Abstract
The role of glycocalyx in blood–brain barrier (BBB) integrity and brain damage is poorly understood. Our study aimed to investigate the impacts of endothelial glycocalyx on BBB function in a rat model of cardiac arrest (CA) and cardiopulmonary resuscitation (CPR). Male Sprague-Dawley rats subjected to 8-min asphyxia CA/CPR. Compared to controls, glycocalyx was mildly injured by CA, severely disrupted by hyaluronidase (HAase) with CA, and mitigated by hydrocortisone (HC) with CA. More importantly, the disruption of glycocalyx caused by HAase treatment was associated with higher BBB permeability and aggravated brain edema at 24 h after return of spontaneous circulation, as well as lower survival rate and poorer neurologic outcome at seventh day. Reversely, less degradation of glycocalyx by HC treatment was accompanied by higher seven-day survival rate and better neurologic outcome. Mechanistically, HAase treatment further increased CA/CPR-induced activation of glia cells and expression of inflammatory factors, whereas HC decreased them in the brain cortex and hippocampus. Glycocalyx degradation results in BBB leakage, brain edema, and deteriorates neurologic outcome after asphyxia CA/CPR in rats. Preservation of glycocalyx by HC could improve neurologic outcome and reduce BBB permeability, apparently through reduced gene transcription-protein synthesis and inflammation.
Keywords: Brain edema, blood–brain barrier, cardiac arrest, endothelium, steroid
Introduction
In spite of great efforts to improve resuscitation methods and post-resuscitation care, high mortality and morbidity still exists in post-cardic arrest (CA) patients with an initial return of spontaneous circulation (ROSC), which is partly due to the post-cardic arrest syndrome (PCAS). Anoxic brain injury, post-CA myocardial dysfunction, systemic ischemia/reperfusion response, and persistent precipitating pathology are considered as the major clinical manifestations of PCAS.1 Among all the pathophysiological processes of PCAS, circulation alterations are prominent, which include microcirculatory dysfunction,2 vascular leakage and accumulation of platelet and leukocyte adhesion to the endothelium.1,3,4
Endothelial glycocalyx, ranging from 200 to 2000 nm in thickness, attracts attention recently because its degradation contributes to tissue edema in ischemia/reperfusion injury.5 As the first defense of blood vessels, it limits the leakage of macromolecules into the vessels and prevents the adhesion of circulating inflammatory cells to the vascular endothelial lining. The core structure of glycocalyx consists of negatively charged mesh of proteoglycans, glycosaminoglycans, and glycoproteins, which comprises a variety of transmembrane and membrane-bound molecules, predominantly hyaluronan (HA), syndecan-1, and heparan sulfate (HS).6 The glycocalyx is quite sensitive to perturbations and has vulnerability to several factors like enzymatic digestion with hyaluronidase (HAase), pronase, heparinase,7 ischemia,5 inflammation,8 trauma,9 diabetes,10 and intravenous fluid mismanagement.11 It has been shown that surface-bound HA is a major determinant of the endothelial glycocalyx permeability and that its degradation from the coronary endothelial surface results in myocardial tissue edema.12 Although poorly studied in the central nervous system, accumulating evidence suggests that the glycocalyx plays an important role in maintaining the barrier function of endothelium due to its matrix-like structure as well as the charge. A prospective clinical trial13 suggests that CA induces glycocalyx degrading stressors and potentially glycocalyx-dependent endothelial responses, indicating a potential important role of this endothelial surface layer in the development of PCAS. However, the pathological role of endothelial glycocalyx in PCAS and blood–brain barrier (BBB) dysfunction remains largely unclear. Nor any reports focused on the alteration of glycocalyx in brain damage after CA/CPR in rats.
In this study, we assessed the endothelial glycocalyx of cerebral capillaries in a rat model of asphyxia CA/CPR to evaluate its impacts on the permeability of BBB and brain edema. To clarify whether glycocalyx degradation can lead to increased BBB permeability, HAase was employed to degrade glycocalyx. Intercellular cell adhesion molecule-1 (ICAM-1), vascular endothelial adhesion factor (VCAM-1), and other adhesion factors were exposed after glycocalyx were destroyed. The expression of matrix metalloproteinase-9 (MMP-9) activities and the destruction of tight junction proteins such as zonula occludens-1 (ZO-1), and occludin protein (Occludin) are closely related to BBB damage. The expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) increased during ischemia and reperfusion. Our results indicated that glycocalyx degradation played a pivotal role in the process of BBB leakage and brain edema after asphyxia CA/CPR. Hydrocortisone (HC) had a protective effect on stability of glycocalyx and improved neurologic outcome.
Material and methods
Ethics statement
Animal experiments were written and performed in accordance with and approved by the Institutional Animal Care and Use Committee of Southern Medical University following the National Guidelines (Guidelines on Administration of Laboratory Animals in China and Guidelines on the Humane Treatment of Laboratory Animals in China) and in accordance with the ARRIVE guidelines.
Animal preparation
Male Sprague-Dawley rats weighing between 350 and 450 g used in the experiments were obtained from the Experimental Animal Center of Southern Medical University. Rats were acclimatized for 5 days prior to study initiation. The animals were housed on a 12-h light/dark cycle with free access to water and food.
CA model
CA/CPR model was performed as described previously.14 Briefly, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (45 mg/kg). Then rats were given orotracheal intubation with a 14G cannula (BD, Suzhou, China), and connected to a rodent ventilator (RWD, Shenzhen, China). Intravascular catheters (24G; BD) were inserted into the left femoral artery and vein for continuous monitoring of dynamic artery blood pressure, measurement of blood gases, infusion of fluid, and drug administration. An equilibration interval of 30 min was allowed to establish steady state conditions. To induce asphyxia CA, vecuronium (0.1 mg/kg) was administrated, leading to respiratory paralysis, and then mechanical ventilator was disconnected to produce complete circulatory arrest. After disconnecting mechanical ventilation, mean arterial pressure and heart rate increased initially followed by progressive bradycardia and hypotension. CA was defined as the onset of mean arterial pressure declined below 20 mmHg. At the end of the 8 min CA, CPR was performed, consisting of resuming mechanical ventilation, administration of epinephrine (0.01 mg/kg) and bicarbonate (1 mEq/kg), and continuous external chest compressions at a rate of 200 compressions per minute until spontaneous pulse was observed in arterial tracing. ROSC was defined as the return of supraventricular rhythm with a mean aortic pressure >50 mmHg for a minimum of 5 min. Rats failed to ROSC within 5 min were excluded from the continuing experiments. Rectal temperatures were monitored and maintained at 37.0℃ ± 0.5℃ with a temperature feedback system (RWD) during the surgery and for another 4 h to avoid spontaneous hypothermia. No additional fluids were administered to the rats during the whole procedure.
