Abstract
Background
Low-profile, self-expandable stents are used to treat wide-neck aneurysms located on the smaller distal intracranial arteries. This study aimed to assess the usefulness of time-of-flight (TOF) and contrast-enhanced (CE) magnetic resonance angiography (MRA) for follow-up after LEO Baby stent (LBS)-assisted coil embolization.
Methods
Twenty-four aneurysms treated with LBS-assisted coil embolization were evaluated. Researchers reviewed TOF MRA and CE MRA images in terms of occlusion and stent patency. Aneurysm occlusion was graded according to Raymond–Roy classification as follows: total occlusion (grade 1), residual neck (grade 2), and residual aneurysm (grade 3). Stent patency was scored as follows: occlusion (1), stenosis (2), and normal (3). Interobserver and intermodality agreement values were determined by weighted kappa (κ) statistics.
Results
Intermodality and interobserver values of TOF MRA and CE MRA with digital subtraction angiography (DSA) were perfect (κ = 1.00, p < 0.001) in terms of aneurysm occlusion. Rate of stent occlusion and stenosis in DSA, TOF, and MRA, respectively, were as follows: 0 and 12.5%, 16.6 and 70.8%, and 0 and 62.5%. Intermodality agreement values of TOF MRA and CE MRA with DSA were insignificant in terms of stent patency (κ = 0.065, p = 0.27; κ = 0.158, p = 0.15, respectively). Interobserver agreement was substantial in both TOF MRA (κ = 0.71, p < 0.001) and CE MRA (κ = 0.64, p = 0.001).
Conclusions
Both TOF and CE MRA techniques have strong concordance with DSA for the detection of aneurysm occlusion status. CE MRA can be used as a first-line noninvasive imaging modality due to its superiority to TOF MRA with respect to the visualization of in-stent signals.
Keywords: Low-profile stent, magnetic resonance angiography, neurointervention, intracranial aneurysm
Introduction
Endovascular therapy of intracranial aneurysms has been widely performed since the International Subarachnoid Aneurysm Trial. The stent-assisted coil embolization technique has widened the indications of endovascular treatment.1 However, residual or recurrent aneurysm filling still remains a problem, necessitating serial imaging follow-up of endovascularly treated aneurysms, usually by magnetic resonance angiography (MRA), as it is a noninvasive follow-up modality. Introduction of low-profile, self-expandable stents to endovascular practice enabled the treatment of wide-neck aneurysms located on the smaller distal intracranial arteries which are more difficult to evaluate with MRA. Among the low-profile intracranial stents, only the LVIS Jr. stent (Microvention, Tustin, CA, USA) has been studied with regard to the applicability of a new non-contrast MRA sequence as an imaging modality for imaging follow-up.2 To our knowledge, the efficacy of either non-enhanced or contrast-enhanced (CE) MRA in the post-treatment imaging of the other frequently used low-profile intracranial stents, the LEO Baby (Balt, Montmorency, France), Acclino (Acandis, Pforzheim, Germany), Atlas (Stryker, Fremont, CA, USA), or LVIS Blue (Microvention, Tustin, CA, USA) stents, is currently unknown. Although all of these stents are made of nitinol, they differ in their strut architecture, platinum content, strut thickness, and the configuration or content of their markers. The susceptibility artifacts secondary to these devices are likely to differ among different stent types. In this study, we evaluated the use of MRA, as a follow-up modality, in those aneurysms treated by the LEO Baby stent (LBS, Balt Extrusion, Montmorency, France), which has 16 nitinol wires and two radiopaque platinum markers. Compared to other low-profile self-expandable intracranial stents,3 the LBS has a higher surface coverage owing to its smaller pore size (approximately 0.9 mm), raising the possibility that impaired luminal visibility may result.
