Short abstract
Ectopic adrenocorticotropic hormone (ACTH) syndrome (EAS) is a condition of endogenous hypercortisolism sustained by an extrapituitary ACTH-secreting tumor. Olfactory neuroblastoma (ONB) is a rare malignant neoplasm of the sinonasal tract and is derived from the olfactory epithelium. Because the paranasal sinus is not a common site of EAS, the development of ONB in patients with EAS is rare. We herein report the first known case of ONB with acquirement of ACTH production during the clinical course as proven by immunohistochemistry. A 50-year-old man diagnosed with ONB was referred to our department in July 2015 because of hypokalemia, hyperglycemia, decreased eosinophil and granulocyte counts, and elevated serum levels of ACTH and cortisol. Although two previous ONB biopsy specimens (2011 and 2014) showed no ACTH immunoreactivity, a newly obtained specimen in August 2015 clearly showed ACTH immunoreactivity. This is the first case of ectopic ACTH syndrome associated with an ONB that acquired the ability to express ACTH during its clinical course as shown by serial immunohistochemical examinations.
Keywords: Ectopic ACTH syndrome, olfactory neuroblastoma, pathological study, adrenocorticotropic hormone (ACTH), immunohistochemistry, Cushing syndrome
Introduction
Ectopic adrenocorticotropic hormone (ACTH) syndrome (EAS) is a cause of endogenous hypercortisolism secondary to an extrapituitary ACTH-secreting tumor. Although most cases of EAS are diagnosed from Cushing symptoms, it is generally difficult to clarify whether the tumor possesses the ability to produce ACTH at the outbreak of the disease or acquires this ability during the disease course.
Olfactory neuroblastoma (ONB) is a rare malignant neoplasm of the sinonasal tract and is derived from the olfactory epithelium.1 Because the paranasal sinus is not a common site for ectopic ACTH secretion, the presence of concurrent ONB and EAS is extremely rare. To the best of our knowledge, only 18 such cases have been reported to date.1 In three of these cases, Cushing symptoms developed after the diagnosis of ONB,2–4 although sequential immunohistochemical examinations were not performed. We herein describe the first case of an ONB that acquired the ability to synthesize ACTH during its disease course as demonstrated by sequential immunohistochemical studies. The immunopathological findings coincided with the occurrence of Cushing syndrome.
Case report
A 50-year-old man was admitted to the Hyogo Medical College Hospital for examination and treatment of an abnormal visual field in mid-July 2011. He was diagnosed with an ONB in the sinonasal cavity. The tumor extended outside of the sinonasal cavity (Kadish stage C), and chemoradiotherapy was performed. No symptoms or signs of Cushing’s syndrome were apparent at that time. Although the size of the primary lesion tended to decrease in 2012, it had extended to the opposite side of the sinonasal cavity (Kadish stage C) by 2014. By January 2015, hypokalemia, hyperglycemia, and decreased eosinophil and granulocyte counts had developed. The patient was subsequently referred to the Department of Diabetes, Endocrinology and Metabolism for further examination of the hypokalemia in mid-July 2015.
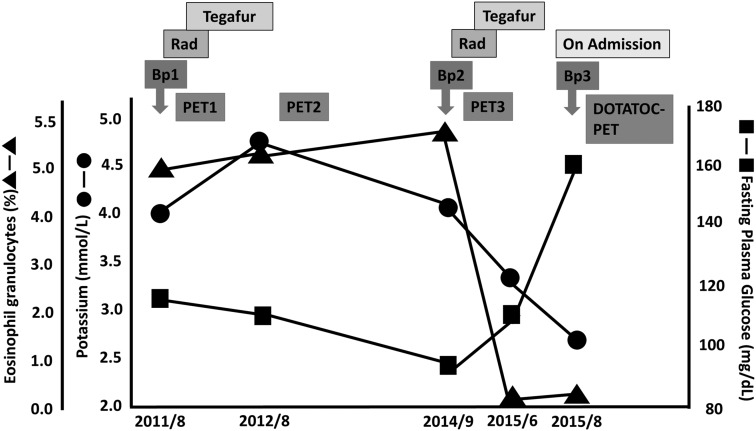
Figure 1 shows changes in the serum concentrations of potassium and glucose and the blood eosinophil count during the clinical course of the disease. From 2011 to 2014, the serum concentrations of potassium and glucose and the blood eosinophil count were almost normal.
Figure 1.
