Short abstract
Objective
Catheter ablation of atrial fibrillation (AF) can lead to thromboembolic complications, especially stroke. We measured the periprocedural serum neuron-specific enolase (NSE) level, which is a biomarker of neuronal injury, after ablation of AF.
Methods
Forty-three patients with paroxysmal AF were prospectively enrolled before radiofrequency ablation. A neurological examination was performed before and after the procedure. The serum NSE level was determined before and at the end of the procedure and at 2, 24, and 48 h after the procedure.
Results
No patients developed new neurological deficits. However, the median (interquartile range) NSE level increased after ablation from 6.7 (3.87) ng/mL at baseline to 11.48 (5.3) ng/mL at 24 h postoperatively. The NSE level exceed the upper reference limit of normal (17 ng/mL) in 14 patients (33%), and these patients were found to have a larger left atrium.
Conclusions
Serum NSE increased in most of the patients undergoing ablation for AF, and it exceeded the normal limit in one-third of the patients. Although NSE is a biomarker of neuronal injury, the clinical importance of this increase after AF ablation and its relationship with the left atrial diameter should be evaluated in a longitudinal study.
Keywords: Atrial fibrillation, pulmonary vein isolation, catheter ablation, serum neuron-specific enolase, cerebral embolism, thromboembolism
Introduction
Catheter ablation of atrial fibrillation (AF), targeting isolation of pulmonary veins (IPV), is an effective therapeutic approach particularly for patients with paroxysmal AF.1,2 However, this procedure can lead to some complications, one of the most striking of which is the occurrence of stroke in 1% of patients.3 Additionally, periprocedural diffusion-weighted cerebral magnetic resonance imaging (DW-MRI) has shown that the IPV procedure can lead to subclinical cerebral embolism in up to 48% of patients.4–8 Previous studies have been performed to evaluate silent cerebral lesions during IPV using DW-MRI as a reference diagnostic method. However, another study suggested that DW-MRI lacks sensitivity, and the authors investigated whether detection of cerebral damage during IPV can be improved by assessing a sensitive biomarker of neuronal injury: protein S100B.9 The authors found that a higher incidence of silent stroke can be revealed by the biomarker-based (protein S100B) approach than by DW-MRI alone. 9
Neuron-specific enolase (NSE) is also a serum biomarker of neuronal injury with a biologic half-life of approximately 24 h. It is an intracytoplasmic enzyme located in neurons and neuroectodermal cells. If neuronal injury occurs and the integrity of the blood–brain barrier is impaired, NSE is released into the cerebrospinal fluid and then into the blood. An increased serum NSE level in the cerebrospinal fluid and blood reportedly has high predictive value in neurocognitive damage or neurological outcomes after stroke, cardiovascular surgery, and cardiac arrest.10–14 However, the literature presents insufficient data about the association between an increased NSE and neurological outcomes during IPV.
This preliminary study was performed to assess the changes in the serum NSE level, which is assumed to be an indicator of neuronal damage, in patients undergoing catheter ablation for AF. To the best of our knowledge, no previous studies have investigated the alterations in the serum NSE level during and after the IPV procedure.
Patients and Methods
Patients
From January 2014 to November 2014, patients with paroxysmal AF diagnosed according to the current guidelines were enrolled before undergoing IPV by radiofrequency catheter ablation (RFA).1,2 The exclusion criteria were severe valvular heart disease, an age of <20 or >80 years, and a thrombus in left atrium (LA) or left atrial appendix (LAA). This prospective study was performed in compliance with the Declaration of Helsinki and was approved by the Human Research and Ethics Committee of Ankara University. All patients provided written informed consent before enrollment.
Periprocedural examinations
Neurological assessment was performed 24 to 48 h before and after the IPV procedure. Considering the biologic half-life and a previous study showing that the maximum level of enolase reached 48 h postoperatively,15 the NSE level was determined before (NSE-pre) and at the end of the procedure (NSE-post) as well as 2, 24, and 48 h after RFA.