Materials and methods
Patients treated with LBS-assisted coil embolization for cerebral aneurysms between May 2013 and May 2015 were retrospectively included in this study. Only those aneurysms coiled with the assistance of a single LBS were included. For each patient, follow-up imaging studies consisting of MRA and digital subtraction angiography (DSA) were evaluated. Our routine protocol involved obtaining a baseline cerebral MRA on the same day as the initial routine follow-up DSA. Once the findings of the MRA were verified by the initial follow-up DSA, the patients were followed up only by MRA. If there was a suggestion of aneurysm recanalization or parent artery stenosis on follow-up MRA, cerebral DSA was repeated. The study was approved by our institutional review board, and informed consent was not obtained due to the retrospective study design.
Digital subtraction angiography
Intra-arterial DSA was performed with a biplane angiographic system (Axiom Artis, Siemens, Erlangen, Germany). Selective injections of a non-ionic iodinated contrast material (İopromid, Ultravist 300 mg I/mL, Bayer HealthCare, Berlin, Germany) into the artery harboring the aneurysm were performed with a power injector, and frontal, lateral, oblique, and working projections were obtained.
Magnetic resonance angiography
MRA examinations were performed on a 1.5 T device (Achieva, Philips Medical System, Best, the Netherlands). The time-of-flight (TOF) MRA scanning parameters were as follows: repetition time (TR)/echo time (TE)/flip angle (FA) = 25 ms/6.9 ms/20°, field of view (FOV) = 230 × 195.5 × 112.5 mm3, acquisition matrix = 480 × 234, reconstruction matrix = 512 × 512, acquisition voxel = 0.48 × 0.84 × 1.5 mm3, reconstruction voxel = 0.45 × 0.45 × 0.75 mm3, and bandwidth = 108.5 Hz. For CE MRA, pre-contrast T1 FFE (fast field echo) images were obtained first, and then 0.1 mL/kg gadobutrol (Gadovist, 1 mmol/L, Bayer HealthCare, Berlin, Germany) was injected at a rate of 2 mL/s. To reduce acquisition time and slice thickness and to increase the matrix, the smallest FOV covering stent and coils were identified. When the common carotid arteries began to enhance on a coronal fluoroscopic sequence, scanning was initiated. Five consecutive dynamic series were obtained for optimal arterial phase imaging and reduction of venous contamination. Pre-contrast images were subtracted from post-contrast images to avoid false residue appearance, which would be due to thrombosed aneurysm. The CE MRA scanning parameters were as follows: TR/TE/FA = 5.5 ms/1.62 ms/40°, acquisition matrix = 384 × 183, reconstruction matrix = 400 × 400, acquisition voxel = 0.6 × 0.94 × 3 mm3, reconstruction voxel = 0.57 × 0.57 × 1.5 mm3, bandwidth = 217 Hz, and k space profile CENTRA (contrast enhanced timing robust angiography).
Image interpretation
Two neuroradiologists (OA and AP) blinded to patient identity and who were not involved in the treatment or follow-up of the patients evaluated DSA images on a commercially available workstation (Syngo workstation, Leonardo, Siemens Medical Solutions, Forchheim, Germany) in random order without any knowledge of magnetic resonance imaging (MRI) examinations. DSA was the gold standard imaging modality.
Two neuroradiologists (SA and SB) separately evaluated TOF and CE MRA images by using source images, maximum intensity projection (MIP) images and multiplanar reformatted (MPR) images on a commercially available workstation (Extended MR Workspace, Philips, Best, The Netherlands), in random order.
Researchers reviewed aneurysms in terms of occlusion and stent patency. Aneurysm occlusion was graded according to Raymond–Roy classification as follows: total occlusion (grade 1), residual neck (grade 2) and residual aneurysm (grade 3).2 Stent patency was scored as follows: occlusion (1), stenosis (2) and normal (3). In cases where there was discordance in DSA or MRA findings, consensus was reached by a collaborative decision of the two neuroradiologists before the analysis of intermodality agreement.
