Abstract
Post-ischemic vasodynamic changes in infarcted brain parenchyma are common and range from hypo- to hyperperfusion. In the present study, appearance of the lenticulostriate arteries (LSAs) on postinterventional 3T time-of-flight (TOF)-MRA suggestive for altered post-stroke vasodynamics following thrombectomy was investigated. Patients who underwent thrombectomy for a proximal MCA occlusion and for whom postinterventional 3T TOF-MRA (median at day 3) was available, were included in this retrospective analysis (n=98). LSA appearance was categorized into presence (LSA-sign+) or absence (LSA-sign−) of vasodilatation in the ischemic hemisphere. Functional outcome was determined using the modified Rankin scale (mRS). LSA-sign+ was observed in 64/98 patients. Hypertension (adjusted OR: 0.171, 95% CI: 0.046–0.645) and preinterventional IV rtPA (adjusted OR: 0.265, 95% CI: 0.088–0.798) were associated with absence of the LSA-sign+. In multivariate logistic regression, LSA-sign+ was associated with substantial neurologic improvement (adjusted OR: 10.18, 95% CI: 2.69–38.57) and good functional outcome (discharge-mRS ≤ 2, adjusted OR: 7.127, 95% CI: 1.913–26.551 and day 90 mRS ≤ 2, adjusted OR: 3.786, 95% CI: 1.026–13.973) after correcting for relevant confounders. For all clinical endpoints, model fit improved when including the LSA-sign term (p<0.05). Asymmetrical dilatation of LSAs following successful thrombectomy indicates favorable neurologic and mid-term functional outcomes. This may indicate preserved cerebral blood flow regulatory mechanisms.
Keywords: Ischemic stroke, thrombectomy, magnetic resonance angiography, lenticulostriate artery, dilatation, striatocapsular
Introduction
Cerebral ischemia, by definition, involves alterations to cerebral blood flow (CBF). In the setting of a large vessel occlusion, relative alterations in CBF create three distinct tissue zones. The ischemic core: a region of critical ischemia resulting in irrecoverable neuronal cell death. The penumbra: a region of hypoperfusion generally maintained by collateral flow, which, if perfusion is not restored, will be recruited by the ischemic core. And the region of benign oligaemia: a region surrounding the penumbra with reduced CBF but able to maintain function and ultimately will survive.1 However, there are also complex changes to CBF, which may persist beyond the restoration of blood flow (e.g. after reperfusion with endovascular thrombectomy). These changes are governed by complex mechanisms at the micro- and macrovascular level.2–5
Vascular responses demonstrate considerable heterogeneity based on the ischemic versus the reperfusion phase, the relative time of the ischemic insult and the type of vessel studied. Post-ischemic vasodynamic alterations vary from relative hyperemia6–8 due to metabolic acidosis or decreased smooth muscle cell activity9 to pronounced hypoperfusion (no reflow phenomenon). The mechanism of the latter is debated. However, smooth muscle cells contraction,10 irreversible pericyte constriction (pericytes rigor mortis),11,12 inflammatory cell and platelet-induced capillary obstruction13,14 or even mechanically induced vessel compression due to neighboring edema15 have been proposed as potential mechanisms. These pathways are of considerable interest. Not only are they potentially important in increasing or limiting clinical benefit of blood flow restoration but also may drive complications such as intracerebral haemorrhage and malignant cerebral oedema.2,6–8,16,17
Thrombectomy is a novel treatment regimen capable of achieving high reperfusion rates at the macrovascular level.18 These high reperfusion rates were considered to be a key factor for the recently reported treatment superiority of thrombectomy when compared to standard medical management.18–21 However, clinical benefit may vary depending on time from symptom-onset to treatment,22 collateralization,23 age24,25 and possibly, post-stroke vascular dysfunction and adaption.26 Post-ischemic changes of vessel tone may have a significant impact on cellular recovery and reperfusion injury17,27 and may even play a mechanistic role in many potential neuroprotective agents.3,26,28
We have repeatedly observed substantial lenticulostriate artery (LSA) dilatation and asymmetry ipsilateral to ischemic injury on routine postinterventional maximum intensity projection 3T time-of-flight magnetic resonance angiography (TOF-MRA) following thrombectomy. As not all patients seemed to exhibit this imaging feature suggestive for altered cerebral vasodynamics, we carried out a systematic retrospective analysis of all cases treated in our department. In particular, we explored incidence, factors associated with its occurrence, whether appearance of LSAs is associated with neurologic and functional outcomes and reviewed potential underlying pathophysiology. We hypothesized that LSA dilatation is associated with improved neurologic recovery and better functional outcome, potentially indicating preserved endothelial and smooth muscle cell function or superior cerebral vasomotor reactivity.
Methods
Patient population and endovascular therapy
All consecutive patients presenting with isolated middle cerebral artery (MCA) occlusion stroke undergoing thrombectomy at the study institution (January 2010–June 2016) were included in this retrospective analysis if they met the following inclusion criteria: (1) available postinterventional 3T MRA-TOF, (2) proximal MCA occlusion, defined as occlusion of the MCA LSAs on preinterventional CT angiography and digital subtraction angiography and (3) complete reperfusion of the M1 segment, including reperfusion of all LSAs as revealed on postinterventional MRI (see study flow chart in supplementary Figure I). Patients generally receive a postinterventional MRI (including an MRA-TOF) if no contraindications were present (e.g. pacemaker) and if clinically feasible (e.g. movement artefacts due to post-stroke delirium). We usually perform a postinterventional MRI to assess infarct extent and to assess the patency of the affected vessels. Written informed consent was waived due to its retrospective nature and according to institutional guidelines. Permission for this retrospective analysis was obtained from the local ethics committee (Ethikkommission der Fakultät für Medizin der Technischen Universität München). The procedures and analyses were in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.29 According to in-house guidelines, all stroke patients with large vessel occlusions were eligible for thrombectomy in the absence of extensive early infarct signs, defined as involving more than one-third of the MCA territory, and if the procedure could begin within 6 h after symptom onset. No further selection criteria were applied (e.g. perfusion imaging criteria or age limit). All patients received preinterventional intravenous recombinant tissue plasminogen activator (IV rtPA) in the absence of contraindication (n = 68, 69.4%).
