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 127.3 (C-6, Ar-); 127.8 (C-5); 129.4 (C-2, Ar-); 130.6 (C-5, Ar-); 131.4 (C-4, Ar-); 134.5 (C-3, Ar-); 135.2 (C-1, Ar-); 143.2 (C=N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.32 (CO-NH). Anal. Calcd. for C19H15ClN2O4 (370.79): C, 61.55; H, 4.08; N, 9.56. Found: C, 61.54; H, 4.07; N, 7.53.
(E)-N´-(2,3-Dihydroxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2d). M.p. 270–274 °C; yield (63%). FT-IR: νmax 3,481; 3,436; 3,332; 3,280; 2,915; 1,719; 1,695; 1,620; 1,541; 1,473; 1,392; 1,265 and 1,153 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.19 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-); 9.27 (s, br., 1H, OH); 11.59 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2 ); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 119.8 (C-4, Ar-); 120.1 (C-1, Ar-); 122.6 (C-5, Ar-); 123.5 (C-6, Ar-); 127.8 (C-5); 143.2 (C=N); 147.5 (C-3, Ar-); 151.4 (C-9); 151.8 (C-2, Ar-); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. for C19H16N2O6 (368.34): C, 61.95; H, 4.38; N, 7.61. Found: C, 61.93; H, 4.36; N, 7.59.
(E)-N´-(2,4-Dihydroxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2e). M.p. 261–262 °C; yield (48%). FT-IR: νmax 3,357; 3,273; 3,169; 3,086; 2,924; 1,716; 1,671; 1,613; 1,512; 1,425; 1,391; 1,264 and 1,152 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-); 10.12 (s, br., 1H, OH); 11.40 (s, br., 1H, OH). 13C- NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2 ); 103.5 (C-3, from Ph); 107.6 (C-8); 108.6 (C-5, Ar-); 111.0 (C-6); 111.3 (C-1, Ar-); 112.8 (C-3); 113.5 (C-9); 127.8 (C-5); 132.5 (C-6, Ar-); 143.2 (C=N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 162.4 (C-4, Ar-); 162.8 (C-2, Ar-); 172.40 (CO-NH). Anal. Calcd. for C19H16N2O6 (368.34): C, 61.95; H, 4.38; N, 7.61. Found: C, 61.93; H, 4.39; N, 7.59.
(E)-N´-(3,4-Dihydroxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2f). M.p. 243–244 °C; yield (38%). FT-IR: νmax 3,393; 3,239; 3,075; 3,071; 2,980; 1,716; 1,694; 1,612; 1,541; 1,509; 1,494; 1,393; 1,262 and 1,159 cm-1. 1H-NMR(δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-); 9.35 (s, br., 1H, OH); 10.14 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2 ); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 116.8 (C-2, Ar‑); 117.6 (C-5, Ar); 123.5 (C-6, Ar-); 127.3 (C-1, Ar-); 127.8 (C-5); 143.2 (C=N); 143.5 (C-3, Ar-); 149.8 (C-4, Ar-); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. for C19H16N2O6 (368.34): C, 61.95; H, 4.38; N, 7.61. Found: C, 61.90; H, 4.35; N, 7.62.
(E)-N´-(2,5-Dihydroxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2g). M.p. 273–274 °C; yield (41%). IR: νmax 3,418; 3,276; 3,075; 2,915; 1,705; 1,686; 1,658; 1,622; 1,611; 1,584; 1,550; 1,512; 1,428; 1,392; 1,288 and 1,149 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.,); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-); 9.26 (s, br., 1H, OH); 11.93 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 116.5 (C-6, Ar-); 117.5 (C-3, Ar-); 119.4 (C-4, Ar-); 120.1 (C-1, Ar-); 127.8 (C-5); 143.2 (C=N); 151.4 (C-9); 151.3 (C-5, Ar-); 152.9 (C-4); 153.8 (C-2, Ar-); 160.3 (C-7); 160.8 (C-2); 172.44 (CO-NH). Anal. Calcd. for C19H16N2O6 (368.34): C, 61.95; H, 4.38; N, 7.61. Found: C, 61.92; H, 4.37; N, 7.62.
