Abstract
Objective
Treatment of middle cerebral artery (MCA) aneurysms has been historically considered as the almost exclusive domain of microsurgical clipping. This retrospective single-center study assesses whether microsurgical clipping or endovascular treatment (i.e. coiling and/or stenting) for MCA aneurysms yielded better occlusion rates and clinical outcome.
Methods
We identified patients with a minimum clinical follow-up of 12 months who had undergone MCA aneurysm repair either by clipping or by endovascular treatment between 2005 and 2015. Aneurysm occlusion rates were assessed by the Raymond-Roy Occlusion Classification (RROC) and patients’ clinical outcome was measured by the modified Rankin Scale (mRS). All patients had been treated in an interdisciplinary treatment concept at a large neurovascular center; both treatment modalities were available at all times.
Results
Ninety-two eligible patients with MCA aneurysms, of whom 21.7% patients were treated for subarachnoid hemorrhages, were included; 38 patients underwent endovascular therapy and 54 clipping. The median age at treatment was 53.5 years (range, 25–79 years) and the median clinical follow-up was 98.5 months (range, 18–213 months). Occlusion rates were significantly higher in the clipping cohort (RROC = 1: 96.3% vs 78.9%; p = 0.04), long-term clinical outcome was better in the endovascular treatment cohort (mRS ≤ 1: 100.0% vs 90.8%; p < 0.01). Permanent treatment-associated morbidity was seen more commonly in the clipping cohort (9.3% vs 0.0%).
Conclusions
Both treatment modalities are associated with excellent clinical and radiological outcome if applied within an interdisciplinary treatment concept. Endovascular aneurysm repair appears to be an attractive treatment alternative compared to clipping with low complication rates for well-selected patients.
Keywords: Endovascular treatment, middle cerebral artery aneurysm, microsurgical clipping, occlusion rate, outcome
Introduction
Treatment of middle cerebral artery (MCA) aneurysms has historically been considered an almost exclusive domain of microsurgical clipping.1–4 This widely established practice has been based on the frequently encountered wide neck of these aneurysms, incorporation of major branches, as well as the favorable location safely accessible with craniotomy.5 With the publication of the International Subarachnoid Aneurysm Trial (ISAT), endovascular coil embolization has been established as an alternative to microsurgical clipping.6,7 However, only 14.1% of aneurysms evaluated in the ISAT were located in the MCA territory. Advances in endovascular technologies such as three-dimensional angiography and adjunctive techniques such as stent-assisted coiling have resulted in improved outcomes; thus, coiling is increasingly utilized for MCA aneurysm repair.7–9 Nonetheless, data on endovascular treatment of MCA aneurysms remain scarce and ongoing evaluation is required to establish appropriate treatment algorithms. Thus far, only two prospective, randomized, controlled studies on ruptured MCA aneurysms are available.6,10 One study, published in 1999, and thus not reflective of current endovascular techniques, concluded that there was no difference in angiographic or clinical outcome comparing both treatment options.10 The ISAT data showed that for lesions for which both modalities were feasible, the clinical outcome measured in disability-free survival free at one year was significantly better for endovascular coiling, while the risk for rebleeding was lower in clipped aneurysms.6 Furthermore, a systematic review on the treatment of only MCA aneurysms came to the conclusion that ruptured aneurysms were treated more safely with coiling, whereas unruptured aneurysm fared better with clipping.7 Another retrospective meta-analysis of unruptured MCA aneurysms found clip ligation to result in a higher rate of complete aneurysm occlusion and a slightly improved long-term outcome.9 Most of the more recent studies found equivalent clinical outcome with either modality, but a more frequent need for retreatment in patients treated with coiling.11,12 In the present study, we performed a retrospective analysis of consecutive series of patients who had undergone treatment of MCA aneurysm by either microsurgical clipping or state-of-the-art endovascular coiling in an interdisciplinary treatment concept at the neurovascular center, University Hospital Salzburg. We assessed the occlusion rates, treatment-associated morbidity and long-term neurological outcome.
