Short abstract
Interdigitating dendritic cell sarcoma (IDCS) is an extremely rare subtype of dendritic cell neoplasms, and current knowledge on this tumor is limited. We herein report a case of an IDCS in a 64-year-old man who presented with a right inguinal mass combined with extensive retroperitoneal, pulmonary, hepatic, renal, and bone marrow infiltration. Because of the advanced stage of the disease, we performed five cycles of chemotherapy, including cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP); doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD); and ABVD combined with cisplatin, and one cycle of radiotherapy. The patient’s inguinal mass became smaller during the treatment, but there was no change in the extent of infiltration at the other sites. The patient died 8 months after the initial diagnosis. We also herein review the etiology, diagnosis, differential diagnosis, treatment, and prognosis of IDCS, and analyze the characteristics of IDCS in Chinese patients.
Keywords: Interdigitating dendritic cell sarcoma, dendritic cell neoplasm, inguinal mass, chemotherapy, radiotherapy, prognosis
Introduction
Interdigitating dendritic cells (IDCs) originate from hematopoietic precursors, and IDC sarcoma (IDCS) is a rare malignant hematopoietic tumor derived from IDCs. The characteristics of IDCS, optimal treatment approaches, and prognostic factors have not been fully clarified.
We herein report a case of an IDCS with extensive infiltration in a 64-year-old patient. He received five cycles of systemic chemotherapy and one cycle of radiotherapy. His prognosis was poor, and he died 8 months after the initial diagnosis. The purpose of this case report and literature review is to increase the current knowledge of IDCS and thus assist physicians in clinical practice.
Case report
Medical history and examinations
This case study was approved by the ethics committee of Shengjing Hospital. The patient’s wife provided written informed consent.
A 64-year-old man was admitted to our hospital with a 3-month history of a painless mass in his right groin. He underwent a right inguinal lymph node biopsy in the general surgery department in February 2016. Examination of the biopsy specimen revealed IDCS, and he was admitted to our department for further evaluation and treatment.
The patient had a productive cough with blood-streaked sputum and occasional upper abdominal discomfort. He had no systemic symptoms such as fever, night sweats, weight loss, or fatigue. Physical examination revealed no palpable superficial lymph nodes anywhere except in the right groin. The right inguinal mass was smooth, hard, and painless with poor mobility and a size of approximately 5 × 4 cm. Positron emission tomography–computed tomography showed multiple enlarged lymph nodes and elevated fluorodeoxyglucose metabolism in the right inguinal area, retroperitoneum along the right iliac artery, and lungs. There were also foci of elevated fluorodeoxyglucose metabolism in the right liver lobe and at the upper pole of the left kidney. Laboratory studies revealed an elevated serum β2 microglobulin level (2.12 mg/L), while the complete blood count, renal and liver function test results, cancer antigen 125 level, lactate dehydrogenase level, and C-reactive protein level were normal. Additionally, hepatitis B virus DNA, Epstein–Barr virus DNA, and human immunodeficiency virus tests were negative. Other laboratory indicators were also within normal limits.
Treatment process and prognostics
On 4 March 2016, the patient received one cycle of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy. However, no response was shown. We administered one cycle of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy the following month (5 April 2016). At this point, ultrasonography showed that the right inguinal and iliac fossa lymph nodes were shrinking, but contrast-enhanced chest computed tomography showed that the number and size of the tumor masses in the lungs were increasing. We administered the ABVD regimen combined with a 4-day course of cisplatin for two cycles beginning on 19 May and 20 June, respectively. Additionally, one cycle of CHOP + etoposide chemotherapy was administered on 8 August 2016. However, the tumor was not sensitive to these agents, and the patient showed no improvement. Finally, one cycle of radiotherapy was performed in October 2016, but again no response was seen. By this time, the patient had hemoptysis, and contrast-enhanced chest computed tomography showed an extensive tumor mass involving the whole lung. Considering the poor condition of the patient after chemotherapy, we proposed palliative care. The patient died of respiratory failure on 13 October 2016, 8 months from the date of diagnosis.
