Abstract
Background and purpose
A frequently reported drawback of ethylene vinyl alcohol copolymer-based liquid embolic agents is the production of artifacts in diagnostic imaging. New embolic agents, such as Precipitating hydrophobic injectable liquid (PHIL; MicroVention, Tustin, CA, USA), are supposed to induce significantly fewer artifacts. The purpose of this study is to assess the degree of artifacts induced by the liquid embolic agents Onyx (Medtronic Neurovascular, Irvine, CA, USA) and PHIL in conventional computed tomography (CT), cone-beam CT and magnetic resonance imaging (MRI) in an experimental in vivo model.
Materials and methods
In 10 pigs the rete mirabile was embolized with Onyx (n = 5) or PHIL (n = 5). After embolization, conventional CT, cone-beam CT and MRI were performed. The degree of artifacts was graded qualitatively (five-point scale; for CT and MRI) and quantitatively (HUs of well-defined regions of interest (ROIs); for CT only).
Results
Artifacts were significantly more severe for Onyx both in the qualitative (e.g. conventional CT: 2 versus 5 (medians); p = 0.008) and in the quantitative image analysis (e.g. cone-beam CT: standard deviation of a ROI near to the embolic agent cast, 94 HU versus 38 HU (medians); p = 0.008). Neither Onyx nor PHIL produced any apparent artifacts in MRI.
Conclusion
PHIL produces fewer artifacts than Onyx in conventional CT and cone-beam CT in an experimental in vivo model.
Keywords: Arteriovenous malformation, artifacts, embolization, liquid embolic agents
Introduction
Either as single treatment or in combination with stereotactic radiation therapy and microneurosurgery, endovascular embolization can be an effective treatment for cerebral arteriovenous malformations (AVMs).1 One of the most frequently used materials for AVM embolization is Onyx (Medtronic Neurovascular, Irvine, CA, USA), a liquid embolic agent (LEA), consisting of ethylene vinyl alcohol copolymer (EVOH), dimethyl-sulfoxide and tantalum powder, the latter causing radiopacity.2 A frequently reported drawback of Onyx is the production of artifacts in diagnostic imaging caused by the inherent tantalum in terms of streak artifacts in conventional and cone-beam computed tomography (CT) and, to a lesser extent, in terms of susceptibility artifacts in magnetic resonance imaging (MRI).3–7 These artifacts can be highly relevant in daily clinical practice as they can limit the diagnosis of peri- and postoperative complications and of residual or recurrent disease.3,4 Furthermore, the planning of subsequent stereotactic radiation therapy can be impeded and the dose and dose distribution of the radiation can be altered.5,7,8
New embolic agents are being introduced in order to enlarge the armamentarium of materials for endovascular embolization and to ultimately improve the outcome of endovascular treatment of AVMs.9–11 Precipitating hydrophobic injectable liquid (PHIL; MicroVention, Tustin, CA, USA) is a new, copolymer-based, nonadhesive, precipitating LEA that uses covalently bound iodine for radiopacity instead of tantalum. Several clinical and preclinical studies demonstrated that PHIL is suitable for endovascular embolization of AVMs and arteriovenous fistulas (AVFs) and, beyond that, described a low degree of artifacts in CT imaging.11–16 This low degree of artifacts was mentioned in all of these studies. However, these artifacts were neither assessed qualitatively or quantitatively nor compared to another LEA in any clinical or preclinical study.
The aim of the present study is the assessment of artifacts in conventional CT, cone-beam CT and MRI, caused by Onyx and PHIL, in an in vivo embolization model.
Materials and methods
Animal procedure
State Animal Care and Ethics Committee approval was obtained. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Ten landrace pigs with a weight of 38–42 kg were used. Anesthesia and sacrifice (two hours after intervention) were performed as described previously.17
Embolization
The rete mirabile (RM) is a fine vascular network, located bilaterally at the cranial base of pigs, consisting of vessels that are similar to human arterioles and has been used as an endovascular embolization model by a number of investigators.9,11,18–20
Embolization of the RM was performed using either Onyx or PHIL.21 Of the different concentrations that are available for these LEAs, the least viscous concentrations (Onyx 18 and PHIL 25%) were used to achieve effective filling of the RM. The required volume of LEA was assessed. The embolization technique, characteristics and results were published previously and were not the focus of this study; they were accordingly not evaluated in this work.21
Imaging
All examinations (conventional CT, cone-beam CT and MRI) were performed with standard settings according to clinical practice.
Conventional CT imaging was performed with a 64-slice multidetector scanner (Somatom Definition Flash; Siemens Healthineers, Erlangen, Germany) applying an automated dose optimization software (CareDose and CarekV; Siemens) with a dose setting for CT dose indexvol of 6.0 mGy and a collimation of 64 × 0.6 mm. CT images were reconstructed in the axial plane by applying an iterative reconstruction software (Admire 3/5; Siemens; reconstruction kernel: I30–30) with a slice thickness of 3 mm and an overlap of 2 mm.