Experimental design
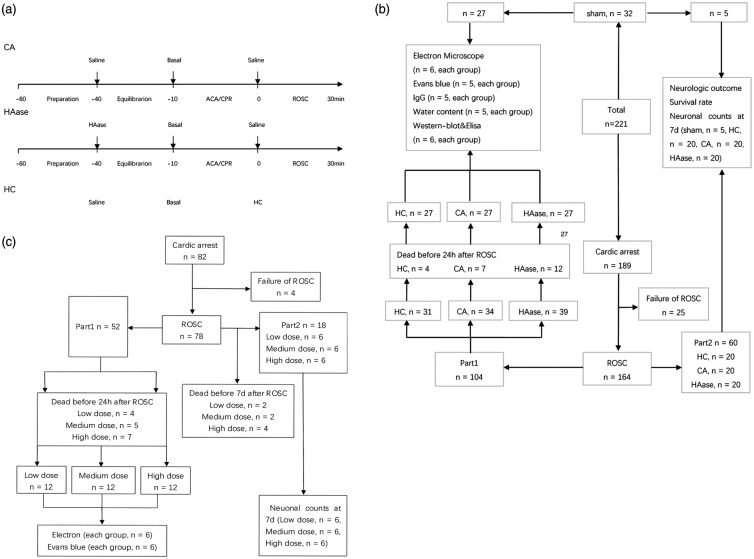
The whole experiment was divided into two parts (Figure 1). Experimental protocols of part 1 were shown in Figure 1(a). After tracheal and vascular intubation, rats were randomized to sham group, CA group, HAase group, or HC group. Rats in HAase group were given a bolus of HAase (2000 units/kg, Type IV-S; Sigma-Aldrich; St. Louis, MO, USA) at the beginning of equilibration, whereas rats in CA group, sham group, and HC group received equivalent volume of saline. After ROSC, rats in HC group were immediately administrated a bolus of HC (30 mg/kg, Sigma) through femoral vein, whereas rats in sham group, CA group, and HAase group received equivalent volume of saline alone. Flow diagram of the experimental groups in part 1 was shown in Figure 1(b).
Figure 1.
Experimental procedure and flow diagram of the study. (a) Experimental procedure and measurements during baseline, ACA, and CPR, and ROSC. (b, c) Flow diagram of the experimental groups. ACA: asphyxia cardiac arrest; CPR: cardiopulmonary resuscitation; ROSC: return of spontaneous circulation; Sham: sham group; CA: cardiac arrest group; HAase: hyaluronidase group; HC: hydrocortisone group; low dose: low dose group; medium dose: medium dose group; high dose: high dose group.
In part 2, rats were divided into three groups and intravenous injected with different doses of HAase before 8-min asphyxia CA/CPR (Figure 1(c)). Rats in high dose group were given a bolus of HAase (2000 units/kg) at the beginning of equilibration, whereas rats in low dose group and medium dose group received 500 units/kg and 1000 units/kg of HAase.
In both parts, electron microscopy imaging, BBB permeability evaluation, brain water content measurement, western blotting, and enzyme-linked immunosorbent assay (ELISA) analyses were performed in rats survived for 24 h after ROSC. Neurologic outcome, survival rate, and neuronal degeneration were evaluated seven days after ROSC.
Electron microscopy
Animals were perfused with a solution containing 2.5% glutaraldehyde, 2% paraformaldehyde, and 2% lanthanum nitrate, according to Vogel et al.,15 to preserve the glycocalyx in brain tissues 24 h after ROSC. Three luminal membrane segments (at least 150 nm distance from each other) of the endothelial cells of each capillary here selected in which the membrane double layer was clearly visible. Then the optical density was measured along a line perpendicular to the membrane, starting at the capillary lumen and continuing through the whole glycocalyx and the membrane double layer. The thickness of the glycocalyx was then defined as the distance between the point at the luminal side at which 50% of the maximal optical density of the glycocalyx was measured and the translucent center of the cytoplasmic membrane.
Determination of glycocalyx components (syndecan-1, HS, and HA)
To measure the concentration of syndecan-1, HA, and HS, 1.0 ml blood sample was collected at 24 h after CA. After centrifugation at 3000 g for 10 min, the clear supernatant was stored at −80℃.
In all groups, samples of serum were used for assessing shedding of syndecan-1, HS, and HA as described in detail elsewhere.16 Syndecan-1 concentrations were determined using an ELISA kit (Diaclone Research, Besancon, France). This kit used a solid phase monoclonal B-B4 antibody and a biotinylated monoclonal B-D30 antibody raised against syndecan-1. According to the manufacturer's instructions, the concentrations of HS were determined using an ELISA kit (Seikagaku Corporation, Tokyo, Japan), which was based on two antibodies specific for HS-related epitopes, and the concentrations of HA were determined using an ELISA kit (R&D Systems, Minneapolis, MN, USA).
Evaluation of BBB permeability by staining with Evans blue (EB) and IgG
BBB permeability was assessed using EB dye17 (Sigma) and IgG (Zhong Shan Jin Qiao; 1:100)18 at 24 h after ROSC.
EB (2% solution of 0.96 kDa dye in saline) were administrated intravenously and allowed to circulate for 60 min. Rats were perfused with saline to remove the residual dye from the vessels until the drainage became colorless. To assess BBB permeability, IgG in the brain was visualized by peroxidase-based immunohistochemistry. Normally, the BBB is not permeable to endogenous IgG, and the IgG leaks into the brain parenchyma when BBB is disrupted. The loss of neuron and BBB damage in the rat models of cardiopulmonary resuscitation (CPR) were the most significant in the CA1 region. To get more information of BBB leakage to macromolecules, we focused on the leakage in the CA1 region.
After weighing, the whole brain was homogenized in 50% trichloroacetic acid. The samples were centrifuged for 30 min at 21,000 g. EB per weight of sample was quantified using the absorbance at 620 nm in the supernatant relative to a series of standard EB solution.
IgG in the hippocampus was visualized by peroxidase-based immunohistochemistry. Endogenous peroxidase activity and non-specific staining were blocked, and sections incubated in biotinylated anti-mouse IgG secondary antibody (1:500, Vector Laboratories) overnight at 4℃. Sections were then incubated with avidin–biotin–peroxidase complex, and color-developed using a diaminobenzidine (DAB) solution (0.05% DAB in 0.005% H2O2). To ensure comparability of DAB staining, all reactions were performed at the same time, with the same batch of DAB, with equal thickness sections exposed to DAB for the same amount of time. IgG staining intensity (using ImageJ) was averaged over five coronal sections. All sections were imaged at the same time with the same settings, with no adjustment to brightness or contrast.
Determination of brain water content
Twenty-four hours after ROSC, animals were normally euthanized when we conducted post-mortem examinations. In order to collect brain samples, we used the decapitation method. Brain samples were divided into cortex and hippocampus and weighed respectively. Then, brain samples were slow dried in an oven (105℃) for 72 h and reweighed to determine the dry weight. Brain water content (%) was calculated as (wet weight-dry weight)/wet weight × 100%.19
Assessment of neurologic outcome and survival rate
Neurologic outcomes were assessed at 24 h, 48 h, 72 h, and 7 days after ROSC in all successfully resuscitated animals. Before the experiment, all animals were familiarized with the neurologic test for three consecutive days and evaluated to ensure normal neurologic function. All evaluations were conducted by two investigators who were masked to animal grouping. Neurologic deficit score (NDS)20 evaluations were performed using previously validated scale, a system consisting of consciousness and breathing, cranial nerves reflexes, motor function, sensory responses, and coordination. A total NDS of 80 was considered as normal, whereas 0 was brain death. Survival was recorded daily.