Detailed pathology findings
The biopsy specimen consisted of two inguinal lymph nodes of 0.6 and 1.5 cm in diameter. Microscopic examination revealed destruction of the lymph node architecture with residual follicles and short spindle-shaped tumor cells forming a woven growth pattern interspersed with occasional giant tumor cells.
Immunophenotypic analysis showed the following results: S100 (+), vimentin (+), CK (−), CD1a (−), CD21 (−), CD23 (−), CD34 (−), CD35 (−), CD117 (−), Melan-A (−), HMB-45 (−), smooth muscle actin (SMA) (−), desmin (−), langerin (−), DOG1 (−), Ki-67 (+) (approximately 40%), and p53 (+) (approximately 70%). Thus, S100 and vimentin staining were positive and all markers for follicular dendritic cell sarcoma (FDCS), Langerhans cell sarcoma, and melanoma were negative. A histopathologic image of a lymph node specimen is shown in Figure 1.
Figure 1.
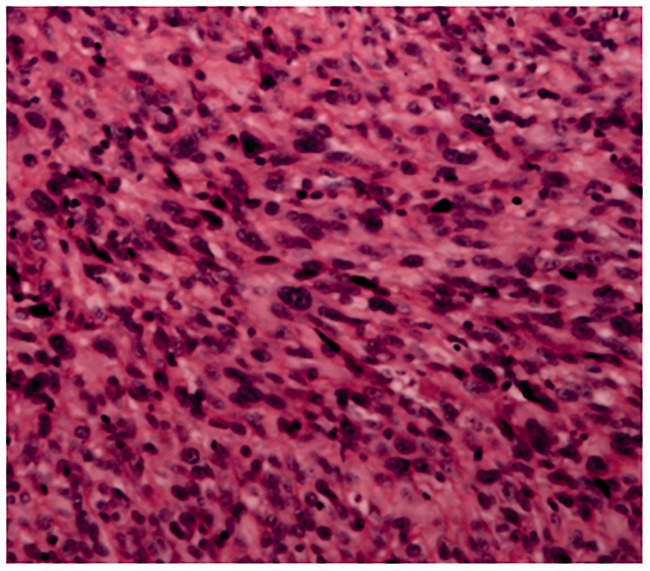
Histopathologic image of the right inguinal lymph node biopsy of the 64-year-old man described in the present report (magnification: ×100).
The bone marrow smears showed 1.60% tumor cells. In summary, the morphological and immunophenotypic features were consistent with IDCS.
Discussion
Various types of dendritic cells are found throughout the body, including Langerhans cells (skin, vagina, stomach, and esophagus), follicular dendritic cells (germinal center of lymph nodes), and IDCs.1 IDCs are mainly found in T-cell zones of lymphoid organs, including the paracortex of the lymph nodes, the lymphatic sheath around the splenic artery, and the follicular portion of mucosa-associated lymphoid tissues. IDCs present antigens to T cells and stimulate T cells. They originate from hematopoietic precursors via conversion of Langerhans cells during migration to the lymph node to capture antigens or through differentiation of myeloid or lymphoid precursor cells.1–3 IDCS is a rare tumor arising from IDCs. Diagnosis of IDCS is challenging, and we still do not have a standard treatment protocol for this tumor.
Saygin et al.3 reported that only 100 cases of IDCS were reported in the English-language literature from 1978 to 2012, of which 40% occurred in Asia, 32% in the United States, 20% in Caucasians, and 5% in Hispanics. IDCS affects a large age range of 1.8 to 88 years (mean, 56.5 years) with a male:female ratio of 1.38:1.00. We reviewed the literature and identified an additional 17 cases of IDCS4–20 that were reported in the English-language literature from 2012 to 2016. The mean age of onset among these cases was 53.5 years (range, 19–87 years), and the male:female ratio was 1.13:1.00. We further identified cases of IDCS reported in the 2005–2016 database of Chinese patients. We searched the Chinese National Knowledge Infrastructure, Chinese Biomedical Literature Database, Chinese Wanfang Database, and Chinese Scientific Journal Database. The following key words were used: “interdigitating dendritic cell sarcoma” and “dendritic cell neoplasm.” Additionally, all articles were included by manual operation, and studies matching the eligible criteria were retrieved for further data extraction and quality assessment. Finally, 45 cases were included.21–52 Among these patients, the mean age at onset was 38.8 years (range, 8 months to 73 years), which is lower than that reported in the above data. Additionally, a slightly higher proportion of men was affected (male:female ratio, 1.25:1.00). Among the Chinese patients, 66.7% (30/45) had lymph node involvement, mainly the cervical (18 patients) and inguinal (8 patients) lymph nodes, and 33.3% (15/45) had extranodal infiltration, mainly affecting the liver (5 patients) and spleen (5 patients).