Statistical analysis
Interobserver and intermodality agreement values were determined by weighted kappa (κ) statistic with a 95% confidence interval: κ = 0–0.2 inadequate agreement; κ = 0.21–0.4 slight agreement; κ = 0.41–0.6 moderate agreement; κ = 0.61–0.8 substantial agreement, κ = 0.81–1.00 almost perfect agreement, and κ = 1.00 perfect agreement. A p-value < 0.05 was considered significant. Statistical analysis was performed with SPSS 23.0 (IBM SPSS Inc., Chicago, IL, USA).
Results
Patient and aneurysm characteristics
Twenty-three patients (10 male, 13 female; mean age: 49 years (range: 23–75 years)) with 24 aneurysms were evaluated in this study. Out of 24 aneurysms, 21 were located in the anterior circulation and three were located in the posterior circulation. Locations of the aneurysms were as follows: 14 on middle cerebral artery (MCA) bifurcation (58.3%, one on early bifurcation of MCA), six on anterior communicating artery (AcoA) (25%), one on anterior cerebral artery (ACA) A2 segment (4.1%), two on superior cerebellar artery (SCA) (8.3%), and one on basilar tip (4.1%). The mean aneurysm size was 7.29 mm (range: 3–12 mm) (Table 1). All of the aneurysms were unruptured and treated with LBS and bare platinum coils.
Table 1.
Patient and aneurysm characteristics.
| Patient/ aneurysm No | Gender | Age | Location | Maximum size (mm) |
|---|---|---|---|---|
| 1/1 | M | 48 | MCA Bif | 6 |
| 2/2 | F | 54 | MCA Bif | 4 |
| 3/3 | M | 61 | AcoA | 3 |
| 4/4 | F | 38 | MCA Bif | 8 |
| 5/5 | F | 41 | MCA Bif | 5 |
| 6/6 | M | 39 | MCA Bif | 5 |
| 7/7 | F | 49 | MCA Bif | 9 |
| 8/8 | F | 51 | MCA Bif | 7 |
| 9/9 | M | 53 | MCA Bif | 10 |
| 10/10 | F | 42 | MCA Bif | 7 |
| 11/11 | M | 68 | MCA Bif | 9 |
| 12/12 | M | 43 | AcoA | 12 |
| 13/13 | F | 36 | MCA Bif | 10 |
| 14/14 | M | 44 | MCA Bif | 12 |
| 15/15 | M | 59 | MCA Bif | 8 |
| 15/16 | M | 59 | AcoA | 8 |
| 16/17 | M | 52 | AcoA | 5 |
| 17/18 | F | 54 | AcoA | 7 |
| 18/19 | F | 75 | MCA Bif | 9 |
| 19/20 | F | 50 | SCA | 9 |
| 20/21 | F | 49 | SCA | 5 |
| 21/22 | M | 23 | AcoA | 3 |
| 22/23 | F | 57 | Basilar tip | 6 |
| 23/24 | F | 42 | Distal ACA | 8 |
M, male; F, female; ACA, anterior cerebral artery; AcoA, anterior communicating artery; Bif, bifurcation; MCA, middle cerebral artery; SCA, superior cerebellar artery.
Aneurysm occlusion
The intermodality agreement was perfect between TOF MRA and DSA (κ = 1.00, p < 0.001). Similarly, the corresponding value was perfect between CE MRA and DSA (κ = 1.00, p < 0.001). Interobserver agreement values of TOF MRA, CE MRA, and DSA were also perfect (κ = 1.00, p < 0.001). Nineteen out of 24 aneurysms were evaluated as complete occlusion. Residual neck was detected in two aneurysms and residual aneurysm was detected in three aneurysms.
Stent patency
The rates of stent occlusion and stenosis in DSA, TOF, and MRA, respectively, were as follows: 0 and 12.5%, 16.6 and 70.8%, 0 and 62.5%.