Methods
Patient selection and treatment decisions
We retrospectively identified all patients who had undergone treatment of an MCA aneurysm at a major neurovascular center between 2005 and 2015; patients with a minimum follow-up of 12 months were eligible for inclusion. Both treatment options, microsurgical clipping as well as coiling, were available at all times. Decisions on the optimal treatment modality were reached in consensus by a multidisciplinary team of neurosurgeons and neurointerventionalists. Multiple parameters were considered to determine treatment decisions such as the patients overall health, anatomical features of the aneurysm, and the presence of subarachnoid hemorrhage (SAH) with or without associated hematoma. Factors that triggered microsurgical clipping in unruptured cases were: younger patients (≤65 years), large or giant aneurysms (diameter > 10 mm), small aneurysms that would possibly require stent placement, contraindication for antiplatelet medication, a low fundus-to-neck ratio (≤2.5), aneurysms in the M2 or M3 segments, and multilobulated aneurysms. Moreover, ruptured aneurysms with associated space-occupying hematoma dictated an open surgical evacuation and aneurysm repair. Endovascular treatment was favored for unruptured aneurysms in older patient, small aneurysms with a high fundus-to-neck ratio, and presence of significant comorbidities. Furthermore, if deemed necessary more advanced techniques were applied for both treatment modalities, consisting of stent-assisted coiling as well as perforator translocation/anastomosis in microsurgically treated patients.
Owing to the strictly retrospective study design, the requirement of a formal written ethics vote was waived by the local ethics committee upon written inquiry by the investigators.
Clinical and radiological follow-up
All pre- and postoperative diagnostic procedures and follow-up examinations were performed at the institution. Clinical and radiographic outcome were assessed in each patient, as well as the presenting symptoms, retreatment rate and procedure-associated complications. In all the surgical cases, the radiological outcome was assessed by an initial postoperative digital subtraction angiography (DSA) during the first year after treatment, and magnetic resonance angiography (MRA) investigations were performed as late follow-up imaging, and to rule out the development of additional, new aneurysms over the years. Patients in the coiling group were followed primarily by MRA; however, DSA was performed in case of suspected recanalization. The clinical outcome was measured with the modified Rankin Scale (mRS).13 Complications that were clinically symptomatic for less than 30 days were considered transient. The occlusion rate of the aneurysms during follow-up was compared with the four-point modified Raymond-Roy Occlusion Scale (RROS): Class 1 complete obliteration, Class 2 residual neck, Class 3 residual aneurysm (Class 3A residual in the center and Class 3B residual in the periphery of the aneurysm sac).14 The hypothesis was tested with a Wilcoxon-Mann-Whitney-test for two independent groups (for location-shift-alternatives), either for mRS of 0–6 or RROS of 1–3.
Results
Patient characteristics
Ninety-two patients (65 females, male:female = 1:1.4) were identified and met the inclusion criteria. The median age at the time of aneurysm treatment was 53.5 years (range, 25–79 years). Aneurysm repair was performed using microsurgical clipping in 54/92 (58.7%) and coiling in 38/92 (41.3%) patients; no patient had treatment for more than a single MCA aneurysm. In case of 1/54 (1.9%) microsurgically treated patients, a transposition with end-to-end anastomosis for preservation of a large perforator vessel, arising from the sac of a large aneurysm, was performed. In this series, no patients had to be treated by clipping because of the need for evacuation of a space-occupying hematoma. Furthermore, in 7/38 (18.4%) patients undergoing endovascular treatment, a stent-assisted coiling was performed. This specific endovascular technique was first utilized in 2011 and used with increasing frequency over the subsequent years until 2015; however, no flow-diverter stents or web devices were applied in this patient series.