Changes in concentrations of serum potassium and glucose and eosinophil count during the clinical course of the disease. The symbols depict potassium (•), fasting plasma glucose (▪), and eosinophils (▲). The medical treatment undertaken is shown at the top: number of chemotherapy treatments (with tegafur), radiation (Rad), and biopsies (Bp) are shown by arrows. PET, positron emission tomography; DOTATOC, 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid-d-Phe1-Tyr3-octreotide.
The patient was a social drinker who had smoked 40 cigarettes a day for 20 years prior to admission. His body weight was 67.0 kg in 2012, 86.0 kg in 2014, and 73.6 kg on admission. His blood pressure was 165/109 mmHg, and his pulse was 80 beats/min. On physical examination, a Cushingoid appearance was apparent, including moon facies, truncal obesity, purpura, and muscle atrophy of the bilateral lower extremities.
Laboratory findings on admission are shown in Table 1. The total white blood cell count was slightly increased; the neutrophil count (both segmented neutrophils and bands) was markedly increased, while the eosinophil count was 0%. The fasting plasma glucose level was elevated. The serum concentration of potassium was low, while the serum concentration of magnesium and renal function test results were normal. Arterial blood gas analysis revealed metabolic alkalosis with respiratory compensation. Both the fractional excretion of potassium and the transtubular potassium gradient were elevated. Endocrinological examinations showed that the serum concentration of ACTH and cortisol and the urinary excretion of cortisol were markedly increased. The serum concentrations of aldosterone and dehydroepiandrosterone sulfate were normal. The urinary excretion of aldosterone and urine 17-ketosteroid was normal. However, the ACTH and cortisol circadian rhythms were deranged (data not shown). Low- and high-dose dexamethasone suppression tests were not performed because the patient’s health was poor. A pituitary tumor was not detected on magnetic resonance imaging of the brain, and tumors were not detected on a computed tomography scan of the chest. Bilateral adrenal hyperplasia was observed. Based on these physical and endocrine findings, the patient was diagnosed with EAS.
Table 1.
Laboratory parameters on admission.
| WBC count | 11,930 | /µL | Na | 139 | mEq/L | Endocrine data | ||
| Neutrophils | 95 | % | K | 2.3 | mEq/L | TSH | 4.59 | µIU/mL |
| Lymphocytes | 1.5 | % | Cl | 92 | mEq/L | Free T4 | 9.90 | pmol/L |
| Monocytes | 2 | % | Ca | 7.7 | mg/dL | ACTH | 39.3 | pmol/L |
| Eosinophils | 0 | % | IP | 2.5 | mg/dL | Cortisol | 2461.9 | nmol/L |
| RBC count | 344 × 104 | /µL | Mg | 2.2 | mg/dL | Aldosterone | 2.66 | pmol/L |
| Hb | 12.3 | g/dL | BUN | 13 | mg/dL | Renin activity | 3.8 | pmol/mL/h |
| Ht | 34.4 | % | UA | 1.7 | mg/dL | DHEA-S | 2.8 | µmol/L |
| Plts | 344 × 104 | /µL | CRE | 0.47 | mg/dL | Urine cortisol | 28,428 | nmol/day |
| TP | 4.9 | g/dL | TC | 173 | mg/dL | Urine aldosterone | <11.8 | nmol/day |
| Alb | 2.8 | g/dL | TG | 168 | mg/dL | 17KS-1 | 1.14 | µmol/day |
| T-bil | 0.6 | mg/dL | HDL-C | 31 | mg/dL | −2 | 5.69 | µmol/day |
| AST | 16 | IU/L | −3 | 8.11 | µmol/day | |||
| ALT | 34 | IU/L | Arterial blood gas | −4 | 8.36 | µmol/day | ||
| LDH | 420 | IU/L | pH | 7.52 | −5 | 5.93 | µmol/day | |
| ALP | 282 | IU/L | PCO2 | 44.7 | Torr | −6 | <0.03 | µmol/day |
| γGTP | 52 | IU/L | PO2 | 96.8 | Torr | −7 | 1.04 | µmol/day |
| FPG | 160 | mg/dL | HCO3− | 35.7 | mEq/L | FEK | 5.7 | |
| HbA1c | 6.1 | % | BE | 11.6 | mEq/L | TTKG | 14.2 | |
WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Ht, hematocrit; Plts, platelets; TP, total protein; Alb, albumin; T-bil, total bilirubin; AST aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; FPG, fasting plasma glucose; γGTP, γ-glutamyl transpeptidase; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; Na, sodium; K, potassium; Cl, chloride; Ca, calcium; IP, inorganic phosphorus; Mg, magnesium; BUN, blood urea nitrogen; UA, uric acid; CRE, creatinine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; HCO3−, bicarbonate; BE, base excess; TSH, thyroid-stimulating hormone; T4, thyroxine; ACTH, adrenocorticotropic hormone; DHEA-S, dehydroepiandrosterone sulfate; 17KS, 17-ketosteroid; FEK, fractional extraction of potassium; TTKG, transtubular potassium gradient.
