Abstract
Moyamoya disease (MMD) is a rare steno-occlusive cerebrovascular disorder. Mechanisms driving the formation of aberrant MMD vessels remain elusive. We collected serum and vessel specimens from MMD and atherosclerotic cerebrovascular disease (ACVD) patients serving as controls due to the same hypoxic stimulus but substantial differences in terms of vascular features. Based on patient material and an in vitro model mimicking ACVD and MMD conditions, matrix metalloproteinase-9 (MMP-9) and vascular-endothelial growth factor (VEGF) were tested for their potential involvement in cerebrovascular disintegration. While serum concentration of both molecules did not significantly differ in both patient groups, excessive collagenase activity and lowered collagen IV protein amount in MMD vessels pointed to a focal MMP-9 activity at the affected vessel sites. We observed overexpressed and autocrinely secreted MMP-9 and VEGF along with disturbances of EC–matrix interactions in MMD but not ACVD serum-treated cEND cells. These seemingly brain-specific effects were partially attenuated by VEGF signaling inhibition suggesting its role in the MMD etiology. In conclusion, our findings support the understanding of the high incidence of hemorrhagic and ischemic events in MMD and provide the basis for novel therapeutic strategies stopping or slowing the development of fragile cerebrovasculature or micro-bleeds characterizing the disease.
Keywords: Moyamoya disease, angiogenesis, endothelial cell, tight junction, cytokine
Introduction
Moyamoya disease (MMD) is a rare and slowly progressing cerebrovascular disease with severe hemodynamic insufficiencies.1 The criteria of MMD diagnosis are mainly based on the unique angiographic appearance resulting from the characteristic vessel network. This hazy vessel network that coined the name of the disease (Moyamoya = ’puff of smoke’) is predominantly located at the distal internal carotid artery and its main branches cannot be observed in any other organ.2 Until now, there is no treatment to prevent or slow the progression of the disease. Without perfusion restoring revascularization techniques (i.e. bypass surgery), the prognosis for strokes of hemorrhagic and ischemic nature is very high.3
Although sharing the same ischemic stimulus, hemodynamic properties and the occurrence of transient ischemic attacks (TIAs), MMD strongly differs from the atherosclerotic cerebrovascular disease (ACVD) in terms of angiographic findings and outcome.4 Compared with ACVD, characterized by mature cerebrovasculature and minimal neovascularization, a highly plastic and simultaneously instable network of collateral peripheral blood vessels that tends to rupture can be observed in patients suffering from MMD. However, mechanisms leading to or driving vascular instability in MMD pathology that develops seemingly irrespectively of the hypoxic milieu are still unknown.
Our recent work reported on angiopoietin-2 (Ang-2) to play a decisive role in the development of vascular instability and permeability in MMD.5 We could show this molecule being overexpressed in MMD vessel specimens and that its autocrine release is stimulated by MMD serum causing disturbed EC barrier properties. In this work, we hypothesize that besides Ang-2 other pro-angiogenic cytokines, such as MMP-9 and VEGF may participate in the destabilization of MMD vessels. EC barrier-destabilizing molecules are implicated in the fetal and postnatal development of the vascular system. Angiopoietins, MMP-9 and VEGF determine vascular plasticity by controlling angiogenic and vasculogenic processes.6,7 By targeting extracellular matrix (ECM) proteins, such as collagen IV, MMPs are involved in new blood vessel formation, vessel branching and EC invasion during development.8,9 Active MMP-9 causes blood vessel destabilization and leakiness by rearranging and digesting cell–cell and cell–matrix contacts.10 Importantly, the biological functions of both, Ang-2 and MMP-9, are closely linked to the bioavailability of VEGF.11,12 Therefore, the effect of the VEGF/VEGF-R2 signaling blocked by two different strategies was tested in the cEND cell culture model for regulating EC–EC and EC–ECM interactions under MMD conditions, revealing its important role, next to Ang-2 in the vascular fragility characterizing MMD pathology.
Materials and methods
Study approval
This study was approved by the local research and Ethics Commission of the Charité–Universitätsmedizin Berlin (reference #EA2/086/09). Informed consent was obtained from all individual participants or the legal representative. Hereby, we followed and strictly adhered to the Ethical Guidelines of the Charité which are in accordance to the Helsinki Declaration of 1975 and its revision of 1983.
Patients
Between 2009 and 2012, unrelated patients with European Caucasian ethnic background diagnosed for MMD (n = 24) and ACVD (n = 13), serving as controls were included in this study. Both patient groups received superficial temporal artery-to-middle cerebral artery (STA-MCA) bypass grafting in our neurosurgical department for treatment of single photon emission computed tomography (SPECT)-confirmed cerebrovascular hemodynamic impairment. Further criteria underlying the diagnosis for MMD were based on magnetic resonance imaging (MRI) and cerebral angiography. The patients had to show at least the following findings: stenosis or occlusion of the intracranial internal carotid artery or proximal portions of the anterior and/or MCA associated with an abnormal moyamoya vessel network in close vicinity of the stenotic or occlusive lesions in the arterial phase classified according to Suzuki scoring.1,13 Patients presenting ACVD, meningitis, systemic vasculitis, acute stroke, Down syndrome and autoimmune diseases such as hyperthyroidism as well as patients with prior skull radiation were excluded from the MMD group. Clinical data for both patient groups were studied and recorded before the bypass surgery. The study population has been described in Supplemental Table 1.
Blood sampling and vessel specimens from revascularization surgery
Blood was obtained by venepuncture using CPT Vacutainer containing Na-citrate as anticoagulant (Becton-Dickinson, Heidelberg, Germany). Serum stored in aliquots at −80℃ until use. Samples of the MCA (M4 arteriotomy side) were obtained during the surgical procedure (STA-MCA bypass surgery) from MMD and ACVD patients. Vessel specimens were shock-frozen in liquid nitrogen and stored at −80℃ until further processing.
Electrophoresis and immunoblotting
Western blot analysis was performed according to standard procedures. Due to the low protein concentration of lysates prepared from MCA samples, an equal lysate volume was subjected per SDS-PAGE lane to analyze collagen IV. For all other Western blot analyses, equal protein amounts were subjected per gel lane. Primary antibodies were: sheep polyclonal collagen IV, rabbit polyclonal GAPDH, biotinylated proMMP-9 (all from R&D Systems, Wiesbaden, Germany), rabbit polyclonal VEGF and HRP-conjugated β-actin (Life Technologies, Karlsbad, CA, USA). Respective secondary antibodies were obtained from Jackson Immuno Res. Laboratories (Suffolk, UK). Densitometric quantification was performed employing ImageJ 1.46e.