On admission, all patients underwent a thorough cardiologic clinical assessment, including a physical examination, electrocardiography, transthoracic echocardiography (TTE), medical history (comorbidities and presence of heart disease), and assessment of the CHA2DS2 VASc score [composite risk score comprising congestive heart failure (1 point), hypertension (1 point), age of ≥75 years (2 points), diabetes (1 point), stroke (2 points), age of ≥65 years (1 point), vascular disease (1 point), and female sex (1 point)]. Transesophageal echocardiography was performed to exclude intracardiac thrombi. Routine biochemical tests including calculation of the international normalized ratio (INR) were also performed.
The same neurologist examined the patients within 24 to 48 h before and after IPV. The clinical outcome was assessed by comparing the preoperative and postoperative neurologic examinations, including assessment of cranial nerves, motor and sensory function, reflexes, and cerebellar function.
An NSE immunoluminometric assay test kit (Roche NSE; Roche, Mannheim, Germany) was used to measure the NSE level. The manufacturer reported that the 95th percentile of the normal serum concentration was <17 ng/mL; the Roche NSE kit measures concentrations of 0.05 to 370 ng/mL.
Blood for NSE measurement was obtained from each patient via venipuncture, allowed to clot for 20 to 30 min at room temperature, and then centrifuged at 800 to 1000 rpm for 15 min. The serum was stored at −20°C and assayed after all samples had been collected. Samples that showed visible hemolysis were not analyzed.
Periprocedural anticoagulation management
In patients taking warfarin, the warfarin was continued before and after the ablation, targeting an INR of 2 to 3; patients with a subtherapeutic INR were given subcutaneous low-molecular-weight heparin. Novel oral anticoagulants were discontinued 12 to 24 h before the procedure and restarted the morning after the procedure. Subcutaneous low-molecular-weight heparin was not used during interruption of the novel oral anticoagulants.
The procedure was performed by femoral vein puncture. An initial bolus of 100 UI/kg unfractionated heparin was given after obtaining peripheral vascular access. Continuous infusion and additional boluses of heparin were administered to maintain the activated clotting time (ACT) above 250 and 300 seconds as suggested by the Venice Chart International Consensus document on AF ablation.16 The ACT was measured every 30 min.
Catheter ablation
All patients underwent anesthetic induction using a combination of midazolam, propofol, and fentanyl. General anesthesia was then maintained using volatile sevoflurane.
IPV was performed using RF energy. An octapolar electrode catheter was placed into the coronary sinus for pacing and recording. The LA was accessed by two echo-guided trans-septal punctures, and each guidewire was introduced into the LA by the trans-septal puncture using an 8-French long sheath (Biosense Webster, Irvine, CA, USA). A circular electrophysiologic catheter was inserted through the long sheath to map the electrical activity of the pulmonary vein ostia (Lasso; Biosense Webster), and an open irrigated-tip ablation catheter (Navistar Thermocool; Biosense Webster) was positioned in the LA. The sheaths were continuously flushed with heparinized solution (3000 UI of heparin in 500 mL NaCl 0.9%) at 180 mL/h. An electroanatomic mapping system (Carto 3; Biosense Webster) via the mapping catheter and a priori computed tomography images were used to obtain reconstructed three-dimensional images of the LA and pulmonary vein ostia. The pulmonary veins and their ostia were encircled by point-by-point circumferential lesions. Radiofrequency energy was applied up to 30 W, and the energy was reduced to 20 to 25 W at the posterior left atrial wall. Saline infusion was continued with the Coolflow Pump (Biosense Webster) to maintain a tip temperature of <45°C. Complete electrical IPV as recorded by the multipolar catheter was accepted as the endpoint of the ablation procedure.
If the AF remained after the ablation, cardioversion was performed to restore sinus rhythm. Post-ablation TTE was performed soon after the procedure to rule out pericardial effusion.
Statistical analysis
We evaluated the within-patient variation of the NSE level during the periprocedural period and recorded whether the NSE level exceeded the upper limit of normal (ULN). Patients were stratified as having a post-procedural maximum NSE level either exceeding the ULN (increasedULN) or not exceeding ULN (stableULN). The two groups were then compared with respect to clinical, echocardiographic, and procedural characteristics.