Cone-beam CT was obtained on a monoplanar angiography system (Artis zee; Siemens) with the following parameters: six-second rotational acquisition generating 396 projections with an angular step of 0.5 degrees for a total coverage of 200 degrees with a pulse length of 5 ms and a dose per frame of 0.36 µGy. Images were reconstructed in the axial plane with a slice thickness of 1 mm.
MRI examinations were performed using a 1.5T MRI system (MAGNETOM Aera; Siemens) with a 12-channel-head-matrix coil. A three-dimensional (3D) T1-weighted (magnetization-prepared rapid gradient echo; matrix: 256 × 240, field of view (FOV): 240 × 200 mm, repetition time (TR): 1.2 ms, echo time (TE): 2.7 ms, flip angle: 12 degrees, slice thickness 1 mm), a 3D T2-weighted (constructive interference in steady state; matrix: 256 × 192, FOV: 200 × 150 mm, TR: 5.2 ms, TE: 2,3 ms, flip angle: 68 degrees, slice thickness: 1 mm) and a 3D susceptibility-weighted sequence ((SWI); matrix: 256 × 186, FOV: 230 × 208 mm, TR: 49 ms, TE: 40 ms, flip angle: 15 degrees, slice thickness: 2 mm) were acquired. Images were reconstructed in the axial plane with a slice thickness of 1 mm (T1- and T2-weighted) or 2 mm (SWI).
Artifacts
Qualitative image analysis
All reconstructed images were reviewed on a picture archiving and communication system work station (CENTRICITY PACS 4.0; GE Healthcare, Barrington, IL, USA) by three readers (D.F.V., C.M.S. and M.A.M. with 5, 12, and 14 years of experience in diagnostic imaging, respectively), blinded to the type of LEA. All analyses were performed on axial reconstructions. For each modality and each animal, all readings were performed on seven images: the image with the most severe artifacts and the three images cranial and caudal to this image, respectively. The observers were allowed to adjust the window. The artifact severity and the feasibility of evaluating the adjacent brain tissue were graded using a five-point Likert scale:22 (1) major artifacts, no depiction of anatomical structures; (2) marked artifacts, no depiction of surrounding tissue and considerably impaired image quality of distant tissue; (3) moderate artifacts, nondiagnostic in the surrounding tissue but fair diagnostic image quality of distant tissue; (4) minor artifacts with good image quality; and (5) no artifacts, full diagnostic image.
Quantitative image analysis
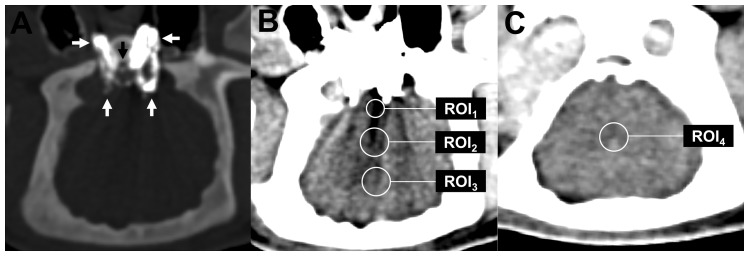
For objective measurement of the degree of artifacts, specific regions of interest (ROIs) were positioned and analyzed on the axial reconstruction with the most severe artifacts or, in the absence of explicit artifacts, on the reconstruction with the RM being most markedly filled, and on an axial reconstruction 20 mm cranial to the RM without any artifacts.22 Accordingly, as illustrated in Figure 1, four different ROIs were defined: ROI1: region with the most severe artifacts; ROI2: region near the RM, defined as 30 mm (referring to the center of the ROI) dorsal to the clivus, on the reconstruction with the most severe artifacts; ROI3: region distal to the RM, defined as 45 mm dorsal to the clivus, on the reconstruction with the most severe artifacts; ROI4 (control ROI): region cranial to the RM (normal brain tissue), not affected by artifacts. For precise assessment of the region with the most severe artifacts, the size of this ROI (ROI1) was set to 20 mm2, while the size of the remaining ROIs was set to 60 mm2.
Figure 1.
Illustration of the quantitative image analysis. (a) Demonstration of the hyperdense LEA cast (Onyx in this example; white arrows) after adjustment of the window (width: 2200 HU, level: 900 HU). In standard brain window ((b), (c)), the LEA cast was difficult to demarcate from the base of the skull because of its high density. (b) and (c) Four specific ROIs were defined in axial reconstructions (standard brain window; level: 80 HU, width: 40 HU): ROI1 in the region with the most severe artifacts, ROI2 near the RM (30 mm dorsal to the clivus (black arrow in (a))), ROI3 distal to the RM (45 mm dorsal to the clivus) and ROI4 cranial to the RM in a region without any apparent artifacts. HU: Hounsfield unit; LEA: liquid embolic agent; RM: rete mirabile; ROI: region of interest.
