Abstract
Introduction
Few liquid embolic materials are available for treatment of arteriovenous malformations. We describe the in vivo experience with the new PHIL low viscosity (LV) liquid embolic agent in a swine rete mirabile model.
Methods
Eight swine were treated. Two animals underwent embolization of a rete with PHIL LV and the contralateral rete with Squid 12 (euthanized the same day). Six animals underwent embolization of the right rete: two with balloon flow arrest (euthanized at 14 d) and four with a microcatheter alone (euthanized at 14 and 90 d). Performance characteristics of the embolic agents were evaluated. Microscopic and histological analysis of the harvested retia was performed. Macroscopic examinations and high contrast digital-based radiographs of the central nervous system were obtained.
Results
We did not experience any technical complication during embolization of each rete. Overall occlusion ability, on/off injection and ease to retrieve the microcatheter/balloon with PHIL LV were optimal. Fluoroscopic visualization of the PHIL LV cast was adequate to optimal. Average embolization time with flow arrest was 9.5 min versus 19.5 min with microcatheter plugging. Embolizations with PHIL LV required less volume and were shorter when compared to Squid 12. Subacute (14 d) and chronic (90 d) microscopic and histological analysis demonstrated minimal inflammatory changes in the perivascular tissues and permanent occlusion of the embolized vasculature.
Conclusion
In this swine rete model, the new PHIL LV embolic agent had an excellent embolization performance. Vessels embolized remained occluded up to 90 d from the procedure with minimal inflammatory changes.
Keywords: AVM, dural arteriovenous fistula, embolic, rete
Introduction
The Precipitating Hydrophobic Injectable Liquid (PHIL) (MicroVention) is a copolymer-based liquid embolic agent with a two-component system. The first component is the organic solvent dimethyl sulfoxide (DMSO). The second component is a liquid embolic agent comprising a copolymer dissolved in DMSO. An iodine component is bonded to the polymer and provides radiopacity for visualization during injection. Upon contact with blood, the liquid embolic precipitates in situ, resulting in an intraluminal polymeric embolus. The occlusion process of the target lesion depends on many variables such as delivery rate, local rate of blood flow and diameter of the parent vessel. These factors will determine the direction of movement and penetration distance of the liquid embolic as it rapidly solidifies to form an outer crust and maintains a liquid center to promote distal propagation.
Ethylene-vinyl alcohol copolymer (EVOH)-based liquid embolic agents such as Onyx (Medtronic) and Squid (Emboflu) have been used extensively in clinical practice. PHIL (25, 30 and 35) has been used in the embolization of arteriovenous malformations (AVMs) and arteriovenous fistulas, among others.1–3 Initial experience in these clinical scenarios has demonstrated its safety and effectiveness.
PHIL is currently available in 25%, 30% and 35% concentrations. A variation in the polymerization process results in a polymer with a shorter chain and a lower molecular weight, producing an embolic with a lower viscosity. We sought to evaluate the preclinical performance of this new PHIL low viscosity (LV) formulation in an in vivo swine model.
Methods
The study had two aims: to evaluate the behavior of the embolic material in vivo in a swine rete mirabile model; and to perform a histological and radiographic analysis of the harvested retia. The study was approved by the Animal Research Committee and swine were managed according to animal standards of the University of California Los Angeles Translational Research Imaging Center. All the procedures were performed under general anesthesia and with surveillance of a certified veterinarian. Conventional angiography was performed via femoral puncture. Using a coaxial approach, we navigated either a Headway Duo microcatheter or a Scepter balloon (MicroVention-Terumo) to the porcine ascending pharyngeal artery (APA), which supplies the rete. Endovascular embolization of each rete was performed as described elsewhere.4
Technical evaluation
A total of eight adult Yucatan pigs underwent embolization. In every embolization, we evaluated the overall occlusion achieved by the embolic material, fluoroscopic visualization of the embolic material, injection rate and on/off control of the embolic material and extraction of the microcatheter or balloon after formation of the embolic plug.
Two animals underwent consecutive embolization of bilateral retia, with PHIL LV and Squid 12, using Headway Duo microcatheters. PHIL LV was injected in a single rete, followed by contralateral rete embolization with Squid 12. Order of embolization was swapped in the second swine. The goal was to embolize the rete without affecting the internal carotid artery (ICA) or the contralateral rete. Each animal was euthanized after the procedure. We compared the performance characteristics of the two embolic materials. Squid is composed of EVOH and has two concentrations: 18 and 12. We chose the lowest concentration, as the Squid 12 formulation is comparable in viscosity to the PHIL LV liquid embolic material.