Intermodality agreement values of TOF MRA and CE MRA with DSA were insignificant in terms of stent patency (κ = 0.065, p = 0.27; κ = 0.158, p = 0.15, respectively). Interobserver agreement was substantial in both TOF MRA (κ = 0.71, p < 0.001) and CE MRA (κ = 0.64, p = 0.001). Additionally, this agreement was perfect in DSA (κ = 1.00, p < 0.001). Table 2 demonstrates stent patency scores. Table 3 shows the specificity, sensitivity, negative predictive value (NPV) and positive predictive value (PPV) of TOF MRA and CE MRA in terms of stent patency. Table 4 shows false positive rates of TOF MRA and CE-MRA in MCA and non-MCA subgroups separately in terms of stent patency. Figures 1–3 are representative images.
Table 2.
Stent patency scores.
| TOF MRA | CE MRA | DSA | |
|---|---|---|---|
| Occlusion | 4 | – | – |
| Stenosis | 17 | 15 | 3 |
| Normal | 3 | 9 | 21 |
Table 3.
Specificity, sensitivity, negative predictive value, and positive predictive value of TOF MRA and CE MRA in terms of visualization of stent lumen.
| TOF MRA | CE MRA | |
|---|---|---|
| Sensitivity | 100% | 100% |
| Specificity | 14,2% | 42,8% |
| Negative predictive value | 100% | 100% |
| Positive predictive value | 14,2% | 20% |
Table 4.
False positive rates of TOF MRA and CE-MRA in MCA and non-MCA subgroups in terms of visualization of stent lumen.
| TOF MRA | CE MRA | |
|---|---|---|
| MCA | 85% | 64% |
| Non-MCA | 60% | 30% |
Figure 1.
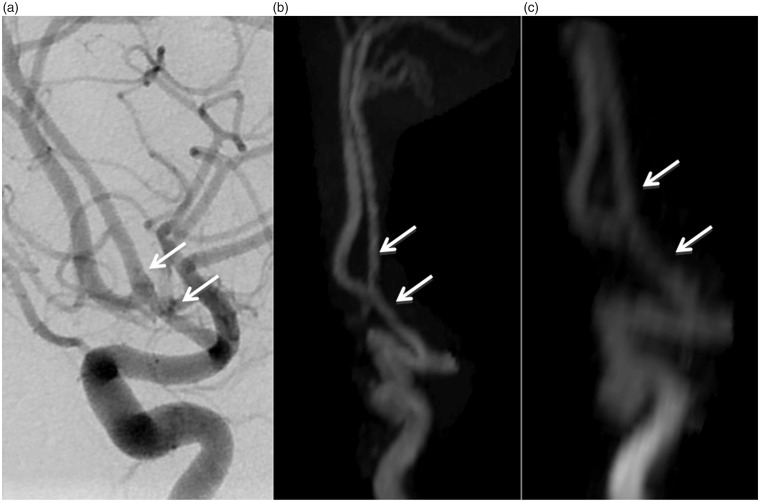
A 23-year-old male patient, AcoA aneurysm treated with LBS-assisted coil embolization. DSA (a), TOF MRA (b), and CE MRA (c) images show total occlusion of the aneurysm. There is no stenosis or occlusion in the stent (arrows).
Figure 2.
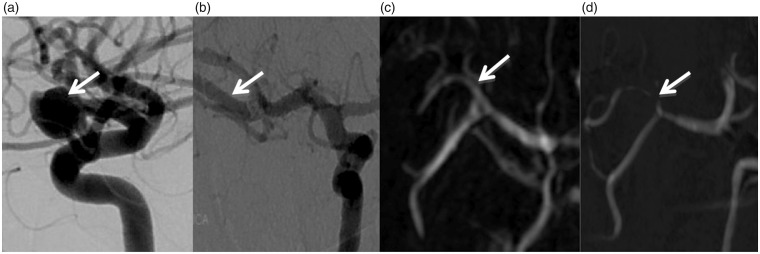
A 39-year-old female patient. Pre-operative DSA (a) shows right MCA bifurcation aneurysm (arrow). Follow-up DSA (b), CE MRA (c), and TOF MRA (d) images depict no residual aneurysm. DSA (b) and CE MRA (c) images show normal parent artery (arrows). TOF MRA (d) image shows severe stenosis and complete signal loss at the stented segment (arrow).