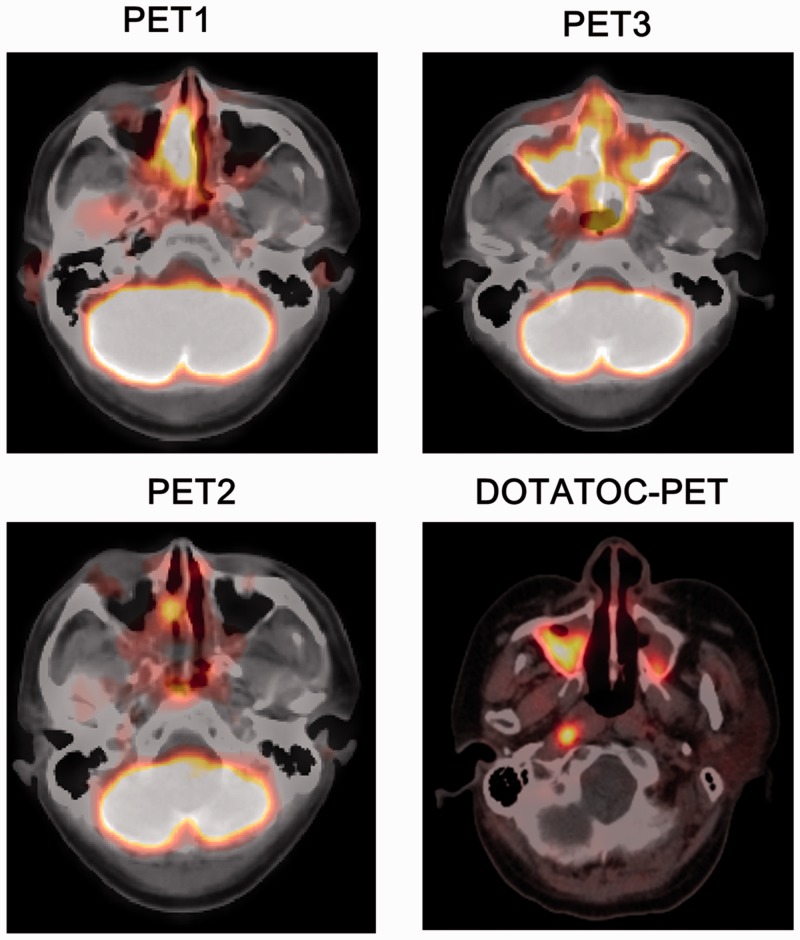
Changes in findings of 18F-fluorodeoxyglucose (FDG) positron emission tomography-(PET) (2011, 2012, and 2015), and of octreotide PET (2015) are shown in Figure 2. Two courses of chemoradiotherapy significantly reduced FDG uptake in the sinonasal cavity (PET2). However, strong uptake of FDG was again noted in the primary lesion in the sinonasal cavity, which had extended to the opposite site on PET3 by 2015, when a Cushingoid appearance had developed. The strong uptake of 68Ga-labelled 1,4,7,10-tetraazacyclododecane-N,N',N″,N0‴-tetraacetic acid-d-Phe1-Tyr3-octreotide (68Ga-DOTATOC) was also observed at the same site in 2015.
Figure 2.
Findings of sequential 18F-fluorodeoxyglucose-PET and DOTATOC-PET. The DOTATOC-PET images shows a delayed scan at approximately 90 min post-injection of DOTATOC. PET, positron emission tomography; DOTATOC, 1,4,7,10-tetraazacyclododecane-N,N',N″,N‴-tetraacetic acid-d-Phe1-Tyr3-octreotide.