The behavior of each embolic agent was scored by the main operators (EAS and TB). The final angiographic fluoroscopic results were evaluated by two blinded neurointerventional surgeons with more than 10 years of experience (CPD and MH). Fluoroscopic visualization was graded with a 1–3 scale as follows: 1 = poor visualization of the embolic material when compared to surrounding bone structures, microcatheter/balloon markers; 2 = adequate visualization; and 3 = optimal visualization of the embolic cast with homogenous distribution of the embolic material within the rete.
Six swine underwent unilateral rete embolization with PHIL LV to evaluate performance characteristics and histological analysis at 14 and 90 d. Approximately 75% of the rete was embolized in each swine, to prevent distal occlusion of the ICA or of the contralateral rete due to embolic material crossing the midline. Two of these six animals were embolized with the flow arrest technique utilizing a Scepter balloon, and were euthanized at 14 d. Reflux of PHIL LV along the APA was allowed in one swine to test if the Scepter balloon can be withdrawn after completion of the embolization. The other four swine were embolized with a Headway Duo microcatheter and were euthanized at 14 and 90 d. The main goal was to compare penetration and performance of PHIL LV using the flow arrest technique with a Scepter balloon versus the standard plug formation with a single lumen microcatheter.
Two different harvesting times were selected to determine subacute (14 d) and chronic (90 d) histological changes after embolization. An angiographic assessment was performed before animals were euthanized. Under general anesthesia, we navigated a diagnostic catheter to the distal common carotid artery. A control angiogram of each vascular territory in each animal was performed to record stability of the embolic material, degree of occlusion and fluoroscopic visualization. Angiographic visualization of the embolic material was blindly scored by two investigators (CPD and MH).
Processing and histopathological analysis
Before processing, the retia and brains were photographed and imaged by capturing high contrast digital-based radiographs (Faxitron X-ray Corp, Model LX-60) to determine the presence of embolic material and extent of penetration throughout the rete vasculature.
The specimens were dehydrated in a graded series of ethanol, cleared in xylene and infiltrated in paraffin. Bilateral cerebral and cerebellar hemispheres, thalamus, mid brain, medulla and basal ganglia were sampled from each brain. The rete paraffin blocks were cut at 5 µm using a rotary microtome and stained with hematoxylin-eosin (H&E) and Movat pentachrome stains. Brain sections were stained with H&E only. All sections were examined by light microscopy to determine extension and occlusion by the embolic agent of the rete vasculature and the extent of induced vascular injury. Brain, lungs, heart, intestines, kidneys, spleen, stomach and prostate samples were grossly examined to assess the effect of embolization on end organs.
Luminal occlusion, vessel wall inflammation, vessel medial wall injury, extravasation of embolic material, vessel lumen recanalization, red blood cell perivascular hemorrhage and arterial wall necrosis were evaluated in every swine after harvesting the brain and rete. A semi-quantitative histology score was assigned to each parameter (Addendum 1). A vessel medial wall injury score was calculated based on the Schwartz method.5 Subacute (14 d) and chronic (90 d) changes were evaluated.
Results
Performance
In total, 10 retia were embolized: 2 with Squid 12 and 8 with PHIL LV. We did not experience any technical difficulties during embolization, such as arterial vasospasm, dissection and microcatheter/balloon occlusion or entrapment. In the two animals that were euthanized immediately after the procedure (Squid and PHIL LV in each rete) we achieved 100% embolization of bilateral retia. In the other eights animals euthanized at 14 and 90 d we achieved approximately a 75% embolization of the retia. Only the rete was embolized and no penetration of the embolic material into the ICA, distal outflow or contralateral rete was documented on fluoroscopy. Immediate fluoroscopic visualization of the PHIL cast was scored by the blinded adjudicators as, on average, 2.1 (adequate). After embolization, all the animals remained asymptomatic and did not experience any neurological or physiological change.