Figure 3.
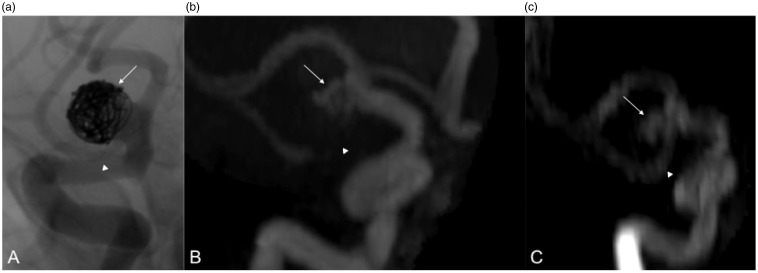
A 68-year-old male patient, right MCA bifurcation aneurysm treated with LBS-assisted coil embolization. DSA (a), TOF MRA (b), and CE MRA (c) images depict residual aneurysm (arrows). TOF MRA (b) image shows complete signal loss and CE MRA (c) image shows stenosis at the stented segment (arrowheads). DSA (a) image shows normal parent artery (arrowhead).
Discussion
This study is the second to assess the ability of MRA to detect residual/recurrent aneurysms and parent artery stenosis/occlusions associated with the endovascular treatment of cerebral aneurysms with new generation intracranial stents and the first to compare CE and non-enhanced MRA for the evaluation of distal aneurysms treated with these devices. To the best of our knowledge, the only study conducted for follow-up evaluations after treatment with low-profile intracranial stents is focused on a newly introduced, non-routine MRA sequence (non-contrast-enhanced “silent scan sequence”), which is not currently available to neuroradiology departments in general; subsequently, the authors did not assess CE MRA in that study. Additionally, notwithstanding the fact that dual stents tend to demonstrate more artifacts as opposed to those treated with a single stent on TOF MRA,3,4 single stent and dual stents were evaluated together in this study to determine the limits of MR visualization with this novel sequence.
Few authors have compared only non-contrast enhanced MRA techniques with DSA after stent-assisted coil embolization.3,5–8 In the studies that evaluated only TOF MRA, it was reported that aneurysm occlusion status was determined effectively by TOF MRA, but it did not adequately show in-stent stenosis.5,6 Takano et al. reported that silent MRA was superior to demonstrate flow in the stent and showed neck remnants more precisely than three-dimensional (3D) TOF MRA.3,7,8
There are even fewer studies reporting the role of CE techniques in the evaluation of stent-assisted coil embolization.9–14 CE MRA was found to be superior to TOF MRA, yet not as good as DSA to assess aneurysm occlusion and parent artery patency and the researchers recommended CE MRA for the evaluation of stent-assisted coil embolization.9,10,11,13 Agid et al. found CE MRA to be an accurate technique to identify remnant aneurysms in stent-assisted coiling,12 and reported that the presence of apparent in-stent stenosis on CE-MRA was often false or exaggerated. Marciano et al. compared CE MRA and TOF MRA with DSA.14 Their results were similar to ours and deduced that TOF MRA and CE MRA showed good accuracy to detect aneurysm remnants, but both MRA techniques were unable to provide a precise evaluation of the stent lumen.
Finally, three series reported the accuracy of MRA in the follow-up of flow diverters, which are variants of braided stents with a higher pore density.4,15,16 All of these latter studies concluded that CE MRA, rather than TOF MRA, is the imaging of choice, regarding MRA follow-up of flow diverters.