In situ gelatinase assay
Gelatinolytic activity was detected by in situ Zymography. Frozen ACVD and MMD MCA specimens were cut and dried at RT for 2 h. The 10 µm slices were incubated at 37℃ for 24 h with 20 µg/mL DQ-Gelatine-FITC in 1 × reaction buffer according to manufacturers’ instructions (EnzChek® Gelatinase/Collagenase Assay Kit, Thermo Fisher Scientific). All images were taken at the same settings using a Kyence BZ9000 microscope.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) assays to test human or mouse VEGF, proMMP-9 and Ang-2 in serum samples as well as in cEND cell supernatants were performed in accordance to manufacturers’ instructions (R&D Systems, Wiesbaden, Germany).
Zymography
Equal amounts of total serum protein mixed with an equal volume of 2 × non-reducing sample buffer were subjected into polyacrylamide gel (10%) supplemented with gelatin (1 mg/mL) and electrophoresed. Gels were treated, as described elsewhere.14 Images of gels captured by HP Laser Jet M1522nf were analyzed with ImageJ 1.46e (NIH, USA). Gelatinase activity was measured by comparative band density.
Cell culture and treatment
The cerebral and myocardial EC lines cEND and MyEND generated from mouse brains and hearts were cultivated over 20 passages as described previously5 and as indicated in the figure legends. CENDs were pretreated with SU5416 (25 µM in DMSO; Sigma-Aldrich, Taufkirchen, Germany) or with Bevacizumab (250 µg/ml; Roche, Basel, Switzerland) for 2 h prior to serum exposition.
Transendothelial electrical resistance measurements
Cells were plated on the top of Transwell chambers (0.4 µm pore size; Greiner Bio-One, Frickenhausen, Germany). Transendothelial electrical resistance (TER) was measured using an assembly containing current-passing and voltage-measuring electrodes (World-Precision Instruments Inc., New Heaven, CT, USA). The resistance of blank filters was subtracted from the one of filters with cells before calculating the final resistance.
Messenger RNA isolation and quantitative real-time PCR
RNA isolation (PureLink RNA Mini Kit, Life Technologies), cDNA synthesis (Onestep RT-PCR Kit, Qiagen, Hilden, Germany) and quantitative real-time PCR (qPCR) (Premix ex Taq Perfect Real Time Kit, Takara Bio, Saint-Germain-en-Laye, France) were performed as previously described.5 Mouse-specific primers were obtained from TIB Molbiol Syntheselabor GmbH, Berlin, Germany. The ABI PRISM 7300 SDS software (Relative quantification study) was used to determine the cycle threshold (CT) for each reaction and expression determined for each gene was normalized to expression of the endogenous housekeeping gene, glyceraldehyde phosphate dehydrogenase (GAPDH). The relative expression intensity was estimated by calculating 2−ΔΔCT for each sample. Specificity of PCR products was checked by melting curve analysis.
In vitro tube formation assay
CEND and MyEND cells (3 × 104 cells per well) were cultivated for in vitro tube formation assay and images were taken as previously described.5 Image analysis for tube parameters was performed by Wimasis.
In vitro cell scratch wounding assay
Scratch wounding assay was performed applying 2D invasion cell culture inserts (24 well, Ibidi, Martinsried, Germany). For each experiment, cEND cells were seeded into both cell culture reservoirs separated by a 500 −µm thick silicone wall adhering to the bottom of the culture plate and grown until confluence. Then, the silicone insert was removed from the surface resulting in two precisely defined cell patches. After the suspended cells were washed for three times with DMEM, the wounded monolayers were conditioned with DMEM containing patients’ serum containing SU5416 or Bevacizumab. Two perpendicular semiopaque marks were placed across each scratch on the external surface of the area covered by cells in each well to standardize quantitative analysis. The repopulation of the wounded areas was documented by phase contrast microscopy (Axiovert, Zeiss). Using ImageJ 1.46e, the size of the denuded area was determined at each time point from digital images.
Immunocytochemistry
Immunostaining was performed in accordance to procedures described previously using the polyclonal goat anti-VE-cadherin (R&D Systems), Alexa488-conjugated rabbit anti-goat (Jackson Immuno Res. Lab) and DAPI to visualize cell nuclei.5 Image acquisition was obtained with a confocal microscope (TCS SP5, Leica Microsystems, Wetzlar, Germany). Confocal images were acquired by LCF AF software (all from Leica Microsystems) using a Z step of 0.1 µm and 63 × 1.4 NA oil immersion objective.
Statistical analysis
Data were obtained from three independent experiments performed in triplicates, as indicated in the figure legends. Values for densitometry and gene expression were averaged to establish a single value for ACVD (control) or MMD serum-incubated cells and compound-treated cells, as indicated. Data were analyzed through GraphPad Prism 6.1 (GraphPad Software) using analysis of variance (ANOVA) with pairwise comparison, Holm–Sidak method assuming significance for *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Results
Enhanced gelatinase activity and reduced collagen IV protein expression in MMD versus ACVD vessel specimens
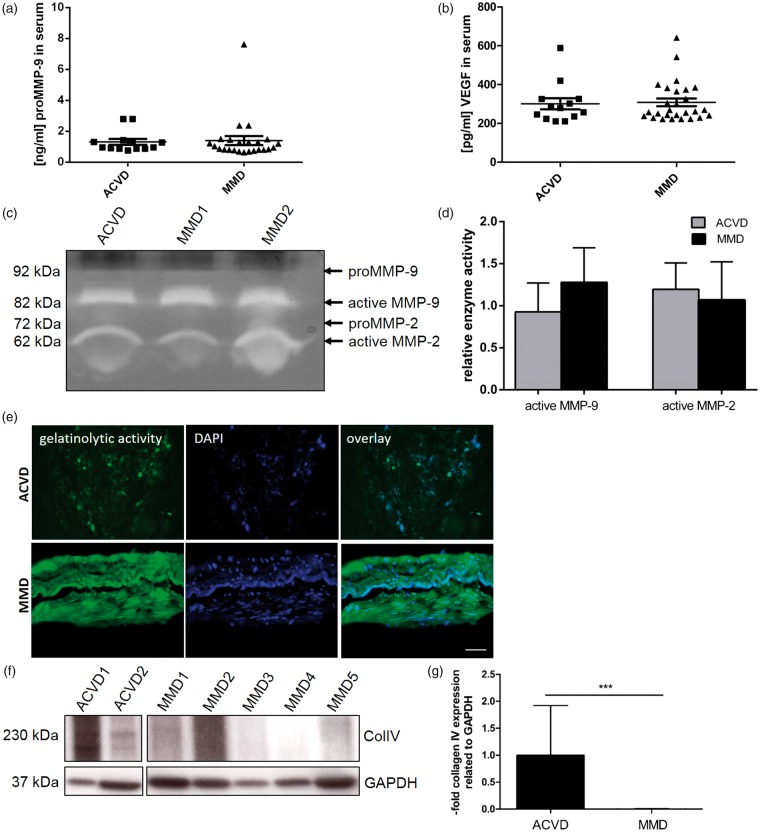
By analogy to our recently published study, we hypothesized that two other factors, MMP-9 and VEGF, with a strong pro-angiogenic potential may be synergistically involved with Ang-2 in the pathological angiogenesis and vascular instability characterizing MMD. Therefore, first, serum concentrations of MMP-9 and VEGF were tested by ELISA, but unexpectedly without finding significant differences (Figure 1(a) and (b)). Also, gelatin degrading properties of active MMP-9 and MMP-2 in serum samples of both patient cohorts did not differ in ACVD versus MMD patients’ serum (Figure 1(c) and (d)). However, a profoundly enhanced gelatinolytic activity could be documented in vessel specimens obtained from MMD versus ACVD patients during the STA-MCA revascularization procedure (Figure 1(e)). In addition, collagen IV being the key molecule in the cerebrovascular basement membrane was significantly lowered in affected MMD versus ACVD vessel specimens (Figure 1(f) and (g)).
