Abstract
Background and purpose
The status of collateral vessels has important clinical implications in acute ischemic stroke. To evaluate which components of ischemic symptoms were predictive of pretreatment collateral status, we tested the hypothesis that sub-item scores from the National Institutes of Health Stroke Scale (NIHSS) are associated with leptomeningeal collateral status in acute ischemic stroke with middle cerebral artery (MCA) occlusion.
Methods
This study included consecutive patients with acute M1 occlusion who underwent revascularization treatment for acute MCA infarction. We evaluated clinical factors and the NIHSS score according to the collateral status assessed by multiphase perfusion computed tomography.
Results
Eighty-six patients were included (48 good collateral status, 38 poor collateral status). The patients with poor collateral status were more likely to have a higher total NIHSS score (18 versus 11, p < 0.001) and atrial fibrillation (65.8% versus 41.7%, p = 0.026) than patients with good collateral flow. In a multiple logistic regression, the NIHSS sub-items such as profound “facial palsy” (score 2 versus 0–1) and “visual field defect” (score 2 versus 0–1) were independently associated with poor collateral status.
Conclusion
Among the NIHSS sub-items, severe facial palsy and visual field defect were associated with poor collateral status in acute MCA stroke with M1 occlusion. Decision on whether to treat these patients endovascularly should be made more cautiously due to the possibility of a poor outcome.
Keywords: Acute ischemic stroke, collateral circulation, endovascular thrombectomy, NIHSS
Introduction
In acute ischemic stroke with the occlusion of a major cerebral artery, retrograde collateral flows maintain cerebral perfusion within the ischemic area until anterograde flow is re-established.1,2 Pretreatment collateral status is an important determinant of clinical outcome for revascularization therapy. Poor collateral flow is associated with a poor recanalization rate, hemorrhagic transformation and infarction growth after revascularization therapy.3–5 Many imaging methods such as conventional angiography, computed tomography (CT) angiography, multiphase perfusion computed tomography (MPCT) and magnetic resonance imaging have been developed and applied to assess collateral status in acute ischemic stroke.1,6–9 However, these imaging studies may take significant time to perform and there is no consensus on the modality that is best suited to judging collateral status. Simpler and faster tools to predict collateral status in acute ischemic stroke are needed for clinicians in an emergency situation. We hypothesized that the most affected area would differ according to the distribution and status of collateral flow in acute ischemic stroke. As a result, the clinical manifestation would also differ.
This study aimed to evaluate the relationship between the National Institutes of Health Stroke Scale (NIHSS) sub-item scores as a proxy for clinical manifestation and retrograde collateral status in patients with acute middle cerebral artery (MCA) infarction who were eligible for revascularization treatment.
Methods
Patient selection and clinical evaluation
We retrospectively analyzed data collected from a consecutive registry of patients who were admitted to the university hospital stroke center for acute ischemic stroke. From July 2005 to December 2013, a total of 135 patients who had acute ischemic stroke in the MCA territory with major vessel occlusion and had received endovascular treatment were analyzed. Inclusion criteria for this study were as follows: (a) presentation within 6 hours of symptom onset; (b) performance of MPCT before endovascular therapy; (c) M1 segment of MCA occlusion on angiography; and (d) NIHSS score of 4 or more at the time of MPCT. This retrospective study was approved by Samsung Medical Center institutional review board, and the requirement for informed consent was waived.
Patients were evaluated based on demographic data, medical history, vascular risk factors and brain imaging results. The NIHSS score was assessed by a neurologist at the time of CT performance.