The role of MRA in the depiction of aneurysm occlusion and stent visualization in the distal circulation where small intracranial stents are utilized is still unknown.17–19 The studies listed above evaluated a handful of distally placed stents with MRA. The braided devices studied by MRA are even lower. Apart from the study evaluating the “silent scan,” the MRA evaluation of only 19 braided stents was reported in the distal circulation in total by several authors,3,4,14 and it is not clear in the literature how reliable MRA, with or without contrast, is for evaluation of these devices. In the present study, we showed that both TOF MRA and CE MRA depicted all residual neck and residual aneurysms demonstrated by DSA. Both MRA techniques were highly sensitive (100%) and had a considerable high NPV (100%), in terms of stent patency, but CE MRA was more specific, and had a higher PPV than TOF MRA. Though intermodality agreement values of these MRA techniques were not sufficient to be used as a substitute for DSA in the follow-up, compared with TOF MRA, CE MRA can be used as a first-step imaging modality due to its high NPV and higher specificity. Additionally, a higher false positive rate in MCA bifurcation aneurysms with TOF MRA (85% in MCA aneurysms versus 60% in non-MCA aneurysms) suggests the use of CE MRA as a first-step imaging modality especially in MCA aneurysms. The reason for the high false positive rate in our series may be the incorporation of the origins of distal MCA branches into the aneurysm dome, a frequent finding in MCA aneurysms.20 In cases treated with stent-assisted coiling, the stents placed within these incorporated branches may be surrounded by the coils used to treat these aneurysms, intensifying the coil-related susceptibility artifact and possibly decreasing the signal intensity within the stent. Our findings indicate that a clear depiction of the patency of the parent artery may obviate the need for a DSA follow-up. Such a follow-up imaging strategy was also suggested by Attali et al. for flow diverters.15 For clinical purposes, if CE MRA depicts in-stent stenosis or stent occlusion, DSA should be carried out in order to rule out in-stent stenosis or stent occlusion due to false positive results. Accordingly, it is possible to avoid performing DSA in a group of patients in whom there are no particular clinical concerns for stent stenosis/occlusion.
Loss of signal in TOF MRA occurs due to the susceptibility artifact caused by stents and coils, the saturation effect, the RF shielding effect of the stent, the turbulent flow, and the intravoxel phase dispersion. The extent of signal loss changes, depending upon thickness of the stent strut, cell design of the stent, stent material, diameter of the stent, tortuosity of parent artery, orientation of the stent, and MRI parameters.10,11,21–23 Open-cell design, thinner stent strut, and nitinol stents are associated with less artifacts and signal loss.6,24 Smaller voxel, shorter TE, shorter TR, wider bandwidth, and a parallel imaging technique reduce magnetic susceptibility artifacts.21,22,25 The radiofrequency (RF) shielding effect of the stent is decreased by a high FA.22 In this study, longer TR, longer TE, and narrower bandwidth of TOF MRA were the reasons for increased susceptibility. The lower FA of TOF MRA increased the RF shielding effect of the stent. These MRI parameters made detection of the signal in the stent difficult with TOF MRA.
Saturation effect, susceptibility artifact, and loss of signal due to turbulent flow are less in the CE MRA than in the TOF MRA. However, venous contamination and contrast enhancement of the vessel wall can result in false positive findings with regard to recurrent aneurysms. Thrombosed aneurysms can be mistaken for residual aneurysm filling due to T1 shortening.11 We avoided this pitfall and minimized venous contamination by dynamic imaging and by subtracting pre-contrast images from post-contrast images.
We believe that the factors which make it difficult to detect in-stent signals are related to the small pore size of LBS and the high surface coverage ratio,26 the smaller parent artery (<3 mm) size, and the two longitudinal radio-opaque platinum markers of the LBS. We also think that the new version of the LVIS Jr. stent, the LVIS Blue, which has specifications that are very similar to the LBS, will demonstrate similar imaging characteristics, except for the markers at both ends, which may cause further signal degradation.