Immunohistochemistry
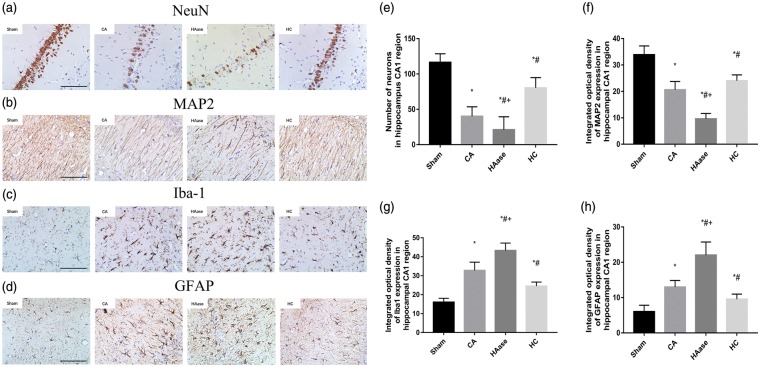
Seven days after ROSC, rats were deeply anesthetized with a lethal dose of sodium pentobarbital (0.1 g/kg) and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and the brain was dissected out. After fixation with the same fixative for overnight, the brain was dehydrated and embedded in paraffin. Immunohistochemistry was performed with an anti-neuronal nuclei antibody (NeuN; Abcam, Cambridge, UK) for detection and measurement of neurons, anti-microtubule-associated protein 2 antibody (MAP2; Sigma-Aldrich, St. Louis, MO) for dendrites, anti-glial fibrillary acidic protein antibody (GFAP, Abcam) for activated astrocytes, and anti-ionized calcium-binding adapter molecule 1 antibody (Iba-1; Wako, Osaka, Japan) for microglia, using the protocol suggested by the manufacturers. Endogenous peroxidase in deparaffinized tissue sections was blocked for 10 min with 3% H2O2 in deionized water, followed by blocking with 10% goat serum diluted in 0.2% Tween-20 in phosphate-buffered saline at room temperature for 1 h. The tissues were then incubated with primary antibody (anti-NeuN, 1:1000; anti-MAP2, 1:1000; anti-IBA-1, 1:500; anti-GFAP, 1:500) at 4℃ overnight. Tissue sections were washed and incubated with secondary antibody (1:1000) for 1 h at room temperature. After washing, sections were incubated with ABC complex for 30 min at room temperature, and then stained with the chromogenic substrate 3,3-diaminobenzidine tetrahydrochloride (DAB) and H2O2, until optimal staining was obtained. In each NeuN-, Iba-1-, MAP2-, and GFAP-stained section, two slide fields were randomly examined using a defined rectangular field area (0.14 mm2). NeuN staining cells were counted automatically, while MAP2, Iba-1, and GFAP staining was reported as the relative intensity of staining (percent) of the area by using Image J (1.49v, NIH, Bethesda, MD). NeuN-stained sections of the CA1 region of hippocampus were examined. Viable neurons were defined as cells showing a distinct nucleus and nucleolus. One observer masked to the experimental protocol counted the number of normal-appearing pyramidal neurons per high-power field (×400). The number of neurons in the CA1 region of hippocampus was quantified as the mean number from five sections (5-μm thickness) per rat (5 rats in sham group, 20 rats in CA group, HC group and HAase group). In each section, the number of neurons was averaged from three random different vision fields in the CA1 region under microscope (×400).
Western blotting
Denatured protein samples from cortex and hippocampus of every rat were resolved on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane (Millipore, Billerica, MA). After blocking, membrane was incubated overnight at 4℃ with antibodies including anti-ICAM-1 (1:500; Abcam, Cambridge, United Kingdom), anti-VCAM-1 (1: 1000; Abcam), anti-MMP-9 (1:1000; Abcam), anti-COX-2 (1:500; Abcam), anti-ZO-1 (1:500; Abcam), anti-Occludin (1:50000; Abcam), and anti-iNOS (1:1000; CWBIO, Beijing, China). Bound primary antibodies were detected with the anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000; CWBIO). All signals were detected by the enhanced chemiluminescence detection method (Millipore). The densities of protein bands were quantified using Image J (National Institutes of Health, Bethesda, MD) and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels.
Statistical analysis
All data were presented as means ± SDs. Continuous data were analyzed with a one-way analysis of variance with Bonferroni correction for post hoc comparison between multiple experimental groups. Difference in survival rate was analyzed using the log-rank testing. SPSS 20.0 (IBM, Armonk, NY) and GraphPad Prism 5.0 (GraphPad, La Jolla, CA) were used for statistical analyses. p < 0.05 was considered statistically significant.
Results
Glycocalyx degradation occurred in the cerebral capillaries of hippocampus after ROSC
The endothelial glycocalyx of cerebral capillaries in hippocampus was assessed with electron microscopic photographs.
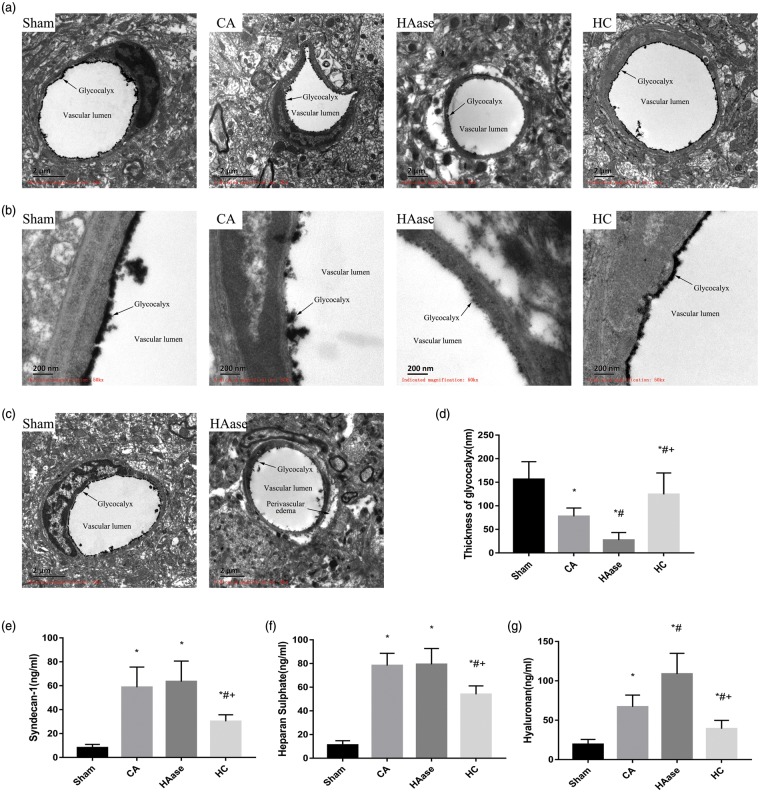
Overall view of glycocalyx was depicted in Figure 2. Sham group without CA/CPR showed an intact glycocalyx. On the contrary, only a residual glycocalyx was visualized in CA group. Few glycocalyx was observed in HAase group, indicating more serious damage than CA group. HC group showed a relatively intact glycocalyx compared to CA group (Figure 2(a) and (b)). Furthermore, HAase treatment significantly increased the pericapillary space (Figure 2(c)).
