Abstract
Investigation of genetic susceptibility to cerebrovascular disease has been of growing interest. A systematic review of human studies assessing neurogenomic aspects of cerebrovascular disease was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. Any association study exploring genetic variants located in the exome associated with one of the major cerebrovascular diseases with at least 500 subjects was eligible for inclusion. Of 6874 manuscripts identified, 35 studies met the inclusion criteria. Most studies of interest focused on ischemic stroke and cerebrovascular occlusive disease. Large cohort genetic association studies on hemorrhagic cerebrovascular disease were less common. In addition to rare, well-established monogenic conditions with significant risk for cerebrovascular disease, a number of genetic variants are also relevant to cerebrovascular pathogenesis as part of a multifactorial process. The 45 polymorphisms identified were located in genes involved in processes related to endothelial and vascular health (15 (33.4%) variants), plasma lipid metabolism (10 (22.2%) variants), inflammation (9 (20%) variants), coagulation (3 (6.7%) variants), and blood pressure modulation (2 (4.4%) variants), and other (6 (13.3%) variants). This work represents a comprehensive overview of genetic variants in the exome relevant to ischemic and hemorrhagic stroke pathophysiology.
Keywords: Cerebrovascular disease, cerebral aneurysm, ischemic stroke, subarachnoid hemorrhage, atherosclerotic cerebrovascular disease
Introduction
Cerebrovascular disease is responsible for approximately 10% of deaths1 and is the leading cause of permanent disability in the United States.2 Genetic predisposition to cerebrovascular disease is well-established. The majority of cerebrovascular disease etiology is multifactorial with a variable amount of genetic predisposition3 involving a complex interplay among lifestyle, environmental factors, and various genes and their alleles, each allele with relatively minimal individual effects.3–5 Conversely, monogenic, or Mendelian, disorders associated with cerebrovascular disease are rare, but have a high penetrance. As awareness of genetic contribution to cerebrovascular disease has grown in recent years, numerous neurogenomic studies have investigated the key genetic markers of these disorders. In 2015, the National Institute of Health launched its Precision Medicine Initiative, a project designed to promote a framework for increased national application of personalized, or precision, medicine.6 The goal of personalized medicine is to tailor treatment and prophylaxis to each individual, patient-specific profile.6 Since the genome contributes substantially to the diversity that exists from patient to patient, the procurement and utilization of patient genetic data is a major focus point within the precision medicine initiative.6 With current genetic sequencing, storing, and analyzing technology, whole-exome sequencing, which primarily targets exons, but may include non-coding regions such as introns, intron-exon boundary regions, untranslated regions, and intergenic regions as a byproduct, is considerably more cost-effective than whole-genome sequencing and more conducive to large scale personalized medicine.7,8 Fifteen years after the completion of the Human Genome Project, next-generation sequencing has matured and costs have continued to decrease. A number of large scale whole-exome sequencing projects are underway and include efforts led by the Broad Institute which has sequenced 360,000 exomes,9 Geisinger’s MyCode Community Health Initiative, the largest health system sequencing project, which has enrolled 150,000 individuals,10 and the United Kingdom Biobank which is scheduled to sequence 500,000 individuals by 2019.11 High-yield variants within the non-coding genetic regions have also been established and are indeed useful to identify cerebrovascular disease predisposition through genome-wide association studies. These include PITX2 (rs6843082),12 ZFHX3 (rs879342),12 and ALDH2 (rs2238151)13 for ischemic stroke, TSPAN2 (rs12122341)13 for cerebrovascular occlusive disease, and PMF1 (rs2984613)14 for hemorrhagic stroke among others. Nevertheless, this review is focused on currently known variants predisposing to or protecting from common cerebrovascular diseases within (italicized) the exome. For completeness, important monogenic disorders are reviewed as well.
Materials and methods
Literature search strategy
A systematic review of studies assessing neurogenomic aspects of cerebrovascular disease was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. The electronic database MEDLINE/PubMed was the primary source for article identification with Google Scholar used as a supplement along with the reference lists of reviewed manuscripts. The MEDLINE/PubMed database was searched from database inception to September 2017. Articles were cross-referenced with phenotype-genotype integrator (PheGenI) and online Mendelian inheritance in man (OMIM®). Appropriate free text and MeSH terms were used to identify all studies and included “genetic” paired with “ischemic stroke,” “intracranial atherosclerosis,” “extracranial atherosclerosis,” “carotid atherosclerosis,” “subarachnoid hemorrhage,” “cerebral aneurysm,” “intracerebral hemorrhage,” “arteriovenous malformation,” “cavernous malformation,” or “moyamoya” (e.g. genetic ischemic stroke, genetic intracranial atherosclerosis, genetic extracranial atherosclerosis). Articles identified using “intracranial atherosclerosis,” “extracranial atherosclerosis,” and “carotid atherosclerosis” search terms were combined as cerebrovascular occlusive disease. Articles were independently appraised and assessed for quality (such as accounting for confounding variables through multivariable analysis or discerning the level of consonance between the conclusions of the articles and the data presented) by two individuals (C.G., S.F.) and reference lists were scanned for additional studies of potential relevance. Correction for multiple comparisons using Bonferroni was favored, but not required as some consider this approach overly conservative in the context of genetic association studies. Discrepancies between reviewers were resolved through discussion until consensus was reached.
Inclusion criteria
Studies eligible for inclusion in this systematic review comprised a minimum of 500 subjects (not counting controls) to minimize the potential for false positive findings in studies of smaller cohorts and to emphasize those of presumably higher impact. Meta-analyses were included with the cumulative number of affected individuals from each included studies representing the total number of subjects. Only variants located in the exome were considered. Studies that failed to show a statistically significant association, investigated variants only in intronic or intergenic locations, analyzed gene–gene, gene-treatment, or gene-environment interaction, or examined a subset of patients with pre-existing risk factors were all excluded. In cases of overlapping cohorts, only the most recent and largest was included. For completion, we contrasted eligible studies to monogenic, or Mendelian, disorders associated with cerebrovascular disease. Such disorders have high penetrance, but their considerably lower prevalence has generally rendered them unable to meet the inclusion criteria as large cohort studies are difficult to conduct.
Results
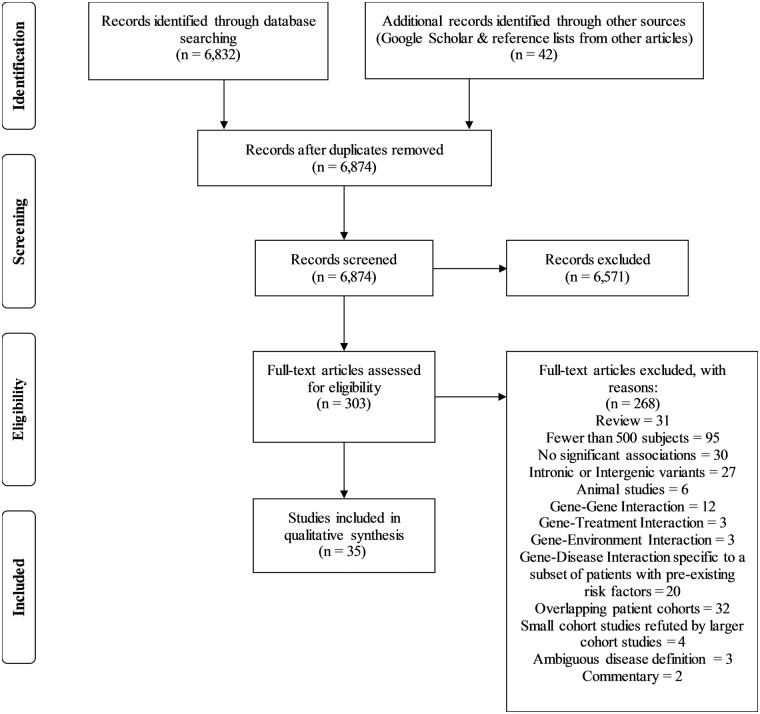
Thirty-five studies met the inclusion criteria using the search strategy (Figure 1). The majority of relevant studies focused on ischemic cerebrovascular disease. A total of 45 genetic variants (43 (95.6%) single nucleotide polymorphisms (SNPs) with rsIDs) qualified for inclusion in this review and are listed in Tables 1 to 6. The individual genes are color coded in the tables based on groups involved in similar functions such as endothelial and vascular health (15 (33.4%) genes; blue), plasma lipid metabolism (10 (22.2%); orange), inflammation (9 (20%); turquoise), coagulation (3 (6.7%); green), blood pressure modulation (2 (4.4%); red), and others (6 (13.3%); no color).
Figure 1.
Flow chart of search strategy.
Table 1.
Ischemic stroke.