(E)-N´-(4-Hydroxy-3-methoxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (2h) [5]. M.p. 253–254 °C; yield (76%). FT-IR: νmax 3,530; 3,481; 3,319; 3,268; 2,914; 1,720; 1,695; 1,618; 1,540; 1,472; 1,390; 1,265 and 1,153 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.82 (3H, s, OCH3); 4.84 (s, 2H, OCH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-); 11.50 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 56.2 (OCH3); 69.10 (OCH2 ); 107.6 (C-8);); 111.0 (C-6); 112.8 (C-3); 113.5 (C‑9); 114.8 (C-2, Ar-); 117.3 (C-5, Ar-); 123.0 (C-6, Ar-); 127.2 (C-1, Ar-); 127.8 (C-5); 143.2 (C=N); 148.1 (C-4, Ar-); 151.4 (C-9); 151.5 (C-3, Ar-); 152.9 (C-4); 160.3 (C-7); 160.7 (C-2); 172.42 (CO-NH). Anal. Calcd. for C20H18N2O6 (382.37): C, 62.82; H, 4.74; N, 7.33. Found: C, 62.79; H, 4.73; N, 7.31.
(E)-N´-(3-Phenoxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-acetohydrazide (2i). M.p. 178–180 °C; yield (72%). FT-IR: νmax 3,526; 3,476; 3,329; 3,288; 2,916; 1,723; 1,690; 1,623; 1,544; 1,470; 1,392; 1,265 and 1,153 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 9H, arom.,); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-). 13C-NMR (δ, ppm): 21.4 (CH3); 56.2 (OCH3); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 116.8 (C-2, Ar-Ph); 117.4 (C-2,6, Ar- PhO); 119.7 (C-4, Ar- Ph); 121.8 (C-4, Ar-PhO); 122.3 (C-6, Ar- Ph); 127.8 (C-5); 128.5 (C-3,5, Ar-PhO); 128.8 (C-5, Ar- Ph); 133.6 (C-1, Ar- Ph); 143.2 (C=N); 151.4 (C-9); 152.9 (C-4); 157.1 (C-1, Ar- PhO); 157.3 (C-3, Ar- Ph); 160.3 (C-7); 160.8 (C-2); 172.40 (CO-NH). Anal. Calcd. for C25H20N2O5 (428.44): C, 70.08; H, 4.71; N, 6.54. Found: C, 70.10; H, 4.72; N, 6.55.
(E)-N´-(4-(Dimethylamino)benzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (2j) [6]. M.p. 260–262 °C; yield (86%). FT-IR: νmax 3,528; 3,471; 3,329; 3,285; 2,912; 1,721; 1,695; 1,621; 1,541; 1,473; 1,392; 1,265 and 1,153 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 2.86 (s, 6H, N(CH3)2); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 4H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-) 13C-NMR, (δ, ppm): 21.4 (CH3); 41.4 (N(CH 3 )2); 69.10 (OCH2 ); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 114.5 (C-3,5, Ar‑); 123.04 (C-1, Ar-); 127.8 (C-5); 130.2 (C-2,6, Ar-); 143.2 (C=N); 151.4 (C-10); 151.8 (C-4, Ar-); 152.8 (C-4,coum.); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. For C21H21N3O4 (379.41): C, 66.48; H, 5.58; N, 11.08. Found: C, 66.50; H, 5.57; N, 11.10.
(E)-N´-(2-Hydroxy-5-nitrobenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazide (2k). M.p. 288–290 °C; yield (90%). FT-IR: νmax 3,536; 3,481; 3,329; 3,268; 2,910; 1,724; 1,690; 1,622; 1,539; 1,474; 1,390; 1,265 and 1,153 cm-1. 1H NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-); 11.59 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 117.1 (C-3, Ar-); 119.6 (C-1, Ar-); 120.1 (C-1, Ar-); 124.5 (C-4, Ar-); 125.7 (C-6, Ar-); 127.8 (C-5); 141.3 (C-5, Ar-); 143.2 (C=N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.8 (C-2); 167.8 (C-2, Ar-); 172.41 (CO-NH). Anal. Calcd. for C19 H15N3O7 (397.34): C, 57.43 ; H, 3.81; N,10.58. Found: C, 57.40; H, 3.82; N, 10.54.