Figure 2.
Electron microscopic views of the cerebral capillaries endothelial glycocalyx in cortex and hippocampus 24 h after CA/CPR. (a) Electron microscopic overview of lanthanum-stained rat cerebral capillaries (bar = 2 μm). (b) Detailed pictures of glycocalyx on cerebral capillaries (bar = 200 nm). Sham group without CA/CPR showed an intact glycocalyx. Only a residual glycocalyx could be visualized in CA group. Little glycocalyx could be visualized in HAase group. A relatively intact glycocalyx could be seen in HC group. (c) The endothelial glycocalyx was nearly completely degraded, and a significant formation of edema was visualized in HAase group. (d) The width of the endothelial glycocalyx (nm, mean ± SD) of cerebral capillaries. Measurements of constitutional parts of the glycocalyx in plasma at 24 h after ROSC. (e) Syndecan-1; (f) heparan sulfate; (g) hyaluronan. Data are presented as mean ± SD. *p < 0.05 vs. sham group. #p < 0.05 vs. CA group. + p < 0.05 vs. HAase group. CPR: cardiopulmonary resuscitation; SD: standard deviation; ROSC: return of spontaneous circulation. Sham: sham group; CA: CA group; HAase: hyaluronidase group; HC: hydrocortisone group.
The width of the endothelial glycocalyx of cerebral capillaries were measured as shown in Figure 2(d). The average width was 156 ± 34 nm in sham group and reduced somewhat to 77 ± 16 nm in CA group (p < 0.05). Only rudimentary glycocalyx was measured 27 ± 15 nm in HAase group (p < 0.05 vs. CA group). On the other hand, HC treatment protected glycocalyx, with thickness of about 124 ± 42 nm (p < 0.05 vs. CA group).
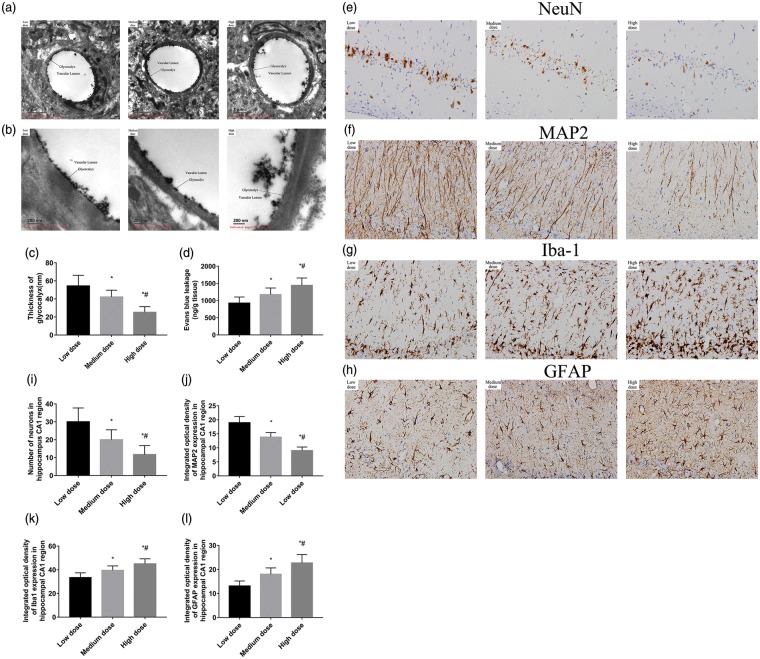
Dose-dependent response of HAase on glycocalyx was also investigated. There was a significant difference in the diameter of glycocalyx in the endothelial cells of the brain among the three different HAase dose groups. The average width was 54 ± 11 nm in low dose group, 42 ± 7 nm in medium dose group, and 25 ± 6 nm in the high dose group. Results revealed that as higher dose of HAase was administered, the more seriously the glycocalyx was degraded (Figure 3(a) to (c)).
Figure 3.
Electron microscopic views of the cerebral capillaries endothelial glycocalyx in cortex and hippocampus 24 h after CA/CPR. (a) Electron microscopic overview of lanthanum-stained rat cerebral capillaries (bar = 2 μm). (b) Detailed pictures of glycocalyx on cerebral capillaries (bar = 200 nm). (c) The width of the endothelial glycocalyx (nm, mean ± SD) of cerebral capillaries. (d) Blood–brain barrier permeability evaluated using Evans blue in the whole brain at 24 h after CA/CPR (n = 6 per group). (e–h) Representative photomicrographs of immunohistochemistry for NeuN, MAP2, Iba-1, and GFAP in the hippocampal CA1 region of the three groups at seventh day after ROSC. All images were captured at ×400 magnification. Scale bar indicates 100 μm, n = 6 for all groups. (i–l) Semi-quantitative results of NeuN, MAP2, Iba-1, and GFAP, respectively. NeuN staining cells are reported as positive number, while MAP2, Iba-1, and GFAP staining as the relative intensity of staining (percent) of the area. Data are presented as mean ± SD. Low dose: low dose group; Medium dose: medium dose group; High dose: high dose group. *p < 0.05 vs. low dose group. #p < 0.05 vs. medium dose group. CA: cardiac arrest; CPR: cardiopulmonary resuscitation; SD: standard deviation; NeuN: neuronal nuclei; MAP2: microtubule-associated protein 2; Iba-1: ionized calcium-binding adapter molecule 1; GFAP: glial fibrillaryacidic protein; ROSC: return of spontaneous circulation.
These data demonstrated that glycocalyx structure was incompletely injured in the model rats, severely disrupted by HAase, and preserved by HC. Moreover, HAase has a dose-dependent effect on the degradation of glycocalyx.
Increased degradation of glycocalyx in serum were detected after ROSC
Serum levels of syndecan-1, HS, and HA indicate the degradation of glycocalyx. In all groups, serum samples collected at 24 h after CA were used for assessing shedding of syndecan-1, HS, and HA.
As shown in Figure 2(e), CA/CPR enhanced shedding of syndecan-1 by approximately seven-fold compared with sham group (58.72 ± 15.40 ng/ml, 8.08 ± 2.60 ng/ml, p < 0.05). Application of HC significantly decreased shedding of syndecan-1 to 30.22 ± 5.04 ng/ml.