| Study | Country(ies) of study | Cases/ controls | Gene | Relevant function | Reference SNP # | Amino acid change | Common name | Associated population | Inheritance model | OR | CI at 95% | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variants associated with ischemic stroke | ||||||||||||
| He et al.20 | China | 500/600 | MTHFR | Endothelial and vascular health (homocysteine metabolism) | rs868014 | *in 3′-UTR* | Chinese Han | Recessive | 1.99 | 1.29–3.88 | 0.001a | |
| Dominant | 1.71 | 1.33–3.56 | <0.001a | |||||||||
| Wei et al.16 | Iraq, India, China, Korea, Romania, Italy, Australia, China, Denmark, Germany, Belgium, Ireland, Japan, North America, Sweden, Austria, Poland, UK, Malaysia, Tunisia, USA, Turkey, Brazil, Hungary, Morocco, Croatia, Turkey, South Korea | 29,637/ 30,880 | rs1801133 | 229A > V | 677C > T | Multiethnic | allelic | 1.32 | 1.25–1.40 | <0.001 | ||
| Recessive | 1.41 | 1.31–1.51 | <0.001 | |||||||||
| Over-dominant | 1.11 | 1.05–1.17 | <0.001 | |||||||||
| Asian | Allelic | 1.43 | 1.34–1.52 | <0.001 | ||||||||
| Recessive | 1.47 | 1.35–1.59 | <0.001 | |||||||||
| Over-dominant | 1.18 | 1.11–1.25 | <0.001 | |||||||||
| Caucasian | Recessive | 1.28 | 1.10–1.48 | 0.002 | ||||||||
| 5674/6793 | rs1801131 | 429E > A | 1298A > C | Multiethnic | Allelic | 1.21 | 1.05–1.38 | 0.006 | ||||
| Recessive | 1.45 | 1.13–1.87 | 0.004 | |||||||||
| Allelic | 1.38 | 1.26–1.53 | <0.001 | |||||||||
| Recessive | 1.86 | 1.42–2.43 | <0.001 | |||||||||
| Over-dominant | 1.27 | 1.10–1.48 | 0.002 | |||||||||
| 29,506/ 32,628 | ApoE | Plasma lipid metabolism | rs429358 | 130C > R | ɛ4 | Multiethnic | Allelic | 1.58 | 1.40–1.79 | <0.001 | ||
| Asian | Allelic | 1.7 | 1.47–1.97 | <0.001 | ||||||||
| Caucasian | Allelic | 1.27 | 1.01–1.60 | 0.04 | ||||||||
| Chen and Hu21 | China | 6190/6248 | Chinese | Additive | 2.19 | 1.90–2.52 | <0.001 | |||||
| Dominant | 2.41 | 2.00–2.89 | <0.001 | |||||||||
| Wei et al.16 | Iraq, India, China, Korea, Romania, Italy, Australia, China, Denmark, Germany, Belgium, Ireland, Japan, North America, Sweden, Austria, Poland, UK, Malaysia, Tunisia, USA, Turkey, Brazil, Hungary, Morocco, Croatia, Turkey, South Korea | 10,198/ 10,821 | eNOS | Endothelial and vascular health | rs1799983 | 298D > E | +894G > T | Multiethnic | Allelic | 1.25 | 1.13–1.38 | 0.003 |
| Recessive | 1.29 | 1.10–1.50 | 0.001 | |||||||||
| Over-dominant | 1.15 | 1.03–1.29 | 0.01 | |||||||||
| Asian | Allelic | 1.45 | 1.23–1.69 | <0.001 | ||||||||
| Recessive | 1.56 | 1.16–2.08 | 0.003 | |||||||||
| Over-dominant | 1.26 | 1.06–1.49 | 0.008 | |||||||||
| Caucasian | Recessive | 1.2 | 1.00–1.43 | 0.001 | ||||||||
| 6501/ 14,101 | AGT | Blood pressure modulation | rs699 | 235M > T | Multiethnic | allelic | 1.33 | 1.17–1.50 | <0.001 | |||
| Recessive | 1.41 | 1.22–1.64 | <0.001 | |||||||||
| Asians | Allelic | 1.4 | 1.24–1.59 | <0.001 | ||||||||
| Recessive | 1.5 | 1.28–1.75 | 0.002 | |||||||||
| 9022/ 9792 | PON1 | Plasma lipid metabolism | rs662 | 192Q>R | Multiethnic | Allelic | 1.14 | 1.06–1.23 | <0.001 | |||
| Recessive | 1.15 | 1.02–1.29 | 0.02 | |||||||||
| Asian | Allelic | 1.15 | 1.04–1.28 | 0.005 | ||||||||
| Recessive | 1.16 | 1.01–1.33 | 0.04 | |||||||||
| Dominant | 0.81 | 0.70–0.94 | 0.005 | |||||||||
| Casas et al.4 | Various | 4588/13,798 | Factor V | Coagulation | rs6025 | 506R > Q | Leiden | Caucasian | Dominant | 1.33 | 1.12–1.58 | 0.03 |
| Au et al.26 | Turkey, Hungary, China | 945/464 | APOA5 | Plasma lipid modulation | rs3135506 | 19S > W | 56C > G | Multiethnic | Over-dominant | 1.97 | 1.23–3.16 | 0.005 |
| allelic | 1.77 | 1.15–2.73 | 0.009 | |||||||||
| Brazil, Russia, Serbia, China | 696/987 | APOB | rs1042031 | 4181E > K | Multiethnic | Over-dominant | 1.88 | 1.46–2.42 | <0.001 | |||
| Allelic | 1.66 | 1.35–2.05 | <0.001 | |||||||||
| Germany, China, Russia, UK | 2873/3146 | ABCA1 | rs2230806 | 219R > K | Multiethnic | Dominant | 1.31 | 1.16–1.48 | <0.001 | |||
| 2052/2106 | Asian | Dominant | 1.37 | 1.19–1.57 | <0.001 | |||||||
| Wang et al.27 | China | 895/883 | APOC3 | rs4520 | *silent* | Silent T-C | Chinese Han | Recessive | 2.05 | 1.28–3.29 | <0.01b | |
| rs5128 | *in 3′-UTR* | Recessive | 0.2 | 0.09–0.43 | <0.01b | |||||||
| Yamada et al.28 | Japan | 1575/9210 | TMPRSS7 | Blood pressure modulation | rs147783135 | *truncated protein* | R692* | Japanese | Additive 1 | 0.38 | 0.17–0.74 | 0.0029c |
| Dominant | 0.37 | 0.16–0.72 | 0.0024c | |||||||||
| PDIA5 | Coagulation | rs2292661 | 391T > M | Additive 1 | 0.35 | 0.14–0.76 | 0.0054c | |||||
| Dominant | 0.35 | 0.14–0.76 | 0.0054c | |||||||||
| CYP4F12 | Inflammation | rs191885206 | 402C>R | Additive 1 | 2.6 | 1.30–4.93 | 0.0082c | |||||
| Dominant | 2.6 | 1.30–4.93 | 0.0082c | |||||||||
| Ma et al.29 | USA, China | 2108/1924 | RAGE | rs2070600 | 82G>S | Multiethnic | Allelic | 1.32 | 1.05–1.65 | <0.05 | ||
| Recessive | 2.2 | 1.74–2.78 | <0.05 | |||||||||
| Dominant | 1.2 | 1.04–1.38 | <0.05 | |||||||||
| Misra et al.34 | India, Germany, UK | 713/948 | PSMA6 | rs1048990 | *in 5′-UTR* | 8C>G | Multiethnic | Recessive | 0.25 | 0.08–0.72 | 0.01 | |
| Kim et al.35 | South Korea | 523/400 | miR-200b | Coagulation | rs7549819 | *non-coding RNA* | South Korean | Recessive | 0.475 | 0.239–0.944 | 0.034d | |
| Kovalevа et al.31 | Russia | 1200/500 | HIF1a | Erythropoiesis | rs11549465 | 582P > S | 1772C > T | Russian | Not specified | 1.603 | 0.01 | |
| Zhu et al.32 | China | 1102/1610 | NLRP3 | Inflammation | rs10754558 | *in 3′-UTR* | Chinese Han | Additive | 1.60 | 1.41–1.73 | <0.001 | |
| Dominant | 1.81 | 1.57–2.11 | <0.001 | |||||||||
| Recessive | 2.01 | 1.65–2.45 | <0.001 | |||||||||
| Sung et al.33 | Taiwan | 914/746 | ALDH2 | Ethanol metabolism | rs671 | 487E > K | *2 allele | Taiwanese | Recessive | 1.84 | 1.10–3.08 | 0.02 |
| Tong et al.36 | China | 648/648 | PPARγ | Plasma lipid modulation | rs1801282 | 12P > A | East Asian | Additive | 0.542 | 0.346–0.850 | 0.008e | |
| Dominant | 0.555 | 0.356–0.864 | 0.009e | |||||||||
| Variants associated with large-vessel ischemic stroke | ||||||||||||
| Malik et al.30 | Australia, Germany, UK, South Asia | 3127/9778 | SERPINA1 | Inflammation | rs6647 | 237V > A | Multiethnic | Not specified | 1.22 | 1.13–1.31 | <0.001 | |
| 3127/9779 | HDAC9 | Epigenetics | rs2023938 | *in 3′-UTR* | Multiethnic | Not specified | 1.28 | 1.16–1.40 | <0.002 | |||
| Monogenic disorders associated with ischemic stroke | ||||||||||||
| Christoffersen et al.38 | Denmark | 2020/57,170 | WRN | DNA repair | rs1346044 | 1367C > R | Danish | Recessive | 1.14 | 1.04–1.25 | 0.02f | |
Adjusted for age, gender, parental smoking, and drinking.
Adjusted for age, gender, BMI, diabetes mellitus, hypertension, history of smoking, history of alcohol use, family history of stroke and hyperlipidemia.
Adjusted for age, sex, hypertension, and diabetes mellitus. Based on Bonferroni's correction.
Derived by multivariate logistic regression adjusted for age, sex, hypertension, diabetes mellitus, hyperlipidemia, and current smoking.
Multivariate logistic regression analysis adjusted for sex, age, hypertension, diabetes, smoking, alcohol drinking, tea drinking, BMI, and waist-hip ratio.
Adjusted for age, sex, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, BMI, hypertension, diabetes, cumulated smoking exposure, heavy alcohol consumption, use of lipid lowering therapy, and physical activity.
OR: odds ratio; CI: confidence interval.
Table 2.
Cerebrovascular occlusive disease.
| Variants associated with study-defined common carotid atherosclerosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country(ies) of study | Patients | Gene | Relevant function | Reference SNP # | Amino acid change | Common name | Associated population | Inheritance model | OR | CI at 95% | p |
| Zhao et al.48a | Japan | 1536 | RYR3 | Endothelial and vascular health | rs2229116 | 731I > V | Japanese | Dominant | 1.45b | 1.13–1.86 | 0.003b | |
| Japanese males | Dominant | 1.63b | 1.17–2.27 | 0.004b | ||||||||
| Lan et al.51 | Taiwan | 593 | MIF | Inflammation | rs755622c | Taiwanese | Dominant | 2.03 | 1.24–3.33 | 0.005 | ||
| Si et al.52d | Japan | 941 | PLA 2 R | rs3749117e | 292M > V | Japanese | Recessive | 1.88 | 1.38–2.86 | 0.0085 | ||
| rs35771982e | 300H > D | |||||||||||
Study defined atherosclerosis as that of the top 25% of atheroma:intimal area; study with statistical power only for elderly.
p-value after adjustment for sex, history of hyperlipidemia, hypertension, diabetes mellitus, drinking, and smoking.
Significant association only found with what study characterized as “severe atherosclerosis” according to study-specific formula that incorporated number of plaques and degree of lumen occlusion.
Study defined atherosclerosis as maxIMT ≥ 1.1 mm.
Polymorphisms show 100% linkage.