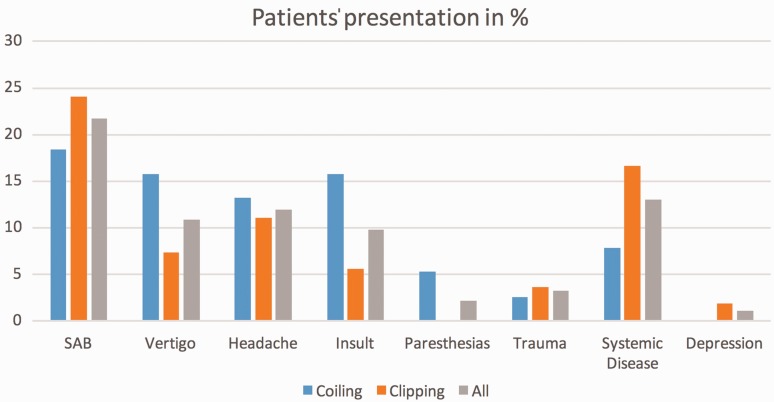
The most common clinical signs and symptoms that led to the diagnosis and treatment of the MCA aneurysm were SAH in 21.7% (World Federation of Neurological Societies scale (WFNS) I (n = 10), WFNS II (n = 3), WFNS III (n = 4), and WFNS IV (n = 3)), headache without SAH in 12%, and vertigo in 10.9% of patients; in 13% of cases the MCA aneurysm was diagnosed incidentally (Figure 1). Among the 20/92 (21.7%) patients who had suffered an SAH, which led to the diagnosis and eventual treatment of the MCA aneurysm, the MCA aneurysm was found to be the cause of the SAH in 11/20 (55.0%) patients, whereas in 9/20 (45.0%) of these patients a different aneurysm was identified as the site of rupture. The median maximum aneurysm diameter measured on preprocedural DSA was 5.5 mm (range, 1.2 mm–23.0 mm). The two treatment groups did not significantly differ in terms of median age at treatment (54 vs 52 years; p = 0.65), aneurysm size (5.0 vs 6.0 mm; p = 0.49), or the proportion of SAH patients (24 vs 18%; p = 0.08) (Table 1).
Figure 1.
Patient signs and symptoms.
SAH: subarachnoid hemorrhage.
Table 1.
Patient population.
| Clipping | Coiling | p value | |
|---|---|---|---|
| Number of patients | 54 (58.7%) | 38 (41.3%) | |
| Median age in years | 54 (range, 36.0–79.0) | 52 (range, 25.0–71.0) | p = 0.65 |
| Median aneurysm size in mm | 5 (range, 1.2–23.0) | 6 (range, 1.5–22.0) | p = 0.49 |
| Female gender | 38 (70.4%) | 27 (71.1%) | |
| Occlusion rate | p = 0.04 | ||
| Raymond-Roy: 1 | 52 (96.3%) | 30 (78.9%) | |
| Raymond-Roy: 2 | 2 (3.7%) | 6 (15.8%) | |
| Raymond-Roy: 3 | 0 (0.0%) | 2 (5.3%) | |
| Clinical outcome | p < 0.01 | ||
| mRS at last follow-up: 0 | 34 (63.0%) | 35 (92.1%) | |
| mRS at last follow-up: 1 | 15 (27.8%) | 3 (7.9%) | |
| mRS at last follow-up: 2 | 1 (1.9%) | 0 (0.0%) | |
| mRS at last follow-up: 3 | 2 (3.7%) | 0 (0.0%) | |
| mRS at last follow-up: 4 | 2 (3.7%) | 0 (0.0%) | |
| mRS at last follow-up: 5 | 0 (0.0%) | 0 (0.0%) | |
| Retreatments | 1 (1.9%) | 4 (10.5%) | |
| Treatment-associated morbidity | 15 (27.8%) | 5 (13.2%) | |
| Permanent morbidity | 4 (7.4%) | 0 (0.0%) | |
| Symptoms | |||
| SAH | 13 (24.1%) | 7 (18.4%) | p = 0.08 |
| Systemic disease | 9 (16.7%) | 3 (7.9%) | |
| Headache | 6 (11.1%) | 5 (13.2%) | |
| Stroke/Transient ischemic attack | 3 (5.6%) | 6 (15.8%) | |
| Vertigo | 4 (7.4%) | 6 (15.8%) | |
| Trauma | 2 (3.7%) | 1 (2.6%) | |
| Paresthesia | 0 (0.0%) | 2 (5.3%) | |
| Depression | 1 (1.9%) | 0 (0.0%) | |
mRS: modified Rankin Scale; Raymond-Roy: Raymond-Roy Occlusion Classification; SAH: subarachnoid hemorrhage.