MPCT and collateral grade
All patients underwent MPCT before undergoing endovascular treatment. MPCT was performed with a helical scanner (High Speed Advantage; General Electric Medical Systems, Milwaukee, Wisconsin, USA), as previously reported.8,10 After pre-contrast CT imaging of the entire brain, contrast-enhanced MPCT images were taken. Non-ionic contrast material (68%, 100 ml; Optiray 320; Mallinckrodt Medical, Pointe-Claire, Quebec, Canada) was administered by a power injector into an antecubital vein (18-gauge intravenous cannula) at a rate of 3 ml/s. Images were then obtained with scan delays of 5 and 14 s (early phase), 23 and 32 s (mid phase), and 41 and 50 s (late phase). Mid-phase MPCT images were sent to a workstation and processed in standardized maximum intensity projection images.
MPCT scan collateral grades were classified as grades 0–3. Grade 0 was defined as no visible collateral or slow collaterals (visible only in the late phase) to the periphery of the occluded MCA territory, grade 1 was defined as relatively rapid collaterals (visible in the early and mid phase) to the periphery of the occluded MCA territory with persistence of some of the defect, grade 2 was defined as collaterals with slow (visible only in late phase) but complete collateral flow to the occluded MCA territory, and grade 3 was defined as relatively rapid (early and mid phase) and complete collateral blood flow to the vascular bed in the entire MCA territory by retrograde perfusion. In this study, grade 0 or 1 was designated as poor and grade 2 or 3 as good collateral flow. Two reviewers (JH and J-WC) assessed the MPCT with knowledge of the symptomatic side and site of occlusion. The opinion of a third assessor (MJL) was sought to resolve any disagreement.
Statistical analysis
We analyzed the differences between the groups using the Pearson χ2 test or linear-by-linear association for categorical variables, and the Student t test or Mann–Whitney U test for continuous variables. In addition, to find independent factors associated with poor collateral flow, logistic regression analyses were performed. We analyzed the data using a logistic regression model in which poor collateral status on MPCT was used as the dependent variable and demographic factors and individual NIHSS scores as independent variables. For this, all NIHSS sub-item scores were dichotomized by the median score. The motor arm and leg categories were given points irrespective of right or left side. Explanatory variables were tested one by one against the dependent variable “poor collateral,” and variables without significant association (p > 0.05) were removed from the model. Regression analysis was performed using a stepwise forward approach. All statistical analyses were performed using commercially available software (SPSS for Windows, version 22.0; SPSS Inc., Chicago, Illinois, USA).
Results
Among the 135 patients in the hyperacute stroke registry, 33 with internal carotid artery occlusion or other vessel territory infarction such as anterior cerebral artery or posterior circulation were excluded. Sixteen patients who had distal MCA occlusion (M2 or M3 segment) were also excluded from this study. Finally, a total of 86 patients with acute MCA infarction and M1 occlusion were enrolled in this study. In terms of the collateral grade, grade 0 was found in 14 (16.3%), grade 1 in 24 (27.9%), grade 2 in 35 (40.7%), and grade 3 in 13 (15.1%) patients. Baseline characteristics according to the collateral status are presented in Table 1. Atrial fibrillation was more frequently observed in the poor collateral group (p = 0.026) and the NIHSS score was lower in the patients with a higher collateral grade (p < 0.001). Other stroke risk factors, premorbid medication and time from symptom onset to the start of CT did not differ between the two groups.
Table 1.
Patient characteristics according to collateral status.