Imaging assessment
Digital subtraction angiography
Thrombolysis in cerebral infarction (TICI) graded reperfusion success was assessed by two neuroradiologists independently on final postprocedural DSA runs. Both readers analyzed the TICI score independently on final angiography run blinded to the respective interventionalist and all clinical data at least two months after the intervention had been performed. Less than 10% of the interventions had been performed by the readers themselves. TICI2b was defined as reperfusion of more than 66% of the initially involved territory, according to the original TICI scale.30 We carefully reviewed all postinterventional DSA control runs for the visibility of the LSAs after successful reperfusion of the M1 segment to ensure reperfusion of the LSAs.
Postinterventional MRI
Postinterventional MRI was usually performed at day 3–4 (median: day 3, interquartile range, IQR: day 2–day 4). MRA-TOF and DWI were acquired at the same time point.
LSA classification and LSA-sign
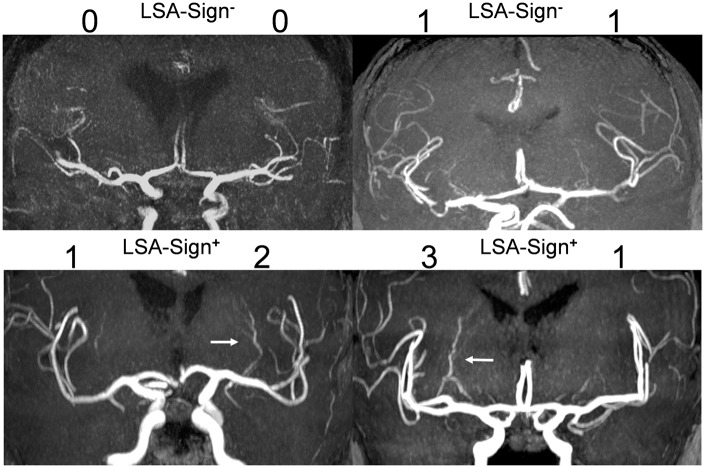
Visibility and dilatation of the MCA LSA were graded on coronal 20 mm maximal intensity projections of a standard MRA TOF (see supplementary Table 1 for sequence details) using the following scale: (0) not visible, (1) visible as faint line, (2) prominent LSA, (3) markedly enlarged vessel diameter comparable to the diameter of a M3/M4-segment artery (see Figure 1 for illustrative cases). The left and right hemispheres were graded blinded to the infarct topology. For this purpose, 20 mm maximum intensity projections were extracted from the local Picture Archiving and Communication System and saved along with the study ID of the patients. The first reader graded left and right LSA appearance. The second reader repeated grading blinded to the grading of the first reader to assess inter-rater reliability. The first reader repeated his grading 14 days later blinded to his first assessment in order to evaluate intra-rater reliability. In cases of discrepancies, a consensus read was performed with both readers and the third more experienced reader who was not involved in the initial grading. Differences between left and right site were calculated using the grade of the ischemic hemisphere minus the grade of the unaffected hemisphere. If LSA grading of the ischemic hemisphere was greater than the assigned grade of the unaffected hemisphere, the overall appearance of the LSAs was rated as asymmetrical LSAs (LSA-sign+ vs. LSA-sign−, see Figure 1). All images and respective grading from the different readers are supported in the supplementary dataset 1. All readers had at least two years of training in neuroradiology (Reader 1: 2 years, Reader 2: 8 years, Reader 3: > 10 years).
Figure 1.
Lenticulostriate artery visibility and dilatation grading.
Classification of lenticulostriate artery visibility and dilatation on ischemic and unaffected side: (0) not visible, (1) visible as faint line, (2) prominent lenticulostriate arteries (LSAs), (3) markedly enlarge vessel diameter comparable to the diameter of an M3/M4-segment artery. Asymmetry in favor of the ischemic side was classified as LSA-sign+. Symmetric lenticulostriate artery visibility without selective dilatation of the lenticulostriate artery within the ischemic area was rated as LSA-sign−. White arrows indicate dilated lenticulostriate arteries.
Other parameters
Recanalization of the respective vessel was graded using the arterial occlusive lesion (AOL) scoring system introduced by the investigators of the IMS I pilot trial.31 The AOL system ranges from 0 to 3, representing a recanalization spectrum from no recanalization to complete recanalization with any distal flow.31 Hemorrhagic infarctions (HI) and parenchymal hematomas (PH) were evaluated according to the European Cooperative Acute Stroke Study criteria.32 In short, PH refers to a homogenous, relatively dense hematoma with subsequent mass effect, while HI refers to heterogeneous hyperdensity occupying an area of the infarcted brain parenchyma. Final infarct volume was semi-automatically segmented using the Insight Segmentation and Registration Toolkit-SNAP's (version 3.2) threshold based segmentation on postinterventional diffusion-weighted imaging.33 Expert review by an experienced neuroradiologist (>3 years of experience) and manual correction were performed to obtain the final segmentations used for subsequent analyses.
Clinical assessment
Patient characteristics and lab findings were extracted by reviewing patients’ medical charts. Symptom-onset to treatment time (SOTT) was defined as interval between symptom onset and first intracranial DSA series. Clinical outcome was assessed by obtaining patients NIHSS, mRS and Barthel index scores at the day of discharge (NIHSS-DIS, mRS-DIS, Barthel-DIS). A NIHSS score of 42 was assigned in cases of death during the acute hospital stay. ‘Substantial neurologic improvement’ was defined as either: (i) difference between admission NIHSS and NIHSS-DIS ≥ 8 (ΔNIHSS ≥ 8) or (ii) NIHSS-DIS ≤ 1, as previously described.34 Good neurologic outcome was defined as NIHSS-DIS < 5. Short-term functional outcome was evaluated using the mRS at the day of discharge sufficiently recorded for 91/98 patients. If available (n=75/98), mid-term functional outcome was assessed by day 90 mRS. mRS ≤ 2 was defined as ‘good functional outcome’.