Clinical and radiological outcome
The median clinical and radiological follow-up after aneurysm treatment was found to be 98.5 months (range, 18–213 months) and 14 months (range, 12–110 months), respectively. Postprocedural mRS as well as long-term mRS at the last date of clinical follow-up were significantly better in the coiling cohort compared to patients who had undergone clipping (postprocedural mRS ≤ 1: 97.3% vs 83.3%; long-term mRS ≤ 1: 100% vs 90.8%; p < 0.01).
Further subgroup analyses for clinical long-term outcome showed the following results: Patients who had suffered an SAH displayed a favorable outcome (mRS ≤ 1) in 95%, and for non-SAH patients a respective favorable outcome was reported in 94.4% (Table 2). To rule out the effects of possible confounding factors (i.e. preprocedural mRS and SAH) on our outcome analyses a multiple regression model including interactions was conducted, which revealed that patients treated by clipping showed worse long-term mRS outcome than patients treated by coiling if the long-term outcome was adjusted for the patients’ respective preprocedural mRS as well as the presence of an SAH (regression coefficient estimate for clipping: 0.5358, standard error: 0.2069, p < 0.01).
Table 2.
Subgroup analyses.
| SAH | Non-SAH | |
|---|---|---|
| Number of patients | 20 (21.7%) | 72 (78.3%) |
| Preprocedural mRS | ||
| mRS preprocedural: 0 | 1 (5.0%) | 60 (83.3%) |
| mRS preprocedural: 1 | 9 (45.0%) | 10 (13.9%) |
| mRS preprocedural: 2 | 3 (15.0%) | 1 (1.4%) |
| mRS preprocedural: 3 | 3 (15.0%) | 1 (1.4%) |
| mRS preprocedural: 4 | 3 (15.0%) | 0 (0.0%) |
| mRS preprocedural: 5 | 1 (5.0%) | 0 (0.0%) |
| Clinical outcome | ||
| mRS at last follow-up: 0 | 13 (65%) | 56 (77.8%) |
| mRS at last follow-up: 1 | 6 (30%) | 12 (16.7%) |
| mRS at last follow-up: 2 | 0 (0.0%) | 1 (13.9%) |
| mRS at last follow-up: 3 | 0 (0.0%) | 2 (2.8%) |
| mRS at last follow-up: 4 | 1 (5.0%) | 1 (13.9%) |
| mRS at last follow-up: 5 | 0 (0.0%) | 0 (0.0%) |
| Permanent treatment-associated morbidity | 1 (5.0%) | 4 (5.6%) |
mRS: modified Rankin Scale; SAH: subarachnoid hemorrhage.
Occlusion rates were better in the clipping cohort with 96.3% of patients achieving an RROC of 1, whereas 78.9% patients did so in the endovascular cohort (p = 0.04); a residual aneurysm was seen in none of the clipped, but in 5.3% of the coiled patients (RROC 3A).