As a marker for the severity of the artifacts, the difference between the mean Hounsfield units (HU) in ROI1–3 (images potentially affected by artifacts) and the mean HU in ROI4 (image not affected by artifacts) was calculated, and defined as ΔMean-ROI1, ΔMean-ROI2 and ΔMean-ROI3, respectively. Since streak artifacts usually consist of very “bright” (high HU) and very “dark” (low HU) areas, mean HU can be normal despite there being severe artifacts. Therefore, the standard deviation (SD) of ROI1–4 was assessed, herein after referred to as SD-ROI1, SD-ROI2, SD-ROI3 and SD-ROI4. Accordingly, ΔROI1–3 and SD-ROI1–4 were compared between Onyx and PHIL for conventional CT and cone-beam CT. Since there were no artifacts in MRI in the qualitative image analysis, no quantitative image analysis of the MRI images was performed.
Statistics
Prism software (version 7.02; GraphPad, La Jolla, CA, USA) was used for data analysis. Quantitative data are presented as medians (lower quartile; upper quartile). For the qualitative image analysis, interreader agreement was assessed by using a weighted κ with a 95% confidence interval.23 The κ values were interpreted as follows: ≤ 0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, very good agreement.24 To evaluate statistical differences between the two study groups, the Mann-Whitney test was performed. P values < 0.05 were considered statistically significant.
Results
All embolization procedures were performed as planned. Examples of postinterventional conventional CT and cone-beam CT are demonstrated in Figure 2. Examples of postinterventional MRI are demonstrated in Figure 3. The volume of required LEA was not significantly different (p = 0.286) between Onyx (0.6 ml (0.45 ml; 0.95 ml)) and PHIL (0.5 ml (0.25 ml; 0.5 ml)).
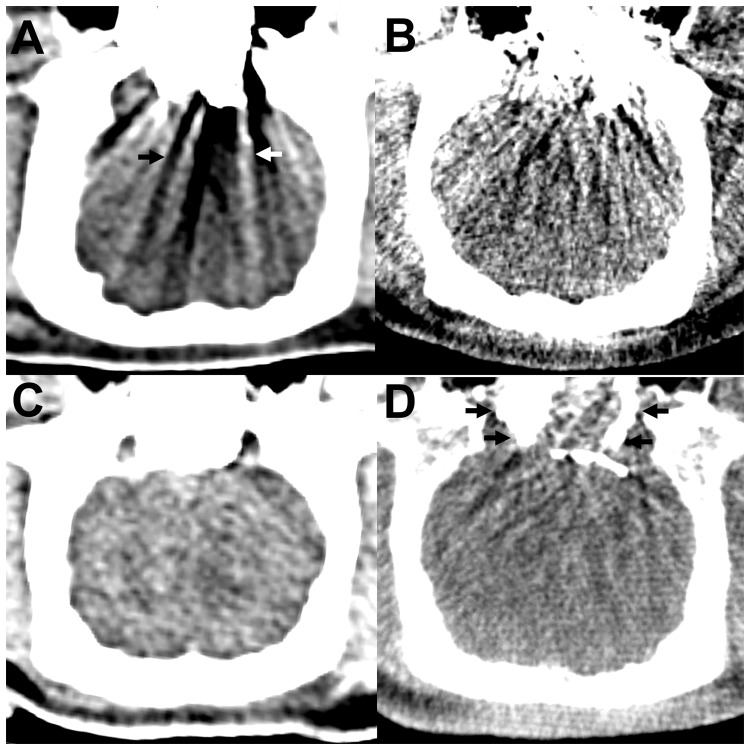
Figure 2.
Artifacts in conventional CT and cone-beam CT. (a) and (b) Conventional CT ((a) window: 80 HU, level: 40 HU), cone-beam CT ((b) window: 330 HU, level: 60 HU), axial reconstructions. For Onyx, there were severe streak artifacts in conventional CT and cone-beam CT, consisting of hypodense (black arrow) and hyperdense (white arrow) streaks. These artifacts were more pronounced in cone-beam CT. (c) and (d) Conventional CT (C; window: 80 HU, level: 40 HU), cone-beam CT (D; window: 330 HU, level: 60 HU), axial reconstructions. PHIL induced only mild artifacts in conventional CT and mild to moderate artifacts in cone-beam CT. Note the hyperdense PHIL cast in cone-beam CT (black arrows). CT: computed tomography; HU: Hounsfield unit; LEA: liquid embolic agent; PHIL: precipitating hydrophobic injectable liquid.