All data were analyzed using the statistical software package SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Categorical variables are reported as count and percentage. Continuous variables are expressed as median (IQR) because the data were non-normally distributed. Categorical variables were assessed by Pearson’s chi-square or Fisher's exact test. The Mann–Whitney U test was used to compare continuous variables.
A P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
This study included 43 patients with paroxysmal AF who were referred for AF ablation. Of these 43 patients, 28 (65.1%) were female, and the median age was 64 (15) years. The baseline characteristics of the patients are listed in Table 1.
Table 1.
Patients’ baseline characteristics (n = 43).
| Age, years | 64 (15) |
| Male/female | 15/28 |
| Hypertension | 24 |
| Diabetes mellitus | 8 |
| Congestive heart failure | 0 |
| History of stroke/transient ischemic attack | 2 |
| Atherosclerotic heart disease | 7 |
| Prosthetic heart valve | 3 |
| Preprocedural oral anticoagulation | |
| No oral anticoagulant | 9 |
| Warfarin | 31 |
| Novel oral anticoagulant | 3 |
Data are presented as median (interquartile range) or n.
The CHA2DS2 VASc score was calculated for each patient and was 0 in 7 patients (16.3%), 1 in 10 patients (23.3%), and ≥2 in 26 patients (60.5%). Seven of the patients with a CHA2DS2 VASc score of 1 were female and <65 years old.
Among all 43 patients, the median (IQR) total procedural time was 160 (60) min, the median minimum ACT value during the procedure was 261 (64) s, the median (IQR) LA size as measured by TTE was 4.3 (0.7) cm, and the median LAA velocity measured via transesophageal echocardiography was 39 (34.45) cm/s.
No overt neurological events indicative of cerebral emboli were observed during the post-RFA neurological examinations.
IPV was successfully performed in all patients. None of the patients developed catheter ablation-related major complications (e.g., vascular access complications, major bleeding, stroke or transient ischemic attack, or cardiac tamponade). Minor bleeding complications without the need for transfusion occurred in one patient.
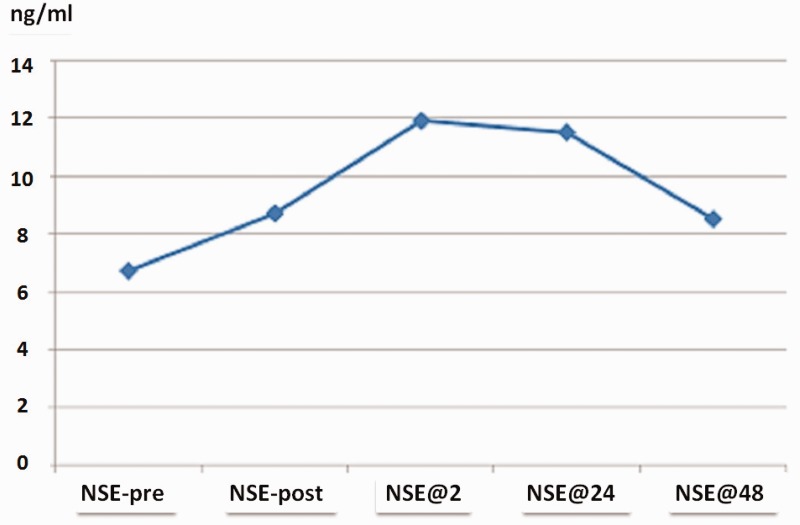
The serum NSE measurements are summarized in Figure 1. The median (IQR) NSE level among all 43 patients increased from 6.70 (3.87) ng/mL to 8.73 (5.76) ng/mL at the end of ablation, to 11.88 (4.33) ng/mL 2 h after ablation, to 11.48 (5.30) ng/mL at 24 h after ablation, and to 8.53 (5.18) ng/mL at 48 h after ablation. Increases in NSE exceeding the ULN occurred in 14 (32.6%) patients.
Figure 1.