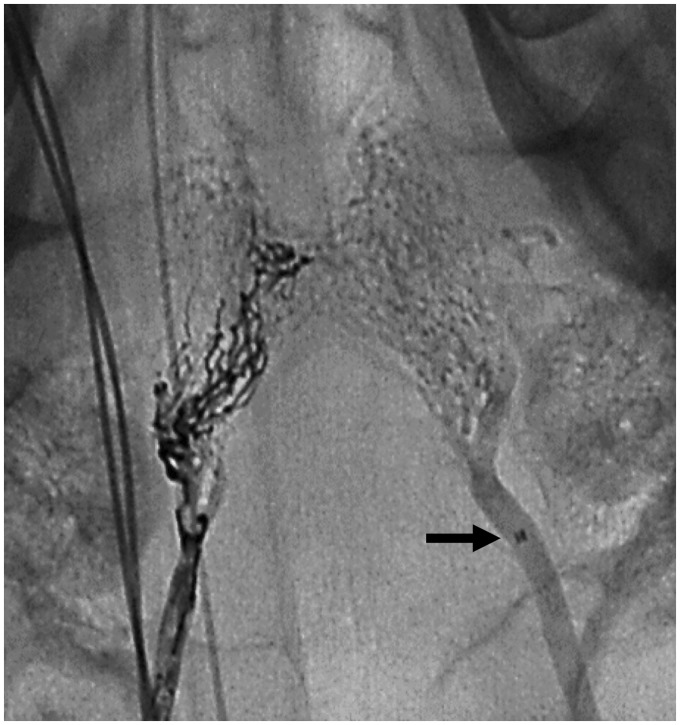
PHIL LV and Squid 12 had similar performances. Overall embolization, control of the injection and ease to retrieve the microcatheter after formation of the embolic plug was similar for both embolic materials (Table 1). PHIL’s fluoroscopic visualization was scored as adequate by one blinded adjudicator and as optimal by the other blinded adjudicator. Squid 12’s fluoroscopic visualization was scored as optimal by both adjudicators (Figure 1). More reflux into the APA was observed with Squid 12. Mean embolization time with PHIL LV was 18.5 min and 22 min with Squid 12 (Table 2). Because Squid 12 had initially more reflux, PHIL was refluxed on purpose in the contralateral rete to recreate an equivalent scenario to Squid in retrieving the microcatheter. We did not experience any difficulty in withdrawing the microcatheter after formation of the embolic cast with Squid 12 and PHIL.
Table 1.
Performance features of embolization.
| PHIL LV and Squid 12 |
PHIL LV Subacute (14 d) |
PHIL LV Chronic (90 d) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Description | Animal 1 PHIL Right rete | Animal 1 Squid Left rete | Animal 2 PHIL Left rete | Animal 2 Squid Right rete | Animal 3 PHIL | Animal 4 PHIL | Animal 5 PHIL Flow arrest | Animal 6 PHIL Flow arrest | Animal 7 PHIL | Animal 8 PHIL |
| Overall occlusion ability | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 3 | 3 |
| Ease of visualization | 2.5 | 3 | 2.5 | 3 | 1.5 | 2 | 3 | 3 | 2 | 3 |
| Ease of on/off control for material injection | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| Ease to extract catheter | 3 | 3 | 3 | 1 | 3 | 3 | 2 | 3 | 3 | 3 |
Overall scores for occlusion ability, ease of on/off control for material injection and ease to extract catheter: 3 = excellent, 2 = acceptable and 1 = unacceptable.
Visualization scores by blinded adjudicators: 3 = optimal, 2 = adequate and 1 = poor.
LV: low viscosity.
Figure 1.
Unsubtracted fluoroscopy of Squid 12 (left) and PHIL LV (right) casts. Note the tip of the microcatheter (arrow) in the distal ascending pharyngeal artery (APA). Both liquid embolics were refluxed along the distal APA.
Table 2.