In the clipping cohort, 5/54 (9.3%) of patients suffered from permanent morbidity due ischemic infarctions, whereas no permanent treatment-associated morbidity was recorded for any of the patients treated with coiling. Of these five patients with permanent morbidity after clipping, one patient had experienced an SAH (WFNS III), whereas the other four patients were allocated to the non-SAH subgroup. Thus, an overall rate of permanent morbidity for all patients of 5.4% was recorded, resulting in a long-term mRS of 2 (n = 1), 3 (n = 2), and 4 (n = 2) in the affected patients. No treatment-associated mortality was observed until the end of follow-up. Retreatment was deemed necessary in 1.9% of the clipping and 10.5% of the coiling patients; resulting in eventual complete occlusion in all retreated aneurysms on follow-up imaging and no additional treatment-associated morbidity. A change of the primarily intended treatment modality for retreatments was not necessary in this series. No patient suffered from hemorrhage or rehemorrhage of the treated MCA aneurysm throughout the follow-up period (Table 3).
Table 3.
Treatment-associated morbidity.
| Morbidity | Coiling (n = 38) | Clipping (n = 54) |
|---|---|---|
| Thromboembolism | 1 (2.6%) | 0 |
| Femoral punction hematoma | 2 (5.2%) | 0 |
| Vasovagal syncopation | 1 (2.6%) | 0 |
| Transient subdural hematoma | 0 | 4 (7.4%) |
| Postoperative ischemia | 0 | 5 (9.3%) |
| Impairment of wound healing | 0 | 5 (9.3%) |
| Postprocedural delirium | 0 | 1 (1.8%) |
Discussion
Treatment of MCA aneurysms has historically been considered as an almost exclusive domain of microsurgical clipping.1–4 Reasons include MCA aneurysm angioarchitecture, possible involvement of MCA branches as well as the relatively straightforward surgical approach via the Sylvian fissure.5 Over recent years, however, advancements in the field of endovascular treatment have led to an expansion of the scope of endovascular treatment options and their applications.6,7 As a consequence of these rapid developments, endovascular treatment is being more commonly applied for treatment of aneurysms for which microsurgical clipping has been previously considered the only or optimal treatment option. Especially, the results of ISAT have shifted favor toward endovascular aneurysm treatment; nonetheless, prospective data specifically for MCA aneurysms are scarce.6,10 Owing to the aforementioned reasons, which often make MCA aneurysms seem unfavorable for endovascular treatment, aneurysms of this specific location were underrepresented in the ISAT.6 The MCA, however, is the second most common location for aneurysms of the anterior circulation; thus, data addressing the topic of possible endovascular treatment options for these aneurysms and comparative analyses to the gold standard of microsurgical clipping are urgently needed. We conducted a retrospective outcome analysis of a series of consecutive patients who had undergone either microsurgical clipping or endovascular treatment of an MCA aneurysm. All treatments were performed at a single neurovascular center, which offered both treatment modalities at all times. The treatment decision for each patient was discussed and agreed on by an interdisciplinary team consisting of neurosurgeons and neurointerventionalists. This crucial aspect should minimize a possible selection bias toward a certain treatment based solely on its availability. When evaluating treatment options of any aneurysm, two main outcome parameters should be addressed: (1) the occlusion rate as surrogate for treatment efficacy either to prevent future hemorrhage or to repair already ruptured aneurysms and thus avert rehemorrhage, and (2) the associated morbidity/mortality rate to allow an appropriate risk/benefit assessment for future patients. Regarding these two parameters our main two findings in this patient series were that occlusion rates were significantly higher in patients who had been treated by microsurgical clipping, whereas the immediate postprocedural and long-term neurological outcome as well as the treatment-associated morbidity rates were significantly superior in the endovascular treatment cohort. Interestingly, these results regarding the long-term neurological outcome persisted when adjusting for the possible confounders of our patients’ preprocedural neurological status and the presence of SAH. Nonetheless, these results have to be interpreted with some caution because of the small sample size. Moreover, the majority of SAH patients were of favorable WFNS grades.
