Short abstract
Back pain is a common clinical symptom. Degeneration of intervertebral discs is one of the most important factors leading to back pain, namely, discogenic back pain. However, at present, the understanding of lumbar intervertebral discs causing back pain is confined to biomechanical and histological studies. The neuropathological mechanism related to discogenic back pain is still not well understood. Many studies have found that as an intervertebral disc degenerates, the peripheral nerve tissues have corresponding structural reorganization, and a series of nerve cells become involved in progression of discogenic back pain. Therefore, study of neural mechanisms that are involved in progression of discogenic back pain will provide additional assistance for treatment of its symptoms. We review the anatomical structure of intervertebral discs and the related neural mechanisms involved in discogenic back pain. We also discuss the current view of neural mechanisms underlying discogenic back pain.
Keywords: Discogenic back pain, disc degeneration, neural mechanism, lumbar intervertebral disc, nerve fiber, nervous system
Introduction
Back pain is one of the most common and compound clinical symptoms. According to the World Health Organization, nearly 33% of people suffer from back pain worldwide,1 and back pain is considered the most common of all types of disabling factors.2 In the United States, the prevalence of chronic back pain is 10.2% among people aged older than 21,3 while the prevalence of back pain has reached 26.1% in China. Back pain ranks third in the world in disabling factors, behind stroke and myocardial ischemia.4 Back pain is also more common than hypertension and diabetes.5 In daily life, the average daily pain expenditure for patients with back pain is 60% more than that of normal people.6 The annual economic loss caused by back pain is 1 to3 billion USD worldwide.7 Back pain has caused a large burden on social and medical resources, and its damage to social and economic development warrants further studies.
In the 1930s, Mixter first reported that lumber intervertebral disc herniation was a cause of back pain, thus opening the “intervertebral disc age”.8 In the 10 years that followed, many researchers began to study intervertebral disc degeneration,9,10 and gradually came to the conclusion that intervertebral disc degeneration might be an important cause of back pain.11 Evidence has shown that approximately 40% of back pain cases can be attributed to lumbar intervertebral disc degeneration,12 and intervertebral disc degeneration constitutes the main cause of chronic back pain.13 Clinically, back pain that is caused by pathological changes of the intervertebral disc, without nerve root injury or segmental instability, is called discogenic back pain. The scientific community has intensely studied the neuroanatomy, cellular mechanisms, and mechanical mechanisms of intervertebral discs. Scientists have gradually realized that in addition to mechanical compression, changes in some biological mechanisms in the process of intervertebral disc degeneration are also important factors that contribute to the symptoms of back pain.14 Unfortunately, these studies have not yet reached an accurate conclusion to date. The questions of “Do intervertebral discs cause pain?” and “Does intervertebral disc degeneration affect the nervous system?” remain unanswered These questions are still the focus of debate in the field of discogenic back pain and make research of discogenic back pain extremely challenging. In this review, we discuss the related neural mechanisms that contribute to discogenic back pain.
Neural distribution of normal intervertebral discs
Researchers have experienced a long process of understanding the distribution of nerve fibers in lumbar intervertebral discs. In the 1930s, with the establishment of microanatomy and neurochemical staining, free nerve endings in the lumbar intervertebral discs were found.15,16 This finding overturned the belief held by some researchers that there was no nerve innervation present in the disc. Subsequently, more complex peripheral structures, such as annular somatic receptors and mechanical stimulators, were gradually found in lumbar intervertebral discs.16 In 1983, Bogduk et al.17 found that the distribution of nerve endings in the intervertebral disc was confined to the outer structure in the fibrous ring of the intervertebral disc. These authors also found that the types of nerve fiber innervation in the different structural regions of the intervertebral disc were different. An example of this finding is that the sinuvertebral nerve and its direct branches innervate the posterior region of the intervertebral disc collectively. The grey communicating branch innervates bilateral regions of the intervertebral disc, and the anterior part of the intervertebral disc is controlled by the sympathetic plexus.17 Additionally, the ascending and descending branches of the sinus nerve also innervate the articular facet joints18 and endplates of the vertebrae,17 and further intertwine into the spinal canal.17,19 The findings of Bogduk et al.17 provided new understanding of the distribution of nerve fibers in intervertebral discs. However, because of the limitations of research conditions at that time, many of the findings were based only on gross observation made at autopsy. Therefore, little is known about the nature of nerve fibers distributed in intervertebral discs and their nerve conduction pathways.