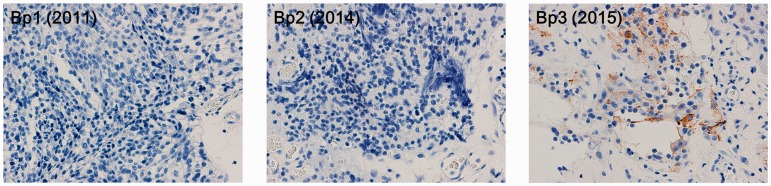
Serial changes in the immunohistochemical reactivity against ACTH at the primary site of the ONB are shown in Figure 3. All Kadish stages were stage C, and the Hyams grade of each biopsy, from Bp1 to Bp3, was grade II. The purpose of Bp1 in 2011 was to diagnose the primary lesion. Although the size of the primary lesion tended to decrease in 2012 by radiotherapy and chemotherapy, it had extended to the opposite side of the sinonasal cavity by 2014. The purpose of Bp2 was to re-evaluate the condition of the primary lesion. In accordance with the development of Cushing’s signs and symptoms in 2015, the purpose of Bp3 was to diagnose EAS. Retrospectively, all tumor cells were negative for ACTH in 2011 and 2014, while the tumor cells became positive for ACTH immunostaining in 2015. Changes in ACTH expression by the tumor cells coincided with a decreased serum potassium concentration and eosinophil count and an elevated fasting plasma glucose level.
Figure 3.
Changes in adrenocorticotropic hormone immunohistochemistry of olfactory neuroblastoma (×400). Bp, biopsy.
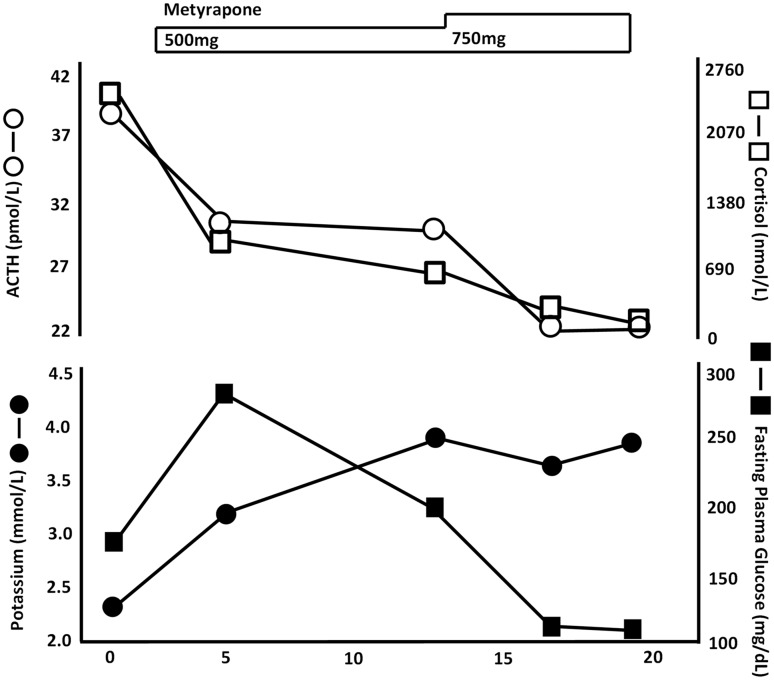
The patient was treated with metyrapone (500–750 mg/day). Changes in the serum concentrations of ACTH, cortisol, potassium, and glucose after treatment are shown in Figure 4. After day 20, the serum concentrations of ACTH and cortisol were markedly decreased (ACTH, 23.5 pmol/L; cortisol, 220.8 nmol/L). The patient’s hypokalemia and hyperglycemia also improved.
Figure 4.
Changes in serum concentrations of ACTH, cortisol, potassium, and glucose during metyrapone administration. The symbols depict ACTH (○), cortisol (□), potassium (•), and fasting plasma glucose (▪). The medical treatment undertaken is shown at the top. ACTH, adrenocorticotropic hormone.
Written informed consent was obtained from the patient for publication of this report and accompanying images. The need for approval by an ethics committee or institutional review board was waived.
Discussion
We have herein reported the first case of an ONB that acquired the ability to express ACTH during the clinical course of the disease as directly demonstrated by immunohistochemical staining of sequential biopsy specimens of the primary lesion.