Similarly, serum HS was detected in the serum as shown in Figure 2(f). CA and HAase treatment induced an approximately eight-fold heightened washout of HS (78.28 ± 9.46, 79.30 ± 12.21 ng/ml) from the glycocalyx compared with sham group (11.12 ± 3.36 ng/ml). HC significantly lowered the release of HS to 53.93 ± 6.53 ng/ml (vs. CA group, p < 0.05).
HA was also detected in the blood as shown in Figure 2(g). CA group induced an approximately three-fold washout of HA from the glycocalyx. Application of HC significantly decreased shedding of HA to 39.22 ± 9.68 ng/ml compared with CA group (66.89 ± 13.77 ng/ml), by contrast, usage of HAase increased the shedding to 108.63 ± 24.07 ng/ml (HC group and HAase group vs. CA group, p < 0.05).
These data were in agreement with the results of electron microscopy, which revealed that glycocalyx was injured in the model rats. HAase selectively degraded HA and had no effect on HS and syndecan-1.
Degradation of glycocalyx increased the permeability of the BBB and aggravated brain edema
Since endothelial glycocalyx is an important contributor to BBB function, we wanted to check whether BBB permeability would be altered after degradation of glycocalyx.
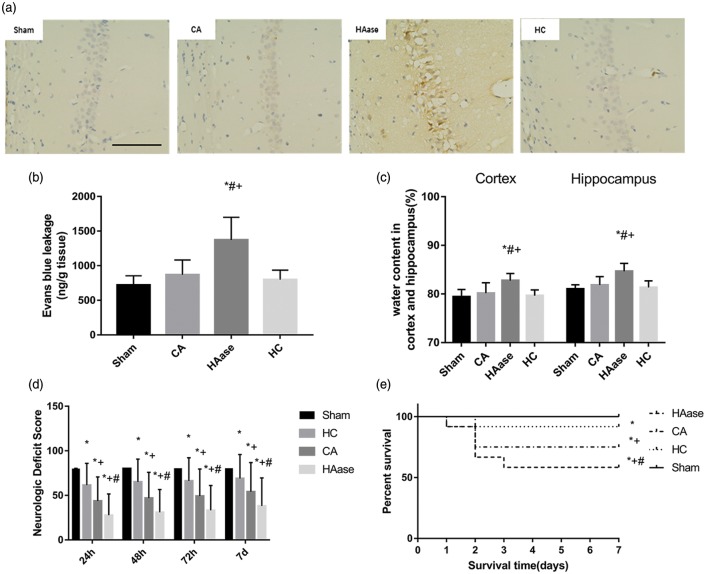
BBB permeability was assessed in the brain using EB and IgG extravasation assays at 24 h after ROSC. IgG in the hippocampus CA1 region was visualized by peroxidase-based immunohistochemistry. There was no significant visible leakage of IgG in CA group, sham group, and HC group. But there was a remarkable reveal in HAase group (Figure 4(a)). There was no significant difference in permeability of EB in CA group, sham group, and HC group. But there was a significant difference in permeability of EB in HAase group compared with the CA group (1371.67 ± 298.35 and 868.33 ± 195.14 ng/g tissue, p < 0.05, Figure 4(b)). In the three different HAase dose groups, the BBB permeability of EB was increased and the HAase has a dose-dependent effect on the EB permeability. There was a significant difference in the EB permeability among these groups. The high dose group rats displayed the highest BBB permeability (Figure 3(d)).
Figure 4.
(a) IgG in the hippocampus was visualized by peroxidase-based immunohistochemistry. There was no significant visible leakage of IgG in CA group, sham group, and HC group. But there was a remarkable reveal in HAase group. Bar, 100 μm. (b) Blood–brain barrier permeability evaluated using Evans blue in the whole brain at 24 h after CA/CPR (n = 5 per group). (c) Water content in the cortex and hippocampus at 24 h after CA/CPR in sham, CA, HC, and HAase groups (n = 5 per group). (d) Neurologic deficit scores of all rats at 24, 48, 72 h, and 7 days after CA/CPR. (e) Survival rate of rats during seven-day follow-up after CA/CPR. Hydrocortisone improved survival and neurologic outcome after CA/CPR. Data are presented as mean ± SD. Sham: sham group; CA: cardiac arrest group; HAase: hyaluronidase group; HC: hydrocortisone group; CPR: cardiopulmonary resuscitation. *p < 0.05 vs. sham group. #p < 0.05 vs. CA group. + p < 0.05 vs. HC group.
The water content of cortical tissue 24 h after ROSC was 79.42% ± 1.37% in sham group. It was not significantly lower than the water content of the HC group (79.67% ± 1.07%) and CA group (80.17% ± 1.95%). But HAase treatment had significantly increased the cortical water content (82.74% ± 1.35%) compared with that in CA group (p < 0.05). Similarly, the water content of the hippocampus was significantly higher in HAase group than in CA group 24 h after ROSC (84.67% ± 1.49% vs. 81.83% ± 1.57%, respectively, p < 0.05).
These data suggested that the abolishment of glycocalyx by HAase accompanied with CA/CPR significantly and dose-dependently increased the BBB permeability of macromolecules.
Degradation of glycocalyx deteriorated the seven-day survival rate and neurologic outcome
There was no significant difference in physiologic variables among four groups at baseline (Supplementary Table 1).
We compared NDS and seven-day survival rate in each group. As shown in Figure 4(d), at 24 h, 48 h, 72 h, and seven-day after ROSC, the NDS of the HAase group were significantly worse than that of CA group (p < 0.05), and the NDS of the HC group were significantly better than that of CA group (p < 0.05).
The survival rate was lowest in HAase group (58.3%, 5 of 12). Nine rats (75%, 9 of 12) survived in CA group compared with 11 rats (91.6%, 11 of 12) in HC group until day 7 at the end of the experiment (p < 0.05, Figure 4(e)). These data suggested that treatment by HAase significantly deteriorated neurologic outcome and the seven-day survival rate. On the contrary, treatment by HC ameliorated them.
Glycocalyx degradation aggravated neuronal death and activation of microglia and astrocytes in the hippocampus CA1 region
The death of neurons and the activation of glial cells were detected by immunohistochemistry in each group.
As depicted in Figure 5(a), the number of NeuN-positive cells was significantly reduced in the CA1 region of CA group compared with sham group and the loss of NeuN-positive cells was mitigated by HC treatment, and exacerbated by HAase treatment (p < 0.05). CA group showed decreased MAP2 immunostaining in the CA1 region compared to sham group, and the loss of MAP2 immunoreactivity was mitigated by HC treatment, and exacerbated by HAase treatment (p < 0.05, Figure 5(b)). The number of Iba-1-positive microglia and GFAP-positive astrocytes were increased in the CA1 region of CA group compared with sham group, and the activity of astrocytes and microglia was mitigated by HC treatment, and exacerbated by HAase treatment (p < 0.05, Figure 5(c) and (d)).
Figure 5.