Median NSE levels of 43 patients. The samples were collected before the procedure (NSE-pre), at the end of ablation (NSE-post), 2 h after ablation (NSE@2h), 24 h after ablation (NSE@24h), and 48 h after ablation (NSE@48h). NSE, neuron-specific enolase.
Table 2 shows the relevant clinical and echocardiographic variables of the study population. No significant differences were found between the increasedULN and stableULN groups in terms of age, sex, comorbidities (hypertension, diabetes mellitus, or history of cerebrovascular accident or atherosclerotic heart disease), LAA velocity, left-ventricular end-diastolic and end-systolic diameters, or a CHA2DS2 VASc score of ≥2. However, the left atrial parasternal diameters were significantly larger in the increasedULN than stableULN group (P = 0.02) (Table 2). Unexpectedly, the coexistence of diabetes mellitus was found to be associated with the absence of an increase exceeding the ULN of NSE (P = 0.04), but the reliability of this finding was limited due to the small number of patients.
Table 2.
Clinical and echocardiographic variables of the increasedULN NSE group and stableULN NSE group.
| StableULN NSE (n = 29) | IncreasedULN NSE (n = 14) | P value | |
|---|---|---|---|
| Female sex | 21 (72%) | 7 (50%) | 0.18 |
| Hypertension | 17 (58%) | 7 (50%) | 0.59 |
| Diabetes mellitus | 8 (27%) | – | 0.04* |
| History of stroke/transient ischemic attack | – | 2 (14%) | 0.10 |
| Atherosclerotic heart disease | 5 (17%) | 2 (14%) | 1.0 |
| CHA2DS2 VASc score of ≥2 | 18 (62%) | 8 (57%) | 0.76 |
| LA diameter, cm | 4.1 (0.62) | 4.5 (0.55) | 0.02* |
| LVEDD, cm | 4.95 (0.37) | 5 (0.6) | 0.75 |
| LVESD, cm | 2.9 (0.65) | 3 (0.2) | 0.32 |
| sPAP, mmHg | 30 (8.75) | 35 (20) | 0.19 |
| LAA velocity, cm/s | 39.2 (33.32) | 32 (44) | 0.86 |
Values are given as n (%) or median (interquartile range).
*P < 0.05
The CHA2DS2 VASc score is a composite risk score comprising congestive heart failure (1 point), hypertension (1 point), age of ≥75 years (2 points), diabetes (1 point), stroke (2 points), age of ≥65 years (1 point), vascular disease (1 point), and female sex (1 point).
NSE, neuron-specific enolase; ULN, upper limit of normal; LA, left atrium; LAA, left atrial appendix; LVEDD, left-ventricular end-diastolic diameter; LVESD, left-ventricular end-systolic diameter; sPAP, systolic pulmonary arterial pressure.
The univariate analysis to identify procedure-related predictors of an increase in NSE included the minimum ACT, minimum systolic and diastolic blood pressures, maximum systolic and diastolic blood pressures, preprocedural cardiac rhythm, electrical cardioversion, left atrial access time, and total procedural time. These procedural parameters were similar between the increasedULN and stableULN groups (Table 3).
Table 3.
Procedural parameters in the increasedULN NSE group and stableULN NSE group.
| StableULN NSE(n = 29) | IncreasedULN NSE(n = 14) | P value | |
|---|---|---|---|
| min. ACT, s | 251 (68.5) | 269 (53.75) | 0.16 |
| min. DBP, mmHg | 50 (7.5) | 50 (15) | 0.41 |
| min. SBP, mmHg | 80 (12.5) | 80 (31.25) | 0.85 |
| max. DBP, mmHg | 80 (17.5) | 80 (1.25) | 0.64 |
| max. SBP, mmHg | 140 (40) | 130 (37.5) | 0.57 |
| Total procedural time, min | 150 (57.5) | 150 (65) | 0.68 |
| Left atrial access time, min | 20 (12.5) | 15 (7.5) | 0.16 |
| Patients with preprocedural atrial fibrillation | 5 (17%) | 1 (7%) | 0.64 |
| Patients requiring electrical cardioversion | 5 (17%) | 6 (42%) | 0.13 |
| min. ACT of <250 s | 13 (44%) | 3 (21%) | 0.14 |
Values are given as n (%) or median (interquartile range).