In this study occlusion rates were assessed by performance of at least one postoperative/postprocedural DSA in all patients; patients with suspected or proven aneurysm remnants underwent serial DSA over the course of follow-up. The occlusion rates were excellent in the clipping and good in the endovascular treatment cohort, with necessary retreatment more commonly performed in patients treated with coiling. Even though all treatment decisions were achieved in interdisciplinary agreement, this may point toward the fact that endovascular treatment might have been favored too aggressively over clipping in some cases. Even though occlusion rates were lower in the endovascular cohort, however, none of these patients suffered from an aneurysmal SAH/rehemorrhage over the course of clinical follow-up. Even though the ISAT did report relevant rates of rehemorrhage after endovascular treatment, we did not record any rebleeds for our 92 patients within a median clinical follow-up of more than eight years.6 Thus, we believe that not every small recanalization or aneurysm remnant after coiling that remains stable over time necessarily requires retreatment since only low rupture rates for these patients have been documented by other groups as well.15
Patients with aneurysm remnants after treatment, however, should be closely followed radiologically. From our experience, DSA remains to be the optimal imaging modality for a thorough radiological evaluation for aneurysm remnants. In this regard it should be taken into consideration that these follow-up examinations may be a relevant stressor for patients and that diagnostic DSAs remain an invasive examination with certain, although very low, procedure-related risks. Although not deemed necessary for our study population, a change of the applied treatment modality for aneurysm remnants (i.e. clipping after coiling or vice versa) has been proven to be feasible if deemed necessary.15–17 When putting our results regarding occlusion rates of both treatments in perspective with previously published data, other groups also found microsurgical clipping to yield higher occlusion rates than endovascular treatment for MCA aneurysms.17 Suzuki et al. reported the following results for endovascular treatment of 115 patients with MCA aneurysms: complete occlusion in 46% of aneurysms, neck remnants in 44%, and incomplete occlusion in 3% as compared to the results of the coiling subgroups in the present study of 79%, 16%, and 5%, respectively.18 Diaz et al. reported a similar outcome for a series of 90 patients undergoing both treatment modalities.12 In our opinion, however, the ongoing development of endovascular techniques and implementation of more sophisticated devices will sooner or later lead to improved occlusion rates and safety for coiled MCA aneurysms as well.19 Whether endovascular embolization can achieve occlusion rates similar to clipping remains to be seen.
The other important factor in any treatment decision is associated morbidity and mortality. In the current study, no treatment-associated mortality was observed; the morbidity and complication rates were, however, higher in the cohort of patients who underwent microsurgical treatment. Five patients suffered from postoperative infarctions leading to permanent treatment-associated morbidity; however, one postoperative ischemia did occur in a patient with a WFNS III SAH and might therefore be possibly disease associated and not treatment associated. In the other four patients, postoperative ischemic complications have to be attributed to the surgical approach as well as the surgical manipulation, and were not deemed to be vasospasm related since they did not occur in SAH patients. On the other hand, no treatment-associated morbidity was recorded for the endovascular treatment cohort. Nonetheless, it may well be possible that there was an intrinsic bias toward clipping for more complex MCA aneurysms, which might at least to some degree explain the observed differences in treatment-associated morbidity between the two cohorts. In our study population, the rate of treatment-associated complications was generally favorable for both treatment modalities. Overall, our treatment-associated complication rates for both treatments were similar to previously published data by other experienced neurovascular centers.20 Nonetheless, complications were seen less commonly in the endovascular group. This may underscore the advantage of the minimally invasive endovascular approach: avoiding possible retractor-induced contusion/ischemia and postoperative craniotomy-associated complications or infections. Patients who had suffered from SAH were, however, although not statistically significant, more common in the clipping cohort. Even though the impact of the possible confounding effects of SAH and preprocedural neurological status on our results was statistically analyzed and our results adjusted accordingly, patients with SAH still represent a completely different subgroup from patients undergoing elective aneurysm repair and therefore the assessment of their neurological outcome as well as treatment-associated complications should be judged differently.