The etiology of IDCS is not clear. Epstein–Barr virus has been implicated as a causative factor in the pathogenesis of FDCS,53 but there is no evidence of an association between viral infection and IDCS.54,55 Nayer et al.56 found that clonal cytogenetic abnormalities and rearrangement might be associated with the development of IDCS. Moreover, three patients developed IDCS following the use of calcineurin inhibitors, tacrolimus, and pimecrolimus.57,58 These drugs show their effect by dampening the responses of T cells to which IDCs present antigens. Dysregulation of the immune system may facilitate malignant transformation of IDCs, but further data are required to determine whether this occurs.3 IDCS may occur in some patients as a result of malignant transformation after radiotherapy and chemotherapy. One systematic review showed that 17% of patients with IDCS had a prior malignancy and had undergone radiotherapy or chemotherapy before being diagnosed with IDCS.4
At present, the diagnosis of IDCS is based mainly on clinical manifestations, cell morphology, and immunohistochemical characteristics. Most patients present with painless lymphadenectasis, while constitutional symptoms such as fever, weight loss, night sweats, and fatigue affect only 25% of patients.3 Microscopically, the neoplastic cells of IDCS are large, fusiform spindle cells with indistinct cell borders, oval central nuclei, micro-eosinophilic cytoplasm, finely dispersed chromatin, and small but prominent nucleoli, and they often form a storiform or whorled fascicular growth pattern. There are always small lymphocytes, plasma cells, or other inflammatory cells surrounding the tumor cells, but they lack melanin, nerve, smooth muscle, or myofibroblast differentiation. A key diagnostic feature is the presence of tumor cells in the subcortical region of the lymph nodes.5,59,60 The main immunohistochemical characteristics are 100% expression of S100 protein and vimentin, frequent CD45RO and CD68 expression, and negative CD1a, CD21, CD23, CD35, Melan-A and HMB-45 expression. Expression of CD4, CD43, CD163, and SMA is variable. Cytokeratins, epithelial membrane antigen (EMA), B-cell markers, T-cell markers, CD30, CD34, ALK, langerin, and myeloperoxidase are negative.4–20
The differential diagnosis of IDCS includes malignant melanoma, malignant peripheral nerve sheath tumor, malignant lymphoma, malignant fibrous histiocytoma, atypical fibrosarcoma, and other types of dendritic cell neoplasms. Melanoma may show nested growth patterns and epithelioid morphology associated with HMB45 and Melan-A positivity, and melanosomes may be present under electron microscopy.61 Malignant peripheral nerve sheath tumor is CD68- and CD45-negative, while EMA/CD30, CD21/CD35/clusterin, factor XIIIa/desmin/vimentin/SMA/cytokeratins, and CD1a can be used to establish the diagnoses of anaplastic large cell lymphoma, FDCS, fibroblastic cell tumor, and malignant Langerhans histiocytosis, respectively.4–20,59