(E)-2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)-N´-[(E)-3-phenylallylidene]acetohydrazide (2l) [6]. M.p. 249–250 °C; yield (46%). FT-IR: νmax 3,530; 3,481; 3,339; 3,288; 2,916; 1,720; 1,695; 1,621; 1,541; 1,473; 1,392; 1,265 and 1,153 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 4.84 (s, 2H, CH2); 5.72 (d, 1H, styryl); 6.24 (s, 1H, H-3); 6.63 (d, 1H, styryl); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 8.52 (s, 1H, HC=N-). 13C-NMR (δ, ppm): 21.4 (CH3); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 126.1 (C-1, Ar-ethenyl); 126.6 (C-2,6, Ar-); 127.8 (C-5); 128.5 (C-4, Ar-); 128.9 (C-3,5, Ar-); 137.2 (C=N); 139.2 (C-2, Ar-ethenyl); 151.4 (C-10); 152.9 (C-4); 160.3 (C-7); 161.0 (C-2); 172.42 (CO-NH). Anal. Calcd. For C21H18N2O4 (362.38): C, 69.60; H, 5.01; N, 7.73. Found. C, 69.59; H, 4.99; N, 7.70.
3.3. General procedure for preparation of N-(2-(substituted)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamides 3a-l [33]
To a solution of (E)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N´-[(E)-3-arylidene]acetohydrazide 2a-l (0.01 mol) in dry 1,4-dioxane (15 mL), freshly distilled mercaptoacetic acid (0.01 mol) and anhydrous ZnCl2 (0.1 g) were added and the mixture was heated under reflux 10 to 12 hours. The solvent was removed (reduced pressure) and residue washed with 5% sodium bicarbonate solution (3 × 20 mL) and water (2 × 20 mL), dried, and recrystallized from an appropriate solvent.
N-(2-(2-Hydroxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3a). M.p. 284–286 °C; yield (72%). FT-IR: νmax 3,418; 3,182; 3,100; 2,906; 2,853; 1,725; 1,681; 1,623; 1,527; 1,489; 1,393; 1,300 and 1,144 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.34 (s, 2H, COCH2S); 4.84 (s, 2H, OCH2); 5.28 (s, 1H, SCHN); 6.24 (s, 1H, C-3); 6.87 (s, 1H, H-8), 6.98 (d, 1H, H-6); 7.25–7.75 (m, 4H, arom.); 8.06 (d, 1H, H-5); 8.33(s, 1H, NH); 10.99 (s, br.,.1H, OH). 13C- NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 69.10 (OCH2); 107.65 (C-8); 111.0 (C-6); 112.8 (C-3); 113.6 (C-10); 116.1 (C-3, Ar-); 118.4 (C-3, Ar-); 121.7 (C-5, Ar-); 127.8 (C-5); 130.5 (C-6, Ar-); 132.4 (C-1, Ar-); 161.2 (C-2, Ar-); 143.2 (CO-N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-4); 172.45 (CO-NH). Anal. Calcd. for C21H18N2O6S (426.44), C, 59.15; H, 4.25; N, 6.57; S, 7.52. Found: C, 59.10; H, 4.27; N, 6.54; S, 7.49.
N-(2-(2-Chlorophenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3b) [26]. M.p. 248–250 °C; yield (82%). FT-IR: νmax 3,280; 2,940; 2,854; 1,722; 1,710; 1,621; 1,580; 1,515; 1,394; 1,295 and 1,146 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.35 (s, 2H, COCH2S); 4.84 (s, 2H, OCH2); 5.29 (s, 1H, SCHN); 6.22 (s, 1H, H-3); 6.99 (s, 1H, H-8); 7.05 (d, 1H, H-6); 7.39–7.75 (m, 4H, arom.); 8.06 (d, 1H, H-5); 8.41 (s, 1H, NH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.65 (C-8); 111.0 (C-6); 112.8 (C-3); 113.4 (C-10); 127.1 (C-5, Ar-); 127.8 (C-5); 129.1 (C-3, Ar-); 130.4 (C-6, Ar-); 132.5 (C-4, Ar-); 133.2 (C-1, Ar-); 134.2 (C-2, Ar-); 143.2 (CO-N); 151.2 (C-19); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. for C21H17Cl N2O5S (444.89), C, 56.69; H, 3.85; N, 6.30; S, 7.21. Found: C, 56.70; H, 3.83; N, 6.31; S, 7.20.