With the development of immunocytochemistry and its application in the field of intervertebral discs, scientists have been able to gain new understanding of the neural structure of the intervertebral disc. Palmgren et al.20 found a sympathetic nerve marker, neuropeptide Y, and sensory nerve marker, substance P, in the outer fibrous ring of lumbar intervertebral discs in normal adults. This finding suggested that the lateral fibrosis of the intervertebral disc was innervated by sympathetic and spinal nerves. With the discovery of some nerve fiber-specific markers, researchers have not only observed the distribution of nerve fibers in an intervertebral disc, but also classified these nerve fibers more accurately. Coppes21 used substance P and calcitonin gene-related peptide to identify nociceptive nerve fibers in the intervertebral disc. Roberts22 used vasoactive intestinal peptide to detect the nerve in the intervertebral disc. Sympathetic fibers in the intervertebral disc were found by tyrosine hydroxylase immunoreactivity in postganglionic fibers.23 Additionally, mechanical receptors, such as Pacinian corpuscles, Ruffini’s corpuscles, and Golgi tendons, are also found in intervertebral discs.22 These receptors are fewer than the normal number found in intervertebral discs and are not normally sensitive to mechanical stimulation.24 However, once the disc has undergone a pathological change, these receptors can be activated and produce corresponding potential changes. This constitutes the body’s perception of changes in disc disease based on electrophysiology of the nerve.25
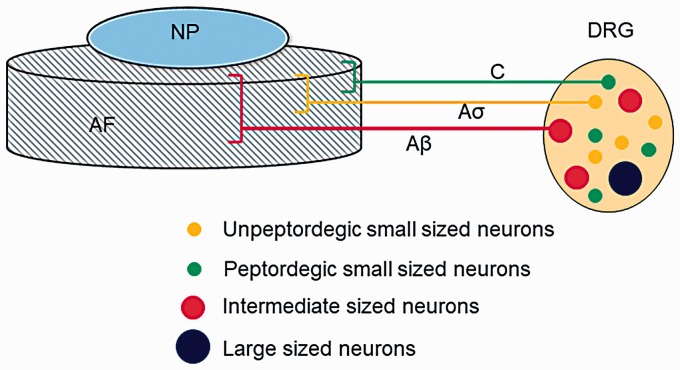
In the 21st century, scientists have a deeper understanding of the nerve structure in intervertebral discs. In 2003, Fagan et al.26 quantitatively found that there was no significant difference in the distribution of nerve densities between the outer fibrous ring and the endplate of a normal intervertebral disc. However, the nerve fibers produced a new distribution in the inner fibrous ring once the intervertebral disc had become diseased. The distribution density of nerve fibers in the lesion was significantly higher than that observed in normal intervertebral discs. Another study showed a source of nerve fibers distributed in the intervertebral disc, which suggested that they all originated from neurons in the dorsal root ganglion (DRG).27 These neurons can be classified in different ways based on the cell diameter, type of neurotransmitter, and the different cytoskeletal components. An example of this classification is that the DRG neurons that innervate the lumbar intervertebral disc are mainly small cell neurons. These neurons can be further divided into peptide-sensitive, nerve growth factor-sensitive neurons and nonpeptide, glial cell-derived neurons according to the different types of transmitters contained in the cell bodies (Figure 1). Both types of small cell neurons can emit fibers to dominate the lumbar intervertebral disc.27 These small cell-derived nerve fibers are mostly nociceptive nerve fibers. These are the type of nerve fibers that form the anatomical basis of discogenic back pain.28
Figure 1.
Schematic representation the origin of sensory nerve fibers that innervate normal intervertebral discs. In the normal intervertebral disc, innervation is restricted to the outer layers of the AF and consists of small nerve fibers (yellow and green) and some large fibers forming mechanoreceptors (red). AF: annulus fibrosus; DRG: dorsal root ganglion; NP: nucleus pulposus.
Nerve conduction anatomical pathway of the intervertebral disc
The nerve conduction pathway of lumbar intervertebral discs is related to the anatomical pathway of nerve fibers that are distributed in the lumbar intervertebral disc to the central nervous system. After Luschka first discovered the sinus nerve in 1850, researchers agreed that the sensory nerve endings in the intervertebral disc were projected by the corresponding segment of DRG neurons.17 This understanding was based on a study of contemporary autopsies.29–31 These studies showed that the sinus nerve at the intervertebral foramen consisted of the corresponding segment of the DRG peripheral process (the spinal sensory nerve fiber) and the sympathetic postganglionic fibers. The sensory nerve endings in the disc came from the sinus nerve and spread through the intervertebral foramen into the spinal canal.29 These findings led to the belief that the sensory nerve fibers in the posterior longitudinal ligament and in the posterior region of the lumbar intervertebral disc were segmentally innervated by the corresponding DRG neurons.