Embolization materials, volume and results.
| Days | Animal number | Embolic material | Location | Catheter | Embolic volume (mL)a | Rete occlusion (%)b | Embolization time (min) |
|---|---|---|---|---|---|---|---|
| Same day | 1 | PHIL LV | R rete | HW Duo | 0.18 | 75–100 | 15 |
| 1 | SQUID 12 | L rete | HW Duo | 0.22 | 75–100 | 8 | |
| 2 | SQUID 12 | R rete | HW Duo | 0.27 | 75–100 | 36 | |
| 2 | PHIL LV | L rete | HW Duo | 0.48 | 75–100 | 22 | |
| 14 d | 3 | PHIL LV | R rete | HW Duo | 0.23 | 75 | 27 |
| 4 | PHIL LV | R rete | HW Duo | 0.18 | 75 | 4 | |
| 5 | PHIL LV | R rete | Scepter XC | 0.17 | 75 | 6 | |
| 6 | PHIL LV | R rete | Scepter XC | 0.13 | 75 | 13 | |
| 90 d | 7 | PHIL LV | R rete | HW Duo | 0.15 | 75 | 37 |
| 8 | PHIL LV | R rete | HW Duo | 0.21 | 75 | 10 |
L: left; LV: low viscosity; HW: Headway; R: right.
The embolic volume refers to the liquid embolic volume (mL) that was injected into the animal. The dead space of the adaptor and microcatheter lumen has been accounted for.
The rete occlusion percentage was assessed angiographically by the operator during the embolization procedure.
Better penetration, controlled injection and reduced reflux were observed with the flow arrest technique compared to just microcatheter plugging. However, both results were optimal. The Scepter balloon was also easily retrieved in the swine with reflux of PHIL LV along the entire length of the APA. In this case, the total embolization time was 13 min. The average embolization time with flow arrest was 9.5 min versus 19.5 min with just microcatheter plugging.
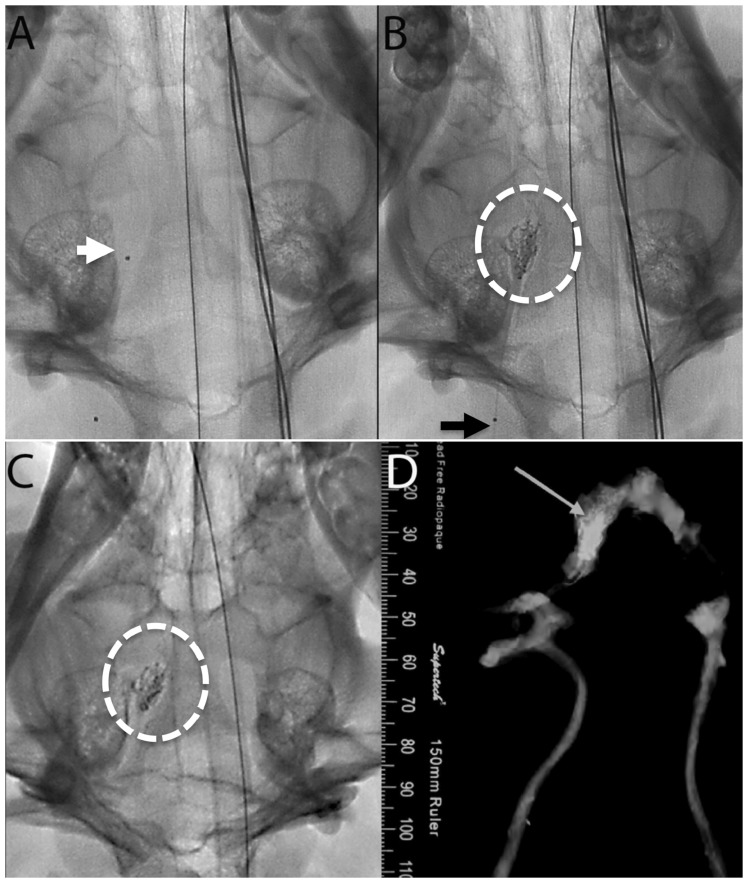
Follow-up angiography performed before the animals were euthanized after 14 and 90 d of the procedure confirmed that there was no distal cast break off, fracture or migration. This was further confirmed by high contrast digital-based radiographs (Figure 2). On average, fluoroscopic visualization of the embolic cast at 14 and 90 d was scored by the blinded adjudicators as 2.5 (adequate to optimal). Ipsilateral and contralateral angiograms of the treated rete demonstrated no evidence of recanalization.
Figure 2.
Pre- (a) and post- (b) embolization unsubtracted fluoroscopy of the right swine rete (white circle). (a) Note the tip of the microcatheter (arrow). (b) Note the proximal marker of the microcatheter (arrow). (c) A 90-d follow-up unsubtracted fluoroscopy demonstrating stability of the PHIL LV cast as compared to the post-embolization cast (b). (d) The rete X-ray (arrow) showed dispersion of the embolic material throughout the rete confirming the angiographic appearance of the embolic cast (b and c).