Statistical analysis
Inter-rater and intra-rater reliability of ordinal variables (i.e. LSA grades and respective LSA grade differences between hemisphere) were assessed using two-way mixed, average-measures, consistency intraclass correlation coefficient to assess the degree that readers provided consistent LSA appearance grades across all patients included. Intra-rater and inter-rater ICCs were in the excellent category for both hemispheres and respective calculated differences between left and right side (all ICC > 0.75). Detailed results regarding the reliability are supported in supplementary data 1 and supplementary Table 2. Inter-rater and intra-rater reliability for categorical classification (i.e. LSA-sign+ vs. LSA-sign−) were evaluated using Cohen's Ƙ. Cohen's Ƙ was 0.844 and 0.864 for inter-rater and intra-rater reliability, respectively. Continuous variables and frequency counts were compared using standard statistical measures (Welch's t-test, Mann-Whitney-U test, Fisher's exact test). Data are shown as median and interquartile range or mean ± standard deviation, if normally distributed. A backward and a forward likelihood-ratio logistic regression model (dependent variable: LSA-sign+) were used to evaluate factors associated with the LSA-sign+. Qualitative agreement between both models was analyzed to evaluate robustness of the statistical association. All baseline and procedural parameters were entered in the model. Clinical impact of the LSA-sign+ was evaluated using multivariate logistic regression models adjusting for potential confounders, including age, admission NIHSS, reperfusion grade, final infarct volume and time to treatment. The respective dependent variables were either good neurologic outcome, substantial neurologic improvement, good functional short-term outcome or good functional mid-term outcome. Cases with missing values (i.e. day 90 mRS) were excluded from analysis of the respective outcome (in this case good functional mid-term outcome). Results from logistic regression models are displayed as adjusted odds ratios (aOR) and corresponding 95% confidence interval (95% CI). Prevalence, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) and corresponding 95% CIs were calculated according to the efficient-score method (corrected for continuity) as outlined by Newcombe.35 Pre- and posttest probabilities were displayed using Fagan nomograms using the ‘Diagnostic test calculator’ (version 2010042101) by Alan Schwartz (http://araw.mede.uic.edu/cgi-bin/testcalc.pl). To compare deviance between nested logistic regression models (with and without the LSA-sign term), analysis of deviance tables was computed, and a chi-square test for reduction of deviance was performed for the different endpoints (i.e. substantial neurologic improvement, good neurologic outcome, good functional short-term outcome and good functional mid-term outcome). All regression models included age, admission NIHSS, reperfusion grade, final infarct volume and time to treatment. To further visualize model performance and to account for overfitting, leave-one-out cross-validated receiver-operating characteristics (LOOCV-ROC) curves were generated using the R package “pROC”. Statistical analysis was performed in R (version 3.2) or IBM SPSS Statistics, release 23.0 (IBM, Armonk, NY, USA).
Results
Ratings of LSA appearance
Distribution of LSA grades for the different readings and respective difference between the hemisphere are denoted in supplementary Table 3. Of 294 hemisphere-blinded ratings (98 for reader 2, for reader 1 in 2 sessions), only four cases were classified as LSA asymmetry in favor of the unaffected hemisphere. In contrast, 194/294 ratings were rated as lenticulostriate asymmetry in favor of the affected hemisphere (referred to as LSA-sign+). This suggests that asymmetry of the LSAs is not a random process affecting both hemispheres (roughly) equally, but rather reflects a functional state of the arteries in the ischemic hemisphere. In consensus (as solved by a third reader), 64/98 patients were rated as LSA+ with a median difference of 1 grade (interquartile range: 0–1).
Study population
In total, 98 patients met the inclusion criteria (mean age: 67.8 ± 16.9 y, 56 female). Median NIHSS on admission and SOTTs were 14 (IQR: 12–17) and 211 (IQR: 173–259) min, respectively. In all patients, it was possible to reperfuse the proximal M1 segment with subsequent reperfusion of all LSAs during the intervention. In most patients, successful reperfusion (TICI2b/3) was achieved (90.8%, n=89), and complete vessel recanalization (AOL 3) as revealed on postinterventional MRA-TOF was observed in 90 patients. No reocclusions were detected. In cases of unsuccessful reperfusion, TICI2a was achieved in seven of nine patients. Substantial neurologic improvement was noted in 59/98 patients (60.2%), while good neurologic outcome was achieved in 42/98 patients (42.9%). Rates of good functional outcome (mRS ≤ 2) were 41.8% (38/91) and 73.3% (55/75) for short- and mid-term, respectively.
Factors associated with the LSA-sign
Comparing baseline and procedural parameters, patients with LSA-sign− were older (73.8 vs. 65.4, p = 0.013) and had a less favorable risk factors profile accordingly (see Table 1). In multivariate backward-likelihood regression modeling, patients with hypertension (aOR: 0.171, 95% CI: 0.046–0.645) and patients receiving bridging IV rtPA (aOR: 0.265, 95% CI: 0.088–0.798) were less likely to be classified as LSA-sign+. The same factors with comparable point-estimates were found to be statistically significant when using a forward likelihood-ratio logistic regression model (see Table 1). The time point of postinterventional MRI did not seem to affect the frequency of detecting LSA-sign+ (median days from thrombectomy to MRI were three in the LSA-sign− group vs. three days in the LSA-sign+ group, p = 0.461 and exclusion in multivariate logistic regression models). Note, however, that in five LSA-sign+ patients, MRA-TOF was repeated within a time range of three to five months and asymmetry of LSAs could not be observed anymore, suggesting LSA dilatation to be a transient post-stroke process. No further imaging was available for LSA-sign− patients.
Table 1.