SNP: single nucleotide polymorphism; OR: odds ratio; CI: confidence interval.
Table 3.
Subarachnoid hemorrhage.
| Variants associated with subarachnoid hemorrhage | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country(ies) of study | Patients/ controls | Gene | Relevant function | Reference SNP # | Amino acid change | Common name | Associated population | Inheritance model | OR | CI at 95% | p |
| Yamada et al.28 | Japan | 265/9158 | COL17A1 | Inflammation | rs117564807 | 919D > N | Japanese | Dominant | 2.2 × 10−8 a | 0–3.7 × 10−4 a | 0.0009a | |
| Additive 1 | 2.2 × 10−8 a | 0–1.1 × 10−4 a | 0.0009a | |||||||||
After adjustment for age, sex, and prevalence of hypertension.
Table 4.
Cerebral aneurysms.
| Variants associated with cerebral aneurysms | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country(ies) of study | Patients/ Controls | Gene | Relevant function | Reference SNP # | Amino acid change | Common name | Associated population | Inheritance model | OR | CI at 95% | p |
| Alg et al.62 | Finland, Netherlands, Germany, pan-Europe, Japan | 4370/14,181 | RRBP1 | Unknown | rs1132274 | 891R > L | Multiethnic | Additive | 1.19 | 1.11–1.28 | <0.001 | |
| China, Japan, Poland | 892/1029 | SERPINA3 | Endothelial and vascular health | rs4934 | 9A > T | Multiethnic | Additive | 1.27 | 1.07–1.50 | 0.0002 | ||
| Dominant | 2.22 | 1.68–2.94 | <0.00001 | |||||||||
| China, Japan, Korea | 812/806 | COL1A2 | rs42524 | 549A > P | East Asian | Additive | 1.69 | 1.11–2.57 | 0.001 | |||
| Dominant | 1.77 | 1.14–2.75 | 0.0009 | |||||||||
| China | 546/2235 | COL3A1 | rs1800255 | 698A > T | Chinese | Additive | 1.4 | 1.14–1.72 | <0.0001 | |||
| Dominant | 1.55 | 1.21–2.00 | <0.00001 | |||||||||
| Yang et al.64 | China, Denmark, Germany, India, Japan, Korea, USA | 1819/1893 | eNOS | rs2070744 | *in 5′-UTR | −786T > C | East Asian | Dominant | 1.277 | 1.019–1.600 | <0.05 | |
| Codominant | 1.294 | 1.025–1.634 | <0.05 | |||||||||
| Fan et al.65 | China | 976/1200 | WWOX | Tumor suppressor | *copy number variation* | CNV-67048 | Chinese | Additive | 1.35 | 1.16–1.57 | 0.000118 | |
| Hu et al.66 | Brazil, Korea, China, Japan, USA | 850/1028 | ENG | Endothelial and vascular health | rs1800956a | 366D > H | Multiethnic | Recessive | 0.65 | 0.45–0.94 | <0.05 | |
Minor allele in Asian populations; major allele in Caucasian and African populations.
Table 5.
Spontaneous intracerebral hemorrhage.
| Study | Country(ies) of study | Patients/ controls | Gene | Relevant function | Reference SNP # | Amino acid change | Common name | Associated population | Inheritance model | OR | CI at 95% | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variants associated witd spontaneous intracerebral hemorrhage | ||||||||||||
| Chen and Hu21 | China | 2018/2143 | APOE | Plasma lipid modulation | rs429358 | 130C > R | ɛ4 | Chinese | Dominant | 2.41 | 1.68–3.47 | 0 |
| Additive | 2.08 | 1.57–2.75 | 0 | |||||||||
| Yamada et al.28 | Japan | 673/9158 | STYK1 | Endothelial and vascular health | rs138533962 | 379R > C | Japanese | Dominant | 111.3 | 33.0–694.6 | <0.001 | |
| Gao et al.72 | China, India, Japan, Turkey | 1828/4067 | MTHFR | Endothelial and vascular health (homocysteine metabolism) | rs1801133 | 229A > V | 677C > T | Multiethnic | Dominant | 1.41 | 1.12–1.78 | 0.003 |
| Recessive | 1.9 | 1.42–2.55 | <0.001 | |||||||||
| Allelic | 1.38 | 1.17–1.62 | <0.001 | |||||||||
| Variants associated with lobar intracerebral hemorrhage | ||||||||||||
| Biffi et al.71 | Austria, Poland, Sweden, USA | 931/3744 | APOE | Plasma lipid modulation | rs7412 | 176R > C | ɛ2 | Caucasian | Allelic | 1.82 | 1.50–2.23 | <0.001 |
| rs429358 | 130C > R | ɛ4 | Allelic | 2.2 | 1.85–2.63 | <0.001 | ||||||
| Variants associated with deep intracerebral hemorrhage | ||||||||||||
| Biffi et al. 71 | Austria, Poland, Sweden, USA | 1085/3657 | APOE | Plasma lipid modulation | rs429358 | 130C > R | ɛ4 | Caucasian | Allelic | 1.21 | 1.08–1.36 | 0.00026 |
Table 6.
Moyamoya disease.
| Variants associated with moyamoya disease | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country(ies) of study | Patients/ controls | Gene | Relevant function | Reference SNP # | Amino acid change | Common name | Associated population | Inheritance model | OR | CI at 95% | p |
| Ma et al.92 | Japan, Korea, China | 421/1214 | RNF213 | Endothelial and vascular health | rs112735431 | 4810R > K | East Asian | Dominant | 92.03 | 54.06–156.65 | <0.00001 | |
| Recessive | 24.58 | 4.50–134.32 | 0.0002 | |||||||||
| Japan | 398/765 | 4859R > K | Japanese | Dominant | 157.53 | 85.37–290.70 | <0.00001 | |||||
| Recessive | 20.08 | 3.73–108.08 | 0.0005 | |||||||||
SNP: single nucleotide polymorphism; OR: odds ratio; CI: confidence interval.
Ischemic cerebrovascular disease
Ischemic stroke
Ischemic stroke constitutes approximately 87% of strokes in the United States. It is the most thoroughly studied cerebrovascular disease from a cerebrovascular neurogenomics perspective (Table 1) and entails a strong genetic component. Numerous alleles have been linked to ischemic stroke but few have been confirmed across ethnicities.
Variants associated with ischemic stroke
The most well-studied susceptibility gene for ischemic stroke, MTHFR, encodes methylenetetrahydrofolate reductase, a critical enzyme in homocysteine metabolism. In homozygous individuals, the 677C > T variant (rs1801133) reduces MTHFR activity to 30%,15,16 and is associated with hyperhomocysteinemia,15 an established risk factor for ischemic stroke.17 In the largest meta-analysis to date consisting of nearly 30,000 Asians and Caucasians, Wei et al.16 found a strong association between the 677C > T variant and ischemic stroke. This finding is consistent with other meta-analyses and was replicated individually in both Asian and Caucasian populations,16 although further evidence is needed for the populations of African descent.18 The relationship between the 677C > T variant and ischemic stroke has also been replicated for both small and large-vessel subtypes.18,19 In their Asian and Caucasian study population, Wei et al.16 were also able to associate ischemic stroke with the 1298A > C variant (rs1801131) of the same gene, which reduces MTHFR activity by an estimated 40%. In a Chinese cohort, He et al.20 discovered a relationship between ischemic stroke and a third MTHFR variant (rs868014), which causes a quantitative decrease in MTHFR.
ApoE, another well-studied susceptibility gene, encodes apolipoprotein E, a cholesterol transporter and ligand that modulates plasma lipid levels.16 In their multiethnic analysis, Wei et al.16 detected an association between one of the minor alleles, ɛ4 (rs429358), and ischemic stroke. Although these findings were replicated individually in both Asian and Caucasian populations, the data suggest that the ɛ4 allele is significantly more threatening in Asians,16 particularly in Chinese individuals.21
eNOS appears to be an additional high-yield susceptibility gene for ischemic stroke. It encodes endothelial nitric oxide synthase, an enzyme on the vascular endothelium that produces nitric oxide, a substance that has various vaso-protective functions including inhibiting platelet aggregation and inducing vasodilation.22 Wei et al.16 included large meta-analyses on three distinct eNOS variants. The +894G > T allele (rs1799983) was evidenced to contribute greatly to ischemic stroke risk in both Asians and Caucasians. Although there is evidence supporting an association between another coding mutation, −786T > C (rs2070744), and ischemic stroke,23 the most extensive meta-analysis on this relationship elucidated no such association.16
Wei et al.16 identified two additional polymorphisms as risk alleles for ischemic stroke: 235M > T (rs699) of AGT and 192Q > R (rs662) of PON1. AGT encodes angiotensinogen, a blood pressure modulator, while PON1 encodes paraoxonase 1, an enzyme involved in lipid metabolism that has anti-atherosclerotic effects. Wei et al.16 identified both 235M > T and 192Q > R as contributors to ischemic stroke risk in their multiethnic study population and Asian sub-population.