Figure 1.
Increased gelatinase activity and reduced collagen IV protein expression in MMD vessel specimens. Quantitative measurements of (a) total MMP-9 (proMMP-9) and (b) VEGF protein in the ACVD (n = 13) and MMD patient cohort (n = 24) were performed by ELISA. No significant differences could be detected between both patient groups. Functional analysis of the enzymatic activity of MMP-9 and MMP-2 in MMD compared with ACVD serum was evaluated by gelatin Zymography (c). After the analysis of nine sera per group, representative protein gels were chosen. Both patient groups did not differ in terms of MMP-9 and MMP-2 activity as indicated by (d) densitometric analyses of all gels performed using ImageJ software and summarized in the graph. Cryosections of human vessel specimens were analyzed for collagenase activity due to manufacturers’ instructions (e). Images are representative. Bar: 50 µm. Vessel specimens were lysed and equal volumes were analyzed for collagen IV protein by Western blot (f). Protein amount per lane was normalized to the expression of GAPDH, the relative collagen IV expression was estimated by densitometrical analysis and expressed in the graph (g), ***P < 0.005, one-way ANOVA.
Elevated intracellular and extracellular MMP-9 and VEGF levels in MMD serum-exposed cEND cells
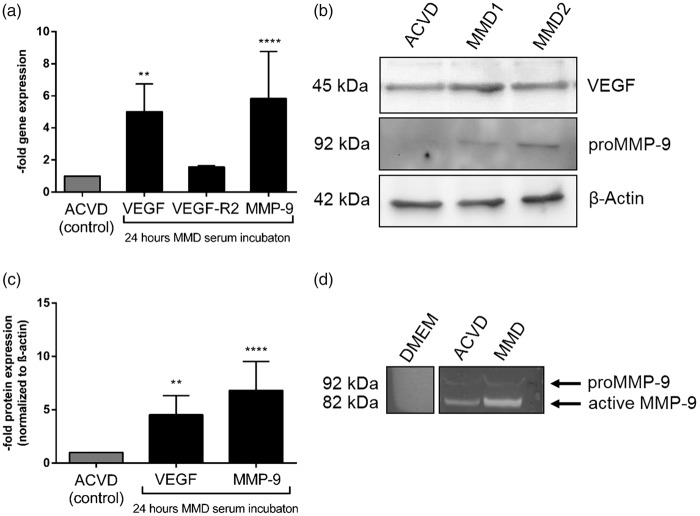
Next, we used the recently described MMD in vitro model of cerebrovascular ECs, cEND cells, to investigate the effect of incubations of serum from both patient groups on the intracellular expression and autocrine release of these molecules. By applying a standardized treatment protocol,5 the influence of ACVD and MMD serum on MMP-9 and VEGF was analyzed by qPCR, Western blot and Zymography assays followed by densitometric quantification (Figure 2). A 24-h treatment of confluent cEND cells maintained with MMD patients’ serum enhanced the gene expression of MMP-9 and VEGF by approximately 5-fold, while transcript level of VEGF-R2 was not attenuated (Figure 2(a) and Supplemental Table 2). MMP-9 and VEGF expression was lower under normal conditions when cENDs were kept in FCS (Supplemental Figure 1(e)). We have also confirmed an overexpression of VEGF and MMP-9 protein (Figure 2(b) and (c)) and found a significantly increased MMP-9 activity in supernatant samples of cENDs incubated in MMD serum versus ACVD serum-treated controls pointing to a role for systemic MMD serum components increasing the gelatinolytic activity in cerebrovasculature, as indicated by densitometric analysis of the protein (Figure 2(d)). The intake of statins at admission by MMD and ACVD patients had no significant influence on the secretion and activity of MMP-9 (Supplemental Figure 2(c) and (e)). Statin intake did also not affect the secretion of Ang-2 into the medium supernatant (Supplemental Figure 2(d)).
Figure 2.
Impact of MMD patients’ serum on intra- and extracellular MMP-9 and VEGF in cENDs. Relative gene expression of VEGF, VEGF-R2 and MMP-9 was assessed in the presence of ACVD versus MMD serum for 24 h by qPCR (a). Protein expression of VEGF and proMMP-9 normalized to β-actin in response to MMD versus ACVD serum was tested by Western blot (b) and densitometry (c). Images are representative. Extracellular secretion of total MMP-9 and VEGF in response to patients’ serum monitored in cENDs using ELISA (d). Values in the graphs are means ± SEM (n = 3 independent experiments performed in triplicates each including at least 5 different pooled patients’ serum of each clinical type) ****P < 0.001, **P < 0.01 versus ACVD-incubated cENDs, one-way ANOVA.