N-(2-(3-Chlorophenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3c). M.p. 242–244 °C; yield (86%). FT-IR: νmax 3,438; 3,200; 3,112; 2,987; 1,723; 1,688; 1,627; 1,516; 1,430; 1,390; 1,264 and 1,136 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.38 (s, 2H, COCH2S); 4.82 (s, 2H, CH2); 5.28 (s, 1H, SCHN); 6.23 (s, 1H, H-3); 6.98 (s, 1H, H-8); 7.02 (d, 1H, H-6); 7.41–7.74 (m, 4H, arom.); 8.09 (d, 1H, H-5); 8.40 (s, 1H, NH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.65 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 127.3 (C-6, Ar-); 127.8 (C-5); 129.4 (C-2, Ar-); 130.6 (C-5, Ar-); 131.4 (C-4, Ar-); 134.5 (C-3, Ar-); 135.2 (C-1, Ar-); 143.3 (CO-N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. for C21H17Cl N2O5S (444.89), C, 56.69; H, 3.85; N, 6.30; S, 7.21.Found: C, 56.62; H, 3.82; N, 6.31; S, 7.24.
N-(2-(2,3-Dihydroxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3d). M.p. 270–271 °C; yield (63%). FT-IR: νmax 3,531; 3,471; 3,309; 3,268; 2,926; 1,725; 1,698; 1,626; 1,551; 1,483; 1,388; 1,260 and 1,143 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.34 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.28 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90–7.75 (m, 3H, arom.); 8.32 (s, 1H, NH); 9.27 (s, br., 1H, OH); 11.59 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 119.8 (C-4, Ar-); 120.1 (C-1, Ar-); 122.6 (C-5, Ar-); 123.5 (C-6, Ar-); 127.8 (C-5); 143.2 (CO-N); 147.5 (C-3, Ar-); 151.4 (C-9); 151.8 (C-2, Ar-); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.43 (CO-NH). Anal. Calcd. for C21H18N2O7S (442.44), C, 57.01; H, 4.10; N, 6.33; S, 7.25. Found: C, 57.00; H, 4.08; N, 6.30; S, 7.23.
N-(2-(2,4-Dihydroxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3e). M.p. 261–262 °C; yield (48%). FT-IR: νmax 3,345; 3,282; 3,172; 3,106; 2,912; 1,726; 1,691; 1,622; 1,510; 1,430; 1,382; 1,274 and 1,141 cm-1. 1H NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.38 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.29 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90–7.70 (m, 3H, arom.); 8.32 (s, 1H, NH); 10.12 (s, br., 1H, OH); 11.40 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.30 (OCH2); 102.7 (C-3, Ar-); 107.9 (C-8); 108.6 (C-5, Ar-); 111.5 (C-6); 111.7 (C-1, Ar-); 112.8 (C-3); 113.6 (C-10); 127.0 (C-5); 132.5 (C-6, Ar-); 143.2 (CO-N); 151.4 (C-9); 152.9 (C-4); 160.4 (C-7); 160.9 (C-2); 162.4 (C-4, Ar-); 162.8 (C-2, Ar-); 172.43 (CO-NH). Anal. Calcd. for C21H18N2O7S (442.44), C, 57.01; H, 4.10; N, 6.33; S, 7.25. Found: C, 57.04; H, 4.97; N, 6.32; S, 7.29.
N-(2-(3,4-Dihydroxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3f). M.p. 243–244 °C; yield (38%). FT-IR: νmax 3,389; 3,209; 3,045; 3,037; 2,974; 1,726; 1,698; 1,612; 1,548; 1,521; 1,489; 1,396; 1,262 and 1,150 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.35 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.28 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.65 (m, 3H, arom.); 8.32 (s, 1H, NH); 9.35 (s, br., 1H, OH); 10.14 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 116.8 (C-2, Ar-); 117.6 (C-5, Ar-); 123.5 (C-6, Ar-); 127.3 (C-1, Ar-); 127.8 (C-5); 143.2 (C-N); 143.5 (C-3, Ar-); 149.8 (C-4, Ar-); 151.4 (C‑9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.42 (CO-NH). Anal. Calcd. for C21H18N2O7S (442.44), C, 57.01; H, 4.10; N, 6.33; S, 7.25. Found: C, 56.99; H, 3.89; N, 6.32; S, 7.24.