| Good collateral status (n = 48) | Poor collateral status (n = 38) | p value | |
|---|---|---|---|
| Age, years | 63.0 ± 15.1 | 65.6 ± 15.5 | 0.431 |
| Gender, male, n (%) | 26 (54.2) | 25 (65.8) | 0.276 |
| Comorbidities, n (%) | |||
| Hypertension | 26 (54.2) | 23 (60.5) | 0.554 |
| Diabetes mellitus | 14 (29.2) | 8 (21.1) | 0.392 |
| Dyslipidemia | 6 (12.5) | 7 (18.4) | 0.447 |
| Current smoker | 6 (12.5) | 5 (13.2) | 0.928 |
| Previous stroke history | 11 (22.9) | 8 (21.1) | 0.836 |
| Atrial fibrillation | 20 (41.7) | 25 (65.8) | 0.026 |
| Coronary heart disease | 2 (4.2) | 4 (10.5) | 0.250 |
| Antithrombotic medication, n (%) | |||
| Antiplatelet agent | 12 (25.0) | 9 (23.7) | 0.888 |
| Anticoagulation | 5 (10.4) | 8 (21.1) | 0.171 |
| Previous statin use, n (%) | 4 (9.3) | 6 (17.6) | 0.279 |
| Left MCA occlusion, n (%) | 21 (43.8) | 18 (47.4) | 0.738 |
| MCA occlusion due to atherosclerosis, n (%) | 17 (35.4) | 5 (13.1) | 0.072 |
| Onset to CT time, minutes | 106.3 ± 65.8 | 98.8 ± 53.9 | 0.559 |
| Baseline NIHSS score | 11 (7–15) | 18 (15–20) | <0.001 |
Values are represented as mean ± standard deviation or median (interquartile range).
CT: computed tomography; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale.
The distributions of individual NIHSS sub-item scores are shown in Table 2. Logistic regression analysis was performed to further evaluate independent predictors in the NIHSS sub-items for poor collateral status (Table 3). We excluded total NIHSS score as an independent predictor to avoid multicollinearity with the NIHSS sub-items. After adjusting for all variables that could be related to collateral status, atrial fibrillation was independently associated with poor collateral flow (odds ratio (OR) 4.431; 95% confidence interval (CI) 1.258–15.601; p = 0.020). In addition, profound facial palsy (score 2 versus 0–1) and visual field defect (score 2 versus 0–1) were independently predictive of a poor collateral status (OR 95.948; CI 8.866–1038.327; p < 0.001 and OR 4.352; CI 1.209–15.660; p = 0.024, respectively).
Table 2.
Baseline National Institutes of Health Stroke Scale sub-item scores.
| Item | NIHSS score |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| #1A Level of consciousness, n (%) | 64 (74.4) | 18 (29.9) | 4 (4.7) | ||
| #1B Questions, n (%) | 39 (44.3) | 10 (11.6) | 37 (43) | ||
| #1C Commands, n (%) | 52 (60.5) | 4 (4.7) | 30 (34.9) | ||
| #2 Gaze, n (%) | 37 (43.0) | 31 (36.0) | 18 (20.9) | ||
| #3 Visual fields, n (%) | 30 (34.9) | 6 (7.0) | 50 (58.1) | ||
| #4 Facial palsy, n (%) | 4 (4.7) | 24 (27.9) | 58 (67.4) | ||
| #5 Motor arm, n (%) | 6 (7.0) | 16 (18.6) | 14 (16.3) | 33 (38.4) | 17 (19.8) |
| #6 Motor leg, n (%) | 8 (9.3) | 24 (27.9) | 19 (22.1) | 30 (34.9) | 5 (5.8) |
| #7 Ataxia, n (%) | 83 (96.5) | 1 (1.2) | 2 (2.3) | ||
| #8 Sensory, n (%) | 30 (34.9) | 44 (51.2) | 12 (14.0) | ||
| #9 Language, n (%) | 45 (52.3) | 4 (4.7) | 5 (5.8) | 32 (37.2) | |
| #10 Dysarthria, n (%) | 5 (5.8) | 49 (57.0) | 32 (37.2) | ||
| #11 Extinction/inattention, n (%) | 53 (61.6) | 8 (9.3) | 25 (29.1) | ||
NIHSS: National Institutes of Health Stroke Scale.
Table 3.