The present study had some limitations. First, the sample size was small. Second, the data were collected retrospectively. Third, 3D DSA, which may be better than two-dimensional DSA for follow-up,27,28 was not obtained routinely. Finally, susceptibility artifacts differ among different coil types; this factor may potentially alter luminal visibility adversely with MRA when certain coils are used. Nevertheless, our study sheds light on the applicability of MRA for the follow-up of aneurysms treated with mini-braided stents, which are becoming the mainstay of endovascular treatment of bifurcation aneurysms, and sets a benchmark to which other types of mini stents can be compared during follow-up.
Conclusions
Both TOF and CE MRA techniques have strong concordance with DSA for the detection of aneurysm occlusion status when used to evaluate aneurysms coiled by LBS assistance and can be used for follow-up of aneurysms treated with LBS-assisted coil embolization. CE MRA can be used as a first-line noninvasive imaging modality in the follow-up, due to its superiority to TOF MRA with respect to the visualization of the in-stent signal.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 2.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001; 32: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 3.Takano N, Suzuki M, Irie R, et al. Non-contrast-enhanced silent scan MR angiography of intracranial anterior circulation aneurysms treated with a low-profile visualized intraluminal support device. Am J Neuroradiol 2017; 38: 1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boddu SR, Tong FC, Dehkharghani S, et al. Contrast-enhanced time-resolved MRA for follow-up of intracranial aneurysms treated with the pipeline embolization device. Am J Neuroradiol 2014; 35: 2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho YD, Kim KM, Lee WJ, et al. Time-of-flight magnetic resonance angiography for follow-up of coil embolization with enterprise stent for intracranial aneurysm: usefulness of source images. Korean J Radiol 2014; 15: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho WS, Kim SS, Lee SJ, et al. The effectiveness of 3 T time-of-flight magnetic resonance angiography for follow-up evaluations after the stent-assisted coil embolization of cerebral aneurysms. Acta Radiol 2014; 55: 604–613. [DOI] [PubMed] [Google Scholar]
- 7.Irie R, Suzuki M, Yamamoto M, et al. Assessing blood flow in an intracranial stent: a feasibility study of MR angiography using a silent scan after stent-assisted coil embolization for anterior circulation aneurysms. Am J Neuroradiol 2015; 36: 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano N, Suzuki M, Irie R, et al. Usefulness of non-contrast-enhanced MR angiography using a silent scan for follow-up after Y-configuration stent-assisted coil embolization for basilar tip aneurysms. Am J Neuroradiol 2017; 38: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubicz B, Levivier M, Sadeghi N, et al. Immediate intracranial aneurysm occlusion after embolization with detachable coils: a comparison between MR angiography and intra-arterial digital subtraction angiography. J Neuroradiol 2007; 34: 190–197. [DOI] [PubMed] [Google Scholar]
- 10.Takayama K, Taoka T, Nakagawa H, et al. Usefulness of contrast-enhanced magnetic resonance angiography for follow-up of coil embolization with the enterprise stent for cerebral aneurysms. J Comput Assist Tomogr 2011; 35: 568–572. [DOI] [PubMed] [Google Scholar]
- 11.Choi JW, Roh HG, Moon WJ, et al. Time-resolved 3D contrast-enhanced MRA on 3.0T: a non-invasive follow-up technique after stent-assisted coil embolization of the intracranial aneurysm. Korean J Radiol 2011; 12: 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agid R, Schaaf M, Farb R. CE-MRA for follow-up of aneurysms post stent-assisted coiling. Interv Neuroradiol 2012; 18: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thamburaj K, Cockroft K, Agarwal AK, et al. A comparison of magnetic resonance angiography techniques for the evaluation of intracranial aneurysms treated with stent-assisted coil embolization. Cureus 2016; 8: e909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marciano D, Soize S, Metaxas G, et al. Follow-up of intracranial aneurysms treated with stent-assisted coiling: comparison of contrast-enhanced MRA, time-of-flight MRA, and digital subtraction angiography. J Neuroradiol 2017; 44: 44–51. [DOI] [PubMed] [Google Scholar]