N-(2-(2,5-Dihydroxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3g). M.p. 278–279 °C; yield (41%). FT-IR: νmax 3,420; 3,281; 3,065; 2,915; 1,708; 1,716; 1,688; 1,648; 1,627; 1,581; 1,554; 1,521; 1,418; 1,390; 1,278 and 1,149 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.38 (s,2H,COCH2S); 4.84 (s, 2H, CH2); 5.28 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.72 (m, 3H, arom.); 8.32 (s, 1H, NH); 9.26 (s, br., 1H, OH); 11.93 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 116.5 (C-6, Ar-); 117.5 (C-3, Ar-); 119.4 (C-4, Ar-); 120.1 (C-1, Ar-); 127.8 (C-5); 143.2 (C-N); 151.4 (C-9); 151.3 (C-5, Ar‑); 152.9 (C-4); 153.8 (C-2, Ar-); 160.3 (C-7); 160.9 (C-2); 172.43 (CO-NH). Anal. Calcd. for C21H18N2O7S (442.44), C, 57.01; H, 4.10; N, 6.33; S, 7.25. Found: C, 57.00; H, 4.08; N, 6.31; S, 7.20.
N-(2-(4-hydroxy-3-methoxyphenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3h) [23]. M.p. 233–234 °C; yield (76%). FT-IR: νmax 3,517; 3,478; 3,329; 3,274; 2,915; 1,723; 1,698; 1,629; 1,536; 1,469; 1,393; 1,255 and 1,145 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.37 (s, 2H, COCH2S); 3.76 (s, 3H, OCH3); 4.84 (s, 2H, CH2); 5.27 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.71 (m, 3H, arom.); 8.32 (s, 1H, NH); 11.59 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 56.2 (OCH3); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 114.8 (C-2, Ar-); 117.3 (C-5, Ar-); 123.0 (C-6, Ar-); 127.2 (C-1, Ar-); 127.8 (C-5); 143.2 (C-N); 148.1 (C-4, Ar-); 151.4 (C‑9); 151.5 (C-3, Ar-); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 172.40 (CO-NH). Anal. Calcd. for C22H20N2O7S (456.47), C, 57.89; H, 4.42; N, 6.14; S, 7.02. Found: C, 57.88; H, 4.39; N, 6.13; S, 7.01.
2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-(3-phenoxyphenyl)thiazolidin-3-yl) acetamide (3i). M.p. 217–218 °C; yield (72%). FT-IR: νmax 3,500; 3,471; 3,329; 3,279; 2,910; 1,720; 1,697; 1,619; 1,531; 1,467; 1,392; 1,264 and 1,151 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.38 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.29 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.76 (m, 9H, arom.); 8.32 (s, 1H, NH). 13C-NMR (δ, ppm), 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 115.7 (C-4, Ar-Ph); 116.8 (C-2, Ar- Ph); 117.4 (C-2,6, Ar- PhO); 121.8 (C‑4, Ar- PhO); 122.3 (C-6, Ar- Ph); 127.8 (C-5); 128.5 (C-3,5, Ar- PhO); 128.8 (C-5, Ar- Ph); 133.6 (C-1, Ar- Ph); 151.4 (C-9); 152.9 (C-4); 157.1 (C-1, Ar- PhO); 157.3 (C-3, Ar- Ph); 160.3 (C-7); 160.9 (C-2); 166.1 (CO-NH); 168.2 (CO-N). Anal. Calcd. for C27H22N2O6S (502.54), C, 64.53; H, 4.41; N, 5.57; S, 6.38. Found: C, 64.50; H, 4.39; N, 5.53; S, 6.39.
N-(2-(4-(Dimethylamino)phenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-acetamide (3j). M.p. 227–228 °C; yield (76%). FT-IR: νmax 3,526; 3,473; 3,329; 3,269; 2,926; 1,729; 1,690; 1,624; 1,539; 1,473; 1,378; 1,260 and 1,150 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 2.86 (s, 6H, N(CH3)2); 3.36 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.29 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90-7.7 (m, 4H, arom.); 8.32 (s, 1H, NH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 41.4(N(CH3)2); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 114.5 (C-3,5, Ar-); 123.04 (C-1, Ar); 130.2 (C‑2,6, Ar); 143.2 (C-N); 151.4 (C-9); 151.8 (C-4, Ar-); 160.2 (C-7); 160.9 (C-2); 172.43 (CO-NH). Anal. Calcd. for C23H23N3O5S (453.51), C, 60.91; H, 5.11; N, 9.27; S, 7.07. Found: C, 60.92; H, 5.09; N, 9.25; S, 7.01.