Multivariable logistic regression analysis for predictors of poor collateral status.
| Crude OR (95% CI) | p value | Adjusted OR (95% CI) | p value | ||
|---|---|---|---|---|---|
| Age, per 1-year increase | 1.012 (0.983–1.041) | 0.426 | |||
| Male gender | 1.627 (0.676–3.917) | 0.277 | |||
| Hypertension | 1.297 (0.547–3.077) | 0.554 | |||
| Diabetes | 0.648 (0.239–1.757) | 0.393 | |||
| Dyslipidemia | 1.581 (0.483–5.170) | 0.449 | |||
| Atrial fibrillation | 2.692 (1.114–6.506) | 0.028 | 4.431 (1.258–15.601) | 0.020 | |
| Previous stroke/TIA | 0.897 (0.320–2.513) | 0.836 | |||
| Onset to CT time, per minute increase | 0.998 (0.991–1.005) | 0.563 | |||
| NIHSS items | |||||
| Level of consciousness | 1–2 versus 0 | 2.253 (0.840–6.047) | 0.107 | ||
| Questions | 1–2 versus 0 | 1.533 (0.647–3.631) | 0.331 | ||
| Commands | 1–2 versus 0 | 2.200 (0.911–5.311) | 0.080 | ||
| Gaze | 1–2 versus 0 | 2.901 (1.177–7.150) | 0.021 | ||
| Visual fields | 2 versus 0–1 | 7.135 (2.601–19.575) | <0.001 | 4.352 (1.209–15.660) | 0.024 |
| Facial palsy | 2 versus 0–1 | 45.571 (6.024–375.684) | <0.001 | 95.948 (8.866–1038.327) | <0.001 |
| Motor arm | 3–4 versus 0–2 | 5.250 (1.994–13.825) | 0.001 | ||
| Motor leg | 3–4 versus 0–2 | 3.702 (1.497–9.156) | 0.005 | ||
| Ataxia | 1–2 versus 0 | 0.999 | |||
| Sensory | 1–2 versus 0 | 2.506 (0.979–6.419) | 0.056 | ||
| Language | 2–3 versus 0–1 | 1.882 (0.302–11.729) | 0.498 | ||
| Dysarthria | 2 versus 0–1 | 2.692 (1.095–6.621) | 0.031 | ||
| Extinction/inattention | 1–2 versus 0 | 1.604 (0.471–5.468) | 0.450 | ||
CI: confidence interval; CT: computed tomography; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; TIA: transient ischemic attack.
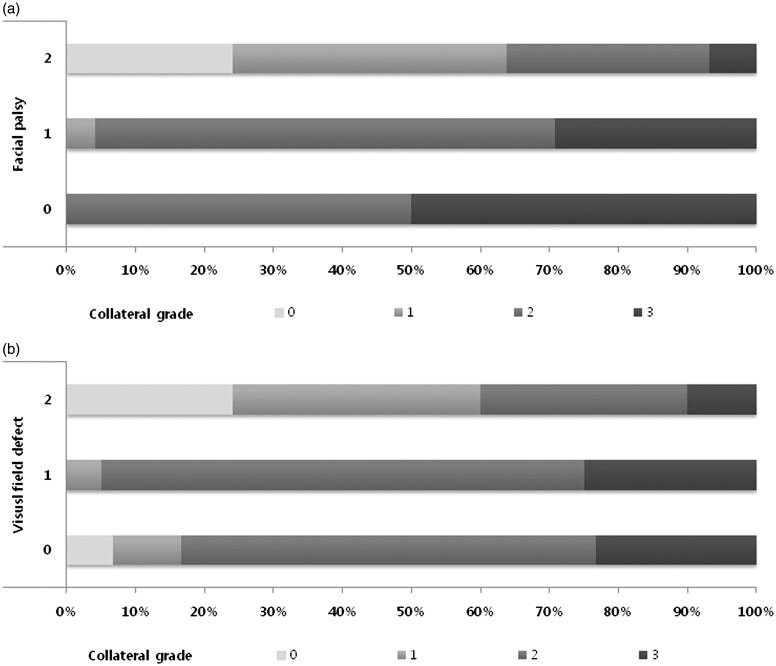
The distribution of the collateral grade with respect to facial palsy and visual field defect grades are shown in Figure 1. There was a more prominent correlation between the collateral grade and facial palsy grade than with visual field defect grade.