Preservation of brain endothelial cell–cell interactions by VEGF signaling block in MMD serum-maintained cENDs
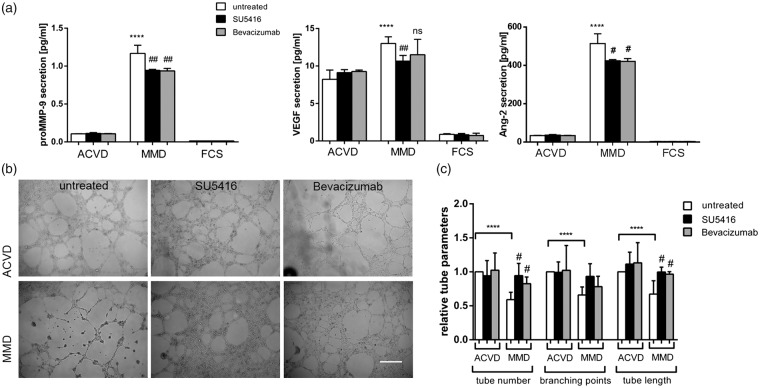
We have recently described reduced EC–EC interaction in response to MMD serum in cEND cells which could be attenuated by the blockade of autocrine Ang-2 secretion.5 To initially determine whether the secretion of MMP-9 and VEGF is influenced by ACVD and MMD serum incubations, cEND cell supernatants were analyzed by ELISA assays. We also analyzed the effect of VEGF signaling block on the release of these molecules and Ang-2. No meaningful regulation of the MMP-9, VEGF and Ang-2 release in response to ACVD serum treatment and VEGF signaling inhibition could be documented. In the presence of MMD serum, the MMP-9 concentration was approximately 6-fold and VEGF protein was 1.6-fold increased in the extracellular compartment. By adding the VEGF-R2 inhibitor SU5416 or the antibody directed to VEGF, Bevacizumab, the induction of MMP-9 was slightly but however statistically significant down-regulated. SU5416 was effective to cause a partial reduction of autocrine VEGF, while no influence on VEGF protein levels in cEND supernatants could be observed after MMD serum application with Bevacizumab. As we have reported recently, Ang-2 was significantly induced by MMD serum compared with ACVD serum treatment in cENDs. By analogy, a down-regulation of Ang-2 secreted into the extracellular compartment was documented in response to both compounds plus MMD serum (Figure 3(a)).
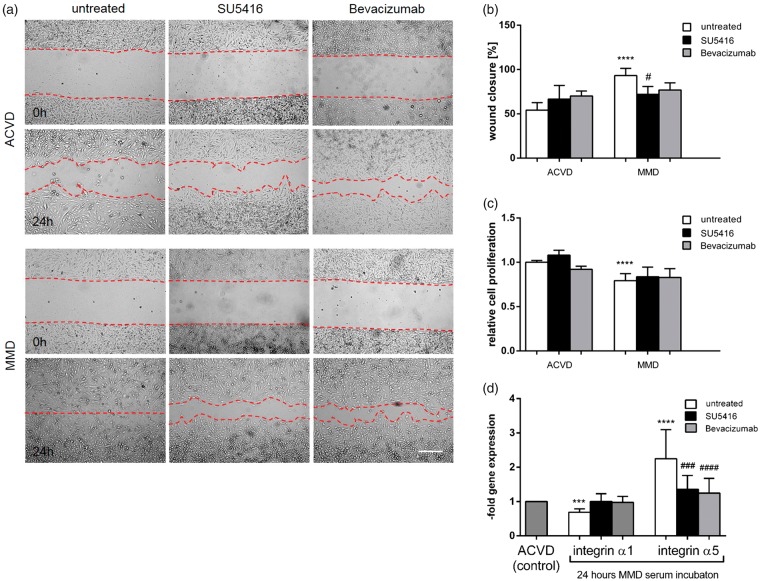
Figure 3.
EC–EC interactions upon blocking VEGF signaling in MMD serum-incubated cENDs. CEND cells were pre-stimulated with SU5416 (25 µM), Bevacizumab (250 µg/ml) or left untreated for 2 h prior to exposition to 2.5% patients’ serum for 24 or 48 h, as indicated. Effects of the compounds on secretion of MMP-9, VEGF and Ang-2 (a). Phenotypic effect of ACVD and MMD serum incubation tested by tube formation assay (b). Representative low magnification images (10×) of tubes formed by serum-conditioned cENDs were chosen. Total tube number, branching points and tube length were assessed by Wimasis Software (c). Values are means ± SEM (n = 3 independent experiments in triplicates each including five different patients’ serum per group performed in triplicates), ****/####P < 0.001, ***/###P < 0.005, **/##P < 0.01, */#P < 0.05, versus cENDs incubated in ACVD serum, one-way ANOVA.
To test cerebro-endothelial cell–cell interactions, we next performed in vitro tube formation assays in patients’ serum-incubated cENDs with and without VEGF signaling inhibiting compounds. The assay displays the ability of ECs to form tube-like structures that depends on the potential of cells to migrate, establish cell–cell and cell–matrix contacts, and to regulate cell survival. Concomitantly to our recently published work, all tube parameters, including tube number, their length and branching points were significantly reduced under MMD versus ACVD conditions. By applying SU5416 or Bevacizumab additionally to MMD serum, a significant increase and reconstitution to the control level of tube number and length could be documented, while no influence of either substance on the number of branching points of the tubes could be observed (Figure 3(b) and (c)).
Restored brain EC barrier properties due to VEGF signaling block in the presence of MMD serum
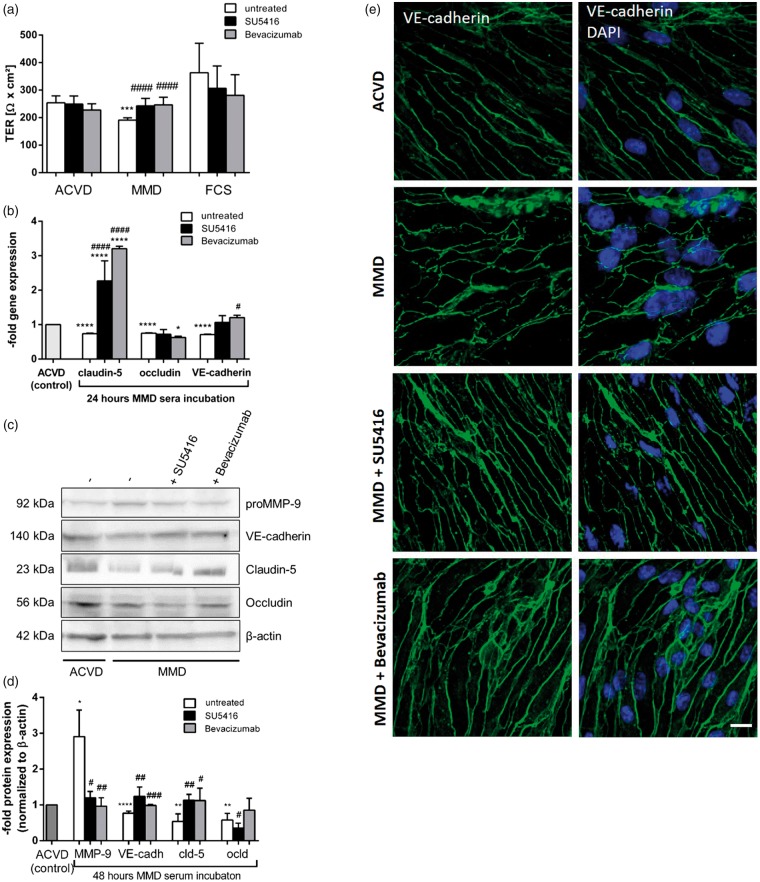
Recently, we focused on the comparison of cENDs exposed to ACVD serum with MMD serum-treated cells. MMD serum caused increased permeability and decreased synthesis of inter-endothelial junction molecules, tight and adherens junctions (TJ and AJ) simultaneously with an induction of an autocrine release of Ang-2.5 Under non-disease conditions, the TER of cEND cells was significantly higher than after incubating the cells with MMD or ACVD serum (Supplemental Figure 1(a)). Blockade of the VEGF signaling cascade has been described to inhibit MMP-9 degrading abilities and is known to restore EC barrier properties. We thus further pursued this observation and tested the effect of SU5416 and of Bevacizumab. Functional analysis of EC barrier properties demonstrated a statistically significant positive effect of SU5416 and Bevacizumab treatment on TER being an indicator of endothelial permeability in MMD serum-stimulated cENDs (Figure 4(a)). Moreover, a transcriptional up-regulation of cell–cell contact molecules by SU5416 or Bevacizumab could be recorded in the presence of MMD serum. Briefly, claudin-5 gene expression was up-regulated by approximately 2.5-fold by VEGF-R2 block and a 3-fold increase due to Bevacizumab treatment compared to the ACVD control. VE-cadherin was restored to the control level by stimulation with both compounds. However, none of the agents could reinforce the expression of occludin being reduced by the exposure to MMD serum by approximately 40% of the control (Figure 4(b)). These results could be reproduced by Western blot analysis where a restoration of claudin-5 and VE-cadherin could be documented, whereas the protein expression of occludin remained down-regulated in response to MMD serum plus compounds (Figure 4(c) and (d)). CEND cells kept in ACVD serum revealed a typical spindle-like elongated EC monolayer morphology with VE-cadherin located predominantly in the membrane (Figure 4(e)). A significantly disintegrated VE-cadherin stain along with a change to a more roundish and disorganized phenotype was observed in the cEND cell monolayer incubated in MMD serum. This characteristic delocalization of VE-cadherin could be abolished by co-treating the cells with both SU5416 or Bevacizumab. After co-stimulation with VEGF signaling blockers, MMD serum-incubated cENDs exhibited the spindle-like elongated morphology being comparable with the one observed in ACVD serum-treated control cells.