Factor V Leiden (rs6025), a relatively common variant in Caucasians, encodes a mutant protein missing an arginine at a key position (506R > Q). In the wild type, this arginine facilitates its interaction with protein C, which functions to cleave Factor V.24 This conferred resistance to cleavage promotes a hypercoagulable state.24 It is estimated that around 5% of Caucasians are heterozygous for Factor V Leiden, but it is uncommon in other races.25 The largest meta-analysis was performed by Casas et al.4 In this study of nearly 5000 subjects, all Caucasians, a strong contribution to ischemic stroke risk was indicated.4
Au et al.26 identified three variants involved in lipid metabolism associated with ischemic stroke in a multiethnic meta-analysis. Among these, the ApoA5 variant 56C > G (rs3135506) was linked to ischemic stroke. The study further elucidated the role of the ApoB 4181E > K variant (rs1042031) in ischemic stroke. The 219R > K variant (rs2230806) of ABCA1 was found to influence ischemic stroke risk in the multiethnic population as well as an Asian sub-population. ABCA1 encodes ATP-binding cassette transporter 1, a protein which collaborates with other apolipoproteins in lipid transport.26
Wang et al. identified two additional apolipoprotein variants in ApoC3 in association with ischemic stroke in a large Chinese Han cohort. ApoC3 encodes apolipoprotein C3, a protein component of very low-density lipoproteins that functions to maintain blood lipid levels.27 One of these variants (rs4520) involves a silent T-C substitution and was suggested to confer increased risk for ischemic stroke in the Chinese Han population; the other, a 3′UTR (rs5128) polymorphism, was actually identified as a protective factor for ischemic stroke in this population.27
In an exome-wide association study of a Japanese cohort, Yamada et al.28 identified three different coding variants associated with ischemic stroke: two protecting alleles involving TMPRSS7 and PDIA5 and a predisposing allele in CYP4F12. TMPRSS7 encodes a serine protease involved in degradation of the extracellular membrane.28 The R692* variant (rs3212335) produces a truncated protein that decreases risk of ischemic stroke incidence.28 PDIA5, which encodes a disulfide isomerase involved in protein folding and thiol-disulfide reactions, has been associated with coagulation.28 The 391T > M polymorphism (rs2292661) was labeled an additional protective factor.28 CYP4F12 encodes a P450 enzyme thought to be an endoplasmic reticulum protein involved in the metabolism of inflammatory mediators.28 The 402C > R polymorphism (rs191885206) was correlated with increased risk of ischemic stroke.28
In a meta-analysis of over 2000 American and Chinese individuals, Ma et al.29 indicated the 82G > S variant (rs2070600) of RAGE as a risk marker for ischemic stroke. RAGE encodes the receptor for AGE, a protein involved in immune defense whose over-activity has been linked with formation of atherosclerotic plaques.29 The polymorphism causes a deviation at codon 82, a key position in AGE-RAGE interaction.29
In an exome-wide association study, Malik et al.30 identified two common polymorphisms in SERPINA1 and HDAC9 that lead to increased risk of ischemic stroke, specifically in the large vessels. The SERPINA1 237V > A polymorphism (rs6647) produces a modified α-1 antitrypsin, an enzyme that antagonizes neutrophil elastase to attenuate the damage associated with inflammation. The HDAC9 variant (rs2023938) involves the 3′UTR and contributes to epigenetic modulation. It is thought that both of these variants increase stroke risk by promoting atherosclerosis.30
Additional exonic variants evidenced to confer risk of ischemic stroke are shown in Table 1 and include the genes HIF1a (rs11549465),31 NLRP3 (rs1810754558),32 ALDH2 (rs671).30,33 Also presented in Table 1 are additional variants that have been shown to be protective against ischemic stroke. They include PSMA6 (rs1048990),34 miR-200b (rs7549819),35 and PPARγ (rs1801282).36
Monogenic disorders associated with ischemic stroke
Monogenic diseases are characterized by high incidence of early-onset, small-vessel stroke, and significant family history.37 As they are considerably less common, large cohort studies are difficult to conduct. Only one study qualified for inclusion. In a Danish study, Christoffersen et al.38 analyzed the relationship between ischemic stroke and Werner syndrome, an autosomal recessive condition caused by a variety of nonsense mutations in WRN and characterized by severely premature aging. WRN encodes a helicase involved in DNA maintenance and repair.38 Christoffersen et al.38 detected a significant, direct correlation between the 1367C > R variant (rs1346044) and ischemic stroke incidence.
The following are monogenic disorders with established correlation to ischemic stroke, but without individual variants that qualified for inclusion. CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is a small-vessel disease due to gain-of-function mutations in NOTCH3,3 most of which are missense involving a cysteine residue in exons 2–6.5 NOTCH3 encodes a transmembrane protein5 and the addition or loss of cysteine residues in key positions of the extracellular domain result in interruption of the vascular basal membrane,3 which leaves these individuals prone to ischemic events.3,5 In the only study of its kind, Chong et al.37 were unable to identify a specific NOTCH3 variant with statistical significance. Its autosomal recessive counterpart, CARASIL is a similar condition as it also predisposes individuals to ischemic episodes. Although it is less thoroughly understood, recent studies have suggested its association with loss-of-function mutations of HTRA1, which encodes a serine protease that antagonizes TGF-β signaling.3 Homocystinuria is caused by a loss-of-function mutation in CBS, which encodes cystathione β-synthase, an enzyme involved in methionine metabolism.5 The resultant elevated plasma homocysteine levels confer an increased risk of ischemic stroke.5
Cerebrovascular occlusive disease: Intracranial and extracranial atherosclerosis
Variants associated with cerebrovascular occlusive disease
Cerebrovascular occlusive disease is a condition of increased cerebral vessel wall thickness of either intracranial vessels, most commonly the intracranial portion of the internal carotid artery, vertebrobasilar artery, and cerebral arteries,39 or the extracranial vessels, the common carotid artery and the extracranial segments of the internal carotid artery.3 Both intracranial and extracranial (carotid) atherosclerosis significantly heighten the risk of ischemic stroke.39 Overall, intracranial atherosclerosis appears to be more common; however, prevalence varies significantly amongst different ethnicities.39 Intracranial stenosis is more typical in people of African, Asian, and Hispanic descent, while Caucasians are particularly susceptible to ischemic stroke due to extracranial atherosclerosis.39 There is evidence to suggest RNF213 as a susceptibility gene for intracranial atherosclerosis in East Asians40 and the 4810R > K allele as a high-risk allele, but neither results are significant enough to be discussed further here.41
Genetic contribution to extracranial atherosclerosis has attracted more attention (Table 2). It is usually measured by levels of carotid plaque and carotid intima media thickness (cIMT). Both have a direct correlation with ischemic stroke incidence, but it is thought that development of each is due to its own unique risk alleles and pathophysiology.3 The fact that traditional vascular risk factors roughly account for only 20% of variance in cIMT and carotid plaque indicates a strong genetic component to extracranial atherosclerosis.3 While most of the known variants linked to extracranial atherosclerosis are non-coding, some exonic variants have been reported. Mutations included in this section met one of three criteria: association with study-defined atherosclerosis, association with cIMT, or association with levels of carotid plaque.
Due to the association between ApoE and ischemic stroke, it is not surprising that ApoE is also a susceptibility gene for extracranial atherosclerosis. The ɛ2 allele (rs7412) was associated with decreased cIMT in a multiethnic study population and is thus indicated as a protective factor for extracranial atherosclerosis;42 this association has been replicated in Caucasians,42 African American,42 and Koreans.43 The ɛ2 minor allele was also found to be inversely correlated with carotid plaque formation in three separate studies of Caucasian populations (Australian,44 Dutch,45 and French46). The Australian study only found an association in women, but its power was undermined by a small sample size.44 Contrarily, the ɛ4 allele (rs429358) has been identified as a risk factor for increased cIMT in African populations47 and a risk factor for carotid plaques in the French population.46
Other variants with considerable evidence for association with cerebrovascular occlusive disease involve RYR3, PCKS9, and GJA4. RYR3 encodes a protein on the membrane of the sarcoplasmic reticulum responsible for the release of calcium into the cytoplasm and, ultimately, regulation of vascular tone.48 Zhao et al.48 identified the 731I > V polymorphism (rs2229116) of RYR3 as a risk factor for carotid atherosclerosis in elderly Japanese individuals – the association was replicated in males only. PCKS9 encodes proprotein convertase subtilisin-like kexin type 9, a serine protease involved in lipid metabolism. Its 670E > G variant (rs505151) was found by Norata et al.49 to be associated with increased cIMT in an American Caucasian study population. GJA4 encodes connexin37, a protein involved in vascular homeostasis; decreased activity of connexin37 has been associated with increased atherosclerotic risk in Taiwanese populations. Leu et al.50 found its 319P > S mutant (rs1764391) to have a direct correlation with cIMT.
Additional mutations evidenced to be associated with extracranial atherosclerosis, all conferring risk, include MIF (rs755622),51 PLA2R (rs3749117 and rs35771982),52 and MMP9 (rs17576).53
Monogenic disorders associated with cerebrovascular occlusive disease
Fabry disease, an X-linked recessive disorder caused by a loss-of-function mutation in α-galactosidase A, is understood to promote cerebrovascular atherosclerosis.3,5 Decreased activity of α-galactosidase A, a lysosomal hydrolase, results in the buildup of glycosphingolipids in cells.3,5 Build-up in vascular endothelial cells is thought to contribute to ischemic stroke.3,5 However, no particular polymorphisms met criteria for inclusion in this study.
Hemorrhagic cerebrovascular disease
Hemorrhagic cerebrovascular disease constitutes the other umbrella of cerebrovascular diseases discussed in this review. Comprising about 13% of strokes in the United States,54 hemorrhagic cerebrovascular conditions include subarachnoid hemorrhage, cerebral aneurysm, spontaneous lobar and deep intracerebral hemorrhage, arteriovenous malformation, and cavernous malformation.
Subarachnoid hemorrhage
Variants associated with subarachnoid hemorrhage
Subarachnoid hemorrhage has the highest mortality, approximately 50%, of any stroke.55 Due to the fact that more than 90% of subarachnoid hemorrhage cases are caused by ruptured cerebral aneurysm,56 predisposition to cerebral aneurysm should also be assumed to precipitate subarachnoid hemorrhage. According to Korja et al.,57 risk for subarachnoid hemorrhage is primarily conferred through lifestyle factors and involves a modest genetic contribution (Table 3).
| Variants associated with common carotid intima media thickness (cIMT) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shin et al.43 | Korea | 10,145 | APOE | Plasma lipid modulation | rs7412 | 176R > C | ɛ2 | Korean | Codominant | −0.011 | −0.015 to −0.007 | 0.005a |
| Natarajan et al.42 | USA, Scotland, Germany, Netherlands, Belgium, Iceland, Finland | 52,869 | Multiethnic | Additive | <0.001 | |||||||
| 44,963 | Caucasian | Additive | <0.001 | |||||||||
| 7906 | African American | Additive | <0.001 | |||||||||
| Volcik et al.47 | USA | 3187 | rs429358 | 130C > R | ɛ4 | African American | Dominant | +0.010 | +0.003 to +0.017 | 0.040b | ||
| Norata et al.49 | USA | 1541 | PCSK9 | rs505151 | 670E > G | Caucasian | Dominant | +0.012 | −0.106 to +0.182 | 0.04 | ||
| Leu et al.50 | Taiwan | 3330 | GJA4 | Endothelial and vascular health | rs1764391 | 319P > S | Taiwanese | Recessive | <0.05c | |||
| Additive | <0.05c | |||||||||||
p-value after adjustment for age, sex, body mass index, smoking, diabetes, hypertension, total cholesterol, HDL cholesterol.
p-value after adjustment for age, sex, smoking, diabetes, hypertension status, body mass index, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and total triglycerides.
p-value after adjustment for age, sex, BMI, blood pressure, waist circumference, smoking, lipid, profiles, history of hypertension, and diabetes.