Baseline and procedural characteristics.
| LSA-sign− (n = 34) | LSA-sign+ (n = 64) | p | Logistic regression: aOR (95% CI) derived from backward- likelihood modela | Logistic regression: aOR (95% CI) derived from forward- likelihood modela | |
|---|---|---|---|---|---|
| Age | 73.84 ± 14.4 | 65.4 ± 17.7 | 0.013 | ||
| Sex, female | 50.0% (17) | 62.5% (40) | 0.284 | ||
| Diabetes (n = 96) | 17.6% (6) | 14.5% (9) | 0.771 | ||
| Hypertension (n = 96) | 88.2% (30) | 66.1% (41) | 0.027 | 0.171 (0.046–0.645), p = 0.009 | 0.191 (0.056–0.649), p = 0.008 |
| Atrial fibrillation (n = 96) | 61.8% (21) | 37.1% (23) | 0.032 | ||
| Previous Stroke (n = 96) | 23.5% (8) | 9.7% (6) | 0.077 | ||
| Admission glucose (mg/dl) | 126 (108–158) | 118 (104–135) | 0.166 | ||
| Hemisphere, left | 50.0% (17) | 40.6% (26) | 0.400 | ||
| Baseline NIHSS | 14 (11–17) | 15 (10–18) | 0.716 | ||
| IV rtPA | 76.5% (26) | 65.6% (42) | 0.358 | 0.265 (0.088–0.798), p = 0.018 | 0.350 (0.128–0.958), p = 0.041 |
| Wake-up stroke | 5.9% (2) | 6.3% (4) | 1.000 | ||
| SOTT (min, n = 92) | 220 (167–272) | 210 (161–260) | 0.290 | ||
| Procedure time (min) | 35 (17–67) | 45 (28–79) | 0.759 | ||
| Successful reperfusion (TICI 2b/TICI 3) | 97.1% (33) | 87.5% (56) | 0.156 | ||
| AOL on postinterventional MRA TOF | 3 (3–3) | 3 (3–3) | 0.345 | ||
| Days to postinterventional MRI | 3 (1–6) | 3 (2–4) | 0.461 |
Other variables were excluded or not included using the backward/forward likelihood-ratio model supported by SPSS (cf. methods).
SOTT: symptom-onset to treatment time; aOR: adjusted odds ratio; 95% CI: 95% confidence interval; AOL: arterial occlusive lesion scoring system; MRI: magnetic resonance imaging (cf. methods).
LSA grades of the unaffected side did not differ between patients with LSA-sign+ and LSA-sign− in any of the readers (p = 0.429 for Reader 1, session 1; p = 0.710 for Reader 1, session 2 and p = 0.851 for reader 2). This suggests that LSA-sign+ and observed asymmetry is not merely reflecting invisibility of the LSAs itself but rather indicates that it is a presumingly active process.
Outcome
Final infarct volume did not differ between LSA-sign+ and LSA-sign− patients (median final infarct volume: 20 mL vs. 18 mL, p = 0.796). Patients with LSA-sign+ showed a more favorable outcome on all prespecified outcome scales (see Table 2). In each multivariate logistic regression model, LSA-sign+ was associated with the respective clinical endpoint, i.e. substantial neurologic improvement (aOR: 4.843, 95% CI: 1.554–14.092, p = 0.007), good neurologic outcome (aOR: 9.711, 95% CI: 2.650–35.594, p = 0.001) and good functional short- and mid-term outcome (aOR: 7.127, 95% CI: 1.913–26.551, p = 0.003 and aOR: 3.786, 95% CI: 1.026–13.973, p = 0.046) after adjusting for age, admission NIHSS, reperfusion grade, SOTT and final infarct volume. The effect was not only seen in the consensus read, but in every grading performed during the study (i.e. for Reader 1 session 1, for Reader 1 session 2 and for Reader 2, see supplementary Table 4). These associations also remained statistically significant if ordinally scaled grade differences between affected and unaffected hemisphere (ranging from 0 to 3) instead of dichotomized asymmetry (LSA-sign+ vs. LSA-sign−) were used as entered variable (see supplementary Table 5). In contrast, however, general visibility of the LSAs was not associated with any of the dichotomized outcome parameters used in the study (see supplementary Table 6).
Table 2.
Outcome.
| LSA-sign− (n = 34) | LSA-sign+ (n = 64) | p | |
|---|---|---|---|
| Final infarct volume, mL | 18 (6–81) | 20 (8–40) | 0.796 |
| HI | 50.0% (17) | 39.1% (25) | 1.000 |
| PH | 2.9% (1) | 1.6% (1) | 0.391 |
| In-hospital mortality | 2.9% (1) | 1.6% (1) | 1.000 |
| NIHSS-DIS | 8 (5–12) | 4 (1–7) | 0.003 |
| Substantial neurologic improvement | 41.2% (14) | 68.8% (44) | 0.010 |
| Good neurologic outcome | 17.6% (6) | 85.7% (36) | <0.001 |
| Barthel index-DIS | 15 (5–55) | 50 (25–75) | 0.010 |
| mRS-DIS (n = 91) | 4 (3–5) | 2 (1–4) | <0.001 |
| Good functional short-term outcome (mRS-DIS≤2, n = 91) | 17.6% (6/34) | 56.1% (32/57) | <0.001 |
| d90 mRS (n = 75) | 2 (1–3) | 1 (1–2) | 0.016 |
| Good function mid-term outcome (d90 mRS ≤ 2, n = 75) | 54.2% (13/24) | 82.4% (42/51) | 0.014 |
HI: hemorrhagic infarction; PH: parenchymal hematoma; DIS: discharge; d90: day 90 after admission.
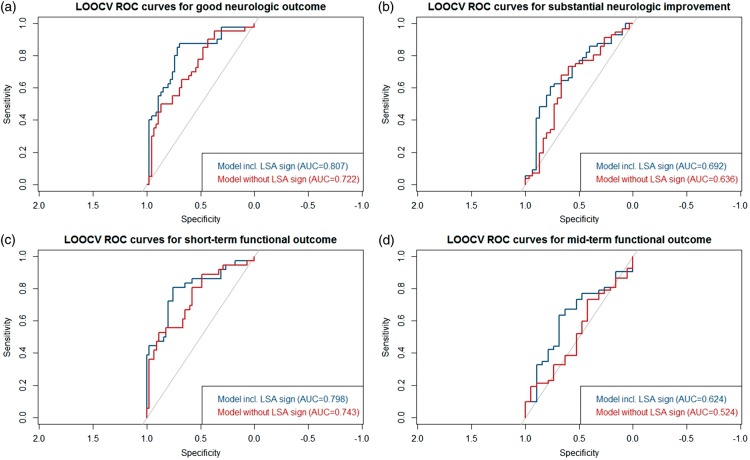
Prediction of clinical endpoints (substantial neurologic improvement, good functional short- and mid-term outcome) was significantly improved in regression models including the LSA-sign term compared to models without (see Table 3), as measured by analysis of deviance. The improved model performance was visualized in LOOCV-ROC curves for all four endpoints (see Figure 2), which all showed a higher area under the curve for models including the LSA-sign term. Sensitivity, specificity and respective PPV/NPV of the LSA-sign+ with regard to all outcome measures are shown in supplementary Table 7 and are displayed in the Fagan nomograms in Figure 3.