Limitations
An obvious limitation of the present study is the retrospective study design and the inability to control for unknown confounding variables. Even though both treatment options were available for all patients and treatment decisions were based on an interdisciplinary approach, a selection toward a certain treatment modality cannot be ruled out completely. This, however, reflects current practice. There are few MCA aneurysms for which there is true equipoise among treatment modalities. Furthermore, the two treatment cohorts revealed a discrepancy with regards to the distribution of SAHs in favor of the coiling cohort (clipping vs coiling: 24% vs 18%), which may have influenced the long-term neurological outcome as mentioned before. On the other hand, there may have been a tendency toward endovascular treatment for patients with significant comorbidities.
Based on these data, it remains difficult to clearly favor endovascular treatment for MCA aneurysms over conventional microsurgical clipping. Nonetheless, both treatment modalities lead to favorable outcome in terms of occlusion rates and neurological outcome. Both options have their place in the treatment of MCA aneurysms, and the days when MCA aneurysm treatment was an exclusive domain of microsurgery have long passed. We believe that endovascular treatment is a viable and attractive, minimally invasive alternative to the established microsurgical clipping, which should deserve further evaluation in future prospective, randomized trials. The optimal treatment algorithm of MCA aneurysms will most likely remain a matter of controversial debate for years to come. For now, we recommend that treatment of any aneurysm should be preferably conducted at a neurovascular center, which can offer both treatments at equal proficiency, and treatment decisions should be based on an interdisciplinary consensus; i.e. cases with elective aneurysm repair may be discussed at an established weekly neurovascular board meeting including neurosurgeons as well as neurointerventionalists/neuroradiologists.21 The inevitable, more widespread implementation of novel endovascular devices in the future will lead to an increase of endovascular treatment of even complex MCA aneurysms; whether this will lead to improved occlusion rates or have an impact on the treatment-associated morbidity remains to be seen, however, and will have to be assessed in future studies.
Conclusion
In this retrospective series of consecutive patients undergoing treatment for MCA aneurysms, either by microsurgical clipping or by endovascular coiling, we found microsurgical clipping to remain the superior treatment option with regards to occlusion rate. Necessary retreatments were more common in the endovascular cohort; none of our patients suffered from hemorrhage or rehemorrhage after treatment. However, the treatment-associated morbidity was significantly lower and long-term neurological outcome was better in the endovascular treatment cohort. Our findings may indicate that coiling is a viable and attractive, minimally invasive treatment option for well-selected patients with certain MCA aneurysms and a favorable angioarchitecture for endovascular treatment. Moreover, we found that, despite possible limitations/bias with regard to patient selection for endovascular treatment, such a procedure was associated with a very low complication rate in our study population. Future data from prospective, randomized studies will be necessary to establish optimal treatment algorithms for patients with MCA aneurysms. Meanwhile, aneurysm repair should be performed at interdisciplinary centers, which can provide both treatment options to achieve optimal clinical results.
Acknowledgment
We would like to thank Mr Georg Zimmermann for providing assistance with the performed statistical analyses.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Monika Killer-Oberpfalzer is in possession of a research grant by MicroVention/Terumo.