Microscopy and histology
Harvested samples were analyzed based on the semi-quantitative histological score assigned to each parameter (Addendum 2). Data was determined by collection of 15 occluded rete cross sections of each treated animal. Scores were made by the histopathologist based on the collection of cross sections. An average score and standard deviation were calculated based on the collection of the 15 cross sections.
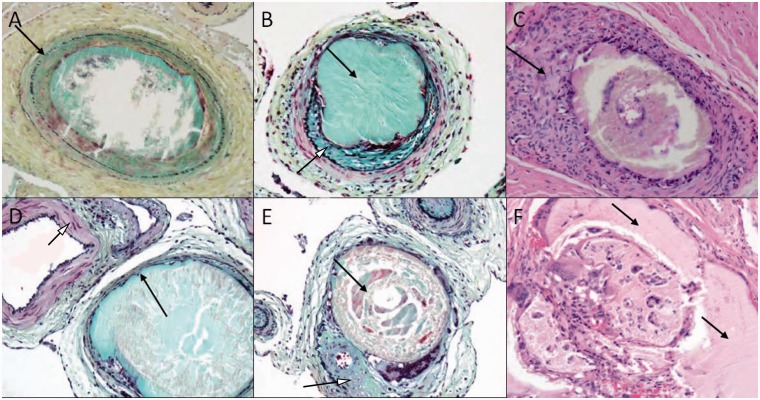
Microscopic analysis of subacute and chronic retia showed distended vessel lumens due to filling with PHIL LV. Although there were frequent disruptions of the internal elastic laminas, only rare medial wall disruptions were visualized. There was no extravasation of the embolic material or red blood cells into the surrounding extravascular tissue in the subacute samples. Occluded vessels showed medial smooth muscle loss with proteoglycan deposition and mild to moderate adventitial fibrosis in most of the samples. Chronic samples exhibited in cross section analysis gross distension with moderate to marked vessel wall attenuation or complete loss of medial smooth muscle. The loss of medial wall and internal elastic lamina was notable in the chronic specimens (Figure 3).
Figure 3.
High power views (50 µm) of retia cross sections demonstrating luminal occlusion with embolic material and blood clot: top row (a–c) shows rete harvested at 14 d and bottom row (d–f) shows rete harvested at 90 d from the procedure. (a) Shows an intact internal elastic lamina (arrow). (b) With luminal embolic material (black arrow) and proteoglycan-rich neointimal ingrowth (white arrowhead). (c) Shows adventitial chronic inflammatory cell infiltration (arrow). (d) With marked medial attenuation of an occluded cross section (black arrow) in comparison to an adjacent patent cross section (white arrowhead). (e) Shows cross sectional occlusion with embolic material (black arrow) and eccentric fibrointimal tissue (white arrowhead). (f) Shows embolic material (arrows) with inflammatory and giant cell infiltration in the lumen. (a, b, d and e) with Movat pentachrome stain; (c and f) with hematoxylin-eosin stain.
Histological analysis of subacute samples showed occasional infiltrating inflammatory cells, giant cells and fibrous tissue ingrowth. There was no difference if the procedure was performed with or without flow arrest. Similar findings were observed in the chronic samples. Cross sections of the occluded APA and proximal rete branches in the chronic specimens showed marked chronic inflammatory reaction with macrophage and giant cell reactivity to the embolic material.
Histological analysis did not demonstrate any significant inflammatory response in the surrounding tissues. Inflammation around the retia vasculature was overall mild in animals euthanized at 14 and 90 d, and generally consisted of intraluminal, medial and adventitial inflammatory cell infiltration (Figure 3).
Gross anatomical examination and high contrast digital-based radiographs of all the swine at 14 and 90 d did not demonstrate embolization of vascular territories beyond the rete or presence of radiodense material in the central nervous system (Figure 2(d)). However, in a subacute brain sample, it was noted in the left cerebellum a single sulcus arteriole branch completely occluded with fragments of foreign material similar to the polymer material present in the contralateral occluded rete.
Gross examination of other harvest organs such as heart, intestines, kidneys, spleen, stomach, prostate and lungs was within normal limits in both the subacute and chronic samples.