Table 3.
Deviance analysis of prediction models for different endpoints.
| Model | AIC | Deviance | d.f. | p |
|---|---|---|---|---|
| Early neurologic improvement | ||||
| W/LSA-term | 100.16 | 88.157 | 80 | |
| W/o LSA-term | 110.03 | 100.03 | 81 | 0.0005708 |
| Discharge mRS≤2 | ||||
| W/p LSA-term | 89.457 | 77.457 | 76 | |
| W/o LSA-term | 10.044 | 90.438 | 77 | 0.0003147 |
| Day 90 mRS≤2 | ||||
| W/LSA-term | 73.13 | 61.313 | 66 | |
| W/o LSA-term | 83.529 | 73.529 | 67 | 0.0004739 |
Note: The Akaike information criterion and deviance is displayed stratified according to two distinct predictions models, one including the variable LSA-sign+ vs. LSA-sign− (LSA-term) and one without the LSA term. Both models included age, admission NIHSS, reperfusion grade, final infarct volume and time to treatment. Data are displayed for three different clinical endpoints (early neurologic improvement, defined as difference between admission and discharge NIHSS ≥8 or discharge NIHSS ≤ 1, modified Rankin scale at discharge ≤2 and modified Ranking scale at day 90 ≤ 2). The p value refers to the comparison of deviance between the model including the LSA-term and the model excluding the LSA-term.
mRS: modified Rankin scale, W/: with; w/o: without; AIC: Akaike information criterion; d.f.: degrees of freedom.
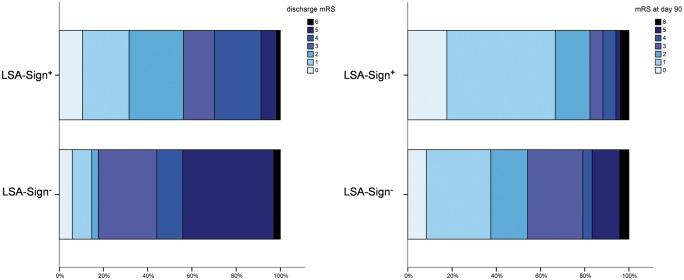
Figure 2.
Functional outcome stratified according to the perf-sign.
Distribution of mRS at discharge (left) and day 90 stratified according to LSA-sign+ vs. LSA-sign−. Patients with LSA-sign+ had significantly higher rates of good functional outcome at discharge and day 90, respectively (mRS ≤ 2, p = 0.001 and p = 0.014).
Figure 3.
Prediction improvement in ROC analysis.
For all endpoints, models including the LSA-sign term (blue) had superior area under the curves as compared to models without the LSA-sign term (red). ROC analysis was performed using leave-one-out cross-validation to account for model overfitting. (a) good neurologic outcome; (b) substantial neurologic improvement; (c) short-term functional outcome; (d) mid-term functional outcome.
ROC: receiver operating characteristic; LOOCV: leave-one-out cross validation; AUC: area under the curve.
Discussion
This study describes a novel vascular imaging finding in patients treated by thrombectomy for proximal MCA obstruction: asymmetrical dilatation of the LSAs of the ipsilateral striatocapsular region (LSA-sign+). Pretreatment with IV rtPA and hypertension were associated with absence of the LSA-sign+. Further, this sign demonstrates evidence for an association with both a favorable neurologic and functional outcome and is independent of other known prognostic factors. Assessment of this finding could therefore improve prediction of outcome of patients and may give insights into the pathophysiology of post-stroke vasodynamics.
Besides neuronal and astrocytic cell damage, ischemic stroke adversely affects the cerebral circulation. The main effect of ischemia and subsequent reperfusion on large diameter and pial arteries seem to be vasodilatory in nature.9,36 This effect was also shown to be time dependent, since longer duration of preceding ischemia and longer timeframes of reperfusion prior to analysis gradually diminish myogenic tone in large caliber and pial cerebral arteries.4,36 In contrast, parenchymal arterioles (PAs), such as the LSAs, are thought to generally preserve their increased basal tone or even constrict after ischemia and subsequent reperfusion.3,5,37 However, these vessels have been shown to be exquisitely sensitive to dilatory signaling from their lining endothelial cells in the setting of ischemia/reperfusion, at least in animal models.5,37 It is therefore reasonable to suggest that PAs such as the LSAs may be capable of responding to ischemia or perhaps more likely, the metabolites and signaling molecules associated with it by vasodilating if they maintain a functioning endothelium. However, in the absence of endothelial cells being able to generate pro-dilatory signals, the default basal tone of the smooth muscle cells of these vessels is generally relatively elevated and this is likely to be accentuated in the setting of ischemia and reperfusion.