Figure 1.
Distribution of collateral grade according to severity of facial palsy (a) and visual field defect (b). Higher National Institutes of Health Stroke Scale sub-item scores were correlated with poorer collateral flow grade.
Discussion
The major finding of this study was that the NIHSS score and atrial fibrillation are associated with collateral flow in acute MCA infarction. This result is consistent with previous studies that examined factors related to collateral flow in acute ischemic stroke.9,11,12 In the present study, we focused on the NIHSS sub-items as a factor of neurological signs and found that facial palsy and visual field defect are related to poor collateral flow in acute MCA infarction with M1 occlusion.
Recent studies about endovascular intervention for acute ischemic stroke demonstrated that successful recanalization failed to improve functional outcome in patients with poor collateral flow.13,14 Adequate retrograde collateral flow can prolong tissue viability and maximize the volume of salvageable tissue until anterograde recanalization of occluded vessel.15,16 Thus, information regarding collateral blood flow has potential clinical applications for deciding upon the appropriate treatment and predicting outcome after endovascular treatment. Pretreatment collateral flow is known to be related to the clinical outcome after endovascular treatment. The ESCAPE trial excluded patients with poor collateral status and showed successful final outcomes.17 Several clinical factors such as atrial fibrillation, old age and tandem occlusion were known as independent predictors for collateral flow.9,16 In our results, atrial fibrillation was related to poor collateral flow in acute MCA infarction with M1 occlusion. This may be because atrial fibrillation-related stroke develops when the large vessel is suddenly blocked by emboli and there is little time for collateral vessels to be established.12
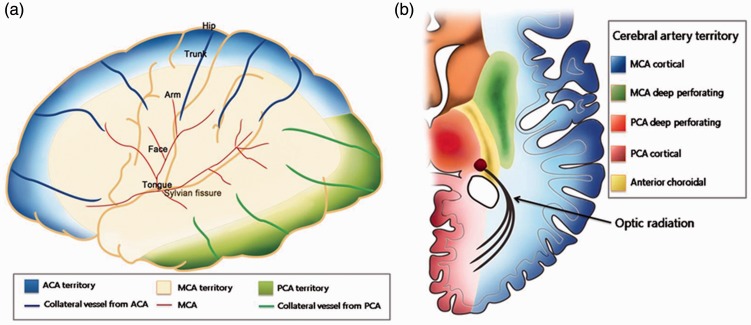
The NIHSS is commonly used to assess the severity of acute ischemic stroke.18 The NIHSS score is known to correlate with collateral status and the severity of the perfusion deficit.1,11 For these reasons, the NIHSS score has been used in thrombolysis clinical trials to include or exclude subjects from active treatment and serially calculated to evaluate clinical status.19 Although the NIHSS is the most widely used scoring system to assess neurologic deficit, the total NIHSS score has limitations in reflecting the status of cerebral tissue and vessels in acute ischemic stroke. Furthermore, the NIHSS sub-item scores differ with the location of the occluded vessel(s).20,21 We focused on MCA occlusion, the most common candidate for intra-arterial revascularization treatment, and found that facial palsy and visual field defect as single items of the NIHSS score were predictors for poor collateral status. These results may be explained by a reduction in distal cerebral perfusion pressure in acute proximal MCA occlusion. Reductions in cerebral perfusion pressure in the cortical MCA territory are most severe at its center (the perisylvian region) and least severe in the watershed areas with other cortical vessels.22,23 The area representing the face in the cortical motor homunculus is located more closely to the perisylvian area than other motor areas (Figure 2(a)). A visual field defect in MCA infarction is usually caused by the involvement of the optic radiation.24 These deep white matter structures, which have fewer anastomoses, are also most severely affected by low blood flow in acute proximal MCA infarction (Figure 2(b)).