With the development of nerve tracer technology, recent research has shown that innervation of lumbar intervertebral discs does not necessarily correspond with innervation of the spinal nerve segment. Only DRGs L1 and L2 were labeled by tracer DRG after injecting horseradish peroxidase and cholera toxin B into the ventral fibrous ring of the L5/6 intervertebral disc in rats.30 This finding suggested that the spinal nerves that innervate lumbar intervertebral discs do not come from the same segment of the spinal nerve, but are innervated by multiple different segments from the DRG. Similarly, Ohtori et al.31 used neural tracer injection at the L5/6 dorsal intervertebral disc area and observed that fluorogold-positive neurons from T13 to L6 might be bilateral DRG neurons. This further proved that a single lumbar intervertebral disc could be influenced by a variety of different segments of DRG neurons with branches. Additionally, Ohtori et al.31 used disc nerve tracer cutting at the L2 and L3 regions of the sympathetic trunk in rats. These authors showed nearly 93% fluorogold-positive nerves at L3 to L6 DRG, and the T13 to L1 DRG also had fluorogold-labeled neurons. These results indicated that the DRG through the sympathetic trunk constitutes an indirect pathway for lumbar disc nerve conduction. Chen et al.32 found that there were sinus nerves from the sympathetic trunk in the posterior part of the intervertebral disc, which further supported Ohtori et al.31 findings. Groen et al.33 showed that the posterior innervation region of the intervertebral disc came from the sinus nerve and that sympathetic nerves were the only component of the sinus nerve. This conclusion contradicted some researchers’ hypothesis that the sinus nerve is a mixed nerve composed of sympathetic nerves and the dorsal branch of the spinal nerve. The main components of the sinus nerve are still controversial. However, existing experimental results have shown that sympathetic nerves and the spinal nerve together constitute the anatomical basis of the nerve conduction pathway of lumbar intervertebral discs.34–36
Changes in nerve fiber distribution in degenerative intervertebral discs
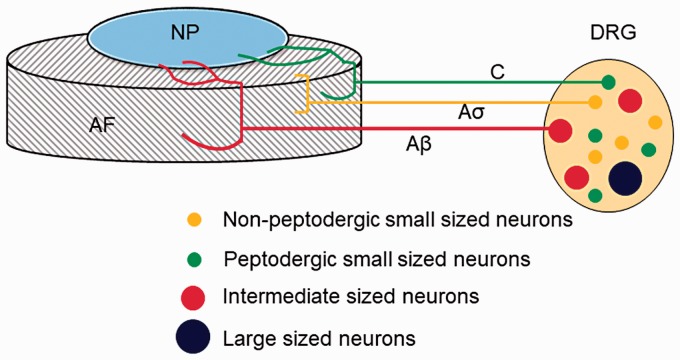
Formation and infiltration of nerve fibers into the inner layer of the fibrous ring and nucleus pulposus during disc degeneration is considered as the main neural mechanism of discogenic back pain. At birth, there are many dense nerve fibers in the endplate and fibrous ring of a newborn’s lumbar intervertebral disc. However, these nerve fibers gradually degenerate to only one third of the outer part of the fibrous ring as they grow older. Finally, the nerve structure is no longer present in the medial region of the fibrous ring of the lumbar intervertebral disc in normal adults.37 However, some nerve endings reappear in a degenerated lumbar disc, and these endings infiltrate deeper into the inner tissue of the intervertebral disc (Figure 2). Ashton et al.38 found that nerve fibers were more deeply infiltrated in painful than in painless intervertebral discs. Peng et al.39 found that nerve fibers can grow along granulation tissue in the fissure of a degenerating intervertebral disc. These nerve fibers can gradually infiltrate the nerve endings in the deep part of the fibrous ring of the deteriorating intervertebral disc, even into the nucleus pulposus. Recently, researchers used Secreted Protein Acidic and Rich in Cysteine (SPARC) gene knockout mice to simulate degeneration of intervertebral discs.40 They also discovered nerve fibers infiltrating into the intervertebral disc, which were identified as nociceptive nerve fibers. On the basis of the above-mentioned findings, some researchers have hypothesized that growth and infiltration of nerve fibers in degenerative intervertebral discs is one of the most important pathological mechanisms of discogenic back pain.