- 15.Attali J, Benaissa A, Soize S, et al. Follow-up of intracranial aneurysms treated by flow diverter: comparison of three-dimensional time-of-flight MR angiography (3D-TOF-MRA) and contrast-enhanced MR angiography (CE-MRA) sequences with digital subtraction angiography as the gold standard. J Neurointerv Surg 2016; 8: 81–86. [DOI] [PubMed] [Google Scholar]
- 16.Patzig M, Forbrig R, Ertl L, et al. Intracranial aneurysms treated by flow-diverting stents: long-term follow-up with contrast-enhanced magnetic resonance angiography. Cardiovasc Intervent Radiol 2017; 40: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 17.Brassel F, Grieb D, Meila D, et al. Endovascular treatment of complex intracranial aneurysms using Acandis Acclino stents. J Neurointerv Surg 2017; 9: 854–859. [DOI] [PubMed] [Google Scholar]
- 18.Shankar JJS, Quateen A, Weill A, et al. Canadian registry of LVIS Jr for treatment of intracranial aneurysms (CaRLA). J Neurointerv Surg 2017; 9: 849–853. [DOI] [PubMed] [Google Scholar]
- 19.Aydin K, Arat A, Sencer S, et al. Stent-assisted coiling of wide-neck intracranial aneurysms using low-profile LEO Baby stents: initial and midterm results. Am J Neuroradiol 2015; 36: 1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topcuoglu OM, Akgul E, Daglioglu E, et al. Flow diversion in middle cerebral artery aneurysms: is it really an all-purpose treatment? World Neurosurg 2016; 87: 317–327. [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Roh HG, Moon WJ, et al. Optimization of MR parameters of 3D TOF-MRA for various intracranial stents at 3.0 T MRI. Neurointervention 2011; 6: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikushima Y, Hashido T, Watanabe Y, et al. Effects of imaging parameters on the quality of contrast-enhanced MR angiography of cerebral aneurysms treated using stent-assisted coiling: a phantom study. Magn Reson Med Sci 2017; 16: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs A, Mohlenbruch M, Hadizadeh DR, et al. Noninvasive imaging after stent-assisted coiling of intracranial aneurysms: comparison of 3-T magnetic resonance imaging and 64-row multidetector computed tomography: a pilot study. J Comput Assist Tomogr 2011; 35: 573–582. [DOI] [PubMed] [Google Scholar]
- 24.Seok JH, Choi HS, Jung SL, et al. Artificial luminal narrowing on contrast-enhanced magnetic resonance angiograms on an occasion of stent-assisted coiling of intracranial aneurysm: in vitro comparison using two different stents with variable imaging parameters. Korean J Radiol 2012; 13: 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada N, Hayashi K, Murao K, et al. Time-of-flight MR angiography targeted to coiled intracranial aneurysms is more sensitive to residual flow than is digital subtraction angiography. Am J Neuroradiol 2004; 25: 1154–1157. [PMC free article] [PubMed] [Google Scholar]
- 26.Akmangit I, Aydin K, Sencer S, et al. Dual stenting using low-profile LEO Baby stents for the endovascular management of challenging intracranial aneurysms. Am J Neuroradiol 2015; 36: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HS, Han MH, Kwon BJ, et al. Postoperative 3D angiography in intracranial aneurysms. Am J Neuroradiol 2004; 25: 1463–1469. [PMC free article] [PubMed] [Google Scholar]
- 28.Serafin Z, Strzesniewski P, Lasek W, et al. Follow-up after embolization of ruptured intracranial aneurysms: a prospective comparison of two-dimensional digital subtraction angiography, three-dimensional digital subtraction angiography, and time-of-flight magnetic resonance angiography. Neuroradiology 2012; 54: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]