References
- 1.Haug T, Sorteberg A, Sorteberg W, et al. Surgical repair of unruptured and ruptured middle cerebral artery aneurysms: Impact on cognitive functioning and health-related quality of life. Neurosurgery 2009; 64: 412–420. [DOI] [PubMed] [Google Scholar]
- 2.Morgan MK, Mahattanakul W, Davidson A, et al. Outcome for middle cerebral artery aneurysm surgery. Neurosurgery 2010; 67: 755–761. [DOI] [PubMed] [Google Scholar]
- 3.Güresir E, Schuss P, Berkefeld J, et al. Treatment results for complex middle cerebral artery aneurysms. A prospective single-center series. Acta Neurochir (Wien) 2011; 153: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 4.Raftopoulos C, Goffette P, Vaz G, et al. Surgical clipping may lead to better results than coil embolization: Results from a series of 101 consecutive unruptured intracranial aneurysms. Neurosurgery 2003; 52: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 5.Jayaraman MV, Do HM, Versnick EJ, et al. Morphologic assessment of middle cerebral artery aneurysms for endovascular treatment. J Stroke Cerebrovasc Dis 2007; 16: 52–56. [DOI] [PubMed] [Google Scholar]
- 6.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002; 360: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 7.Zijlstra IA, Verbaan D, Majoie CB, et al. Coiling and clipping of middle cerebral artery aneurysms: A systematic review on clinical and imaging outcome. J Neurointerv Surg 2016; 8: 24–29. [DOI] [PubMed] [Google Scholar]
- 8.Brinjikji W, Lanzino G, Cloft HJ, et al. Endovascular treatment of middle cerebral artery aneurysms: A systematic review and single-center series. Neurosurgery 2011; 68: 397–402. [DOI] [PubMed] [Google Scholar]
- 9.Smith TR, Cote DJ, Dasenbrock HH, et al. Comparison of the efficacy and safety of endovascular coiling versus microsurgical clipping for unruptured middle cerebral artery aneurysms: A systematic review and meta-analysis. World Neurosurg 2015; 84: 942–953. [DOI] [PubMed] [Google Scholar]
- 10.Vanninen R, Koivisto T, Saari T, et al. Ruptured intracranial aneurysms: Acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology 1999; 211: 325–336. [DOI] [PubMed] [Google Scholar]
- 11.Ruan C, Long H, Sun H, et al. Endovascular coiling vs. surgical clipping for unruptured intracranial aneurysm: A meta-analysis. Br J Neurosurg 2015; 29: 485–492. [DOI] [PubMed] [Google Scholar]
- 12.Diaz OM, Rangel-Castilla L, Barber S, et al. Middle cerebral artery aneurysms: A single-center series comparing endovascular and surgical treatment. World Neurosurg 2014; 81: 322–329. [DOI] [PubMed] [Google Scholar]
- 13.Newcommon NJ, Green TL, Haley E, et al. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin scale. Stroke 2003; 34: 377–378. [DOI] [PubMed] [Google Scholar]
- 14.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015; 7: 496–502. [DOI] [PubMed] [Google Scholar]
- 15.Kim BM, Kim DJ, Kim DI, et al. Clinical presentation and outcomes of coil embolization of remnant or recurred intracranial aneurysm after clipping. Neurosurgery 2010; 66: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 16.Bendok BR, Ali MJ, Malisch TW, et al. Coiling of cerebral aneurysm remnants after clipping. Neurosurgery 2002; 51: 693–698. [PubMed] [Google Scholar]
- 17.Rubino PA, Mura J, Kitroser M, et al. Microsurgical clipping of previously coiled aneurysms. World Neurosurg 2014; 82: 203–208. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Tateshima S, Jahan R, et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: Angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery 2009; 64: 876–888. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Pan R, Wang H, et al. Clipping versus coiling for ruptured intracranial aneurysms: A systematic review and meta-analysis. Stroke 2013; 44: 29–37. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz C, Pfefferkorn T, Ebrahimi C, et al. Long-term neurological outcome and quality of life after World Federation of Neurosurgical Societies grades IV and V aneurysmal subarachnoid hemorrhage in an interdisciplinary treatment concept. Neurosurgery 2017; 80: 967–974. [DOI] [PubMed] [Google Scholar]
- 21.Davis BW, Stuart MJ, Jayapratap P, et al. Routine multidisciplinary cerebrovascular meetings do not reduce aneurysm clipping case load: A cohort study. ANZ J Surg 2016; 86: 594–597. [DOI] [PubMed] [Google Scholar]