No precise treatment guidelines have yet been developed for IDCS because this tumor is uncommon and prospective clinical data are limited. The current treatment of IDCS includes surgery, radiotherapy, chemotherapy, and targeted therapy. Radical surgery has been the main treatment for patients with local disease. Chemotherapy is usually recommended as the main treatment for advanced IDCS. Several chemotherapeutic regimens have been reported, including CHOP; ABVD; ifosfamide, carboplatin, and etoposide (ICE); dexamethasone, cisplatin, and high-dose cytarabine (DHAP); and etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH).61–64 However, none has shown a consistent effect, although some studies have associated ABVD with an improved prognosis.6,65,66
In one study, the median survival time for patients with metastatic disease was 9 months (range, 0.25–72 months).3 The median survival rates were not reported for patients with local disease because 82% were still alive at the last follow-up. The overall survival rates at 1 and 2 years for patients with local disease were 84.8% and 38.5%, and those for patients with metastatic disease were 68.1% and 15.8%, respectively.3 The authors of a small retrospective study found that mitotic counts, percent necrosis, and nuclear pleomorphism may serve as prognostic factors, but no further study has been performed to confirm these findings.62 Other factors that have been associated with a poor prognosis include younger age, larger tumor diameter, higher expression of Ki-67, p53 positivity, extranodal disease, and abdominal tumor infiltration.3,60 In the present study, we performed a thorough search of published articles regarding IDCS in Chinese from 2005 to 2016. We found 45 reported cases of IDCS in China.21–52 We used the chi-square test to analyze the association between clinicopathological features (age, sex, histological features, intra-abdominal involvement, and cytokeratin positivity) and adverse outcomes (local recurrence, distant metastasis, and death). We found no significant association with sex, mitotic counts, age, or tumor size. The latter two findings differ from those reported by Saygin et al.,3 but our finding that abdominal involvement, high Ki-67, extranodal disease, and advanced clinical stage were associated with a poor prognosis are in agreement with the study by Saygin et al.3 (Table 1).
Table 1.
Univariate analysis of factors associated with adverse outcomes in 45 Chinese patients with interdigitating dendritic cell sarcoma.
| Prognostic factors | Positive events/Total number | P value |
|---|---|---|
| Age | 0.085 | |
| ≥40 | 11/28 | |
| <40 | 17/28 | |
| Sex | 0.772 | |
| Male | 17/28 | |
| Female | 11/28 | |
| Tumor size | 0.114 | |
| ≥3 cm | 9/23 | |
| <3 cm | 14/13 | |
| Mitosis | 0.262 | |
| ≥5/10 HPF | 7/21 | |
| <5/10 HPF | 14/21 | |
| Intra-abdominal involvement | 0.008 | |
| Present | 7/25 | |
| Absent | 18/25 | |
| Presentation | 0.019 | |
| Nodal | 22/28 | |
| Extranodal | 6/28 | |
| Clinical stage | 0.012 | |
| Early | 20/28 | |
| Advanced | 8/28 | |
| Ki-67 | 0.043 | |
| ≥20% | 6/17 | |
| <20% | 11/17 |
In summary, we have presented a rare case of IDCS in a 64-year-old man. We have gained a better understanding of IDCS after reviewing its etiology, diagnosis, treatment, and prognosis. We also analyzed the characteristics of IDCS in Chinese patients with a literature review. However, the extreme rarity of this tumor still hinders clinical research; thus, well-designed prospective clinical trials are needed to guide physicians who encounter patients with this disease.
Declaration of conflict of interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81600115).