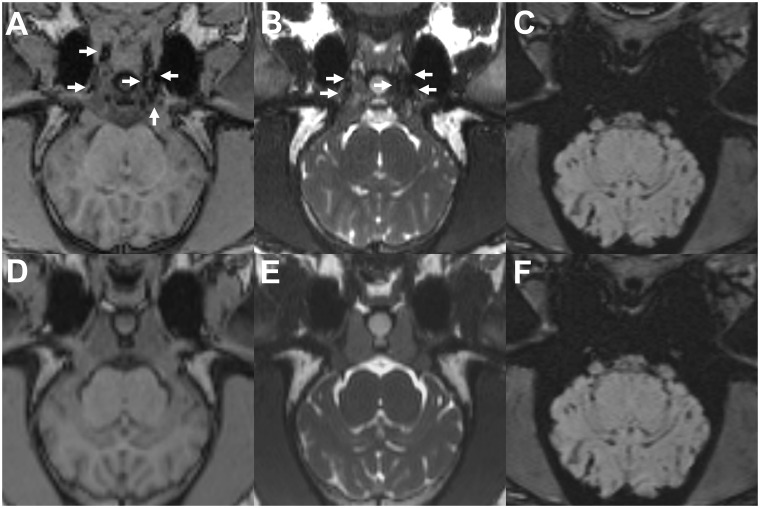
Figure 3.
Onyx and PHIL in MRI. Group Onyx ((a)–(c)) and Group PHIL ((d) and (e)); T1-weighted ((a) and (d)), T2-weighted ((b) and (e)) and susceptibility-weighted ((c) and (f)) images; axial reconstructions. Note the absence of any apparent artifacts in T1- and T2-weighted images. Onyx was visible as hypointense material in T1- and T2-weighted sequences (white arrows). In the susceptibility-weighted sequences, the LEA cast was not definable because of artifacts caused by the osseous, partially pneumatized skull base. PHIL could not be identified in MRI. LEA: liquid embolic agent; MRI: magnetic resonance imaging; PHIL: precipitating hydrophobic injectable liquid.
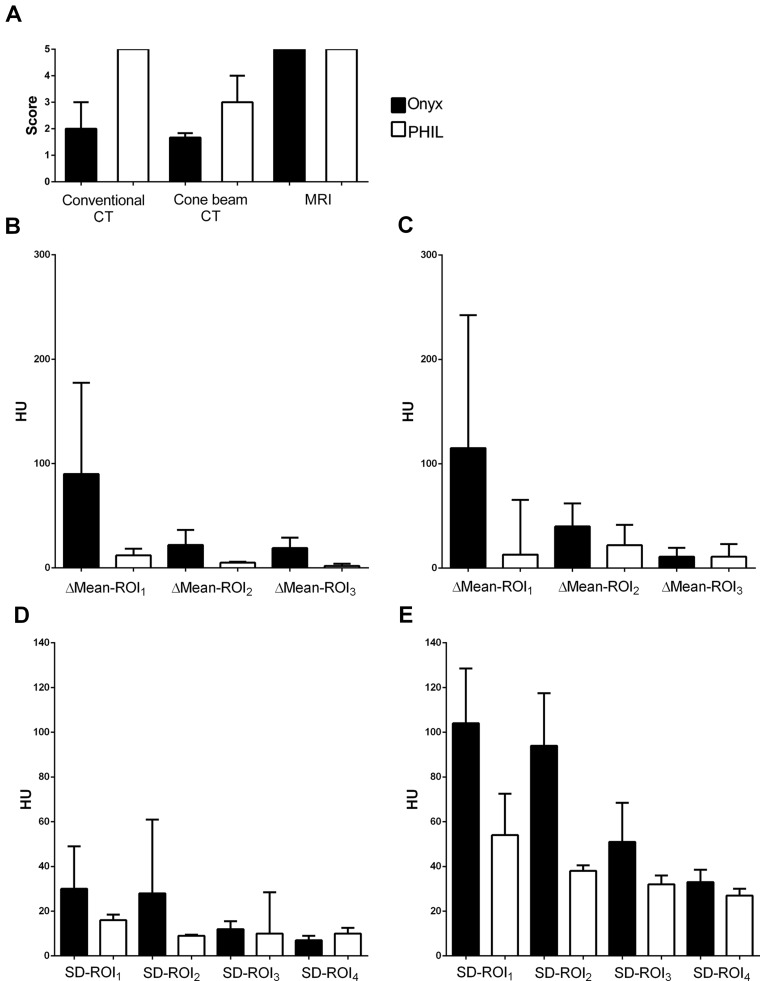
The results of the qualitative image analysis are illustrated in Figure 4. There was very good agreement (90% concordance, κ = 0.907 (range: 0.697–1.000)) for conventional CT and good agreement (70% concordance, κ = 0.726 (range: 0.514–0.939)) for cone-beam CT in the scoring of the artifacts in the qualitative image analysis. In conventional CT, artifact severity was graded higher (p = 0.008) for Onyx (2 (2; 3), all readers) than for PHIL (5 (5; 5), all readers). Also in cone-beam CT, artifacts were graded as more intense (p = 0.008) for Onyx (1 (1; 2), reader 1; 2 (1; 2), reader 2; 2 (1; 2), reader 3) when compared with PHIL (3 (3; 3), reader 1; 3 (3; 4), reader 2; 3 (3; 4), reader 3). In MRI, in T1- and T2-weighted sequences for both LEAs all readers did not detect any artifacts (a score of 5 for all images). In SWI, the LEA cast was not definable because of artifacts caused by the skull base.