Figure 2.
Schematic representation of innervation of degenerated intervertebral discs. In degenerated intervertebral discs, sensory nerve fibers that arise from neurons in the DRG enter the inner layers of the AF and even the NP. Thin myelinated Aδ fibers and unmyelinated C fibers originate from small neurons (yellow and green) in DRGs. The myelinated Aβ fibers (red) arise from intermediate neurons, and the Aδ or C fibers originate from small peptidergic neurons or non-peptidergic neurons. AF: annulus fibrosus; DRG: dorsal root ganglion.
Cytokines that are produced during the process of intervertebral disc degeneration are important factors in inducing nerve endings to grow into degenerative intervertebral discs.41 There is a small amount of neural growth factor and brain-derived neurotrophic factor (BDNF) in the nucleus pulposus and fibrous ring cells of normal intervertebral discs.42,43 However, expression of BDNF and nerve growth factor in degenerative discs of patients with discogenic back pain is considerably increased.42,44 Additionally, some protein receptors associated with nerve growth, such as tyrosine kinase A, can be released into the matrix via autocrine signals from degenerated intervertebral disc cells. This further alters the distribution of nerve endings in a degenerating intervertebral disc.45 Changes in inflammatory levels in degenerative intervertebral discs also affect secretion of neurotrophic factors. Purmessur et al.44 showed that interleukin-1 beta and tumor necrosis factor-alpha could stimulate human-derived nucleus pulposus cells to secrete nerve growth factor and BDNF in in vitro experiments. Richardson et al.46 found that matrix metalloproteinases could further degrade the extracellular matrix of a degenerated intervertebral disc, thereby promoting nerve fibers into the nucleus pulposus and producing pain. Burke et al.47 found that elevated inflammatory mediators in degenerative intervertebral discs can act on the nociceptors of the sinus nerve, directly resulting in changes in the electrical mechanism of the nerve. This reduces the excitatory threshold, making it more likely to produce nerve impulses. However, the interactions between various protein factors are complex, and some specific mechanisms need further examination in the future.
Effect of degenerative intervertebral discs on nervous system excitability
Many studies have confirmed that production of discogenic back pain is related to the electrophysiological state of the nerve as follows. Takahashi et al.48 found that electrical stimulation of the L5/6 intervertebral disc could induce action potentials in the L2 nerve root in rats. This finding indicates that there are certain receptors in the intervertebral disc that can convert an external electrical signal into a bioelectrical signal for conduction. After mechanical and chemical stimulation of the L5/6 intervertebral disc in rats, mechanical stimulation could not induce an action potential in the L2 nerve; only chemical stimulation could induce a change in the L2 nerve action potential.49 Therefore, mechanical stimulation cannot induce pain, and inflammation in the intervertebral disc causes transmission of noxious sensory impulses. Burke et al.47 reported that elevated inflammatory mediators in degenerative intervertebral discs could act on intra-disc nociceptors and directly caused electrophysiological changes in nerve fibers. This was characterized by a lower threshold for nerve excitation and an increased likelihood for producing nerve impulses. Le Maitre et al.50 found that interleukin-1 stimulated production of additional inflammatory factors in lumbar intervertebral discs, and this led to a series of inflammatory cascades. This finding suggests that inflammatory cells and mediators are important initiators of pain. These studies showed an inflammatory nerve mechanism of discogenic back pain caused by degenerative intervertebral discs. Although the diagnosis and treatment of discogenic back pain can be achieved by a nerve root block or discography technique, its effect is still controversial.
In addition to electrophysiological activities, nerve excitability is also related to the metabolic state of nerve cells. Some new studies have found that discogenic back pain is accompanied by changes in the metabolic level of central nervous cells. Loggia et al.51 studied patients with chronic back pain as experimental subjects. The activated microglia cells were labeled with 11C-PBR28 in vivo. The stoichiometric concentrations of 11C-PBR28 in the brain of patients with chronic back pain were significantly higher than those in patients without back pain. These results suggest that microglia in the brain may play a role in the progression of back pain. In animal experiments, Miyagi et al.52 found that the process of intervertebral disc degeneration caused by acupuncture injury was accompanied by accumulation and activation of glial cells in the posterior horn of the spinal cord. They further used a mouse model and spontaneously caused degeneration of lumbar intervertebral discs. In this model, the activity of glial cells in the posterior horn of the lumbar spinal cord was also increased. This phenomenon was closely related to the behavior of back pain in mice.40 These results suggest that discogenic back pain is associated with disc degeneration, which affects the metabolic levels of nerve cells. Current studies on disc degeneration and back pain have focused on intervertebral disc tissue. The mechanism of intervertebral disc degeneration and nervous system functional changes still needs to be explored.