Discussion
We report the performance of PHIL LV in an in vivo swine rete model. PHIL LV is based on a biocompatible copolymer instead of EVOH and has the lowest viscosity of the PHIL line. Overall, PHIL LV demonstrated an excellent technical performance in all the evaluated parameters. Histological and microscopic analysis at 14 and 90 d demonstrated a 100% occlusion rate without evidence of recanalization or of significant inflammatory changes in the surrounding tissue.
The AVM rete swine model has been validated with different embolic agents.6,7 In this in vivo experience, embolization of rete with PHIL LV demonstrated excellent distal penetration of the target vascular territory, ease of plug formation and no entrapment of the microcatheter or balloon. One study by Vollherbst et al. compared Onyx to PHIL in an in vitro AVM model and found a similar embolization pattern of the artificial nidus.8 However, lower quantities of PHIL compared with Onyx were required to cover the same nidal volume. This may explain why, in our experience, the average embolization time with Squid was longer (22 min) when compared to PHIL LV (18.5 min) in embolizing each rete. Moreover, in general we required larger volumes of Squid compared to PHIL (Table 2). Squid and Onyx require prolonged injections to achieve a proximal plug due to their relatively long precipitation process. Both EVOH-based liquid embolic agents penetrate and fill the vascular lumen with a lava-layering effect instead of a forward “column” movement like the one exhibited by PHIL. Squid and PHIL LV demonstrated similar embolization properties, such as overall occlusion, fluoroscopic visualization and microcatheter extraction.
Fluoroscopic visualization of the PHIL LV cast was scored as adequate to optimal (Table 1) in every scenario: immediately after embolization and in follow-up angiograms performed at 14 and 90 d of the procedure. Cast formation was uniform because PHIL does not have tantalum and precipitates evenly throughout the vessel network. The microcatheter or balloon distal markers can easily be seen even within large embolization casts (Figure 1).3 Precise localization of the microcatheter/balloon tip during embolization facilitates reflux control, assessment of venous penetration and nidal embolization in treatment of AVMs and complex arteriovenous fistulas.2,9
Gentric et al. also described their experience with PHIL and Onyx 18 in embolizing swine rete.10 However, their study was mainly focused in comparing balloon flow arrest versus microcatheter plugging, instead of in describing the behavior of each embolic agent. We also encountered easier plug formation and shorter embolization times when we used flow arrest with the Scepter balloon instead of forming a plug with the microcatheter. Flow arrest is recommended to increase nidal penetration and injection control during embolization.11 The embolization time with PHIL LV was shorter (9.5 min) when we used balloon arrest in the APA when compared to no flow arrest (19.5 min). Other performance properties such as penetration of the target vascular territory, ease of plug formation and no entrapment of the balloon, did not change when compared to microcatheter plugging. Microscopic and histological findings were similar in both scenarios.
PHIL LV did not show clinical or histological evidence of any adverse reaction in the swine rete model. After embolization animals remained asymptomatic up to 90 d from the procedure. Vollherbst et al. described signs of mild inflammation after 7 d of embolization with PHIL 25 and Onyx 18.4 We encountered similar histological findings at 14 and 90 d from the procedure. We harvested the embolized retia up to 90 d from the procedure and confirmed the permanent occlusion of the treated vessels by PHIL LV, with minimal inflammatory changes in surrounding tissue. It is unclear if the mild inflammatory findings are related to the presence of the embolic material or the intra-arterial infusion of DMSO as has been described by other authors.12 Moreover, mild and usually focal periadventitial inflammation has also been described in both control and embolized retia. This finding is commonly observed in swine and may be related to the continuity of the nasal venous circulation with the cavernous sinus.13
This study has several limitations. First, the main operators were not blinded at the time of embolization and their appraisal of the behavior of the embolic materials can have been biased. Second, although the study was not intended to compare PHIL LV versus other embolic agents, we only injected two retia with Squid 12, limiting the comparison we can make with this EVOH-based embolic agent.
Conclusions
In this swine rete model, the new PHIL LV embolic agent had an excellent embolization performance. Vessels embolized with PHIL LV remained occluded up to 90 d from the procedure with minimal inflammatory changes. Vascular occlusion was corroborated with angiographic, macroscopic and histologic analysis.