Figure 4.
Restoring effect on brain EC barrier function mediated by VEGF signaling blockage in cENDs exposed to MMD serum. CEND cells were maintained in ACVD and MMD and co-treated with Bevacizumab, SU5416 or left untreated for 48 h. The effect of ACVD and MMD serum on TER of cENDs in the presence or absence of Bevacizumab or SU5416 was determined and expressed in absolute TER values (Ω × cm2, a). Gene expression of claudin-5 and occludin, and VE-cadherin in response to human serum and VEGF signaling blockade was analyzed by real-time qPCR (b). Western blot analysis (c) followed by densitometry (d) to elucidate the effect of VEGF signaling block on MMD serum-induced TJ and AJ reduction. Values are means ± SEM (n = 3 independent experiments each including five different patients’ serum per group performed in triplicates), ****/####P < 0.0001, ***/###P < 0.001, **/##P < 0.01, */#P < 0.05, versus cENDs incubated in ACVD serum, one-way ANOVA. Cells stained for VE-cadherin (green) and DAPI to visualize cell nuclei observed by confocal microscopy (e). Bar: 10 µm, n = 3 independent experiments performed in triplicates each including five different pooled patients’ sera per group.
Taken together, the exposure of cEND cells to MMD serum showed reciprocal effects on the expression and secretion of barrier-destabilizing cytokines and cell–cell contacts, while the simultaneous stimulation of cEND cells with VEGF signaling blockers represses the expression of MMP-9 and synergistically reestablishes EC barrier properties.
Lowered MMD serum-induced cell–matrix disturbances by inhibiting the VEGF signaling pathway
By the scratch migration assay, the motility of a wounded cEND cell monolayer was tested in response to patients’ serum with and without blockage of the VEGF signaling path. While the scratch was completely closed in MMD serum-incubated samples after 24-h treatment, the cEND cell monolayer sealed by approximately 50% of the starting point of ACVD serum exposure. Blockade of the VEGF signaling path by SU5416 or Bevacizumab did not significantly influence the migration rate of the cells in response to ACVD serum treatment. However, while the migration rate of MMD serum-incubated cEND cell monolayers was observed to be statistically decreased by SU5416 reaching almost the value of the ACVD control monolayers, we were not able to detect any difference after Bevacizumab application (Figure 5(a) and (b)). Next, MTT assays were performed to initially test if the increased migration of MMD serum-treated cENDs depends on an increased cell proliferation rate and to document the influence of VEGF signaling block (Figure 5(c)). A 48-h treatment of cEND cells with MMD versus ACVD serum induced a decreased proliferation rate, which could not be increased by treating the cells with VEGF and VEGF-R2 interfering compounds.
Figure 5.
Increased cEND cell migration and deregulated integrin expression by MMD serum irrespectively of cell proliferation. CEND cell migration was measured in response to MMD versus ACVD serum and upon VEGF signaling block using SU5416 or Bevacizumab over 24 h (a). The repopulation of the wounded areas was documented by phase contrast microscopy. The size of the denuded area was determined from digital images, analyzed using ImageJ 1.46e and depicted in the graph (b). CEND cell proliferation in response to MMD versus ACVD serum at 48 h analyzed by MTT assay (c). Effect of MMD versus ACVD serum with or without SU5416 or Bevacizumab on α1 and α5 integrin receptor gene expression tested by real-time qPCR (d). Values from all graphs are means ± SEM (n = 3 independent experiments each including five different pooled patients’ serum per group performed in triplicates), ****/####P < 0.001, ***/###P < 0.005, **/##P < 0.01, */#P < 0.05, versus cENDs incubated in ACVD serum, one-way ANOVA.
We then speculated that MMD serum-mediated up-regulation of autocrine MMP-9 protein could influence the expression of integrin α1, the subunit of the major collagen receptor integrin α1β1, on vascular endothelium responsible for EC adhesion to the matrix ligand collagen type IV. A 24-h incubation of cEND cells with MMD serum led to a 0.7 ± 0.2-fold down-regulation of integrin α1 subunit when compared to ACVD serum-treated control cEND cells. However, by exposing the cells to VEGF signaling inhibiting substances, no significant changes of integrin α1 could be observed (Figure 5(d)). Moreover, elevated levels of the α5 integrin subunit were assessed by qPCR (2.3 ± 0.9) revealing a deregulated interaction between cEND cells and their basement membrane caused by MMD serum incubation. A significantly reduced expression of this molecule was measured in cENDs treated with SU5416 or Bevacizumab in the presence of MMD serum (Figure 5(d)).