While it is understood that a genetic component to subarachnoid hemorrhage risk exists and while there have been efforts to identify these genetic markers, few studies have had sufficient statistical power to indicate associations between specific exonic mutations and subarachnoid hemorrhage. The most note-worthy finding in this area was discovered by Yamada et al. in a study previously discussed in detail due to its outcomes relevant to ischemic stroke. Yamada et al. discovered a strong link between a COL17A1 polymorphism, 919D > K (rs117564807), and incidence of subarachnoid hemorrhage in their Japanese study population, less than 500 cases but still included in Table 3.28 COL17A1 encodes a domain of collagen 17, involved in the anchoring of the epidermis into the basement membrane but also believed to contribute to cerebrovascular inflammation.28 The 919D > K allele was found to be highly protective against incidence of subarachnoid hemorrhage.28
Monogenic disorders associated with subarachnoid hemorrhage
The following are monogenic disorders with known association with subarachnoid hemorrhage, but without particular polymorphisms that qualified for inclusion. Neurofibromatosis 1, a disease characterized by defective production of neurofibromin, is thought to be caused by deregulated signaling of Ras proteins, which play a critical role in vascular morphology.58 Neurofibromatosis has been observed to increase risk of both subarachnoid hemorrhage and moyamoya disease.58
Ehlers-Danlos syndrome type IV is an autosomal dominant disorder caused by aberrant COL3A1, type III procollagen. Mutation usually occurs in the glycine residues in the three amino acid pattern of collagen and is often de novo.5,59 Ehlers-Danlos syndrome is understood to increase the risk of both cerebral aneurysm formation and subarachnoid hemorrhage.59 Fabry disease, previously discussed for its association with cerebrovascular occlusive disease, is also understood to predispose individuals to subarachnoid hemorrhage.60
Cerebral aneurysms
Variants associated with cerebral aneurysms
It is evidenced that cerebral aneurysms are present in approximately 2% of the general population.61 While cerebral aneurysms are generally asymptomatic, approximately 0.7%–1.9% of cerebral aneurysms lead to subarachnoid hemorrhage.62 A considerable amount of research exists dedicated to identifying genetic markers for cerebral aneurysm and, appropriately, various variants in the exome have indeed been linked to cerebral aneurysm formation (Table 4).
| Variants associated with presence of carotid plaques | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slooter et al.45 | Netherlands | 1194 | APOE | Plasma lipid modulation | rs7412a | 176R > C | ɛ2 | Dutch | Codominant | 0.6 | 0.4–0.8 | 0.003b |
| Beilby et al.44 | Australia | 551 | rs7412 | Caucasian | Codominant | 0.4c | 0.17–0.91 | 0.03 | ||||
| Debette et al.46d | France | 5856 | rs7412 | French | Dominant | 0.79 | 0.66–0.95 | 0.01c | ||||
| rs429358 | 130C > R | ɛ4 | Recessive | 2.12 | 1.27–3.53 | 0.004e | ||||||
| Panayiotou et al.53 | Cyprus | 762 | MMP9 | Endothelial and vascular health | rs17576 | 279R > Q | Cypriot | Dominant | 1.5 | 1.08–2.10 | 0.016f | |
Significant association only found with presence of more than three carotid plaques.
p-value after adjustment for age, sex, and total and HDL cholesterol.
OR from generalized linear model after adjustment for age, LDL-C level, smoking pack-years, hypertension, systolic blood pressure, history of all diabetes, and history of vascular disease.
Study only included individuals age 65 and older.
p-value after adjustment for −219G/T SNP, vascular risk factors, history of vascular event, and center.
p-value after adjustment for age, sex, smoking in pack-years, hypertension, diabetes, body mass index, and total cholesterol.
A multiethnic meta-analysis by Alg et al.62 identified four single-nucleotide polymorphisms resulting in missense mutations in RRBP1, SERPINA3, COL1A2, and COL3A1. RRBP1 encodes ribosome binding protein-1. No potential mechanism for its involvement with cerebral aneurysm formation is yet obvious, but the rs1132274 variant was shown to be strongly associated in a large multiethnic population.63 SERPINA3, COL1A2, and COL3A1 all encode proteins that function in the extracellular matrix with COL1A2 and COL3A1 contributing to the tensile strength of the arterial walls.55,63 Thus, it is thought that their variants, 9A > T (rs4934), 549A > P (rs42524), and 698A > T (rs1800255), respectively, may confer susceptibility to cerebral aneurysm formation by compromising the arterial wall.62
In a large meta-analysis collecting studies from various countries aimed toward discerning the relationship between different eNOS alleles and cerebral aneurysm formation, Yang et al.64 concluded that the −786T > C polymorphism (rs2070744) in the 5′UTR, distinct from the +894G > T variant associated with ischemic stroke, confers risk of cerebral aneurysm formation in eastern Asians but not Caucasians. In a study of Chinese patients by Fan et al.,65 a coding copy number variation (CNV-67048) in WWOX was identified as a risk allele for cerebral aneurysm formation. WWOX encodes human WW domain-containing oxidoreductase, a tumor suppressor protein that functions in protein degradation, transcription, and RNA splicing among other roles.65 Hu et al.66 identified polymorphisms of ENG, a gene encoding a vessel wall maintenance protein called endoglin, as a risk factor in aneurysm pathogenesis. The 366D > H polymorphism (rs1800956), which is the major allele in Caucasians and Africans, but the minor allele in Asians,67 was found to be protective against cerebral aneurysm formation.66
The majority of relevant studies on variants associated with cerebral aneurysm appear to be focused on East Asian populations. There is little evidence on aneurysm-associated variants for non-east Asian races; although there is some support for the aforementioned SERPINA3 and COL1A2 polymorphisms as risk factors for cerebral aneurysm formation in Caucasians.62,68,69
Monogenic disorders associated with cerebral aneurysms
The following are monogenic disorders known to precipitate cerebral aneurysm formation, but without individual variants that met the study inclusion criteria. Marfan syndrome is an autosomal dominant disorder caused by various gain-of-function mutations in FBN1,5 which encodes fibrillin 1.70 Mutation in this extracellular matrix protein can result in damage of the vessel wall.5 Although neurovascular events are rare, Marfan syndrome is thought to be associated with cerebral aneurysm.5 Additionally, as previously discussed, Ehlers-Danlos syndrome predisposes individuals to both cerebral aneurysm formation and subarachnoid hemorrhage.59 Alagille’s syndrome similarly predisposes individuals to cerebral aneurysm formation, but will be discussed later for its association with spontaneous intracerebral hemorrhage.58 Autosomal dominant polycystic kidney disease involves a variant of either polycystin-1 or polycystin-2, both of which are transmembrane proteins that have roles in endothelial metabolism.59 The disease is usually acquired via a second-hit mechanism which confers risk of cerebral aneurysm.59 Loeys-Dietz syndrome is a connective tissue disorder that propagates in a dominant fashion, but also has a very high rate of de novo mutation (roughly two-thirds of cases).59 It can be caused by loss-of-function mutations of three known proteins: TGF-β receptor type I, TGF-β receptor type II, and SMA- and MAD-related protein 3. All three mutations result in rampant TGF-β signaling which is thought to promote formation of aneurysms as 20% of patients with Loeys-Dietz syndrome have reported aneurysms in the neck or intracranial arteries.59
Spontaneous intracerebral hemorrhage
Variants associated with spontaneous intracerebral hemorrhage
Spontaneous intracerebral hemorrhage accounts for approximately 10% of strokes in the United States. There are two types differing in both location and pathology. Lobar intracerebral hemorrhage involves the cortices of the cerebrum and amyloid accumulation has been identified as the predominate pathophysiology.55 Contrarily, deep intracerebral hemorrhage involves the basal ganglia, thalamus, and cerebellum, and its surrounding regions and is usually caused by hypertension.55
It is estimated that heritability accounts for nearly half of total intracerebral hemorrhage risk.55 Three coding variants (Table 5), involving ApoE and MTHFR, are supported with particularly strong evidence to be associated with intracerebral hemorrhage.55 In a large study of Caucasians, Biffi et al.71 found associations between the ApoE ɛ2 allele and increased risk of lobar intracerebral hemorrhage and between the ApoE ɛ4 allele and increased risk of both lobar and deep intracerebral hemorrhage. Additionally, results from a large, multiethnic study performed by Gao et al.72 suggests a conferred predisposition to nonspecific spontaneous intracranial hemorrhage by the MTHFR 677C > T allele. There is evidence to support the replication of associations of both APOE ɛ4 and MTHFR 677C > T in east Asian populations.73,74
Monogenic disorders associated with spontaneous intracerebral hemorrhage
The following are monogenic disorders that cause intracerebral hemorrhage, but without particular variants qualifying for study inclusion. Alagille’s syndrome is caused by mutations in various NOTCH signaling molecules, the vast majority involving Jagged-1 and Notch2.59 It is inherited dominantly, but with significantly high rates of de novo mutation (60% of patients).59 NOTCH signaling is essential for proper systemic embryonic development and has a key function in ensuring proper vascular morphology.59 Alagille’s syndrome has been associated with development of cerebral aneurysm and spontaneous intracerebral hemorrhage.59
Arteriovenous malformation
Variants associated with arteriovenous malformations
Brain arteriovenous malformation75 is a rare vascular disorder with a prevalence of 0.015–0.018%.76 It is characterized by capillary maldevelopment which results in direct communication between the arterial and venous systems.77 They typically manifest clinically as hemorrhages or seizures77 before the age of 40.76 Hemorrhage is the major concern for patients with a cerebral arteriovenous malformation78 and, while damage varies among patients, approximately 75% of those who survive the initial hemorrhage have perceptible neurologic deficits.77 The genetic influence on arteriovenous malformation formation is still largely unknown. A considerable genetic component is evidenced,79 yet a strong familial pattern has not yet been established80,81 and, to our knowledge, no specific genetic markers have been identified in large studies. There is evidence to suggest that common genetic polymorphisms vary little in their contribution to arteriovenous malformation.82
Monogenic disorders associated with arteriovenous malformation
Hereditary hemorrhagic telangiectasia, an autosomal dominant disorder caused by variants in ENG, ALK1, and SMAD4, often presents with multiple cerebral arteriovenous malformations.83 All three genes encode constituents of transforming growth factor-β signaling, which has been demonstrated to play a role in vascular homeostasis.84 However, no particular polymorphisms met criteria for study inclusion.