The lack of vasodilatation of PAs may have important clinical repercussion because regions supplied by PAs (e.g. basal ganglia) lack a collateral supply.38 At the same time, the striatocapsular region harbors eloquent white matter tracts whose ischemic cell death is associated with more severe neurologic disability,39 and the lack of vasodilatation of PAs has previously been suggested to contribute to infarct growth.5,40 We report LSA vessel dilatation, which is conspicuous on TOF-MRA and demonstrates evidence for an association with neurologic improvement and good functional outcomes. However, it does not appear to be associated with smaller infarcts based on median ischemic core volumes. Therefore, the mechanism by which this observation correlates with improved outcomes is unclear. Animal studies generally report relative increased tone in PAs following ischemia and reperfusion in the hyperacute reperfusion period, typically ∼30 min post reperfusion.2,3,37 We observed asymmetrical dilatation of the lenticulostriate vessels (LSA-sign+) at the first few days post reperfusion and it is tempting to suggest the observation we report relates to preservation or restoration of lenticulostriate endothelial cell function. Although infarct volumes were not different between LSA-sign+ and LSA-sign− patients, that does not necessarily imply that respective ischemia in this region is equally severe.41,42 Infarct severity may cause ischemia of the relatively more resistant white matter tracts in the striatocapsular region43 which could explain the clinical impact. The internal capsule at the level supplied by the LSAs is a relatively small volume structure39 which would not necessarily be reflected by different infarct volumes. Hence, occurrence of the LSA-sign− may indicate more severe ischemia potentially accountable for vessel wall damage and endothelial cell dysfunction causing the lack of vasodilatation. Moreover, enhanced cerebral vasomotor reactivity to stress has been associated with a favorable outcome in human cerebrovascular cohorts.44,45 Patients with LSA-sign+ were younger and had fewer vascular risk factors (especially hypertension, see logistic regression model) known to diminish cerebral vasomotor reactivity accordingly.46–48
An association between pretreatment with IV rtPA and the lack of vasodilatation of LSA (LSA-sign−) is of interest, as there is an ongoing scientific debate if the bridging approach is of any benefit in the era of fast and successful reperfusion brought by subsequent thrombectomy.49 A recent study has suggested that preinterventional IV rtPA potentiates vessel wall damage brought by stent-retrievers.50 Hence, one may also speculate that vessel wall damage of the MCA may impact integrity and function of the relatively small orifices of the LSAs (e.g. induced by vessel wall swelling or postinterventional vessel inflammation). Further studies may elucidate if the rates of LSA-sign+ differ between types of revascularization devices (e.g. aspiration vs. stent-retriever) or are associated with the number of maneuvers required for reperfusion. Further, animal studies have suggested that rtPA is not only associated with direct neurotoxic effects but also affects endothelial and vessel wall function and integrity.51 Thus, pretreatment with IV rtPA may directly influence vasomotor reactivity of LSAs following ischemia.
Although vasodilatation does not necessarily reflect hyperemia, it is likely that the observed vasodilatation is accompanied by an increased blood flow. Post-ischemic hyperperfusion has been described as both, harmful, promoting edema,52 hemorrhagic transformations53 and secondary infarct growth54 and as a rather benign imaging sign, suggesting early and complete reperfusion and favorable tissue outcome.7,8,17,55 As LSA-sign+ was significantly associated with higher rates of neurologic improvement and better functional outcome, another, albeit tentative, hypothesis may be considered: hyperperfused tissue was found to be metabolically more active which may reflect an overshoot of oxygen consumption, which was interpreted as a rebound of protein and neurotransmitter synthesis.7,17 This theory implies that vasodilatation is a more active process guided by increased metabolic demands of tissue repair processes via the neurovascular unit.17 Such theories are of rising interest as a ‘Lenticulostriate artery and Lenciulostriate artery Neural complex’ was recently proposed.56 This complex highlights the close pathophysiological interaction between vascular tone of the LSAs and the functional tissue state of the circumferential deep grey matter.56
With the advent of neuroprotective treatment regimens, vasodilatation of the MCA LSAs could increase accessibility of potential agents in a vulnerable and clinically eloquent subcortical tissue after successful MCA recanalization, which may otherwise potentially be limited due to the increased myogenic basal tone of PAs. Depending on when the vasodilatation occurs and can be observed, assessment of this sign could guide future therapeutic decisions regarding initiation of neuroprotective therapies. Further studies are thus warranted to assess the exact time points of vasodilatation in the striatocapsular region.
The clinical outcome of patients following thrombectomy varies considerably. To improve post-stroke treatment of patients, a more reliable prediction of outcome is highly desirable. Several factors have been shown to influence clinical outcome beyond known baseline prognostic effectors (infarct severity, age, risk factor profile), including reperfusion success,18,57,58 collaterals23 and time-to-treatment,22 and lately also post-stroke vasodynamics, as discussed above. The LSA-sign+ described here may indicate patients with a favorable outcome following thrombectomy (Table 2, Figure 4). This association was observable despite successful thrombectomy (thought to be the predominant predictor of outcome18) was achieved more often in LSA-sign− patients (97.1% vs. 87.5%), further pointing towards the importance of yet unknown factors influencing the clinical course. We hypothesize that the post-ischemic vasodilatation visualized in the LSA-sign+ reflects a beneficial vascular adaptation and ischemic resistance and therefore contributes to the more favorable outcome of these patients. Nonetheless, future studies investigating post-ischemic vascular processes and perfusion in more detail and with respect to the LSA-sign are warranted to substantiate this notion.
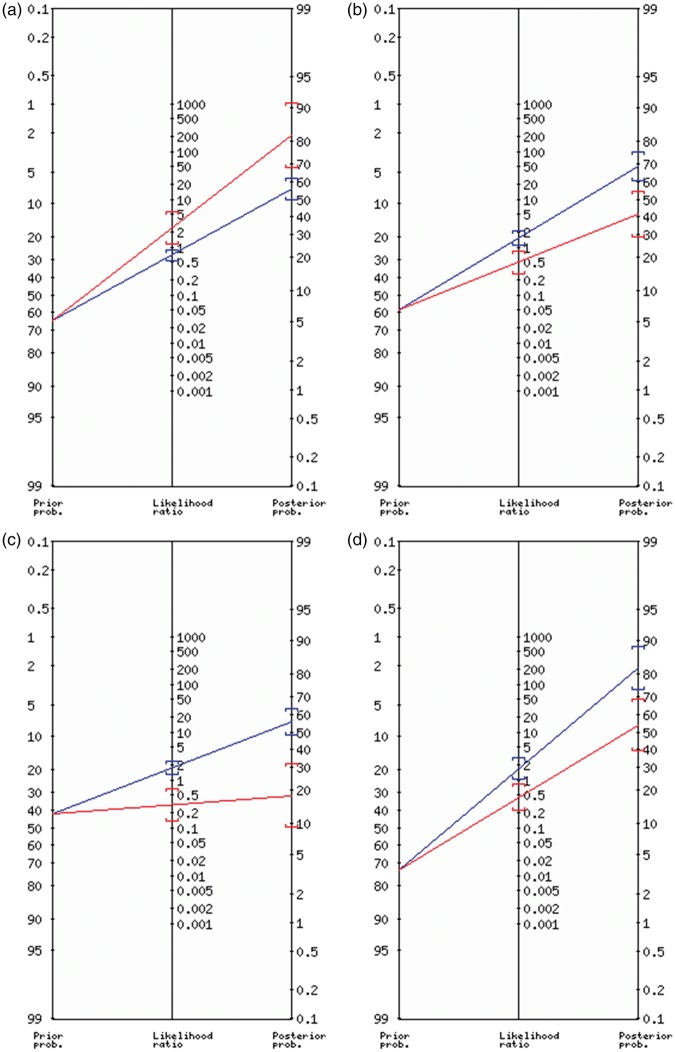
Figure 4.