N-(2-(2-Hydroxy-5-nitrophenyl)-4-oxothiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetamide (3k). M.p. 288–290 °C; yield (90%). FT-IR: νmax 3,468; 3,459; 3,329; 3,218; 2,910; 1,720; 1,692; 1,625; 1,536; 1,469; 1,386; 1,260 and 1,147 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.37 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.28 (s, 1H, SCHN); 6.24 (s, 1H, H-3); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.90–7.82 (m, 3H, arom.); 8.32 (s, 1H, NH); 11.54 (s, br., 1H, OH). 13C-NMR (δ, ppm): 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-10); 117.1 (C-3, from Ph); 120.1 (C-1, from Ph); 124.5 (C-4, from Ph); 125.7 (C-6, from Ph); 127.8 (C-5); 141.3 (C-5, from Ph); 143.3 (C-N); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C-2); 167.8 (C-2, from Ph); 174.0 (CO-NH). Anal. Calcd. for C21H17N3O8S (471.44), C, 53.50; H, 3.63; N, 8.91; S, 6.80. Found: C, 53.47; H, 3.64; N, 8.90; S, 6.79.
(E)-2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-oxo-2-styrylhiazolidin-3-yl)acetamide (3l). M.p. 249–250 °C; yield (46%). FT-IR: νmax 3,489; 3,416; 3,309; 3,292; 2,906; 1,728; 1,695; 1,621; 1,543; 1,468; 1,389; 1,254 and 1,131 cm-1. 1H-NMR (δ, ppm): 2.40 (s, 3H, CH3, C-4); 3.36 (s, 2H, COCH2S); 4.84 (s, 2H, CH2); 5.26 (s, 1H, SCHN); 6.13 (d, 1H, styryl); 6.24 (s, 1H, H-3); 6.41 (d, 1H, styryl); 6.73 (s, 1H, H-8); 6.85 (d, 1H, H-6); 7.20 (d, 1H, H-5); 6.94–7.70 (m, 5H, arom.); 8.32 (s, 1H, NH). 13C-NMR (δ, ppm) 21.4 (CH3); 32.92 (COCH2S); 47.81 (SCHN); 69.10 (OCH2); 107.6 (C-8); 111.0 (C-6); 112.8 (C-3); 113.5 (C-9); 126.1 (C-1, styryl); 126.6 (C-2,6, Ar-); 127.8 (C-5); 128.5 (C-4, Ar-); 128.9 (C-3,5, Ar-); 137.2 (C-N); 139.2 (C-2, styryl); 151.4 (C-9); 152.9 (C-4); 160.3 (C-7); 160.9 (C‑2); 174.4 (CO-NH). Anal. Calcd. for C23H20N2O5S (436.48), C, 63.29; H, 4.62; N, 6.42; S, 7.35. Found: C, 63.27; H, 4.61; N, 6.39; S, 7.31.
3.4. Evaluation of antioxidant activity
The antioxidant activity of tested coumarin derivatives was evaluated by the phosphomolybdenum method according to the procedure in [34]. This method is based on the reduction of Mo(VI) to Mo(V) by the tested compounds followed by formation of a green phosphate/Mo(V) complex at acid pH. An aliquot of sample solution (100 μL, 2 mM in DMSO) is mixed with the reagent solution (1 mL, 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The samples are incubated in a water bath at 95 °C for 90 minutes. Samples are cooled to room temperature and the absorbance was measured at 695 nm. The antioxidant activity was expressed relative to the antioxidant activity of same concentration of ascorbic acid.
4. Conclusions
In this study a series of Schiff’s bases (E)-N-2-aryliden-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetohydrazides 2a-l and novel N-(2-(substituted phenyl)-4-oxo-thiazolidin-3-yl)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)acetamides 3a-l were synthesized and evaluated for their antioxidant activity by phosphomolybdenum method. The 1,3-thiazolidine-4-one derivatives containing the coumarin moiety were synthesized by cyclocondensation of the Schiff's bases and mercaptoacetic acid. Compounds which are indicated as already known are resynthetized and the analytical data obtained for these compounds were comparable, but slightly different from those of other authors. For all the novel compounds structures were elucidated by the means of various spectral methods. Two of the Schiff’s bases (2g, 2d) and three of 1,3-thiazolidine-4-ones (3g, 3d, 3e) proved to have better antioxidant activity in comparison with ascorbic acid. In conclusion, it is evident that the substituents on the phenyl ring have a great influence on antioxidant activity.