Supplemental Material
Supplemental material for In vivo evaluation of the new PHIL low viscosity in a swine rete mirabile model by Edgar A Samaniego, Colin P Derdeyn, Minako Hayakawa, David Hasan and Santiago Ortega-Gutierrez in Interventional Neuroradiology
Acknowledgment
We thank Tom Burke (TB) of MicroVention for presenting a preliminary version of this study on 17 October 2017 as an oral abstract at the World Federation of Interventional and Therapeutic Neuroradiology meeting in Budapest, Hungary, and for participating as main operator in some of the embolizations and follow-up angiograms.
Contributors
EAS: acquisition of data, study design, data analysis, manuscript preparation, critical revision of the manuscript and guarantor of the study. MH and CPD: blinded adjudicators. All authors reviewed and approved the manuscript.
Declaration of conflicting interests
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EAS is a consultant for MicroVention. CPD has stock options with Pulse and has received an honorarium from Bayer. MH, DH and SO-G declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lamin S, Chew HS, Chavda S, et al. Embolization of intracranial dural arteriovenous fistulas using PHIL liquid embolic agent in 26 patients: a multicenter study. AJNR Am J Neuroradiol 2017; 38: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samaniego EA, Kalousek V, Abdo G, et al. Preliminary experience with Precipitating Hydrophobic Injectable Liquid (PHIL) in treating cerebral AVMs. J Neurointerv Surg 2016; 8: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 3.Varadharajan S, Ramalingaiah AH, Saini J, et al. Precipitating hydrophobic injectable liquid embolization of intracranial vascular shunts: initial experience and technical note. J Neurosurg 2017; epub ahead of print, DOI: 10.3171/2017.6.JNS16447. [DOI] [PubMed]
- 4.Vollherbst DF, Otto R, von Deimling A, et al. Evaluation of a novel liquid embolic agent (precipitating hydrophobic injectable liquid (PHIL)) in an animal endovascular embolization model. J Neurointerv Surg 2018; 10: 268–274. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992; 19: 267–274. [DOI] [PubMed] [Google Scholar]
- 6.Derdeyn CP, Graves VB, Salamat MS, et al. Collagen-coated acrylic microspheres for embolotherapy: in vivo and in vitro characteristics. AJNR Am J Neuroradiol 1997; 18: 647–653. [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber BB, Wakhloo AK, Siekmann R, et al. Acute and chronic swine rete arteriovenous malformation models: effect of ethiodol and glacial acetic acid on penetration, dispersion, and injection force of N-butyl 2-cyanoacrylate. AJNR Am J Neuroradiol 2005; 26: 1707–1714. [PMC free article] [PubMed] [Google Scholar]
- 8.Vollherbst DF, Sommer CM, Ulfert C, et al. Liquid embolic agents for endovascular embolization: evaluation of an established (Onyx) and a novel (PHIL) embolic agent in an in vitro AVM model. AJNR Am J Neuroradiol 2017; 38: 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samaniego EA, Fisher M, Hasan D, et al. Embolization of palpebral and orbito-frontal fistulas: technical and anatomical considerations in treating high-flow superficial skin lesions with liquid embolics. J Neurointerv Surg 2018; 10: 240–244. [DOI] [PubMed] [Google Scholar]
- 10.Gentric JC, Raymond J, Batista A, et al. Dual-lumen balloon catheters may improve liquid embolization of vascular malformations: an experimental study in swine. AJNR Am J Neuroradiol 2015; 36: 977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapot R, Stracke P, Velasco A, et al. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol 2014; 41: 87–91. [DOI] [PubMed] [Google Scholar]
- 12.Chaloupka JC, Huddle DC, Alderman J, et al. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete embolization model. AJNR Am J Neuroradiol 1999; 20: 401–410. [PMC free article] [PubMed] [Google Scholar]
- 13.Chaloupka JC, Vinuela F, Vinters HV, et al. Technical feasibility and histopathologic studies of ethylene vinyl copolymer (EVAL) using a swine endovascular embolization model. AJNR Am J Neuroradiol 1994; 15: 1107–1115. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for In vivo evaluation of the new PHIL low viscosity in a swine rete mirabile model by Edgar A Samaniego, Colin P Derdeyn, Minako Hayakawa, David Hasan and Santiago Ortega-Gutierrez in Interventional Neuroradiology