Fagan nomogram of LSA-sign+ for different clinical endpoints.
Left axis, pretest probability; middle axis, likelihood ratio with 95% confidence interval (brackets); right axis, posttest probability with 95% confidence interval (brackets) for the respective endpoints: (a) good neurologic outcome; (b) substantial neurologic improvement; (c) good functional short-term outcome; (d) good functional mid-term outcome; Red indicates LSA-sign+, blue indicates LSA-sign−.
This is a retrospective study and thus prone to biases. Firstly, a considerable number of patients were excluded owning to missing postinterventional MRA. Although patients generally receive a postinterventional MRI at our department, there are many factors that may impede feasibility (e.g. MRI contraindication, post-stroke delirium, and early transfer back to the referring hospital). Therefore, patients with potentially more severe comorbidities and patients at risk for poorer outcome were more likely to receive postinterventional imaging limited to non-contrast CT. Hence, a positive selection bias for patients with a more favorable outcome cannot be excluded. Secondly, we are unable to draw firm conclusions regarding the duration of the vasodilatative changes observed. Although preliminary analysis of five cases with long-term follow-up MRA suggests, that the effect is transient, a systematic investigation is warranted. Thirdly, the sign observed is of purely structural nature and conclusions about the potential functional and pathophysiological relevance (e.g. perfusion) are tentative and have to be evaluated in future studies. Fourthly, 23 patients were lost to follow-up regarding the evaluation of their mid-term functional outcome. However, as an association between the LSA-sign and outcome was observed regarding all other prespecified outcome variables, it seems unlikely that the observed effect regarding mid-term functional outcome is artificial. Especially, substantial neurologic improvement has been shown as a more sensitive parameter regarding the detection of treatment effects in acute ischemic stroke as opposed to other dichotomized outcome scales.34
Conclusion
Relevant vasodilatative changes of MCA LSAs following endovascularly treated ipsilateral MCA occlusions commonly occur and are a prognostic imaging sign. Assessment of this sign may improve prediction of patients' outcome beyond known procedural and imaging characteristics. This observation may reflect phenotypic resistance to ischemic stress, more active repair or relatively less severe hypoperfusion. Future studies addressing the pathophysiological background and external validation of the prognostic capability are warranted.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: BW and JK received a scholarship from the TUM (KKF).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Study design, study conception: JK, BW, TBB; initial manuscript draft: JK; interventional procedure and image analysis: JK, KK, JFK; clinical analysis: SW; statistical analysis plan: JK, BW, KK; data interpretation: JK, KK, NM, ASG, SW, CZ , JFK, BW, TBB; revising the manuscript for important intellectual content: JK, KK, NM, ASG, SW, CZ, JFK, BW, TBB. BW, TBB contributed equally to this article.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Manning NW, Campbell BC, Oxley TJ, et al. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke 2014; 45: 640–644. [DOI] [PubMed] [Google Scholar]
- 2.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke 2015; 10: 143–152. [DOI] [PubMed] [Google Scholar]
- 3.Cipolla MJ, Chan SL, Sweet J, et al. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke 2014; 45: 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke 2002; 33: 2094–2099. [DOI] [PubMed] [Google Scholar]
- 5.Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation 2008; 15: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidwell CS, Saver JL, Mattiello J, et al. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans. Neurology 2001; 57: 2015–2021. [DOI] [PubMed] [Google Scholar]
- 7.Marchal G, Furlan M, Beaudouin V, et al. Early spontaneous hyperperfusion after stroke. A marker of favourable tissue outcome?. Brain 1996; 119(Pt 2): 409–419. [DOI] [PubMed] [Google Scholar]
- 8.Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab 1999; 19: 467–482. [DOI] [PubMed] [Google Scholar]
- 9.Maneen MJ, Hannah R, Vitullo L, et al. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke 2006; 37: 894–899. [DOI] [PubMed] [Google Scholar]
- 10.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 12.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Zoppo GJ, Schmid-Schonbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 14.Thomas WS, Mori E, Copeland BR, et al. Tissue factor contributes to microvascular defects after focal cerebral ischemia. Stroke 1993; 24: 847–853. discussion 847. [DOI] [PubMed] [Google Scholar]
- 15.Ito U, Hakamata Y, Kawakami E, et al. Temporary [corrected] cerebral ischemia results in swollen astrocytic end-feet that compress microvessels and lead to delayed [corrected] focal cortical infarction. J Cereb Blood Flow Metab 2011; 31: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onoda K, Kuroda Y, Yamamoto Y, et al. Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovasc Dis 2011; 31: 6–11. [DOI] [PubMed] [Google Scholar]
- 17.Bivard A, Krishnamurthy V, Stanwell P, et al. Spectroscopy of reperfused tissue after stroke reveals heightened metabolism in patients with good clinical outcomes. J Cereb Blood Flow Metab 2014; 34: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning NW, Chapot R, Meyers PM. Endovascular stroke management: key elements of success. Cerebrovasc Dis 2016; 42: 170–177. [DOI] [PubMed] [Google Scholar]
- 19.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 20.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins BT, Lundeen TF, Norwood KM, et al. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 2007; 50: 202–211. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 23.Berkhemer OA, Jansen IG, Beumer D, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke 2016; 47: 768–776. [DOI] [PubMed] [Google Scholar]
- 24.Kleine JF, Boeckh-Behrens T, Prothmann S, et al. Discrepancy between early neurological course and mid-term outcome in older stroke patients after mechanical thrombectomy. J Neurointerventional Surg 2016; 8: 671–676. [DOI] [PubMed]
- 25.Beumer D, Rozeman AD, Lycklama ANGJ, et al. The effect of age on outcome after intra-arterial treatment in acute ischemic stroke: a MR CLEAN pretrial study. BMC Neurol 2016; 16: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res 2016; 7: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CY, Chang C, Cheung WM, et al. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: evaluation with contrast-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab 2008; 28: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland BA, Papadakis M, Chen RL, et al. Cerebral blood flow alteration in neuroprotection following cerebral ischaemia. J Physiol 2011; 589: 4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WMA General Assembly. World Medical Asssociation Declaration of Helsinki. Ferney-Voltaire, France: World Medical Association, 1964, p. 0.