Footnotes
Sample Availability: Samples of all compounds are available from the authors.
References and Notes
- 1.Fylaktakidou K.C., Hadjipavlou-Litina D.J., Litinas K.E., Nikolaides D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 2.Mladenova R., Ignatova M., Manolova N., Petrova T., Rashkov I. Preparation, characterization and biological activity of Schiff base compounds derived from 8-hydroxyquinoline-2-carboxaldehyde and Jeffamines ED. Eur. Polym. J. 2002;38:989–999. doi: 10.1016/S0014-3057(01)00260-9. [DOI] [Google Scholar]
- 3.Walsh O.M., Meegan M.J., Prendergast R.M., Nakib T.A. Synthesis of 3-acetoxyazetidin-2-ones and 3-hydroxyazetidin-2-ones with antifugal and antifungal and antibacterial activity. Eur. J. Med. Chem. 1996;31:989–1000. [Google Scholar]
- 4.Singh K., Barwa M.S., Tyagi P. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur. J. Med. Chem. 2006;41:147–153. doi: 10.1016/j.ejmech.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Ahluwalia V.K., Anand S., Goyal M.: Neghipur., G.A. Synthesis of N-benzylidene derivatives of 7-hydroxy-4-methyl/phenyl coumarin as potenial fungicides and bactericides. Bokin Bobai. 1983;11:622–626. [Google Scholar]
- 6.Sengupta A.K., Sen S., Srivastava V. Synthesis of coumarin derivatives as possible antifungal and antibacterial agents. J. Ind. Chem. Soc. 1989;66:710–716. [Google Scholar]
- 7.Panneerselvam P., Nair R.R., Vijayalakshmi G., Subramanian E.H., Sridhar S.K. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents. Eur. J. Med. Chem. 2005;40:225–229. doi: 10.1016/j.ejmech.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar S.K., Saravan M., Ramesh A. Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur. J. Med. Chem. 2001;36:615–623. doi: 10.1016/S0223-5234(01)01255-7. [DOI] [PubMed] [Google Scholar]
- 9.Pandeya S.N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2-yl]thiosemicarbazide. Eur. J. Pharmacol. Sci. 1999;9:25–31. doi: 10.1016/S0928-0987(99)00038-X. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Hussen A.A.A. Synthesis and spectroscopic studies on ternary bis-Schiff-base complexes having oxygen and/or nitrogen donors. J. Coord. Chem. 2006;59:157–176. doi: 10.1080/00958970500266230. [DOI] [Google Scholar]
- 11.Karthikeyan M.S., Prasad D.J., Poojary B., Subramanya Bhat K., Holl B.S., Kumari N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006;14:7482–7489. doi: 10.1016/j.bmc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Sharma B.M., Parsania M.V., Baxi A.J. Synthesis of some azetidinones wih coumarinyl moiety and their antimicrobial activity. Org. Chem. 2008;4:304–308. [Google Scholar]
- 13.Husain M.I., Shukla M.A., Agarwal S.K. Search for potent anthelmintics. Part VII. Hydrazones derived from 4-substituted 7-coumarinyloxyacetic acid hydrazides. J. Ind. Chem. Soc. 1979;56:306–307. [Google Scholar]
- 14.Thangadurai T.D., Gowri M., Natarajan K. Synthesis and characterization of ruthenium(III) complexes containing monobasic bidentate Schiff bases and their biological acivities. Synth. React. Inorg. Met. Org. Chem. 2002;32:329–343. doi: 10.1081/SIM-120003211. [DOI] [Google Scholar]
- 15.Pulici M., Quartieri F. Traceless Solid-Phase Synthesis of 2-Amino-5-alkylidene-thiazol-4-ones. Tetrahedron Lett. 2005;46:2387–2391. doi: 10.1016/j.tetlet.2005.02.059. [DOI] [Google Scholar]
- 16.Brown F.C. 4-Thiazolidinones. Chem. Rev. 1961;41:464–511. [Google Scholar]
- 17.Manojkumar P., Ravi T.K., Subbuchettiar G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009;59:159–170. doi: 10.2478/v10007-009-0018-7. [DOI] [PubMed] [Google Scholar]