References
- 1.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity 2007; 26: 741–750. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzwajg M, Canque B, Gluckman JC. Human dendritic cell differentiation pathway from CD34+ hematopoietic precursor cells. Blood 1996; 87: 535–544. [PubMed] [Google Scholar]
- 3.Saygin C, Uzunaslan D, Ozguroglu M, et al. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol 2013; 88: 253–271. [DOI] [PubMed] [Google Scholar]
- 4.Jiang YZ, Dong NZ, Wu DP, et al. Interdigitating dendritic cell sarcoma presenting simultaneously with acute myelomonocytic leukemia: report of a rare case and literature review. Int J Hematol 2013; 97: 657–666. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Niglio SA, Jo VY, et al. Interdigitating dendritic cell sarcoma presenting in the skin: diagnosis and the role of surgical resection, chemotherapy and radiotherapy in management. Rare Tumors 2014; 6: 5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyogoku C, Seki M, Ogawa S, et al. Complete remission in systemic skin interdigitating dendritic cell sarcoma after ABVD chemotherapy. J Clin Exp Hematop 2015; 55: 33–37. [DOI] [PubMed] [Google Scholar]
- 7.Parada D, Pena KB, Gil I, et al. Interdigitating dendritic cell sarcoma presenting in the nasal region. Pathol Res Pract 2012; 208: 368–371. [DOI] [PubMed] [Google Scholar]
- 8.Radovic S, Doric M, Zujo H, et al. Interdigitating dendritic cell sarcoma of the liver and lung: a case report with morphological and immunohistochemical features of tumor. Bosn J Basic Med Sci 2012; 12: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo D, Xiao WZ, Hu M, et al. Interdigitating dendritic cell sarcoma complicated with paraneoplastic pemphigus after surgery of resection. Chin Med J (Engl) 2013; 126: 1797. [PubMed] [Google Scholar]
- 10.Lee EJ, Hyun DW, Cho HJ, et al. A rare case of interdigitating dendritic cell sarcoma in the nasal cavity. Case Rep Otolaryngol 2013; 2013: 913157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RL, Boisot S, Ball ED, et al. A case of interdigitating dendritic cell sarcoma/histiocytic sarcoma–a diagnostic pitfall. Int J Clin Exp Pathol 2014; 7: 378–385. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Liu B, Song N, et al. Interdigitating dendritic cell sarcoma presenting in the kidney combined with retroperitoneal leiomyosarcoma: A case report and literature review. Oncol Lett 2014; 7: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helbig G, Wichary R, Pajak J, et al. Sustained remission after ABVD treatment for interdigitating dendritic cell sarcoma. Contemp Oncol (Pozn) 2015; 19: 83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutter G, Hofer S, Tzankov A, et al. Intracranial interdigitating dendritic cell sarcoma: first case report. Neurosurgery 2015; 77: E979–E983. [DOI] [PubMed] [Google Scholar]
- 15.Khashab T, Sehgal L, Medeiros LJ, et al. Spontaneous regression of interdigitating dendritic sarcoma in a patient with concurrent small lymphocytic lymphoma. BMJ Case Rep 2015. pii: bcr2014209014. doi: 10.1136/bcr-2014-209014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Malley DP, Agrawal R, Grimm KE, et al. Evidence of BRAF V600E in indeterminate cell tumor and interdigitating dendritic cell sarcoma. Ann Diagn Pathol 2015; 19: 113–116. [DOI] [PubMed] [Google Scholar]
- 17.Pokuri VK, Merzianu M, Gandhi S, et al. Interdigitating dendritic cell sarcoma. J Natl Compr Canc Netw 2015; 13: 128–132. [DOI] [PubMed] [Google Scholar]