Most ONBs are nonfunctioning tumors; however, several previous reports suggested that ONB might be associated with paraneoplastic syndrome. Endocrinological and neurological syndromes have been described in association with ONB, such as EAS, syndrome of inappropriate antidiuretic hormone secretion, catecholamine secretion, hyperprolactinemia, and humoral hypercalcemia of malignancy.1 The most plausible explanation is that the primary ONB lesion changes its functional phenotype. Along with clinical inspection, the presence of Cushing’s symptoms, hyponatremia, hypokalemia, or hypercalcemia is useful for diagnosis of functional changes in an ONB. Conversely, a previous report described the incidental diagnosis of ONB in patients with these symptoms or electrolyte abnormalities.5 Magnetic resonance imaging, computed tomography, and PET have been shown to be useful for detecting the tumor origin.
ONB accompanied by EAS and Cushing’s symptoms is rare. To the best of our knowledge, only 18 such cases have been reported to date.1 In 15 of these cases, Cushing’s symptoms or hypokalemia developed before or simultaneously with the ONB diagnosis. In addition, the corticotropin-releasing hormone (CRH) test and the low- and high-dose dexamethasone suppression tests were performed to distinguish EAS. Octreotide or ketoconazole in addition to surgery, radiotherapy, and chemotherapy were used therapeutically to suppress hypercortisolemia in several cases. Most reported cases of ONB with EAS had a favorable outcome; only two patients died, probably because the clinical manifestations of Cushing’s syndrome led to earlier diagnosis and treatment in most patients.
Of all reported ONB cases with EAS, three other cases involved the development of Cushing’s symptoms after a diagnosis of ONB,2–4 as in the present case, and only two of these cases were reported in the English-language medical literature (Cases 1 and 2 in Table 2). The time from the onset of ONB to the diagnosis of EAS differed in each case, ranging from 2 to 15 years. In Case 1,2 the patient underwent craniofacial resection with postoperative radiation therapy for ONB. The tumor relapsed in the cervical lymph nodes, and the patient was subsequently successfully treated with chemoradiotherapy; however, Cushing’s symptoms developed 15 years later. In Case 2,3 the patient had been diagnosed with an ONB but refused a curative operation. Cushing’s symptoms developed 2 years later. In Cases 1 and 2 and in the present case, the ONB was resistant to chemoradiotherapy and subsequently recurred; similar clinical symptoms also developed. However, the serum ACTH and cortisol concentrations and the urinary cortisol concentration were markedly different. In Cases 1 and 2, the serum ACTH concentration was much higher than that in the present case, while the serum cortisol concentration was similar to or lower than that in the present case. The tumors in patients with EAS process the ACTH precursor, proopiomelanocortin (POMC), in an aberrant manner, producing low levels and altered molecular forms of POMC and thus releasing high concentrations of “big ACTH” and less intact ACTH in the circulation.6 As a result, the plasma ACTH level may be influenced by the existence of “big ACTH,” which may contribute to the large differences in ACTH levels found in each respective patient with EAS. In the present case, although the serum ACTH and cortisol levels prior to November 2014 were not measured, EAS was thought to have developed simultaneously with the hypokalemia, hypoeosinophilia, hyperglycemia, and Cushing’s symptoms. A previous report showed that the serum potassium concentration was significantly inversely associated with the serum cortisol concentration in patients with EAS.7
Table 2.
Previous case reports of EAS that developed after diagnosis of ONB.
| Patient | Age (years) | Sex | Time from onset of ONB to diagnosis of EAS | Symptoms | ACTH(pmol/L) | Cortisol(nmol/L) | Urinary cortisol(nmol/day) | Treatment for ONB | Relapse of ONB | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | Male | 15 years | Fatigue, confusion, severe hypertension, muscle atrophy, hyperglycemia, hypokalemia, metabolic alkalosis | 100.1 | 2428.8 | 42,120 | Craniotomy | Yes | [2] |
| 2 | 66 | Female | 2 years | Systemic edema, fatigue, moon face, truncal obesity, thin skin with purpura and hirsutism, hypokalemia, metabolic alkalosis | 189.6 | 1802.2 | 13,924 | Refused craniotomy | Yes | [3] |
| 3 | 48 | Female | 28 months | Cushing like-syndrome (details unknown) | – | – | – | Craniotomy | Yes | [4] |
| 4 | 50 | Male | 4 years | Moon face, truncal obesity, purpura, muscle atrophy, hypertension, hyperglycemia, hypokalemia, metabolic alkalosis | 39.3 | 2461.9 | 28,428 | Radiation and chemotherapy | Yes | Present case |
EAS, ectopic adrenocorticotropic hormone syndrome; ACTH, adrenocorticotropic hormone; ONB, olfactory neuroblastoma.