Conclusions
In conclusion, discogenic back pain is a common clinical symptom. Degenerated intervertebral disc cells and inflammatory cell infiltrates can stimulate reconstruction of nerve fibers of internal intervertebral discs and cause back pain at the same time. Generally, pain is a process that is signaled from the injured site or lesion and conducted to the central nervous system to produce the perception of pain. Many studies have shown that sympathetic and spinal nerves play a role in nerve signal transduction in discogenic back pain. Changes in nervous system excitability throughout the course of discogenic back pain have gradually drawn the attention of researchers. In the future, a full understanding of the specific mechanism of nerves throughout the course of discogenic back pain needs to be investigated. This will allow new methods and approaches for the treatment of discogenic back pain.
Acknowledgements
We would like to acknowledge the language help provided by Wanli Dong, MS.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was partially supported by the National Natural Science Foundation of China (NSFC, 81772311).
References
- 1.Gureje O, Korff MV, Simon GE, et al. Persistent pain and well-being: a World Health Organization study in primary care. JAMA 1998; 280: 147–151. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich GE. Low back pain. Bull World Health Organ 2003; 81: 671–676. [PMC free article] [PubMed] [Google Scholar]
- 3.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med 2009; 169: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay SI, Abajobir AA, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv Y, Tian W, Liu Y, et al. A cross-sectional study on low back pain among adults in Beijing. Chinese Journal of Orthopaedics 2013; 33: 60–64. [Google Scholar]
- 6.Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004; 29: 79–86. [DOI] [PubMed] [Google Scholar]
- 7.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 2006; 88(Suppl 2): 21–24. [DOI] [PubMed] [Google Scholar]
- 8.Mixter WJ. Rupture of the lumbar intervertebral disk: an etiologic factor for so-called “sciatic” pain. Ann Surg 1937; 106: 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epps PG. A case of degeneration of the intervertebral disc following lumbar puncture: (section of orthopædics). Proc R Soc Med 1942; 35: 220–221. [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen AK. Disc degeneration: a distinct clinical entity. J Nerv Ment Dis 1950; 112: 262–263. [PubMed] [Google Scholar]
- 11.Lindblom K. Diagnostic puncture of intervertebral disks in sciatica. Acta Orthop Scand 1948; 17: 231–239. [DOI] [PubMed] [Google Scholar]
- 12.Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009; 34: 934–940. [DOI] [PubMed] [Google Scholar]
- 13.Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000; 25: 487–492. [DOI] [PubMed] [Google Scholar]
- 14.Mertens P, Blond S, David R, et al. Anatomy, physiology and neurobiology of the nociception: a focus on low back pain (part A). Neurochirurgie 2015; 61: S22–S34. [DOI] [PubMed] [Google Scholar]
- 15.Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament - fourth and fifth lumbar level. Arch NeurPsych 1940; 44: 100–103. [Google Scholar]
- 16.Malinský J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). Acta Anat (Basel) 1959; 38: 96–113. [PubMed] [Google Scholar]
- 17.Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat 1981; 132(Pt 1): 39–56. [PMC free article] [PubMed] [Google Scholar]
- 18.Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg 1979; 51: 172–177. [DOI] [PubMed] [Google Scholar]
- 19.Groen GJ, Baljet B, Drukker J. The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien) 1988; 92: 39–46. [DOI] [PubMed] [Google Scholar]
- 20.Palmgren T, Gronblad M, Virri J, et al. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976) 1999; 24: 2075–2079. [DOI] [PubMed] [Google Scholar]
- 21.Coppes MH, Marani E, Thomeer RF, et al. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976) 1997; 22: 2342–2349. [DOI] [PubMed] [Google Scholar]
- 22.Roberts S, Eisenstein SM, Menage J, et al. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976) 1995; 20: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 23.Bjurholm A, Kreicbergs A, Terenius L, et al. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst 1988; 25: 119–125. [DOI] [PubMed] [Google Scholar]
- 24.Walsh TR, Weinstein JN, Spratt KF, et al. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am 1990; 72: 1081–1088. [PubMed] [Google Scholar]