- 18.Shan SJ, Meng LH, Lu R, et al. Primary cutaneous interdigitating dendritic cell sarcoma: a case report and review of the literature . Am J Dermatopathol 2015; 37: 639–642. [DOI] [PubMed] [Google Scholar]
- 19.Lupato V, Romeo S, Franchi A, et al. Head and neck extranodal interdigitating dendritic cell sarcoma: case report and review of the literature. Head Neck Pathol 2016; 10: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen CM, Cassarino D. Primary cutaneous interdigitating dendritic cell sarcoma: a case report and review of the literature . Am J Dermatopathol 2016; 38: 628–631. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZG, Ye MF, Diao XW, et al. [ Interdigitating dendritic cell sarcoma: a report of two cases with review of literature]. J Diag Pathol 2005; 12: 426–428. doi: 10.3969/j.issn.1007-8096.2005.06.008 [Google Scholar]
- 22.Xiao HL, Wang D, Li ZP, et al. [ Interdigitating dendritic cell sarcoma: a clinicopathologic observation]. J Diag Pathol 2006; 13: 40–42. doi: 10.3969/j.issn.1007-8096.2006.01.010 [Google Scholar]
- 23.Xu HP, Chen JF, Gao WG, et al. [ A case report of interdigitating dendritic cell sarcoma in mediastinum lymph node]. J Diagn Concepts Pract 2006; 5: 535–536. doi: 10.3969/j.issn.1671-2870.2006.06.021 [Google Scholar]
- 24.Gao MT, Liu B, Li L, et al. [ Infant left axillary lymph node interdigitating dendritic cell sarcoma: a case report]. Chin J Pediatr surg 2007; 28: 672. doi: 10.3760/cma.j.issn.0253-3006.2007.12.018 [Google Scholar]
- 25.Zhao Y, Liu YH, Xiao W, et al. [ Clinicopathological observation of interdigitating dendritic cell sarcoma]. Journal of Taizhou Polytechnical Cortege 2008; 8: 24–26. doi: 10.3969/j.issn.1671-0142.2008.05.012 [Google Scholar]
- 26.Gan MF, Lu HS, Zhang JW, et al. [Interdigitating dendritic cell sarcoma/tumor: a study of 3 cases]. Chin J Pathol 2008; 37: 676–679. doi: 10.3321/j.issn:0529-5807.2008.10.007 [PubMed] [Google Scholar]
- 27.Li LD, Yu L, Li QL, et al. [ Pulmonary interdigitating dendritic cell sarcoma with cervical lymph node metastasis: a report of one case and literature review]. Modern Oncology 2008; 16: 1687–1689. doi: 10.3969/j.issn.1672-4992.2008.10.014 [Google Scholar]
- 28.Wang BL, Zheng JA, Liu M. [ A case of interdigitating dendritic cell sarcoma]. Chin J Radiol 2008; 42: 217. doi: 10.3321/j.issn:1005-1201.2008.02.028 [Google Scholar]
- 29.Qian Z, Xing CP, Liu B. [ Interdigitating dendritic cell sarcoma: a case report with review of literature]. Modern Oncology 2009; 17: 1333–1335. doi: 10.3969/j.issn.1672-4992.2009.07.050 [Google Scholar]
- 30.Chen MF, Ma LY, Xu C. [ A case of interdigitating dendritic cell sarcoma of the lung]. Chin J Postgrad Med 2009; 32: 76–77. doi: 10.3760/cma.j.issn.1673-4904.2009.13.033 [Google Scholar]
- 31.Fan YJ, Li XQ, Du GY, et al. [ Interdigitating dendritic cell sarcoma in the inguinal lymph node: a case report with review of literature]. Modern Oncology 2009; 17: 325–328. doi: 10.3969/j.issn.1672-4992.2009.02.054 [Google Scholar]
- 32.Zhang XR, Chen GQ, Yu G, et al. [ Interdigital dentritic cell sarcoma: two cases report and literature review]. J Diag Pathol 2010; 17: 431–434. doi: 10.3969/j.issn.1007-8096.2010.06.012 [Google Scholar]
- 33.Zhang YL, Deng HJ, Yin ZQ. [ Clinicopathological observation of interdigitating dendritic cell sarcoma in the neck: a case report and literature review]. Lab Med Clin 2010; 7: 1105–1106. doi: 10.3969/j.issn.1672-9455.2010.11.046 [Google Scholar]
- 34.Wu RJ, Ma XD, Wu WQ. [ A case of interdigitating dendritic cell sarcoma with bone marrow infiltration]. J Fujian Med Univ 2011; 45: 385–386. doi: 10.3969/j.issn.1672-4194.2011.05.021 [Google Scholar]