Our patient developed Cushing’s syndrome long after the onset of ONB for several possible reasons. The most plausible explanation is that the primary ONB lesion changed its functional phenotype, acquiring characteristics that allowed it to produce and secrete ACTH. In a previous case of EAS associated with thymic carcinoid, the tumor shifted its phenotype from the production of CRH to ACTH during the clinical course of the disease.8 In another case of EAS, neither ACTH nor CRH could be detected.9 A previous review showed that 14 of 16 ACTH-secreting ONBs were positive for ACTH as shown by immunohistochemical staining, while 2 were negative for ACTH staining despite the fact that the patients showing apparent features of Cushing’s syndrome. A second possible reason why our patient developed Cushing’s syndrome long after ONB is that the tumor may have secreted POMC or pro-ACTH, later shifting to produce ACTH during the clinical course of the disease. Glucocorticoid is known to affect POMC gene expression and pro-ACTH and ACTH secretion in patients with EAS.10 Finally, a third reason is that the tumor may have cyclically produced ACTH. A previous report emphasized that cyclic Cushing’s syndrome is observed in most patients with EAS.11 Although the median interval from the onset of ONB to the development of Cushing’s syndrome is 35 days, a case involving an interval of almost 6 years (2160 days) has also been reported.1 In the present case, the possibility that the tumor is still undergoing a cycle of ACTH secretion after 3 years cannot be completely excluded. Thus, careful follow-up for the appearance of Cushing’s symptoms is required in this patient.
In conclusion, we experienced a rare case of ONB that acquired the ability to express ACTH during its clinical course as demonstrated by sequential immunopathological studies of biopsy specimens.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Kunc M, Gabrych A, Czapiewski P, et al. Paraneoplastic syndromes in olfactory neuroblastoma. Contemp Oncol 2015; 19: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mintzer DM, Zheng S, Nagamine M, et al. Esthesioneuroblastoma (olfactory neuroblastoma) with ectopic ACTH syndrome: a multidisciplinary case presentation from the Joan Karnell Cancer Center of Pennsylvania Hospital. Oncologist 2010; 15: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo BK, An JH, Jeon KH, et al. Two cases of ectopic adrenocorticotropic hormone syndrome with olfactory neuroblastoma and literature review. Endocr J 2008; 55: 469–475. [DOI] [PubMed] [Google Scholar]
- 4.Reznik M, Melon J, Lambricht M, et al. Neuroendocrine tumor of the nasal cavity (esthesioneuroblastoma). Apropos of a case with paraneoplastic Cushing's syndrome. Ann Pathol 1987; 7: 137–142. [PubMed] [Google Scholar]
- 5.Parrilla C, Lucidi D, Petrone G, et al. Idiopathic SIADH in young patients: don't forget the nose. Acta Otorhinolaryngol Ital 2017; 37: 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzolo M, Reimondo G, Ali A, et al. Ectopic ACTH syndrome: molecular bases and clinical heterogeneity. Ann Oncol 2001; 12(Suppl 2): S83–S87. [DOI] [PubMed] [Google Scholar]
- 7.Doi M, Sugiyama T, Izumiyama H, et al. Clinical features and management of ectopic ACTH syndrome at a single institute in Japan. Endocr J 2010; 57: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 8.Ozawa Y, Tomoyasu H, Takeshita A, et al. Shift from CRH to ACTH production in a thymic carcinoid with Cushing's syndrome. Horm Res 1996; 45: 264–268. [DOI] [PubMed] [Google Scholar]
- 9.Saeger W, Reincke M, Scholz GH, et al. Ectopic ACTH- or CRH-secreting tumors in Cushing's syndrome. Zentralbl Pathol 1993; 139: 157–163. [PubMed] [Google Scholar]
- 10.Liu J, Heikkila P, Voutilainen R, et al. Pheochromocytoma expressing adrenocorticotropin and corticotropin-releasing hormone; regulation by glucocorticoids and nerve growth factor. Eur J Endocrinol 1994; 131: 221–228. [DOI] [PubMed] [Google Scholar]
- 11.Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing's syndrome: a clinical challenge. Eur J Endocrinol 2007; 157: 245–254. [DOI] [PubMed] [Google Scholar]