Neuronal death and activation of microglia and astrocytes after CA/CPR. (a–d) Representative photomicrographs of immunohistochemistry for NeuN, MAP2, Iba-1, and GFAP in the hippocampal CA1 region of the sham group and the experimental groups at seventh day after ROSC. All images were captured at × 400 magnification. Scale bar indicates 100 μm, n = 5 for sham group, and n = 20 for experimental groups. (e–h) Semi-quantitative results of NeuN, MAP2, Iba-1, and GFAP, respectively. NeuN staining cells are reported as positive number, while MAP2, Iba-1, and GFAP staining as the relative intensity of staining (percent) of the area. Data are presented as mean ± SD. Sham: sham group; CA: cardiac arrest group; HAase: hyaluronidase group; HC: hydrocortisone group; CPR: cardiopulmonary resuscitation; NeuN: neuronal nuclei; MAP2: microtubule-associated protein 2; Iba-1: ionized calcium-binding adapter molecule 1; GFAP: glial fibrillaryacidic protein; ROSC: return of spontaneous circulation; SD: standard deviation. *p < 0.05 vs. sham group. #p < 0.05 vs. CA group. + p < 0.05 vs. HC group.
These data suggested that HAase treatment increased CA/CPR-induced neuron loss and activation of glia cells, whereas HC could decrease them in the brain cortex and hippocampus.
Representative photomicrographs of immunohistochemistry for NeuN, MAP2, Iba-1, and GFAP in the hippocampal CA1 region of the three different doses groups at seventh day after ROSC were shown in Figure 3(e) to (h). There were significant differences in neuronal loss and glial activation in the hippocampus CA1 region among the high, medium, and low dose groups, suggesting that degradation of glycocalyx aggravated brain injury after asphyxia CA/CPR.
Degradation of glycocalyx exacerbated CA/CPR-induced increases of inflammatory factors expression
We further explored the molecular mechanism of neuron loss and BBB damage after degradation of glycocalyx.
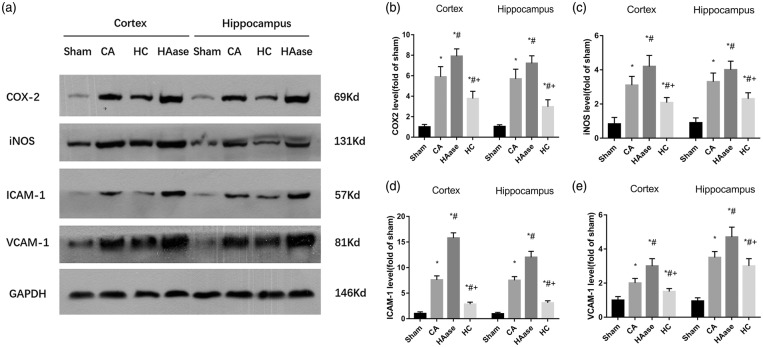
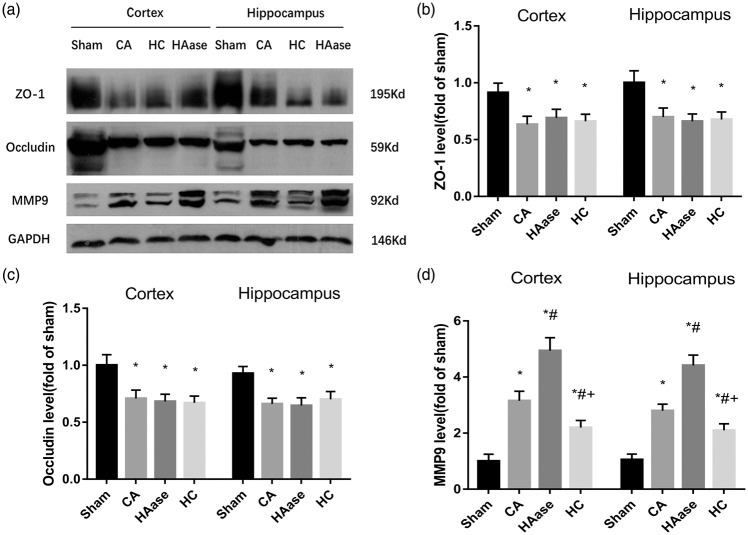
As shown in Figure 6, a significant increase in VCAM-1, ICAM-1, iNOS, and COX-2 protein was detected in the cortex and hippocampus at 24 h after ROSC in the CA group when compared with the sham group (p < 0.05). Importantly, HAase treatment was associated with significantly higher levels of VCAM-1, ICAM-1, iNOS, and COX-2 protein in the cortex and hippocampus compared with the CA group (p < 0.05). HC treatment was associated with significantly lower levels of VCAM-1, ICAM-1, iNOS, and COX-2 protein in the cortex and hippocampus compared with the CA group (p < 0.05). All these are induced by stress/inflammation and HC reduces these by blocking nuclear events. As shown in Figure 7, a significant decrease in ZO-1, Occludin protein was detected in the cortex and hippocampus at 24 h after ROSC in the CA group when compared with the sham group (p < 0.05). However, there were no significant differences in ZO-1, Occludin protein in the cortex and hippocampus between CA group, HAase group and HC group (p < 0.05). Similarly, MMP-9 activities significantly increased in the cortex and hippocampus at 24 h after ROSC in the CA group when compared with the sham group, upper and lower levels of MMP-9 separately existed in HAase group and HC group (p < 0.05).
Figure 6.
Representative western blot pictures of COX-2, iNOS, ICAM-1, and VCAM-1 are shown (n = 6 rats per group) (a). Quantification of the blot densities (b–e). Results are expressed as percentage of the sham group. Data are presented as mean ± SD. COX-2: cyclooxygenase-2; iNOS: inducible nitric oxide synthase; ICAM-1: intercellular cell adhesion molecule-1; VCAM-1: vascular endothelial adhesion factor; SD: standard deviation; Sham: sham group; CA: cardiac arrest group; HAase: hyaluronidase group; HC: hydrocortisone group. *p < 0.05 vs. sham group. #p < 0.05 vs. CA group. + p < 0.05 vs. HAase group.
Figure 7.
Representative western blots for ZO-1, Occludin levels, and MMP-9 activities in the cortex and hippocampus at 24 h after CA/CPR (n = 6 per group) are shown (a). Quantification of the blot densities (b–d); Results are expressed as percentage of the sham group. Data are presented as mean ± SD. ZO-1: zonula occludens-1; Occludin: occludin protein; MMP-9: matrix metalloproteinase-9; CPR: cardiopulmonary resuscitation; SD: standard deviation; Sham: sham group; CA: cardiac arrest group; HAase: hyaluronidase group; HC: hydrocortisone group. *p < 0.05 vs. sham group. #p < 0.05 vs. CA group. + p < 0.05 vs. HAase group.
These data suggested that HAase treatment increased CA/CPR-induced expressions of inflammatory factors, whereas HC could decreased them in the cortex and hippocampus.