Cavernous malformation
Cavernous malformations are caused by maldeveloped parenchyma,85 which leads to a compromised, leaky blood–brain barrier in the affected area.86 Similarly to arteriovenous malformations, cavernous malformations almost always occur in the central nervous system and commonly lead to hemorrhage and seizure.86,87 However, cavernous malformations involve vessels of lower pressure and flow;76,87 thus, although prevalence is higher at 0.5%,76 resultant hemorrhages are not nearly as severe as those attributed to arteriovenous malformation76 and only 20–30% of patients become symptomatic.85 Mutations in three genes, aptly named CCM1, CCM2, and CCM3, that encode proteins forming a trimeric protein complex, are thought to cause more than 90% of familial cavernous malformation; many mutations have been identified but are believed to behave in a monogenic fashion.85,86 Similarly to arteriovenous malformation, various mutations have been observed, but no large studies were found for inclusion in this review.
Moyamoya disease
Moyamoya disease is a pathology with both ischemic and hemorrhagic manifestations. It begins as a vaso-occlusive disorder of the terminal portions of the internal carotid arteries and the Circle of Willis;3,5 this occlusion of the cerebral vasculature precipitates hypoxia-induced angiogenesis.88,89 The occluded vessels are predisposed to ischemic events, while the abnormal, hypoxia-induced collateral vessels are susceptible to rupture and, thus, can cause hemorrhage.90 Moyamoya disease is caused by various mutations with different hereditary patterns and is named accordingly.3 Moyamoya disease is extremely rare in Caucasians, but more common in Asian populations, particularly in Japanese individuals.89
Moyamoya has been associated with various mutations in case reports and studies of low power. The most thoroughly scrutinized susceptibility gene is RNF213 (Table 6), encoding a ubiquitously-expressed cytosolic ubiquitin ligase, which has been shown to play a role in angiogenesis89,91 Ma et al.92 identified two risk alleles in their meta-analysis of East Asians: the 4810R > K polymorphism (rs112735431) and the 4859R > K polymorphism. There is evidence that these variants contribute to Moyamoya through aberrant vessel wall remodeling.89
Discussion
This review summarizes the most salient associations between coding polymorphisms and cerebrovascular disease. Most of these polymorphisms were found in genes involved in processes related to endothelial and vascular health, plasma lipid metabolism, inflammation, coagulation, and blood pressure modulation. The primary objective of this review is to assemble current knowledge in the field of cerebrovascular neurogenomics, which is expected to become an important aspect of personalized medicine, particularly as data from ongoing large exome sequencing projects in health systems and population health studies will become available. Knowledge of genetic links to cerebrovascular disease could be used to identify and guide susceptible individuals to appropriate prophylactic and therapeutic strategies. One aspect of this would involve studies focused on developing a more precise understanding of the level of contribution specific alleles confer as well as the interplay between different alleles and clinical factors in precipitating cerebrovascular disease. Leveraging genomic data for these more common forms of cerebrovascular disease will be advanced by the creation and validation of multifactorial risk scores that include information from multiple genes, necessitated by the relatively small risk contributed by each individual variant, which ultimately could be used in conjunction with clinical presentation to develop prophylactic and therapeutic protocols.
Genetic risk scores to predict cerebrovascular disease
Rare monogenic cerebrovascular disorders aside, the contribution of individual alleles to the development of cerebrovascular diseases is marginal at best. Coupled with clinical variables, however, genetic risk scores incorporating a large number of genetic polymorphisms may accurately identify patients at risk. Such polygenic risk scores are calculations that demonstrate disease risk based on the presence of pre-selected polymorphisms that have been associated with disease risk in prior studies.93 They provide a specific advantage in that they can help predict risk of adverse events in patients without monogenic diseases of high penetrance. Polygenic risk scores are still in their infancy, but have shown promise in predicting presentation of certain diseases such as schizophrenia, multiple sclerosis, cardiovascular disease, and rheumatoid arthritis.94 A thorough and well-calibrated polygenic risk score assessing risk alleles, each with low penetrance, could be used to calculate the risk for cerebrovascular disease in an individual. One drawback of polygenic risk scores is that they likely lack reproducibility across ethnic groups and, as shown in this review, there is little genetic data outside European and Asian ethnicities.93 Additionally, polygenic risk scores have yet to prove clinical relevance, but it is expected that this will be achieved with further development, even if only proving helpful to patients who score on either tail of a normal distribution and not for patients with only marginally significant scores.93 Next-generation polygenic risk scores may not be limited to a select number of high yield polymorphisms, but rather incorporate all genetic variants characterizing health and disease states based on the idea that a few strong signals, as well as several weaker signals, may collectively be informative. Recent work on ischemic stroke in the Japanese population has shown promising results of such “true” polygenic risk scores demonstrating superiority over the traditional weighted multilocus genetic risk scores.95
Clinical ischemic stroke risk factors and their genetic counterparts
Most polymorphisms in this review mirror clinical risk factors for ischemic stroke. Age is the most significant risk factor for stroke, with elevated age increasing risk of all stroke subtypes96; however, there is evidence to suggest that aging interacts with stroke risk to varying degrees depending on stroke subtype.97 A 2017 study of Netherlands patients suggested that aging is a more significant contributor to risk for ischemic stroke than hemorrhagic stroke.97 This relationship is potentially reflected in the early-onset ischemic strokes seen in Werner syndrome, a disease of premature aging. Additionally, it is well established that sex also plays a significant role in determining risk of particular cerebrovascular disease subtypes.98–100 It has been demonstrated that, globally, men have a higher risk of ischemic stroke than women, while hemorrhagic stroke risk seems to not be significantly tied to sex.100 Only one polymorphism in this review was established to have specificity for sex: rs2229116 of RYR3 which confers a greater risk of common carotid atherosclerosis in Japanese males than in Japanese females. Thus, another area of further development is the influence of non-modifiable risk factors other than ethnicity, such as age and sex, on the relationship between certain polymorphisms and cerebrovascular disease. Other clinical but modifiable risk factors for stroke include hypertension, hypercoagulable states, inflammatory states, and dyslipidemia.101 These increase risk of cerebrovascular incidents by atheroma, or plaque, formation in ischemic stroke and increased vessel wall stress in hemorrhagic stroke. Polymorphisms included in this review encode proteins associated with all these processes. Other well-represented as functional groups are genes involved in endothelial and vascular health and include vessel remodeling and extracellular matrix homeostasis, which are particularly important in hemorrhagic stroke and represented by genes encoding structural proteins like collagens and the protease inhibitor α1-antitrypsin (SERPINA3). In fact, 39 (86.7%) of the 45 variants included in this review have direct, established relationships to at least one of the following processes: endothelial and vascular health, plasma lipid metabolism, inflammation, coagulation, and blood pressure modulation. Given the aforementioned known risk factors and pathophysiology of stroke, it is not surprising that variants in such genes would be involved in stroke risk. This review can help identify additional gaps in our current understanding of cerebrovascular neurogenomics. Further efforts are especially warranted to replicate these variants across ethnicities, understand the interaction between these polymorphisms and sex, and identify additional relevant coding variants in cerebrovascular diseases with little current genomic literature, such as moyamoya disease. Of the 42 distinct variants included in this review, only 1 was associated with a monogenic disease. This was not a surprise as the relative infrequency of stroke due to monogenic disease renders large cohort studies difficult to conduct. Future work in this area will be particularly helpful as identification of monogenic forms of cerebrovascular disease in patients could provide immediate clinical impact. Potential clinical application of such data is modeled well in the use of aspirin to prevent colorectal cancer in those with predisposing monogenic disorders. A study by Burn et al.102 indicated that regular aspirin may indeed reduce the risk of development of colorectal cancer in patients with Lynch syndrome, a disease with autosomal dominant propagation. If, for example, a similar practice reduces the risk of ischemic stroke in patients with homocystinuria, there may be a greater demand for more accurate genetic screening of the disease.
Limitations
There are a number of limitations that should be highlighted. In order to summarize the literature of high-leverage, coding polymorphisms, this review focuses on specific mutations that have well-documented associations with cerebrovascular disease. Thus, we do not claim this review to be an exhaustive list of relevant polymorphisms. Many lower-leverage alleles have likely been omitted through our screening process. Namely, alleles of extremely low-frequency but significant contribution to cerebrovascular disease may have been excluded as only studies with 500 or more case subjects were eligible. The threshold of 500 was set after the initial review of the literature. There were 95 studies excluded due to a sample size of less than 500 subjects as there was concern that those studies were underpowered to support the conclusions drawn. There were 2 studies, however, that were included with fewer than 500 subjects because they were very close to this threshold and demonstrated remarkably high association with their respective diseases; with that, we felt omission could not be justified. The other option to provide a comprehensive review in an already lengthy manuscript was to set certain odds ratio or confidence interval thresholds. This, however, was abandoned because the effect of an allele on a specific disease is frequently robust, but overall very small, as indicated above. Additionally, due to the focus on individual genetic markers, studies on gene-gene, gene-environment, and gene-disease interactions specific to a subset of patients with pre-existing risk factors were excluded. Susceptibility genes for cerebrovascular disease were not included if they lacked strong evidence for a specific mutation. For example, COL4A1 has been identified as a likely susceptibility gene for hemorrhagic stroke;103 however, no sizeable studies of human cohorts have been performed to identify specific disease-relevant mutations in this gene and, thus, no COL4A1 risk alleles are included in this review. Finally, studies that did not provide a definition of atherosclerosis or report cIMT or plaque formation were discarded and, thus, it is possible that some polymorphisms evidenced for association with cerebrovascular occlusive disease were excluded.