- 25.Yamashita T, Minaki Y, Oota I, et al. Mechanosensitive afferent units in the lumbar intervertebral disc and adjacent muscle. Spine (Phila Pa 1976) 1993; 18: 2252–2256. [DOI] [PubMed] [Google Scholar]
- 26.Fagan A, Moore R, Vernon Roberts B, et al. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976) 2003; 28: 2570–2576. [DOI] [PubMed] [Google Scholar]
- 27.Garcia Cosamalon J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat 2010; 217: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitroulias A, Tsonidis C, Natsis K, et al. An immunohistochemical study of mechanoreceptors in lumbar spine intervertebral discs. J Clin Neurosci 2010; 17: 742–745. [DOI] [PubMed] [Google Scholar]
- 29.Jackson HC, Winkelmann R, Bickel WH. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg Am 1966; 48: 1272–1281. [PubMed] [Google Scholar]
- 30.Morinaga T, Takahashi K, Yamagata M, et al. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine (Phila Pa 1976) 1996; 21: 1848–1851. [DOI] [PubMed] [Google Scholar]
- 31.Ohtori S, Takahashi Y, Takahashi K, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine (Phila Pa 1976) 1999; 24: 2295–2299. [DOI] [PubMed] [Google Scholar]
- 32.Chen JD, Hou SX, Peng BG, et al. Anatomical study of human lumbar spine innervation. Zhonghua Yi Xue Za Zhi 2007; 87: 602–605. [PubMed] [Google Scholar]
- 33.Groen GJ, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat 1et al. 0; 188: 282–296. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura S, Takahashi K, Takahashi Y, et al. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine (Phila Pa 1976) et al. ; 21: 917–924. [DOI] [PubMed] [Google Scholar]
- 35.Takebayashi T, Cavanaugh JM, Kallakuri S, et al. Sympathetic afferent units from lumbar intervertebral discs. J Bone Joint Surg Br et al. ; 88: 554-557. [DOI] [PubMed] [Google Scholar]
- 36.Teske W, Boudelal R, Zirke S, et al. Anatomical study of preganglionic spinal nerve and disc relation at different lumbar levels: Special aspect for microscopic spine surgery. Technol Health Care et al. ; 23: 343–350. doi: 10.3233/THC-150914. [DOI] [PubMed] [Google Scholar]
- 37.Roberts S, Evans EH, Kletsas D, et al. Senescence in human intervertebral discs. Eur Spine J 2006; 15(Suppl 3): 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashton IK, Roberts S, Jaffray DC, et al. Neuropeptides in the human intervertebral disc. J Orthop Res 1994; 12: 186–192. [DOI] [PubMed] [Google Scholar]
- 39.Peng B, Wu W, Hou S, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br 2005; 87: 62–67. [PubMed] [Google Scholar]
- 40.Miyagi M, Millecamps M, Danco AT, et al. ISSLS Prize winner: Increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976) 2014; 39: 1345–1354. [DOI] [PubMed] [Google Scholar]
- 41.Lee JM, Song JY, Baek M, et al. Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res 2011; 29: 265–269. [DOI] [PubMed] [Google Scholar]
- 42.Krock E, Currie JB, Weber MH, et al. Nerve growth factor is regulated by toll-like receptor 2 in human intervertebral discs. J Biol Chem 2016; 291: 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krock E, Rosenzweig DH, Chabot-Dore AJ, et al. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med 2014; 18: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther 2008; 10: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navone SE, Marfia G, Canzi L, et al. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J Orthop Res 2012; 30: 1470–1477. [DOI] [PubMed] [Google Scholar]
- 46.Richardson SM, Doyle P, Minogue BM, et al. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther 2009; 11: R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke JG, Watson RW, Mccormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br 2002; 84: 196–201. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi Y, Sato A, Nakamura SI, et al. Regional correspondence between the ventral portion of the lumbar intervertebral disc and the groin mediated by a spinal reflex. A possible basis of discogenic referred pain. Spine (Phila Pa 1976) 1998; 23: 1853–1858; discussion 1859. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi Y, Hirayama J, Nakajima Y, et al. Electrical stimulation of the rat lumbar spine induces reflex action potentials in the nerves to the lower abdomen. Spine (Phila Pa 1976) 2000; 25: 411–417. [DOI] [PubMed] [Google Scholar]
- 50.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 2005; 7: R732–R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain 2015; 138: 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyagi M, Ishikawa T, Orita S, et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine (Phila Pa 1976) 2011; 36: 2260–2266. [DOI] [PubMed] [Google Scholar]