Figure 4.
Qualitative and quantitative image analysis. Illustration of the results of the qualitative (a) and of the quantitative ((b)–(e)) image analysis. (b) Mean HU of the ROIs, conventional CT; (c) mean HU of the ROIs, cone-beam CT; (d) SD of the ROIs, conventional CT; (e) SD of the ROIs, cone-beam CT. Bars: median; whiskers: interquartile range. Black bars: Onyx; white bars: PHIL. In the qualitative image analysis there was a higher degree of artifacts for Onyx when compared with PHIL in conventional and cone-beam CT. Similarly, both markers for artifact severity (mean HU and SD of the HU of the specific ROIs) indicated more severe artifacts for Onyx. For both LEAs, artifacts were more pronounced in cone-beam CT than in conventional CT. CT: computed tomography; HU: Hounsfield unit; LEA: liquid embolic agent; PHIL: precipitating hydrophobic injectable liquid; ROI: region of interest.
The results of the quantitative image analysis are summarized in Table 1 and illustrated in Figure 4. The difference between the mean HU in the reconstruction with the most severe artifacts and the reconstruction without any apparent artifacts (ΔMean-ROI1–3) was significantly higher for Onyx for all ROIs on conventional CT and for the ROI in the region with the most severe artifacts (ΔMean-ROI1) only on cone-beam CT. The SD of the ROIs was significantly higher for Onyx for ROI1 and ROI2 on conventional CT and for ROI1, ROI2 and ROI3 on cone-beam CT.
Table 1.
Quantitative image analysis.
| Conventional CT | |||||||
|---|---|---|---|---|---|---|---|
| Mean-ROI1 |
ΔMean-ROI1 |
Mean-ROI2 |
ΔMean-ROI2 |
Mean-ROI3 |
ΔMean-ROI3 |
Mean-ROI4 |
|
| Onyx | −49 (−71; −5) | 90 (50; 121) | 19 (10; 22) | 22 (19; 31) | 34 (31; 38) | 19 (7; 22) | 44 (41; 45) |
| PHIL | 54 (27; 54) | 12 (11; 18) | 48 (40; 49) | 5 (1; 5) | 45 (40; 45) | 2 (1; 3) | 43 (42; 45) |
| p valuea | – | 0.008 | – | 0.008 | – | 0.016 | – |
| SD-ROI1 |
SD-ROI2 | SD-ROI3 | SD-ROI4 | ||||
| Onyx | 30 (29; 45) | 28 (12; 38) | 12 (9; 15) | 7 (7; 8) | |||
| PHIL | 16 (15; 17) | 9 (7; 9) | 10 (9; 16) | 10 (9; 10) | |||
| p valuea | 0.040 | 0.032 | 0.587 | 0.238 | |||
| Cone-beam CT | |||||||
| Mean-ROI1 |
ΔMean-ROI1 |
Mean-ROI2 |
ΔMean-ROI2 |
Mean-ROI3 |
ΔMean-ROI3 |
Mean-ROI4 |
|
| Onyx | −77 (−100; −53) | 115 (102; 175) | 16 (5; 26) | 40 (22, 44) | 44 (40; 56) | 11 (6; 19) | 49 (38; 60) |
| PHIL | 92 (55; 115) | 13 (11; 59) | 69 (22; 76) | 22 (21; 31) | 58 (33; 114) | 11 (10; 23) | 48 (44; 91) |
| p valuea | – | 0.032 | – | 0.595 | – | 0.579 | – |
| SD-ROI1 |
SD-ROI2 | SD-ROI3 | SD-ROI4 | ||||
| Onyx | 104 (83; 127) | 94 (78; 110) | 51 (46; 55) | 33 (28; 33) | |||
| PHIL | 54 (54; 59) | 38 (38; 40) | 32 (31; 34) | 27 (26; 29) | |||
| p valuea | 0.032 | 0.008 | 0.008 | 0.159 | |||
Data (in HU) presented as median (lower quartile; upper quartile). aMann-Whitney test; for the mean HU values of the ROIs, only ΔMean-ROI1–3 (difference between Mean-ROI1–3 and Mean-ROI4, respectively) were statistically compared.