- 30.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 31.Khatri P, Neff J, Broderick JP, et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke 2005; 36: 2400–2403. [DOI] [PubMed] [Google Scholar]
- 32.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999; 30: 2280–2284. [DOI] [PubMed] [Google Scholar]
- 33.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006; 31: 1116–1128. [DOI] [PubMed] [Google Scholar]
- 34.Kerr DM, Fulton RL, Lees KR, et al. Seven-day NIHSS is a sensitive outcome measure for exploratory clinical trials in acute stroke: evidence from the Virtual International Stroke Trials Archive. Stroke 2012; 43: 1401–1403. [DOI] [PubMed] [Google Scholar]
- 35.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: 857–872. [DOI] [PubMed] [Google Scholar]
- 36.Cipolla MJ, Lessov N, Hammer ES, et al. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke 2001; 32: 1658–1664. [DOI] [PubMed] [Google Scholar]
- 37.Cipolla MJ, Sweet JG, Gokina NI, et al. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab 2013; 33: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura N, Rosidi NL, Iadecola C, et al. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab 2010; 30: 1914–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konishi J, Yamada K, Kizu O, et al. MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 2005; 64: 108–113. [DOI] [PubMed] [Google Scholar]
- 40.Ngai AC, Nguyen TS, Meno JR, et al. Postischemic augmentation of conducted dilation in cerebral arterioles. Stroke 2007; 38: 124–130. [DOI] [PubMed] [Google Scholar]
- 41.Fiehler J, Knab R, Reichenbach JR, et al. Apparent diffusion coefficient decreases and magnetic resonance imaging perfusion parameters are associated in ischemic tissue of acute stroke patients. J Cereb Blood Flow Metab 2001; 21: 577–584. [DOI] [PubMed] [Google Scholar]
- 42.Wardlaw JM, Keir SL, Bastin ME, et al. Is diffusion imaging appearance an independent predictor of outcome after ischemic stroke?. Neurology 2002; 59: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 43.Bristow MS, Simon JE, Brown RA, et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab 2005; 25: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 44.Portegies ML, de Bruijn RF, Hofman A, et al. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke 2014; 45: 42–47. [DOI] [PubMed] [Google Scholar]
- 45.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001; 32: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 46.Moghaddasi M, Mamarabadi M, Habibi AH. A comparison of cerebral vasomotor reactivity in diabetic and nondiabetic Iranian patients. J Res Med Sci 2010; 15: 50–53. [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda H, Matsumoto M, Handa N, et al. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial Doppler method. J Hypertens 1994; 12: 191–197. [PubMed] [Google Scholar]
- 48.Kario K, Ishikawa J, Hoshide S, et al. Diabetic brain damage in hypertension: role of renin-angiotensin system. Hypertension 2005; 45: 887–893. [DOI] [PubMed] [Google Scholar]
- 49.Chandra RV, Leslie-Mazwi TM, Mehta BP, et al. Does the use of IV tPA in the current era of rapid and predictable recanalization by mechanical embolectomy represent good value? J Neurointerventional Surg 2016; 8: 443–446. [DOI] [PubMed]
- 50.Renu A, Laredo C, Lopez-Rueda A, et al. Vessel wall enhancement and blood-cerebrospinal fluid barrier disruption after mechanical thrombectomy in acute ischemic stroke. Stroke 2017; 48: 651–657. [DOI] [PubMed] [Google Scholar]
- 51.Gravanis I, Tsirka SE. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin Ther Targets 2008; 12: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundt TM, Jr, Waltz AG. Cerebral ischemia and reactive hyperemia. Studies of cortical blood flow and microcirculation before, during, and after temporary occlusion of middle cerebral artery of squirrel monkeys. Circ Res 1971; 28: 426–433. [DOI] [PubMed] [Google Scholar]
- 53.Yu S, Liebeskind DS, Dua S, et al. Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke. J Cereb Blood Flow Metab 2015; 35: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wegener S, Artmann J, Luft AR, et al. The time of maximum post-ischemic hyperperfusion indicates infarct growth following transient experimental ischemia. PloS One 2013; 8(5): e65322, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marchal G, Serrati C, Rioux P, et al. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: relation to outcome. Lancet 1993; 341: 925–927. [DOI] [PubMed] [Google Scholar]
- 56.Hu R, Feng H. Lenticulostriate artery and lenticulostriate-artery neural complex: new concept for intracerebral hemorrhage. Curr Pharm Des 2017; 23(15): 2206–2211. [DOI] [PubMed] [Google Scholar]
- 57.Kleine JF, Wunderlich S, Zimmer C, et al. Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerventional Surg 2017; 9: 117–121. [DOI] [PubMed]
- 58.Yoo AJ, Simonsen CZ, Prabhakaran S, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke 2013; 44: 2509–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.