Endothelial barrier properties are preserved in myocardial ECs kept in MMD serum
Our recently published work described MMD serum incubations affecting cEND cells in a brain-specific manner by testing endothelial features in HuVEC cells.5 In analogy, a non-cerebral EC line derived from mouse hearts was applied to confirm our hypothesis. The gene expression of angiogenic factors, some of their respective receptors and cell–cell contact molecules were tested in MyEND cells, compared with data obtained for cENDs and summarized in Supplemental Table 2. There were no statistically significant changes in the gene expression of the tested molecules confirming that the effects gained by the incubation of cerebrovascular cENDs in MMD versus ACVD patients’ serum are brain specific.
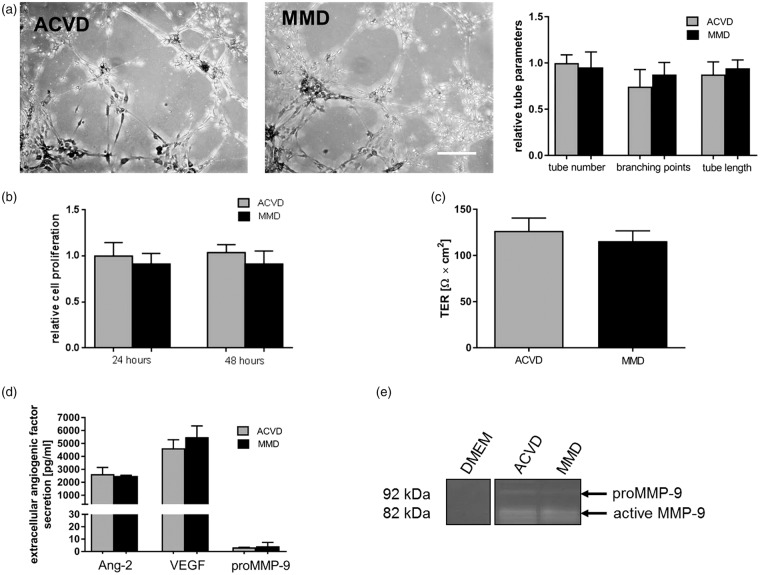
We have also investigated functional effects on MyEND cells in the presence of MMD against ACVD serum by applying tube formation and MTT proliferation assay. As depicted in Figure 6(a) and (b), there were no significant differences in the tube-forming ability of MyEND cells and their proliferation rate caused by MMD serum incubation versus ACVD control treatment. There were also no statistically significant effects documented by measuring the TER of MyEND cells under serum treatment (Figure 6(c)). In accordance with our hypothesis and comparably to the results gained in HuVEC cells, described previously, no relevant differences in Ang-2, MMP-9 and VEGF secretion into the cell culture supernatant were observed (Figure 6(d)). In addition, no significant alterations in extracellular MMP-9 activity in MMD versus ACVD serum-incubated MyEND cells were detectable in gelatinase Zymography assay (Figure 6(e)).
Figure 6.
Effects of MyEND cell incubations with ACVD versus MMD serum EC barrier properties.MyEND cells were incubated in analogy to cENDs for 24 or 48 h. The effect of ACVD and MMD patients’ serum incubation on MyEND cell phenotype and cell–cell interactions was tested by Matrigel tube formation assay (a). Low magnification images (10 × magnification) of tube formation in response to ACVD and MMD serum are representative. Total number of tubes, branching points and tube length was assessed by Wimasis Image Analysis and depicted by the graph. MyEND cell proliferation was analyzed by MTT assay (b). EC barrier properties were proven by TER (c). Secretion of human Ang-2, VEGF and proMMP-9 protein into the supernatant was analyzed by ELISA (d). Gelatinolytic activity of MMP-9 evaluated in cell culture supernatants by gelatinase zymography (e). Values are means ± SEM (n = 3 independent experiments each including 5 different serum per group), one-way ANOVA (Holm–Sidak method) was used as statistical test.
Discussion
In our present work, we investigated the role of pro-angiogenic and EC barrier-disintegrating molecules VEGF and MMP-9, which additionally to Ang-2 are involved in the development of the hazy vascular network observed in MMD patients. Since MMD and ACVD patients exhibit a similar occurrence of ischemic episodes and a comparable localization of cerebral infarctions, all experiments were conducted with samples from ACVD patients as a control. We show that gelatinase activity is significantly stronger and the essential substrate of MMP-9, collagen IV is markedly affected in MMD vessel specimens compared to those obtained from ACVD patients. Although not regulated in serum, MMP-9 and VEGF were strongly overexpressed and secreted by cEND cells maintained with MMD serum, similarly to what we observed for Ang-2.5 Moreover, by blocking VEGF signaling, we saw a partial reduction of the MMD serum-mediated effects improving the EC–EC and EC–ECM function in our in vitro model. Applying an EC line derived from mouse myocardium, we were able to confirm our hypothesis on a brain-specific influence of MMD serum.
Overexpression of Ang-2 has been implicated in the activation of signals leading to a disassembly of the junctional complex under physiological pro-angiogenic conditions and in a number of cerebrovascular disorders.11 By analogy, we hypothesized further that other pro-angiogenic mediators may interplay with the so far identified Ang-2 to be regulative for the pathological angiogenesis characterizing MMD. Therefore, the involvement of MMP-9 and VEGF in the disruption EC barrier and hemorrhage was tested in this work in vessel specimens from MMD and ACVD patients as well as in the cEND cell culture model mimicking the disease conditions. To our knowledge, we are the first providing evidence for the focal digestive activity of gelatinases in the affected vasculature of MMD patients, which was accompanied by the loss of the ECM component collagen IV. Mediators of the EC barrier disruption include MMPs, a group of zinc-containing endopeptidases.15 MMP-9, known to be elevated under inflammatory conditions and to drive angiogenesis, requires the degradation and remodeling of the ECM. MMP-9 acts either on ECM or on non-ECM substrates, including inter-endothelial TJs, cell-surface and ECM-bound growth regulators, releasing them from stores.16,17 The molecule was also hypothesized to be involved in the development of the rete mirabile characterizing MMD.17 However, until today, the expression and activity of MMP-9 in MMD patients’ material were exclusively shown in relation to healthy donors not taking in account the hypoxic conditions being present in MMD cerebrovasculature being a strong pro-angiogenic stimulus, per se.18,19
The pro-angiogenic and vascular barrier-de-arranging action of Ang-2 and MMP-9 is strongly dependent on the bioavailability of VEGF.11,20 Thus, functional impairments mediated by MMD serum condition could be partially abolished by inhibiting the VEGF signaling pathway. Neutralization of VEGF was achieved by Bevacizumab treatment or by blocking the VEGF-R2 using SU5416, that was shown to be an effective and non-toxic strategy to control growth of angiogenesis-dependent tumors.21 Both strategies efficiently attenuated extracellular elevation of MMP-9 and Ang-2. In addition, claudin-5 and VE-cadherin overcame completely the down-regulative effect of MMD serum on their expression, while stimulations with Bevacizumab or SU5416 were not effective to preserve the expression of occludin in response to MMD serum, suggesting the down-regulation of occludin not to be mediated by VEGF-R2.