CT: computed tomography; HU: Hounsfield unit; PHIL: precipitating hydrophobic injectable liquid; ROI: region of interest; SD: standard deviation.
Artifacts were more pronounced in cone-beam CT than in conventional CT; however, no direct comparison of these two modalities with respect to artifact severity was performed.
Discussion
A variety of embolic agents is currently available for the endovascular treatment of vascular pathologies, each with specific advantages and disadvantages with regard to the particular pathology to be treated (e.g. different requirements for high-flow or low-flow lesions, AVMs or AVFs and with regard to caliber and length of feeding and draining vessels).25 The treatment success of cerebral AVMs has considerably improved in the last decade, which is, among other factors, often attributed to the introduction of the EVOH-based LEA Onyx.2,25–27 Despite the potential advantages of Onyx over other LEAs, there are still several shortcomings reported by interventionalists, one of these being the production of imaging artifacts, especially in peri- and postprocedural CT imaging.3,4
PHIL, a LEA that was introduced to the market in 2015, is based on a biocompatible copolymer and uses covalently bound iodine for radiopacity, instead of tantalum, the latter being admixed to Onyx. As initially indicated, several studies demonstrated the feasibility, safety, efficacy and, albeit with only short observation times, the biocompatibility of PHIL, although the number of cases (experiments and patients) was small in these studies.11–16 All of these studies mentioned the low degree of artifacts caused by PHIL, however, without qualitatively or quantitatively measuring these artifacts and without any direct comparison to other LEAs.
In this experimental in vivo study we demonstrated that PHIL produces fewer artifacts than Onyx in conventional and cone-beam CT, evaluated by applying subjective image analysis, performed by three experienced radiologists, and quantitative HU measurements. Quantitatively, the observed streak artifacts were represented by low or high HU values and by a high spread of the HU in specific ROIs in proximity to the LEA cast.
The underlying reason for the artifact production by Onyx and PHIL in CT imaging is the high absorption of photons by the admixed high atomic number materials tantalum (anatomic number of 73) for Onyx and iodine (atomic number of 53) for PHIL, resulting in beam hardening, scatter and noise, which are visible as dark and bright streaks in the reconstructed CT image.4,8,28 The higher degree of artifact production by Onyx can accordingly be explained by the higher atomic number of tantalum.
Both LEAs did not produce any apparent artifacts in MRI. This finding is in line with the literature. Only a few studies have described a limited diagnostic evaluation due to artifacts of Onyx in MRI, with the most pronounced artifact in diffusion-weighted sequences, while most studies observed no or only negligible artifacts caused by Onyx.3,4,6,7 For PHIL, to the best of our knowledge, the presence of MRI artifacts was not evaluated before.
Peri- and postinterventional procedure-related intracranial hemorrhage is one of the most feared complications of the treatment of cerebral AVMs, occurring in 0% to 12% of cases, with most series being concentrated between 6% and 9%.2,26,29 In the case of suspected procedure-related hemorrhage, cone-beam CT (mainly peri-procedural) and conventional CT (mainly post-procedural) are the imaging modalities of choice. As initially indicated, after AVM embolization with Onyx, because of severe streak artifacts, the evaluation of the most relevant CT images, namely the images at the level of the embolized AVM, can be significantly restricted or even impossible.3,4,7,8
After endovascular embolization, angiography represents the gold-standard imaging modality for the evaluation of treatment success.25 The aforementioned artifacts do not impair the diagnostic accuracy of angiography. However, if performing an angiography is not possible (e.g. because of refusal by the patient, advanced renal insufficiency or severe contrast agent allergy), MRI is the imaging modality of choice for the further evaluation of the AVM.4,6 In MRI, susceptibility artifacts caused by magnetic field inhomogeneities, induced by foreign implants, can impede the assessment of the AVM, such as AVM-related aneurysms, (residual) arterial blood flow within the nidus or within the draining veins or contrast enhancement of the nidus.4,6,7 However, the findings of this experimental study suggest that the radiological evaluation of MRI images is not impeded by Onyx or PHIL.