- 35.Bai M, Zhu MG, Feng XD, et al. [ Cervical lymph node interdigitating dendritic cell sarcoma: a case report and review of the literature]. Clinical Misdiagnosis and Mistherapy 2011; 24: 36–38. doi: 10.3969/j.issn.1002-3429.2011.01.022 [Google Scholar]
- 36.Chen EC, Ma JB, Song YP. [One case of lymph node interdigitating dendritic cell sarcoma]. Zhonghua Xue Ye Xue Za Zhi 2011; 32: 888 [in Chinese, English abstract]. doi: 10.3760/cma.j.issn.0253-2727.2011.12.024 [PubMed] [Google Scholar]
- 37.Liu AN, Qu HJ, Lang ZQ, et al. [ Interdigitating dendritic cell sarcoma within the inguinal lymph nodes secondary to autoimmune hemolytic anemia: a case report and literature review]. Chin J Clinicians 2011; 5: 4873–4875. doi: 10.3877/cma.j.issn.1674-0785.2011.16.058 [Google Scholar]
- 38.Tang WJ, Gao YJ, Chen L, et al. [ A case report of interdigitating dendritic cell sarcoma in children]. Chin J Evid Based Pediatr 2011; 6: 158–160. doi: 10.3969/j.issn.1673-5501.2011.02.016 [Google Scholar]
- 39.Tong LL, Yan X, Wang JB, et al. [ A case of interdigitating dendritic cell sarcoma]. J Clin Exp Pathol 2011; 27: 1266–1267. doi: 10.3969/j.issn.1001-7399.2011.11.036 [Google Scholar]
- 40.Xing YX, Li H, Chen RX, et al. [Extranodular the finger-like protrusion of dendritic cell sarcoma/tumor: clinicopathological observation]. Anhui Medical and Pharmaceutical Journal 2012; 16: 337–338. doi: 10.3969/j.issn.1009-6469.2012.03.026 [Google Scholar]
- 41.Li Q, Yang J, Cheng G, et al. [ A case report of renal interdigitating dendritic cell sarcoma]. Chin J Urol 2012; 33: 862. doi: 10.3760/cma.j.issn.1000-6702.2012.11.017 [Google Scholar]
- 42.Qiu K, Mao YR, Zou JJ, et al. [ Interdigitating dendritic cell sarcoma/tumor: a case report and literature review]. J Clin Exp Pathol 2010; 26: 497–498. doi: 10.3969/j.issn.1001-7399.2010.04.031 [Google Scholar]
- 43.Mao RJ, Zhu XZ, Li QM, et al. [ A case of granulomatous interdigitating dendritic cell sarcoma]. Chin J Pathol 2012; 41: 134–136. doi: 10.3760/cma.j.issn.0529-5807.2012.02.018 [PubMed] [Google Scholar]
- 44.Wang LJ, Pan P. [ Interdigitating dendritic cell sarcoma of cervical lymph node: case report]. Chin J Med Imaging Technol 2012; 28: 1654 http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyxyxjs201209010 [Google Scholar]
- 45.Wang ZQ, Zhang FL, Wang ZM, et al. [ Clinicopathological analysis of interdigitating dendritic cell sarcoma of the cervical lymph node]. J Clin Exp Pathol 2012; 28: 95–97. doi: 10.3969/j.issn.1001-7399.2012.01.030 [Google Scholar]
- 46.Zhang H, An HM, Zhao P. [ A case of interdigitating dendritic cell sarcoma with pulmonary metastasis]. Chin J Oncol 2014; 36: 879–880. doi: 10.3760/cma.j.issn.0253-3766.2014.11.017 [Google Scholar]
- 47.Fan SH, Li XD, Nong ZL, et al. [ Scrotal swelling as main manifestation of IDCS in the bilateral inguinal lymph nodes]. Chin J Human Sexuality 2014; 50: 11–13. doi: 10.3969/j.issn.1672-1993.2014.07.004 [Google Scholar]
- 48.Pan MH, Gong QX, Fan QH, et al. [Interdigitating dendritic cell sarcoma/tumor: a clinicopathologic study]. Chin J Pathol 2014; 43: 99–102. doi: 10.3760/cma.j.issn.0529-5807.2014.02.006 [PubMed] [Google Scholar]
- 49.Gu Y, Zhou P, Tan XD. [ Interdigitating dendritic cell sarcoma in children: a case report and literature review]. J China Pediatr Blood Cancer 2014; 19: 316–319. doi: 10.3969/j.issn.1673-5323.2014.06.009 [Google Scholar]