Mechanisms leading to EC migration are very complex and there are diverse pathways to initiate and progress cell motility. We saw that enhanced cEND cell migration caused by MMD serum was not dependent on increased cell proliferation. Moreover, ECs initiate migration by digesting the ECM and by changing the expression of cell–matrix contacts, such as integrins. The classical scratch wound healing assay as well as the tube formation assay clearly displayed deregulated cell–cell or the cell–matrix interactions in cENDs maintained in MMD versus ACVD serum. Therefore, we furthermore speculated that alterations in endothelial cell–matrix adhesion mediated by integrins in response to MMD serum stimulus may be crucially involved in the EC disturbance observed in the disease. ECM integrin receptors control adhesion to and migration toward ECM thereby regulating angiogenesis,22 i.e. the matrix-binding integrin α1 receptor representing one of the major collagen IV receptors on brain ECs was shown to participate in the control of cerebrovascular integrity and to be lost, i.e. in focal cerebral ischemia.23 As it was observed in integrin α1 knockout mice, its expression is inversely correlated with MMP levels.24 Interestingly, lowered integrin α1 expression was associated with a reduced proliferation of ECs grown on collagen IV.25 Both facts were simultaneously observed in cENDs under MMD conditions. Concomitantly, we observed an enhancement of the integrin α5 subunit, that is also known as one of the pro-angiogenic integrins.22 An overexpression of integrin α5 seems to play an important role in EC migration and in the ability to form tube-like structures.22,26 By treating the cells with SU5416 or Bevacizumab additionally to MMD serum, the overexpression of integrin α5 could be prevented without significantly affecting the down-regulation of integrin α1 further implicating, at least in part, the involvement of the VEGF signaling in the control of cell–matrix interactions in MMD. However, the detailed mechanism of this phenomenon remains to be elucidated.
Finally, by applying the same experimental setup for brain and non-brain EC cultures, we were able to determine differential effects on the expression profile of molecules involved in the regulation of angiogenesis in the presence of MMD and ACVD serum. We could already describe these observations in our previous work where HuVEC cells were used for this purpose. Since the cultivation (cell line versus primary ECs), host (mouse versus human) and function (micro- versus macro-vascular ECs) of cEND and HuVEC cells differ very much, we herein confirmed the brain-specificity of the observed effects in the mouse myocardial cell line, MyEND.27 One explanation for our results pointing to brain specificity would be that divergent signaling mechanisms are involved in EC barrier regulation through a differential expression profile of pro- and anti-angiogenic factors in cerebral and non-cerebral ECs. This fact seems not be surprising, since the aberrant MMD collateralization pattern and micro-hemorrhages resulting from the pathological vessel fragility in MMD are observed exclusively in the brain.28,29 The results are also concomitant with findings of others reviewing all EC-specific genes being differentially or unevenly expressed throughout the vascular tree.30 Even small differences in the expression status of cellular contacts, growth or angiogenic factors between ECs may therefore account for large differences in environmentally induced gene programs.31 It is therefore very likely that the combination of both intrinsic and extrinsic factors determines the phenotypic heterogeneity of ECs. In line with these facts, pro- and anti-angiogenic therapy should be targeted to specific vascular beds displaying the diseased phenotype.32 The evaluation and identification of such novel site-specific therapies will, however, depend on our understanding of the proximate mechanisms underlying EC heterogeneity also in the context of MMD.
Taken together, MMD represents a rare and clinically heterogeneous disease. The access to adequate animal models and to affected tissue from patient lesions is limited. The cEND cell line is therefore proposed as an useful experimental approach to investigate mechanisms underlying the instability and plasticity characterizing MMD. Based on this in vitro model, we found evidence for so far unidentified signals or factors in MMD patients’ serum that actively and brain-specifically stimulate the expression of different vessel-destabilizing mediators. Elevation of these factors, released in different concentrations and their interplay seems to be regulative for EC barrier destabilization observed in MMD. So far, our results support MMP-9, VEGF and Ang-2 to control the autocrine-mediated EC–EC and EC–matrix interactions strongly differing from those observed in brain ECs kept in ACVD serum. The potential mechanisms behind the vascular fragility in MMD is depicted in Figure 7. The inhibition of the Ang-2/tie-2 signaling rather than blockade of VEGF/VEGFR-2 restored EC function,5 suggesting VEGF to play a lesser role, however, to be a new promising target to prevent micro-bleeds or hemorrhagic stokes in MMD.
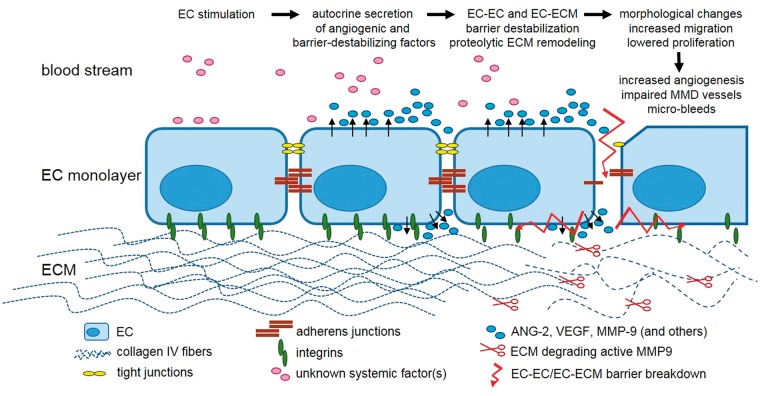
Figure 7.
Potential mechanism behind the development of fragile MMD cerebrovasculature. ECs stimulated by so far undetermined serum factors circulating in the blood stream of MMD patients excessively secrete pro-angiogenic mediators, such as Ang-2, MMP-9 and VEGF in an autocrine manner. These factors influence the EC–EC and EC–ECM barrier by disrupting inter-endothelial junctions and deregulating the expression of integrins. Increased proteolytic activity of MMP-9 induces ECM remodeling through the degradation of collagen IV. Morphological changes along with an increased migration and lowered proliferation are followed by an excessive angiogenesis mediating the development of the cerebrovascular fragility and potential micro-bleeds.