Another important aspect for which imaging artifacts of LEAs can be relevant is postembolization stereotactic radiation therapy. Owing to the abovementioned artifacts in treatment-planning CT, delineation of the AVM nidus and accordingly of the target volume can be impeded and consequently cause errors in dose calculations, which need to be identified and resolved during treatment planning.5,7,8 Furthermore, image distortion, induced by foreign materials such as Onyx, can influence the automated dose calculation of the treatment planning system, potentially leading to over- or underdosing of the AVM.5,7,8
Even though the lower degree of artifacts in CT imaging can be seen as an advantage of PHIL over Onyx, and preclinical and preliminary clinical studies demonstrated that PHIL can be an effective LEA, clinical studies with a sufficient number of patients and sufficient follow-up times are needed to define the value of PHIL for endovascular embolization of AVMs and AVFs.
There are some limitations in our study. First, the number of trials was small; however, the findings were consistent in the different analyses for the two embolic agents. Second, the assessment of artifacts near the RM is impeded because of its proximity to the skull base (absorbance of artifacts in the ventral direction and production of artifacts by the skull base). Third, even though the volume of required LEA was not significantly different, the higher volume of Onyx could have led to significantly more artifacts. Fourth, the transferability of an experimental in vivo study to clinical practice is generally limited.
Conclusion
In this experimental in vivo study, PHIL produced fewer artifacts than Onyx on conventional and cone-beam CT, which could facilitate the diagnosis of peri- and postprocedural hemorrhage and subsequent stereotactic radiation therapy.
Acknowledgments
State Animal Care and Ethics Committee approval was obtained. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Footnotes
*C.M.S. and M.A.M. contributed equally to this work.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.F.V. has received travel support outside this work from MicroVention and Stryker GmbH & Co. KG. H.U.K. reports grants, personal fees and nonfinancial support from Siemens, personal fees from Boehringer Ingelheim, personal fees and nonfinancial support from Bayer, personal fees from GSK, personal fees from Novartis, personal fees from Astra Zeneca, personal fees from Philips, and personal fees from Bracco, outside the submitted work. M.B. reports board membership for DSMB Vascular Dynamics; consultancy for Roche, Guerbet, and Codman; grants/grants pending from DFG, Hopp Foundation, Novartis, Siemens, Guerbet, Stryker, and Covidien; and payment for lectures (including service on speakers bureaus) from Novartis, Roche, Guerbet, Teva, Bayer, and Codman. M.A.M. has received consulting honoraria, speaker honoraria, and travel support outside this work from Codman, Covidien/Medtronic, MicroVention, Phenox, and Stryker. R.O., T.D. and C.M.S. have nothing to declare.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This study was technically supported by MicroVention (Tustin, CA, USA) and Medtronic Neurovascular (Irvine, CA, USA) without any influence on data acquisition, analysis or writing of the manuscript.
References
- 1.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med 2007; 356: 2704–2712. [DOI] [PubMed] [Google Scholar]
- 2.Pierot L, Cognard C, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: Results of a prospective, multicentre study (BRAVO). Eur Radiol 2013; 23: 2838–2845. [DOI] [PubMed] [Google Scholar]
- 3.Ayad M, Eskioglu E, Mericle RA. Onyx: A unique neuroembolic agent. Expert Rev Med Devices 2006; 3: 705–715. [DOI] [PubMed] [Google Scholar]
- 4.Saatci I, Cekirge HS, Ciceri EF, et al. CT and MR imaging findings and their implications in the follow-up of patients with intracranial aneurysms treated with endosaccular occlusion with Onyx. AJNR Am J Neuroradiol 2003; 24: 567–578. [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts DA, Balter JM, Chaudhary N, et al. Dosimetric measurements of Onyx embolization material for stereotactic radiosurgery. Med Phys 2012; 39: 6672–6681. [DOI] [PubMed] [Google Scholar]
- 6.Loy DN, Rich KM, Simpson J, et al. Time-of-flight magnetic resonance angiography imaging of a residual arteriovenous malformation nidus after Onyx embolization for stereotactic radiosurgery planning. Technical note. Neurosurg Focus 2009; 26: E13. [DOI] [PubMed] [Google Scholar]
- 7.Shtraus N, Schifter D, Corn BW, et al. Radiosurgical treatment planning of AVM following embolization with Onyx: Possible dosage error in treatment planning can be averted. J Neurooncol 2010; 98: 271–276. [DOI] [PubMed] [Google Scholar]