Discussion
In this study, we observed the pathophysiological role of glycocalyx degradation on brain injury and BBB dysfunction during PCAS in the model of CA/CPR in rats. Our result showed that degradation of glycocalyx aggravated brain injury compared with CA group and caused an increase in BBB permeability, resulting in vasogenic brain edema. On the contrary, protection of the glycocalyx reduced brain damage. The smallest diameter of glycocalyx was observed in HAase group by electron microscopy, which indicated that glycocalyx degradation was the most significant. The diameter of glycocalyx was negatively correlated with the degree of neuron loss, neuroinflammation activation, and BBB dysfunction. Our data suggest that the integrity of the glycocalyx has a significant impact on the BBB permeability and pathophysiology of brain damage after CA/CPR, so its preservation could be a new strategy of cerebral protection.
The unique structure and location of the glycocalyx determine the fatal role in maintaining the low BBB permeability.21 However, the status of endothelial glycocalyx in cerebral capillaries and its contribution to BBB dysfunction and brain edema after ischemia insult has never been reported anywhere. In this study, we checked the relationship between the glycocalyx and BBB after CA/CPR. Although undergoing notable ischemia/perfusion injury, studies have shown that there is no significant brain edema and BBB damage in the rat model CPR. The result of Erika et al. shown that there was no BBB leakage over 24 h to conventional small or large molecule tracers after 9-min of asphyxial CA.22 In our study, BBB leakage was not seen 24 h after CA/CPR in CA group with moderate glycocalyx degradation. IgG and EB leakage and prominent brain edema were observed in HAase group with severest glycocalyx degradation. A study shows that hydroxyethyl starch extravasation after hypoxia/reoxygenation did not increase statistically significantly, which may be due to less severe endothelial cell perturbation.23 In addition, we treated rats with different doses of HAase before CA/CPR. In high dose group, the glycocalyx were degraded most seriously, and the BBB EB permeability was the highest. After HAase treatment in healthy rats, severe glycocalyx degradation and increased BBB permeability were also observed. Therefore, it could be speculated that severe disruption of glycocalyx might be the key factor to increase the permeability of BBB. The mechanism of increased BBB permeability after the destruction of glycocalyx was as below. First, the glycocalyx plays an important role in maintaining the low permeability of BBB due to its matrix-like structure as well as the charge. Yuan et al. found that the permeability of positively charged ribonuclease was four times as large as that of negatively charged a-lactalbumin in intact rat pial microvessels.24 Second, endothelial glycocalyx may mediate the transcellular transport of endothelial cells. One study indicated that the negative charge of the surface glycocalyx played a pivotal role in transcellular transport of nanoparticles of diameters ranging from 20 to 100 nm.25 Third, after glycocalyx degradation, inflammatory factors such as MMPs and vascular endothelial growth factors (VEGFs) were increased, causing disruption of tight junctions and BBB hyperpermeability. Since the high mortality in HAase group is due to status epilepticus and severe paralysis, we speculate that glycocalyx degradation worsen brain damage by aggravating BBB damage and brain edema within the perivascular space after CA/CPR. In addition, HS and syndecan-1 were not statistically significantly different between CA group and HAase group, suggesting that HAase could affect glycocalyx permeability in the absence of other glycosaminoglycan degradation and associated gross increases in capillary wall permeability.26
In CA group rats of our study, glycocalyx was mildly injured and no detectable BBB leakage was noticed, but neuronal necrosis and inflammatory activation were observed. The mechanism of delayed neurons loss and glial activation in hippocampus CA1 area after CPR were complex, autophagic, and apoptosis of neurons may be involved in Cui et al.27 Some researchers showed that BBB didn't damage in 9-min asphyxia CPR model,22 which implied that neurons loss may stem from hypoxia, apoptosis, and dilatation of the cell itself, not only the degradation of glycocalyx. Therefore, many other mechanisms, not just BBB leakage and neuroinflammation, mediate neuronal injury after CPR.
Glial activation and upregulation of cell adhesion molecules and proinflammatory cytokines were found in the cortex and hippocampus in rats suffering CA/CPR. We found significant higher expression of ICAM-1, VCAM-1, COX-2, iNOS, and MMP-9 in the cortex and hippocampus 24 h after CA/CPR in HAase group. This supported the hypothesis that glycocalyx damage led to endothelial cell activation28 and excessive activation of inflammation.29 The adhesion molecules such as VCAM-1 and ICAM-1 are relatively short and are harbored within the glycocalyx, so they do not have chance to contact the circulating blood cells under physiological circumstances.30 However, when the glycocalyx is structurally degraded under ischemia/reperfusion stimulation, the activated adhesion molecules are largely exposed. ICAM-1 and VCAM-1 promote inflammation by mediating leukocytes adhesion to the endothelial cells and eventually migration into brain tissue.31 Moreover, results from Karli et al. suggested that the glycocalyx lead to an increase in leukocyte adhesion by an increase of ICAM-1 expression and NF-κB activity. The NF-κB regulated expressions of iNOS and COX-2.32 Proinflammatory cytokines such as VCAM-1 and free radicals promote the production of MMPs, a well-established destructive mediator of BBB both in cerebral ischemia and inflammation studies. In our study, we did observe significantly increased MMP-9 in HAase group. The NF-κB regulated expressions of MMP9. Glycocalyx can also be shed by MMPs,33,34 particularly MMP-9 and MMP-2. Considerable evidence suggest that MMPs have the ability to modify components of the endothelial glycocalyx and thus facilitate the shedding of its constituents under pathological conditions. Taken together, ours and other studies support that glycocalyx degradation trigger the BBB dysfunction and neuroinflammatory activation, local inflammation, and coagulation further destruct the glycocalyx. Glycocalyx degradation, BBB dysfunction, brain edema, and inflammation are all the factors work as a vicious cycle contributing to the development of pathophysiology of the PCAS.
This study further confirmed the protective effect of HC on the glycocalyx against brain injury after CA/CPR. Previous studies have revealed that HC can preserve the glycocalyx in the face of ischemia/reperfusion29 or TNF-α mediated inflammation.35 HC can limit inflammatory damage to the glycocalyx by suppressing cytokines and chemokines production, as well as by preventing the migration of inflammatory cells and mast cell degranulation. HC seems to have a multifactorial impact on the post-ischemia reperfused injury. Tsai et al. reported that glucocorticoid administered during CPR significantly increased ROSC rate.36 Yang et al. found that glucocorticoid inhibits ICAM-1 and MMP-9 expression and hence reduces brain edema in intracerebral hemorrhagic rats.37 Importantly, our study provided direct evidence that glycocalyx in cerebral capillaries was protected by HC after CPR (Figure 2(a) and (b)). Administration of HC decreased neuron loss and increased the survive rate at day 7 after CPR. Together, our study demonstrates that HC is an active intervention strategy for the treatment of CA/CPR. The detailed mechanisms of the glycocalyx-protective role of HC remain unclear and deserve further investigation.