- 18.Verma A., Saraf S.H. 4-Thiazolidinone – A biologically active scaffold. Eur. J. Med. Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Look G.C., Schuilck J.R., Homes C.P., Chinn J.P., Gordon E.M., Gallop M.A. The identification of cyclooxygenase-1 inhibitors from 4-thiazolidine combinatorial. Bioorg. Med. Chem. Lett. 1996;6:707–712. doi: 10.1016/0960-894X(96)00097-2. [DOI] [Google Scholar]
- 20.Anders C.J., Bronson J.J., D'Andrea S.V., Despande M.S., Falk P.J., Grant-Young K.A., Harte W.E., Ho H.T., Misco P.F., Robertson J.G., Stock D., Sun Y., Walsh A.W. 4-Thiazolidinones: Novel inhibitors of the bacterial enzyme MurB. Bioorg. Med. Chem. Lett. 2000;10:715–717. doi: 10.1016/s0960-894x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 21.Barreca M.L., Chimiri A., De Luca L., Monforte A.M., Monforte P., Rao A., Zappala M., Balzarini J., De Clercq E., Pannecouque C., Witvrouw M. Discovery of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV-1 agents. Bioorg. Med. Chem. Lett. 2001:1793–1796. doi: 10.1016/s0960-894x(01)00304-3. [DOI] [PubMed] [Google Scholar]
- 22.Diurno M.V., Mazzoni O., Piscopo E., Calignano P.E., Giordano F., Bolognese A. Synhesis and antihistaminic activity of some thiazolidin-4-ones. J. Med. Chem. 1992;35:2910–2912. doi: 10.1021/jm00093a025. [DOI] [PubMed] [Google Scholar]
- 23.Bonde C.G., Gaikwad N.J. Synhesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Biorg. Med. Chem. 2004;12:2151–2161. doi: 10.1016/j.bmc.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Kucukguzel S.G., Oruc E.G., Rolas S., Sahin F., Ozbek A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002;37:197–206. doi: 10.1016/S0223-5234(01)01326-5. [DOI] [PubMed] [Google Scholar]
- 25.Shyam R., Tiwari I.C. Synthesis and spectra of 3-benzyl(or p-tolyl)-5-methyl-2-(substituted benzothiazol-2′-ylimino)-4-thiazolidones. Bull. Chem. Soc. Japan. 1977;50:514–516. [Google Scholar]
- 26.Taranalli A.D., Bhat A.R., Srinivas S., Saravanan E. Evaluation of analgesic and anti-inflammatory activities of thiazolidinone derivatives. J. Cell Tiss. Res. 2007;7:1057–1059. [Google Scholar]
- 27.Vicini P., Zani F., Cozzini P., Doytchinova I. Hydrazones of 1,2-benzisothiazole hydrazides: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002;37:353–364. doi: 10.1016/s0223-5234(02)01378-8. [DOI] [PubMed] [Google Scholar]
- 28.Zani F., Vicini P., Incerti M. Synthesis and antimicrobial properties of 2-(benzylidene-amino)-benzo[d]isothiazol-3-ones. Eur. J. Med. Chem. 2004;39:135–140. doi: 10.1016/j.ejmech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Vicini P., Geronikaki A., Anastasia K., Incerti M., Zani F. Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg. Med. Chem. 2006;14:3859–3864. doi: 10.1016/j.bmc.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Butvin P., Mitkova M., Svetlik J., Havranek E. Complex Formation of Thiazolidine-2,4-dicarboxylic Acid with Selected Divalent and Trivalent Metal Ions. Chem. Pap. 2002;56:174–177. [Google Scholar]
- 31.Cacic M., Trkovnik M., Cacic F., Has-Schön E. Synthesis and antibacterial acivity of some derivatives of (7-hydroxy-2-oxo-2H-chromen-4-yl) acetic acid hydrazide. Molecules. 2006;11:134–147. doi: 10.3390/11010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H.C., Tsai S.H., Chen C.S., Chang Y.C., Lee C.M., Lai Z.Y., Lin C. M. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem. Pharmacol. 2008;75:1416–1425. doi: 10.1016/j.bcp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Cacic M., Molnar M., Balic T., Draca N., Rajkovic V. Design and synthesis of some thiazolidin-4-ones based on (7-hydroxy-2-oxo-2H-chromen-4-yl) acetic acid. Molecules. 2009;14:2501–2513. doi: 10.3390/molecules14072501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]