Conclusion
This systematic review summarizes studies associating exonic variants with cerebrovascular diseases for the neurosurgical audience. Further studies can build off this review to form polygenic risk scores in order to set clinically relevant parameters for stroke prophylaxis and treatment and also broaden our understanding of cerebrovascular neurogenomics, namely by replicating risk alleles across different ethnicities, recognizing sex-specific genetic markers, and identifying risk alleles for disease that have not been thoroughly examined.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis 2013; 1: 1–2. [Google Scholar]
- 2.Koton S, Schneider ALC, Rosamond WD, et al. Stroke incidence and mortality trends in us communities, 1987 to 2011. JAMA 2014; 312: 259–268. [DOI] [PubMed] [Google Scholar]
- 3.Della-Morte D, Pacifici F, Rundek T. Genetic susceptibility to cerebrovascular disease. Curr Opin Lipidol 2016; 27: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casas JP, Hingorani AD, Bautista LE, et al. Meta-analysis of genetic studies in ischemic stroke: thirty-two genes involving approximately 18 000 cases and 58 000 controls. Arch Neurol 2004; 61: 1652–1661. [DOI] [PubMed] [Google Scholar]
- 5.Francis J, Raghunathan S, Khanna P. The role of genetics in stroke. Postgrad Med J 2007; 83: 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015; 372: 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 2010; 11: nrg2779. [DOI] [PubMed] [Google Scholar]
- 8.Warr A, Robert C, Hume D, et al. Exome sequencing: current and future perspectives. G3 2015; 5: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broad Institute sequences its 100,000th whole human genome on National DNA Day. Broad Institute, www.broadinstitute.org/news/broad-institute-sequences-its-100000th-whole-human-genome-national-dna-day (2018, accessed 14 May 2018).
- 10.System GH. MyCode Community Health Initiative hits another milestone; 150,000 signed up to largest health system sequencing project, www.prnewswire.com/news-releases/mycode-community-health-initiative-hits-another-milestone-150000-signed-up-to-largest-health-system-sequencing-project-300473302.html (accessed 14 May 2018).
- 11.Regeneron to Lead $50M Exome Sequencing Consortium with UK Biobank. GEN, www.genengnews.com/gen-news-highlights/regeneron-led-50m-exome-sequencing-consortium-with-uk-biobank-modeled-on-geisinger-partnership/81255353 (accessed 14 May 2018).
- 12.Traylor M, Farrall M, Holliday EG, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE Collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol 2012; 11: 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulit SL, McArdle PF, Wong Q, et al. The NINDS stroke genetics network: a genome-wide association study of ischemic stroke and its subtypes. Lancet Neurol 2016; 15: 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcone GJ, Malik R, Dichgans M, et al. Current concepts and clinical applications of stroke genetics. Lancet Neurol 2014; 13: 405–418. [DOI] [PubMed] [Google Scholar]
- 15.Frosst P, Blom H, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 16.Wei LK, Au A, Menon S, et al. Polymorphisms of MTHFR, eNOS, ACE, AGT, ApoE, PON1, PDE4D, and ischemic stroke: meta-analysis. J Stroke Cerebrovasc Dis 2017; 26: 2482–2493. [DOI] [PubMed] [Google Scholar]
- 17.Ashjazadeh N, Fathi M, Shariat A. Evaluation of homocysteine level as a risk factor among patients with ischemic stroke and its subtypes. Iran J Med Sci 2013; 38: 233–239. [PMC free article] [PubMed] [Google Scholar]
- 18.Cui T. MTHFR C677T mutation increased the risk of Ischemic Stroke, especially in large-artery atherosclerosis in adults: an updated meta-analysis from 38 researches. Int J Neurosci 2016; 126: 10–19. [DOI] [PubMed] [Google Scholar]
- 19.Rutten-Jacobs LCA, Traylor M, Adib-Samii P, et al. Association of MTHFR C677T genotype with ischemic stroke is confined to cerebral small vessel disease subtype. Stroke 2016; 47: 646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He W, Lu M, Li G, et al. Methylene tetrahydrofolate reductase (MTHFR) rs868014 polymorphism regulated by miR-1203 associates with risk and short term outcome of ischemic stroke. Cell Physiol Biochem 2017; 41: 701–710. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Hu Z. ApoE Polymorphisms and the risk of different subtypes of stroke in the Chinese population: a comprehensive meta-analysis. Cerebrovasc Dis 2016; 41: 119–138. [DOI] [PubMed] [Google Scholar]
- 22.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006; 113: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Misra S, Kumar P, et al. Association between endothelial nitric oxide synthase gene polymorphisms and risk of ischemic stroke: a meta-analysis. Neurol Ind 2017; 65: 22. [DOI] [PubMed] [Google Scholar]
- 24.Rees DC. The population genetics of factor V Leiden (Arg506Gln). Br J Maematol 1996; 95: 579–586. [DOI] [PubMed] [Google Scholar]
- 25.Van Cott EM, Khor B, Zehnder JL. Factor V Leiden. Am J Hematol 2016; 91: 46–49. [DOI] [PubMed] [Google Scholar]
- 26.Au A, Griffiths LR, Irene L, et al. The impact of APOA5, APOB, APOC3 and ABCA1 gene polymorphisms on ischemic stroke: evidence from a meta-analysis. Atherosclerosis 2017; 265: 60–70. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Yin X, Li L, et al. Association of apolipoprotein C3 genetic polymorphisms with the risk of ischemic stroke in the Northern Chinese Han Population. PLoS One 2016; 11: e0163910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada Y, Sakuma J, Takeuchi I, et al. Identification of six polymorphisms as novel susceptibility loci for ischemic or hemorrhagic stroke by exome-wide association studies. Int J Mol Med 2017; 39: 1477–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma W-Q, Qu Q-R, Zhao Y, et al. Association of RAGE gene Gly82Ser polymorphism with coronary artery disease and ischemic stroke. Medicine 2016; 95: e5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik R, Dau T, Gonik M, et al. Common coding variant in SERPINA1 increases the risk for large artery stroke. Proc Natl Acad Sci U S A 2017; 114: 3613–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovaleva EV, Doronin BM, Morozov VV, et al. [The HIF1a polymorphism is a diagnostic marker of ischemic stroke]. Zh Nevrol Psikhiatr Im S S Korsakova 2016; 116: 10–13. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Z, Yan J, Geng C, et al. A Polymorphism within the 3′UTR of NLRP3 is associated with susceptibility for ischemic stroke in Chinese population. Cell Mol Neurobiol 2016; 36: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung Y-F, Lu C-C, Lee J-T, et al. Homozygous ALDH2*2 is an independent risk factor for ischemic stroke in Taiwanese men. Stroke 2016; 47: 2174–2179. [DOI] [PubMed] [Google Scholar]
- 34.Misra S, Kumar P, Kumar A, et al. Genetic association between inflammatory genes (IL-1α, CD14, LGALS2, PSMA6) and risk of ischemic stroke: a meta-analysis. Meta Gene 2016; 8: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Choi GH, Ko KH, et al. Association of the single nucleotide polymorphisms in microRNAs 130b, 200b, and 495 with ischemic stroke susceptibility and post-stroke mortality. PLoS One 2016; 11: e0162519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong Y, Cai L, Han S, et al. Functional genetic variants of PPARγ and PPARα encoding peroxisome proliferator-activated receptors and susceptibility to ischemic stroke in Chinese Han population. Cerebrovasc Dis 2016; 41: 96–99. [DOI] [PubMed] [Google Scholar]
- 37.Chong M, O’Donnell M, Thijs V, et al. Mendelian genes and risk of intracerebral hemorrhage and small-vessel ischemic stroke in sporadic cases. Stroke 2017; 48: 2263–2265. [DOI] [PubMed] [Google Scholar]
- 38.Christoffersen M, Frikke-Schmidt R, Nordestgaard BG, et al. Genetic variation in WRN and ischemic stroke: general population studies and meta-analyses. Exp Gerontol 2017; 89: 69–77. [DOI] [PubMed] [Google Scholar]
- 39.Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1: 158–159. [DOI] [PubMed] [Google Scholar]
- 40.Bang OY, Chung J-W, Cha J, et al. A polymorphism in RNF213 is a susceptibility gene for intracranial atherosclerosis. PLoS One 2016; 11: e0156607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyawaki S, Imai H, Shimizu M, et al. Genetic variant RNF213 c.14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke 2013; 44: 2894–2897. [DOI] [PubMed] [Google Scholar]
- 42.Natarajan P, Bis JC, Bielak LF, et al. Multiethnic exome-wide association study of subclinical atherosclerosis. Circ Cardiovasc Genet 2016; 9: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin M-H, Choi J-S, Rhee J-A, et al. APOE polymorphism and carotid atherosclerosis in Korean population: the Dong-gu study and the Namwon study. Atherosclerosis 2014; 232: 180–185. [DOI] [PubMed] [Google Scholar]
- 44.Beilby JP, Hunt CCJ, Palmer LJ, et al. Apolipoprotein E gene polymorphisms are associated with carotid plaque formation but not with intima-media wall thickening: results from the Perth Carotid Ultrasound Disease Assessment Study (CUDAS). Stroke 2003; 34: 869–874. [DOI] [PubMed] [Google Scholar]
- 45.Slooter AJC, Bots ML, Havekes LM, et al. Apolipoprotein E and carotid artery atherosclerosis: the Rotterdam study. Stroke 2001; 32: 1947–1952. [DOI] [PubMed] [Google Scholar]
- 46.Debette S, Lambert J-C, Gariépy J, et al. New insight into the association of apolipoprotein E genetic variants with carotid plaques and intima-media thickness. Stroke 2006; 37: 2917–2923. [DOI] [PubMed] [Google Scholar]
- 47.Volcik KA, Barkley RA, Hutchinson RG, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol 2006; 164: 342–348. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C, Ikeda S, Arai T, et al. Association of the RYR3 gene polymorphisms with atherosclerosis in elderly Japanese population. BMC Cardiovasc Disord 2014; 14: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norata GD, Garlaschelli K, Grigore L, et al. Effects of PCSK9 variants on common carotid artery intima media thickness and relation to ApoE alleles. Atherosclerosis 2010; 208: 177–182. [DOI] [PubMed] [Google Scholar]
- 50.Leu H-B, Chung C-M, Chuang S-Y, et al. Genetic variants of connexin37 are associated with carotid intima-medial thickness and future onset of ischemic stroke. Atherosclerosis 2011; 214: 101–106. [DOI] [PubMed] [Google Scholar]
- 51.Lan M-Y, Chang Y-Y, Chen W-H, et al. Association between MIF gene polymorphisms and carotid artery atherosclerosis. Biochem Biophys Res Commun 2013; 435: 319–322. [DOI] [PubMed] [Google Scholar]
- 52.Si NV, Fujioka D, Watanabe K, et al. Phospholipase A2 receptor gene polymorphisms alter its functions and present a genetic risk of an increased intima-media thickness of the carotid artery. J Atheroscler Thromb 2016; 23: 1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panayiotou AG, Griffin MB, Tyllis T, et al. Association of genotypes at the matrix metalloproteinase (MMP) loci with carotid IMT and presence of carotid and femoral atherosclerotic plaques. Vasc Med 2013; 18: 298–306. [DOI] [PubMed] [Google Scholar]
- 54.Carpenter AM, Singh IP, Gandhi CD, et al. Genetic risk factors for spontaneous intracerebral haemorrhage. Nat Rev Neurol 2016; 12: 40–49. [DOI] [PubMed] [Google Scholar]
- 55.Tromp G, Weinsheimer S, Ronkainen A, et al. Molecular basis and genetic predisposition to intracranial aneurysm. Ann Med 2014; 46: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Mao P, Xie C, et al. Apolipoprotein E polymorphism and the risk of intracranial aneurysms in a Chinese population. BMC Neurol 2017; 17: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korja M, Silventoinen K, McCarron P, et al. Genetic epidemiology of spontaneous subarachnoid hemorrhage. Stroke 2010; 41: 2458–2462. [DOI] [PubMed] [Google Scholar]
- 58.Barak T, Cheng Y, Youngblood MW, et al. Genetics of intracranial aneurysms. In: Winn HR (ed) Youmans and Winn Neurological Surgery. Philadelphia, PA: Elsevier, 2017, pp.3198–3206.e4.