- 50.Li JP, Li B, Zhang WK, et al. [Skin interdigitating dendritic cell sarcoma with bone marrow metastasis: a case report and literature review]. Chin J Postgrad Med 2014; 37: 77–78. doi: 10.3760/cma.j.issn.1673-4904.2014.11.031 [Google Scholar]
- 51.Tao HB, Li K, Ding YY, et al. [ A case of thoracic vertebrae interdigitating dendritic cell sarcoma]. Chin J Radiol 2016; 50: 704–705. doi: 10.3760/cma.j.issn.1005-1201.2016.09.015 [Google Scholar]
- 52.Li CF, Zhao WG, Wang GN, et al. [ Two cases of interdigitating dendritic cell sarcoma: a clinicopathologic study]. J Clin Exp Pathol 2016; 32: 344–346. doi: 10.13315/j.cnki.cjcep.2016.03.028 [Google Scholar]
- 53.Choe JY, Go H, Jeon YK, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: a report of six cases with increased IgG4-positive plasma cells. Pathol Int 2013; 63: 245–251. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Deng Y, Zhang X, et al. Interdigitating dendritic cell sarcoma following adult liver transplantation: case report and literature review. Pathol Oncol Res 2011; 17: 397–402. [DOI] [PubMed] [Google Scholar]
- 55.Barwell N, Howatson R, Jackson R, et al. Interdigitating dendritic cell sarcoma of salivary gland associated lymphoid tissue not associated with HHV-8 or EBV infection. J Clin Pathol 2004; 57: 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nayer H, Murphy KM, Hawkins AL, et al. Clonal cytogenetic abnormalities and BCL2 rearrangement in interdigitating dendritic cell sarcoma. Leuk Lymphoma 2006; 47: 2651–2654. [DOI] [PubMed] [Google Scholar]
- 57.Gordon MK, Kraus M, van Besien K. Interdigitating dendritic cell tumors in two patients exposed to topical calcineurin inhibitors. Leuk Lymphoma 2007; 48: 816–818. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q, Liu C, Lei L, et al. Interdigitating dendritic cell sarcoma involving bone marrow in a liver transplant recipient. Transplant Proc 2010; 42: 1963–1966. [DOI] [PubMed] [Google Scholar]
- 59.Pileri SA, Grogan TM, Harris NL, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the international lymphoma study group based on 61 cases. Histopathology 2002; 41: 1–29. [DOI] [PubMed] [Google Scholar]
- 60.De Pas T, Spitaleri G, Pruneri G, et al. Dendritic cell sarcoma: an analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol 2008; 65: 1–7. [DOI] [PubMed] [Google Scholar]
- 61.Kairouz S, Hashash J, Kabbara W, et al. Dendritic cell neoplasms: an overview. Am J Hematol 2007; 82: 924–928. [DOI] [PubMed] [Google Scholar]
- 62.Gaertner EM, Tsokos M, Derringer GA, et al. Interdigitating dendritic cell sarcoma. A report of four cases and review of the literature. Am J Clin Pathol 2001; 115: 589–597. [DOI] [PubMed] [Google Scholar]
- 63.Fonseca R, Yamakawa M, Nakamura S, et al. Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma: a review. Am J Hematol 1998; 59: 161–167. [DOI] [PubMed] [Google Scholar]
- 64.Dalia S, Jaglal M, Chervenick P, et al. Clinicopathologic characteristics and outcomes of histiocytic and dendritic cell neoplasms: the moffitt cancer center experience over the last twenty five years. Cancers 2014; 6: 2275–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olnes MJ, Nicol T, Duncan M, et al. Interdigitating dendritic cell sarcoma: a rare malignancy responsive to ABVD chemotherapy. Leuk Lymphoma 2002; 43: 817–821. [DOI] [PubMed] [Google Scholar]
- 66.Lee SY, Lee SR, Chang WJ, et al. Successful treatment of disseminated interdigitating dendritic cell sarcoma with adriamycin, bleomycin, vinblastine, and dacarbazine chemotherapy. Korean J Hematol 2012; 47: 150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