Figure 2.
Topography of leptomeningeal collateral flow (a) and the optic radiation (b).
ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery.
This study has several limitations. We used MPCT to evaluate collateral status. Although digital angiography is the gold standard to assess collateral vessels, this invasive approach is not routinely used and frequently omitted to save time prior to acute endovascular treatment. MPCT is a rapid and simple method to simultaneously exclude hemorrhagic stroke and examine vessel status. Second, despite statistical significance, there was a wide confidence interval in the logistic regression model. The number of patients in the study was rather low which leads to these statistical results. Due to the small number of subjects and many NIHSS subtypes, we could not perform stratification analysis according to lesion side. Fortunately, there was no difference in the lesion side depending on the degree of collateral flow. Another major shortcoming of our study with respect to its application to the clinical field is the narrow inclusion criteria. We included patients with MCA occlusion in this study similar to recent endovascular studies. Therefore, there may be restrictions on application in patients who are suspected of posterior circulation occlusion. This limitation should be addressed in future studies. Lastly, clinical outcome was not analyzed. Most endovascular treatments in this study did not use a retrievable stent that has an excellent and rapid recanalization result. We thought that the real effect of collateral flow on clinical outcome could be biased by the pre-retrievable stent era.
Additional studies using a stent retrieval device are needed to confirm our results and find the relationship between the NIHSS sub-items and clinical outcomes after endovascular treatment.
In conclusion, profound facial palsy and visual field defect in the NIHSS sub-items are associated with poor collateral flow in proximal MCA occlusion with infarction.
Acknowledgment
None.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors report no disclosures.
References
- 1.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009; 132: 2231–2238. [DOI] [PubMed] [Google Scholar]
- 2.Shuaib A, Butcher K, Mohammad AA, et al. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 2011; 10: 909–921. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008; 79: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 2235–2239. [DOI] [PubMed] [Google Scholar]
- 6.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–137. [DOI] [PubMed] [Google Scholar]
- 7.Lee KY, Latour LL, Luby M, et al. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology 2009; 72: 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Noh HJ, Yoon CW, et al. Multiphasic perfusion computed tomography as a predictor of collateral flow in acute ischemic stroke: comparison with digital subtraction angiography. Eur Neurol 2012; 67: 252–255. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Son JP, Ryoo S, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann Neurol 2014; 76: 356–369. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Lee KH, Na DG, et al. Multiphasic helical computed tomography predicts subsequent development of severe brain edema in acute ischemic stroke. Arch Neurol 2004; 61: 505–509. [DOI] [PubMed] [Google Scholar]
- 11.Marks MP, Lansberg MG, Mlynash M, et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke 2014; 45: 1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu HT, Campbell BC, Christensen S, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis 2010; 30: 389–395. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015; 313: 1451–1462. [DOI] [PubMed] [Google Scholar]
- 14.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebeskind DS, Tomsick TA, Foster LD, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014; 45: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke 2015; 46: 3302–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999; 53: 126–131. [DOI] [PubMed] [Google Scholar]
- 19.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Toyoda K, Uehara T, et al. Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology 2008; 70: 2371–2377. [DOI] [PubMed] [Google Scholar]
- 21.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005; 36: 2121–2125. [DOI] [PubMed] [Google Scholar]
- 22.Symon L, Pasztor E, Branston NM. The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: an experimental study by the technique of hydrogen clearance in baboons. Stroke 1974; 5: 355–364. [DOI] [PubMed] [Google Scholar]
- 23.Muir KW, Buchan A, von Kummer R, et al. Imaging of acute stroke. Lancet Neurol 2006; 5: 755–768. [DOI] [PubMed] [Google Scholar]
- 24.Rowe FJ, Wright D, Brand D, et al. A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. BioMed Res Int 2013; 2013: 719096. [DOI] [PMC free article] [PubMed] [Google Scholar]