Supplemental Material
Supplemental material, Supplementary Figure 1 for Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease by Kinga G Blecharz-Lang, Vincent Prinz, Małgorzata Burek, Dietmar Frey, Tobias Schenkel, Susanne M Krug, Michael Fromm and Peter Vajkoczy in Journal of Cerebral Blood Flow & Metabolism
Supplemental Material
Supplemental material, Supplementary Figure 2 for Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease by Kinga G Blecharz-Lang, Vincent Prinz, Małgorzata Burek, Dietmar Frey, Tobias Schenkel, Susanne M Krug, Michael Fromm and Peter Vajkoczy in Journal of Cerebral Blood Flow & Metabolism
Supplemental Material
Supplemental material, Supplementary Tables for Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease by Kinga G Blecharz-Lang, Vincent Prinz, Małgorzata Burek, Dietmar Frey, Tobias Schenkel, Susanne M Krug, Michael Fromm and Peter Vajkoczy in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors are grateful to Irina Kremenetskaia and Elisabeth Wilken for excellent technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from Deutsche Forschungsgemeinschaft (DFG: VA 244/10-1 and FOR 2325 NVI).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KGBL, VP, MB, DF, TS, SMK, MF and PV designed the experiments, collected, analyzed and interpreted the data and KGBL wrote the manuscript. All authors critically revised the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20: 288–299. [DOI] [PubMed] [Google Scholar]
- 2.Czabanka M, Acker G, Jussen D, et al. Collateralization and ischemia in hemodynamic cerebrovascular insufficiency. Acta Neurochir 2014; 156: 2051–2058. [DOI] [PubMed] [Google Scholar]
- 3.Vajkoczy P. Moyamoya disease: collateralization is everything. Cerebrovasc Dis 2009; 28: 258. [DOI] [PubMed] [Google Scholar]
- 4.Schubert GA, Czabanka M, Seiz M, et al. Perfusion characteristics of Moyamoya disease: an anatomically and clinically oriented analysis and comparison. Stroke 2014; 45: 101–106. [DOI] [PubMed] [Google Scholar]
- 5.Blecharz KG, Frey D, Schenkel T, et al. Autocrine release of angiopoietin-2 mediates cerebrovascular disintegration in Moyamoya disease. J Cereb Blood Flow Metab 2017; 37: 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999; 5: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 7.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248. [DOI] [PubMed] [Google Scholar]
- 8.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 2001; 21: 1104–1117. [DOI] [PubMed] [Google Scholar]
- 9.Johnson C, Sung HJ, Lessner SM, et al. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res 2004; 94: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer AT, Bürgers HF, Rabie T, et al. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab 2010; 30: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A 2002; 99: 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Q, Su H, Palmer D, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke 2011; 42: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg 1997; 99(Suppl 2): S238–S240. [PubMed] [Google Scholar]
- 14.Zozulya A, Weidenfeller C, Galla HJ. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res 2008; 1189: 1–11. [DOI] [PubMed] [Google Scholar]
- 15.Rempe RG, Hartz AM, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab 2016; 36: 1481–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lischper M, Beuck S, Thanabalasundaram G, et al. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res 2010; 1326: 114–127. [DOI] [PubMed] [Google Scholar]
- 17.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem 1999; 274: 21491–21494. [DOI] [PubMed] [Google Scholar]
- 18.Fujimura M, Watanabe M, Narisawa A, et al. Increased expression of serum Matrix Metalloproteinase-9 in patients with moyamoya disease. Surg Neurol 2009; 72: 476–480; discussion 480. [DOI] [PubMed] [Google Scholar]
- 19.Kang HS, Kim JH, Phi JH, et al. Plasma matrix metalloproteinases, cytokines and angiogenic factors in moyamoya disease. J Neurol Neurosurg Psychiatry 2010; 81: 673–678. [DOI] [PubMed] [Google Scholar]
- 20.Thanabalasundaram G, Pieper C, Lischper M, et al. Regulation of the blood-brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res 2010; 1347: 1–10. [DOI] [PubMed] [Google Scholar]
- 21.Vajkoczy P, Menger MD, Vollmar B, et al. Inhibition of tumor growth, angiogenesis, and microcirculation by the novel Flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. Neoplasia 1999; 1: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva R, D’Amico G, Hodivala-Dilke KM, et al. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol 2008; 28: 1703–1713. [DOI] [PubMed] [Google Scholar]
- 23.Tagaya M, Haring HP, Stuiver I, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab 2001; 21: 835–846. [DOI] [PubMed] [Google Scholar]
- 24.Pozzi A, Moberg PE, Miles LA, et al. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A 2000; 97: 2202–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murikipudi S, Methe H, Edelman ER. The effect of substrate modulus on the growth and function of matrix-embedded endothelial cells. Biomaterials 2013; 34: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Befani C, Liakos P. Hypoxia upregulates integrin gene expression in microvascular endothelial cells and promotes their migration and capillary-like tube formation. Cell Biol Int 2017; 41: 769–778. [DOI] [PubMed] [Google Scholar]
- 27.Förster C, Waschke J, Burek M, et al. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J Physiol 2006; 573(Pt 2): 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita M, Tanaka K, Kishikawa T, et al. Moyamoya disease associated with renovascular hypertension. Hum Pathol 1984; 15: 191–193. [DOI] [PubMed] [Google Scholar]
- 29.Ellison PH, Largent JA, Popp AJ. Moya-moya disease associated with renal artery stenosis. Arch Neurol 1981; 38: 467. [DOI] [PubMed] [Google Scholar]
- 30.Aird WC, Edelberg JM, Weiler-Guettler H, et al. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol 1997; 138: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aird WC. Endothelial cell heterogeneity. Crit Care Med 2003; 31(4 Suppl): S221–S230. [DOI] [PubMed] [Google Scholar]
- 32.Regan ER, Aird WC. Dynamical systems approach to endothelial heterogeneity. Circ Res 2012; 111: 110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Figure 1 for Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease by Kinga G Blecharz-Lang, Vincent Prinz, Małgorzata Burek, Dietmar Frey, Tobias Schenkel, Susanne M Krug, Michael Fromm and Peter Vajkoczy in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, Supplementary Figure 2 for Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease by Kinga G Blecharz-Lang, Vincent Prinz, Małgorzata Burek, Dietmar Frey, Tobias Schenkel, Susanne M Krug, Michael Fromm and Peter Vajkoczy in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, Supplementary Tables for Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease by Kinga G Blecharz-Lang, Vincent Prinz, Małgorzata Burek, Dietmar Frey, Tobias Schenkel, Susanne M Krug, Michael Fromm and Peter Vajkoczy in Journal of Cerebral Blood Flow & Metabolism