- 8.Giantsoudi D, De Man B, Verburg J, et al. Metal artifacts in computed tomography for radiation therapy planning: Dosimetric effects and impact of metal artifact reduction. Phys Med Biol 2017; 62: R49–R80. [DOI] [PubMed] [Google Scholar]
- 9.Kulcsár Z, Karol A, Kronen PW, et al. A novel, non-adhesive, precipitating liquid embolic implant with intrinsic radiopacity: Feasibility and safety animal study. Eur Radiol 2017; 27: 1248–1256. [DOI] [PubMed] [Google Scholar]
- 10.Izaaryene J, Saeed Kilani M, Rolland PH, et al. Preclinical study on an animal model of a new non-adhesive cyanoacrylate (Purefill®) for arterial embolization. Diagn Interv Imaging 2016; 97: 1109–1116. [DOI] [PubMed] [Google Scholar]
- 11.Vollherbst DF, Otto R, von Deimling A, et al. Evaluation of a novel liquid embolic agent (precipitating hydrophobic injectable liquid (PHIL)) in an animal endovascular embolization model. J Neurointerv Surg 2018; 10: 268–274. [DOI] [PubMed] [Google Scholar]
- 12.Koçer N, Hanımoğlu H, Batur Ş, et al. Preliminary experience with precipitating hydrophobic injectable liquid in brain arteriovenous malformations. Diagn Interv Radiol 2016; 22: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leyon JJ, Chavda S, Thomas A, et al. Preliminary experience with the liquid embolic material agent PHIL (precipitating hydrophobic injectable liquid) in treating cranial and spinal dural arteriovenous fistulas: Technical note. J Neurointerv Surg 2016; 8: 596–602. [DOI] [PubMed] [Google Scholar]
- 14.Lamin S, Chew HS, Chavda S, et al. Embolization of intracranial dural arteriovenous fistulas using PHIL liquid embolic agent in 26 patients: A multicenter study. AJNR Am J Neuroradiol 2017; 38: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaniego EA, Kalousek V, Abdo G, et al. Preliminary experience with precipitating hydrophobic injectable liquid (PHIL) in treating cerebral AVMs. J Neurointerv Surg. Epub ahead of print 27 January 2016. DOI: 10.1136/neurintsurg-2015-012210. [DOI] [PubMed] [Google Scholar]
- 16.Vollherbst DF, Sommer CM, Ulfert C, et al. Liquid embolic agents for endovascular embolization: Evaluation of an established (Onyx) and a novel (PHIL) embolic agent in an in vitro AVM model. AJNR Am J Neuroradiol 2017; 38: 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollherbst D, Bertheau RC, Fritz S, et al. Electrochemical effects after transarterial chemoembolization in combination with percutaneous irreversible electroportation: Observations in an acute porcine liver model. J Vasc Interv Radiol 2016; 27: 913–921. [DOI] [PubMed]
- 18.Xu M, Xu H, Qin Z. Animal models in studying cerebral arteriovenous malformation. Biomed Res Int 2015; 2015: 178407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ning X, Zhao C, Pang J, et al. Experimental study of temperature-sensitive chitosan/beta-glycerophosphate embolic material in embolizing the basicranial rete mirabile in swines. Exp Ther Med 2015; 10: 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haussen DC, Ashour R, Johnson JN, et al. Direct continuous measurement of draining vein pressure during Onyx embolization in a swine arteriovenous malformation model. J Neurointerv Surg 2015; 7: 62–66. [DOI] [PubMed] [Google Scholar]
- 21.Vollherbst DF, Otto R, von Deimling A, et al. Evaluation of a novel liquid embolic agent (precipitating hydrophobic injectable liquid (PHIL)) in an animal endovascular embolization model. J Neurointerv Surg 2018; 10: 268–274. [DOI] [PubMed]
- 22.Weiß J, Schabel C, Bongers M, et al. Impact of iterative metal artifact reduction on diagnostic image quality in patients with dental hardware. Acta Radiol 2017; 58: 279–285. [DOI] [PubMed] [Google Scholar]
- 23.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions, 3rd ed Hoboken, N.J.: J. Wiley, 2003. , p.xxvii, 760 p. [Google Scholar]
- 24.Altman DG. Practical statistics for medical research, Boca Raton, FL: Chapman & Hall/CRC, 1999. p. xii, 611 p. [Google Scholar]
- 25.Gross BA, Du R. Diagnosis and treatment of vascular malformations of the brain. Curr Treat Options Neurol 2014; 16: 279. [DOI] [PubMed] [Google Scholar]
- 26.van Rooij WJ, Jacobs S, Sluzewski M, et al. Curative embolization of brain arteriovenous malformations with Onyx: Patient selection, embolization technique, and results. AJNR Am J Neuroradiol 2012; 33: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross BA, Albuquerque FC, Moon K, et al. Evolution of treatment and a detailed analysis of occlusion, recurrence, and clinical outcomes in an endovascular library of 260 dural arteriovenous fistulas. J Neurosurg 2017; 126: 1884–1893. [DOI] [PubMed] [Google Scholar]
- 28.Barrett JF, Keat N. Artifacts in CT: Recognition and avoidance. Radiographics 2004; 24: 1679–1691. [DOI] [PubMed] [Google Scholar]
- 29.Panagiotopoulos V, Gizewski E, Asgari S, et al. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol 2009; 30: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]