Several limitations exist here should be taken into consideration in the future study. First, it is believed that endothelial glycocalyx is degraded within a few minutes after hypoxia/ischemia injury. However, we only observed the similarity in brain endothelial glycocalyx between 6 h and 24 h after CA/CPR, the dynamic changes of glycocalyx in the early phase after CA/CPR was not available. Second, brain injury after CA/CPR results in obvious vasogenic brain edema indicated by brain imaging in human beings. However, our current and previous studies did not observe obvious brain edema in rats after 8 or 10 min CA/CPR, suggesting a difference between human and rats. Different animals other than rat might be better model for the fair evaluation of glycocalyx and brain edema. Third, it is worth to find out the detail mechanism how glycocalyx degradation trigger the inflammatory response and destruction of the BBB.
In summary, our data, for the first time, demonstrate that perturbation of the endothelial glycocalyx in a rat model of asphyxia CA/CPR, severe glycocalyx degradation increase the permeability of BBB, is a potential pathologic component after ROSC. In the clinical treatment of PCAS, attention should be paid to the situations, which may disturb the glycocalyx, such as complicated infection, iatrogenic excessive fluid infusion, and hyperglycemia. Protection of this endothelial surface layer may be a potential therapeutic strategy for the treatment of PCAS. The glycocalyx needs to be studies in a similar manner in experimental forebrain hypoperfusion and stroke.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundation of China (No. 81271521, 81471339).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
JZ planned the study, wrote most of the manuscript, and performed the main experiments. SP, JY, YH, and YG coordinated study efforts and revised the manuscript. XL provided technical support. JZ and XL established the CA/CPR model and performed Western blotting experiments. All authors read and approved the final manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008; 118: 2452–2483. [DOI] [PubMed] [Google Scholar]
- 2.Dokken BB, Hilwig WR, Teachey MK, et al. Glucagon-like peptide-1 (GLP-1) attenuates post-resuscitation myocardial microcirculatory dysfunction. Resuscitation 2010; 81: 755–760. [DOI] [PubMed] [Google Scholar]
- 3.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002; 106: 562–568. [DOI] [PubMed] [Google Scholar]
- 4.Fink K, Schwarz M, Feldbrugge L, et al. Severe endothelial injury and subsequent repair in patients after successful cardiopulmonary resuscitation. Critical Care (London, England) 2010; 14: R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell D, Jacob M, Hofmann-Kiefer K, et al. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res 2009; 83: 388–396. [DOI] [PubMed] [Google Scholar]
- 6.Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv: Eur J Physiol 2007; 454: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landsverk SA, Tsai AG, Cabrales P, et al. Impact of enzymatic degradation of the endothelial glycocalyx on vascular permeability in an awake hamster model. Crit Care Res Pract 2012; 2012: 842545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol 2004; 286: H1672–H1680. [DOI] [PubMed] [Google Scholar]
- 9.Chignalia AZ, Yetimakman F, Christiaans SC, et al. The glycocalyx and trauma: A review. Shock 2016; 45: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuurbier CJ, Demirci C, Koeman A, et al. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol (1985) 2005; 99: 1471–1476. [DOI] [PubMed] [Google Scholar]
- 11.Jacob M, Saller T, Chappell D, et al. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol 2013; 108(3): 347, . [DOI] [PubMed] [Google Scholar]
- 12.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res 2003; 92: 592–594. [DOI] [PubMed] [Google Scholar]
- 13.Grundmann S, Fink K, Rabadzhieva L, et al. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation 2012; 83: 715–720. [DOI] [PubMed] [Google Scholar]
- 14.Huang K, Gu Y, Hu Y, et al. Glibenclamide improves survival and neurologic outcome after cardiac arrest in rats. Crit Care Med 2015; 43: e341–e349. [DOI] [PubMed] [Google Scholar]
- 15.Vogel J, Sperandio M, Pries AR, et al. Influence of the endothelial glycocalyx on cerebral blood flow in mice. J Cereb Blood Flow Metab 2000; 20: 1571–1578. [DOI] [PubMed] [Google Scholar]
- 16.Yini S, Heng Z, Xin A, et al. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol Scand 2015; 59: 160–169. [DOI] [PubMed] [Google Scholar]
- 17.Valable S, Montaner J, Bellail A, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab 2005; 25: 1491–1504. [DOI] [PubMed] [Google Scholar]
- 18.Chen B, Friedman B, Cheng Q, et al. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke 2009; 40: e666–e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzialowski I, Weber J, Doerfler A, et al. Brain tissue water uptake after middle cerebral artery occlusion assessed with CT. J Neuroimag 2004; 14: 42–48. [PubMed] [Google Scholar]
- 20.Neumar RW, Bircher NG, Sim KM, et al. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 1995; 29: 249–263. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Fu BM. An electrodiffusion model for the blood-brain barrier permeability to charged molecules. J Biomech Eng 2011; 133: 021002. [DOI] [PubMed] [Google Scholar]
- 22.Tress EE, Clark RS, Foley LM, et al. Blood brain barrier is impermeable to solutes and permeable to water after experimental pediatric cardiac arrest. Neurosci Lett 2014; 578: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annecke T, Fischer J, Hartmann H, et al. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br J Anaesth 2011; 107: 679–686. [DOI] [PubMed] [Google Scholar]
- 24.Yuan W, Li G, Zeng M, et al. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. Microvasc Res 2010; 80: 148–157. [DOI] [PubMed] [Google Scholar]
- 25.Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J Microencapsul 2013; 30: 49–54. [DOI] [PubMed] [Google Scholar]
- 26.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol 1999; 277: H508–H514. [DOI] [PubMed] [Google Scholar]
- 27.Cui D, Shang H, Zhang X, et al. Cardiac arrest triggers hippocampal neuronal death through autophagic and apoptotic pathways. Sci Rep 2016; 6: 27642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Berg BM, Nieuwdorp M, Stroes ES, et al. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep 2006; 58(Suppl): 75–80. [PubMed] [Google Scholar]
- 29.Chappell D, Dorfler N, Jacob M, et al. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010; 34: 133–139. [DOI] [PubMed] [Google Scholar]
- 30.Chappell D, Jacob M, Paul O, et al. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res 2009; 104: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich JB. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol 2002; 128: 58–68. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Chung JH, Yoon JS, et al. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-kappaB in LPS-stimulated RAW264.7 cells and mouse liver. J Ginseng Res 2013; 37: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Wu C, Korpos E, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep 2015; 10: 1040–1054. [DOI] [PubMed] [Google Scholar]
- 34.Lipowsky HH. Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discov Today Dis Models 2011; 8: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chappell D, Hofmann-Kiefer K, Jacob M, et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol 2009; 104: 78–89. [DOI] [PubMed] [Google Scholar]
- 36.Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA 2013; 310: 270–279. [DOI] [PubMed] [Google Scholar]
- 37.Yang JT, Lee TH, Lee IN, et al. Dexamethasone inhibits ICAM-1 and MMP-9 expression and reduces brain edema in intracerebral hemorrhagic rats. Acta Neurochirurg 2011; 153: 2197–2203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.