- 59.Castori M, Voermans NC. Neurological manifestations of Ehlers-Danlos syndrome(s): a review. Iran J Neurol 2014; 13: 190–208. [PMC free article] [PubMed] [Google Scholar]
- 60.Kolodny E, Fellgiebel A, Hilz MJ, et al. Cerebrovascular involvement in Fabry disease: current status of knowledge. Stroke 2015; 46: 302–313. [DOI] [PubMed] [Google Scholar]
- 61.Rinkel GJE, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998; 29: 251–256. [DOI] [PubMed] [Google Scholar]
- 62.Alg VS, Sofat R, Houlden H, et al. Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. Neurology 2013; 80: 2154–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kataoka H. Molecular mechanisms of the formation and progression of intracranial aneurysms. Neurol Med Chir 2015; 55: 214–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang C, Qi Z, Shao C, et al. Association between three eNOS polymorphisms and intracranial aneurysms risk. Medicine 2015; 94: e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan J, Sun W, Lin M, et al. Genetic association study identifies a functional CNV in the WWOX gene contributes to the risk of intracranial aneurysms. Oncotarget 2016; 7: 16104–16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu X, Fang Y, Li Y, et al. Role of endoglin insertion and rs1800956 Polymorphisms in intracranial aneurysm susceptibility. Medicine 2015; 94: e1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joo S-P, Lee J-K, Kim T-S, et al. A polymorphic variant of the endoglin gene is associated with increased risk for intracranial aneurysms in a Korean population. Surg Neurol 2008; 70: 39–44. [DOI] [PubMed] [Google Scholar]
- 68.Slowik A, Borratynska A, Turaj W, et al. α1-Antichymotrypsin gene (SERPINA3) A/T polymorphism as a risk factor for aneurysmal subarachnoid hemorrhage. Stroke 2005; 36: 737–740. [DOI] [PubMed] [Google Scholar]
- 69.Gläsker S, Schatlo B, Klingler J-H, et al. Associations of collagen type I α2 polymorphisms with the presence of intracranial aneurysms in patients from Germany. J Stroke Cerebrovasc Dis 2014; 23: 356–360. [DOI] [PubMed] [Google Scholar]
- 70.FBN1 fibrillin 1 [Homo sapiens (human)] – Gene – NCBI, www.ncbi.nlm.nih.gov/gene/2200 (accessed 12 July 2017).
- 71.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010; 68: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao S, Li H, Xiao H, et al. Association of MTHFR 677T variant allele with risk of intracerebral haemorrhage: a meta-analysis. J Neurol Sci 2012; 323: 40–45. [DOI] [PubMed] [Google Scholar]
- 73.Zhang R, Wang X, Tang Z, et al. Apolipoprotein E gene polymorphism and the risk of intracerebral hemorrhage: a meta-analysis of epidemiologic studies. Lipids Health Dis 2014; 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu X, Tao C, Xie Z, et al. The MTHFR C677T polymorphism and risk of intracerebral hemorrhage in a Chinese Han population. Med Sci Monit 2016; 22: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greene AK, Orbach DB. Management of arteriovenous malformations. Clin Plast Surg 2011; 38: 95–106. [DOI] [PubMed] [Google Scholar]
- 76.Ortega-Barnett J, Mohanty A, Desai SK, et al. Neurosurgery. In: Townsend CM, Beauchamp RD, Evers BM, et al. (eds) Sabiston textbook of surgery. Philadelphia, PA: Elsevier, 2016, pp.1900–1937.
- 77.Solomon RA, Connolly ESJ. Arteriovenous malformations of the brain. N Engl J Med 2017; 376: 1859–1866. [DOI] [PubMed] [Google Scholar]
- 78.Sturiale CL, Puca A, Sebastiani P, et al. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand? Brain 2013; 136: 665–681. [DOI] [PubMed] [Google Scholar]
- 79.Ramey WL, Martirosyan NL, Zabramski JM, et al. A hierarchical model for the development of cerebral arteriovenous malformations. Clin Neurol Neurosurg 2014; 126: 126–129. [DOI] [PubMed] [Google Scholar]
- 80.Beijnum J van, Worp HB van der, Algra A, et al. Prevalence of brain arteriovenous malformations in first-degree relatives of patients with a brain arteriovenous malformation. Stroke 2014; 45: 3231–3235. [DOI] [PubMed] [Google Scholar]
- 81.van Beijnum J, van der Worp HB, Schippers HM, et al. Familial occurrence of brain arteriovenous malformations: a systematic review. J Neurol Neurosurg Psychiatr 2007; 78: 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weinsheimer S, Bendjilali N, Nelson J, et al. Genome-wide association study of sporadic brain arteriovenous malformations. J Neurol Neurosurg Psychiatr 2016; 87: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H, Nelson J, Krings T, et al. Hemorrhage rates from brain arteriovenous malformation in patients with hereditary hemorrhagic telangiectasia. Stroke 2015; 46: 1362–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bertolino P, Deckers M, Lebrin F, et al. Transforming growth factor-β signal transduction in angiogenesis and vascular disorders. Chest 2005; 128: 585S–590S. [DOI] [PubMed] [Google Scholar]
- 85.Louvi A and Günel M. Genetics of cerebral cavernous malformations. In: Winn HR (ed) Youmans and Winn Neurological Surgery. Philadelphia, PA: Elsevier, 2017, pp.3547–3553.e5.
- 86.Kim J. Introduction to cerebral cavernous malformation: a brief review. BMB Rep 2016; 49: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baranoski JF, Kalani MYS, Przybylowski CJ, et al. Cerebral cavernous malformations: review of the genetic and protein-protein interactions resulting in disease pathogenesis. Front Surg 2016; 3: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukui M, Kono S, Sueishi K, et al. Moyamoya disease. Neuropathology 2000; 20: 61–64. [DOI] [PubMed] [Google Scholar]
- 89.Bersano A, Guey S, Bedini G, et al. Research progresses in understanding the pathophysiology of Moyamoya disease. Cerebrovasc Dis 2016; 41: 105–118. [DOI] [PubMed] [Google Scholar]
- 90.Smith ER and Scott RM. Moyamoya disease. In: Winn HR (ed) Youmans and Winn Neurological Surgery. Philadelphia, PA: Elsevier, 2017, pp.1766–1772.e3.
- 91.Ohkubo K, Sakai Y, Inoue H, et al. Moyamoya disease susceptibility gene RNF213 links inflammatory and angiogenic signals in endothelial cells. Sci Rep 2015; 5: srep13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma J, Liu Y, Ma L, et al. RNF213 polymorphism and Moyamoya disease: a systematic review and meta-analysis. Neurol Ind 2013; 61: 35. [DOI] [PubMed] [Google Scholar]
- 93.Lewis CM, Vassos E. Prospects for using risk scores in polygenic medicine. Genome Med 2017; 9: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 2013; 9: e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hachiya T, Kamatani Y, Takahashi A, et al. Genetic predisposition to ischemic stroke: a polygenic risk score. Stroke 2017; 48: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crocco TJ and Meurer WJ. Stroke. In: Marx J, Walls R, Hockberger R (eds) Rosen’s emergency medicine: concepts and clinical practice. Philadelphia, PA: Elsevier, 2013 pp.1241–1255.e3.
- 97.Hauer AJ, Ruigrok YM, Algra A, et al. Age-specific vascular risk factor profiles according to stroke subtype. J Am Heart Assoc 2017; 6: e005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park HJ, Kim SK, Park H-K, et al. Association between paraoxonase gene polymorphisms and intracerebral hemorrhage in a Korean population. J Mol Neurosci 2015; 57: 410–416. [DOI] [PubMed] [Google Scholar]
- 99.Palm F, Urbanek C, Wolf J, et al. Etiology, risk factors and sex differences in ischemic stroke in the Ludwigshafen Stroke Study: a population-based stroke registry. Cerebrovasc Dis 2012; 33: 69–75. [DOI] [PubMed] [Google Scholar]
- 100.Barker-Collo S, Bennett DA, Krishnamurthi RV, et al. Sex differences in stroke incidence, prevalence, mortality and disability-adjusted life years: results from the global burden of disease study 2013. Neuroepidemiology 2015; 45: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376: 112–123. [DOI] [PubMed] [Google Scholar]
- 102.Burn J, Gerdes A-M, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378: 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ratelade J, Mezouar N, Domenga-Denier V, et al. Severity of arterial defects in the retina correlates with the burden of intracerebral haemorrhage in COL4A1-related stroke. J Pathol 2018; 244: 408–420. [DOI] [PubMed] [Google Scholar]