NSE, neuron-specific enolase; ULN, upper limit of normal; ACT, activated clotting time; DBP, diastolic blood pressure; max., maximal; min., minimal; SBP, systolic blood pressure
Discussion
The present study shows the changes in the serum NSE level during the periprocedural period of AF ablation. An increase in the median (IQR) NSE level occurred after the procedure from a baseline value of 6.7 (3.87) ng/mL to 11.48 (5.3) ng/mL at 24 h. Additionally, an increase in the NSE level exceeding the ULN was observed in 14 (32.6%) of the 43 patients, and this proportion is similar to the incidence of subclinical embolism established in previous studies, in which the reported prevalence was 48%.4–8 We can speculate that an increase in the serum NSE level during IPV may be a sign of silent cerebral microembolism. We therefore investigated various factors that may be associated with an NSE increase. Patient-related factors are unchangeable but can be used for individualized risk assessment, and procedural factors can be modified.
In the present study, the CHA2DS2 VASc scores were similar between the two groups. All patients underwent RFA with effective anticoagulation. Previous studies have established the association of CHADS2/CHA2DS2 VASc scores with the occurrence of cerebrovascular accident during IPV,7,17 but several studies showed no relationship between these scores and procedure-related thromboembolism.4,18–20
Because our study patients had heterogeneous anticoagulation schemes, a statistical analysis between the NSE level and anticoagulation method was not performed.
Although Gaita et al.21 showed that the risk of subclinical cerebrovascular accident increased 2.75-fold in patients requiring cardioversion to obtain sinus rhythm during IPV, our findings regarding the association of cardioversion and the NSE level are in line with previous studies showing no relationship between cardioversion and silent embolism.4,22
In contrast to the study by Sramko et al.,9 who reported that a longer procedural time was associated with an increased incidence of thromboembolism, we found no association between the procedural time and increase in NSE. This supports previous studies showing that the procedural time did not affect the incidence of cerebral embolism in patients undergoing AF ablation.22,23
Similar to previous studies showing that cerebral embolic events during ablation are independent of age,4,22 we also found no correlation between age and an increase in NSE.
The most striking point of the present study was the significant relationship between a larger LA and an increase in NSE. This is similar to the results reported by Müller et al.7 and Sramko et al.,9 who showed that the LA diameter measured by TTE was significantly greater in patients with embolism. However there are also conflicting data displaying no link between stroke and LA diameter.19,23
Study limitations
The main limitation of our study was the absence of DW-MRI examination in the pre-ablation and post-ablation periods. Clinic cerebral embolism was investigated only by neurological examinations; neurocognitive tests could not be performed because of the patients’ low educational levels. Therefore, we cannot definitively conclude that subclinical neurologic damage was present in patients with an increased NSE level. Additionally, all procedures were performed under general anesthesia; therefore, anesthesia-related neuronal damage could not be distinguished. Another limitation was the absence of a control group. Finally, the reliability of our results is limited by the small number of patients.
Conclusion
To the best of our knowledge, this study is the first to interpret the effect of AF ablation on the serum NSE level, which is a surrogate marker of neuronal damage. Our study showed that the NSE level increased in most of the patients undergoing IPV and exceeded the ULN in at least one-third of the patients. Obtaining a further understanding of the correlation between the serum NSE level and RFA-related cerebral embolism will require a large number of patients and the performance of DW-MRI. Our findings will serve as a baseline for future investigations of the clinical usefulness and cut-off value of this biomarker for prediction of poor neurological outcomes after IPV. These preliminary results indicate that patients with a large LA diameter may have an increased risk of cerebral embolism during IPV.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by Ankalab Laboratory (Ankara, Turkey) as a promotional campaign of this laboratory. The serum neuron-specific enolase levels were measured by Ankalab Laboratory free of charge.
References
- 1.Camm AJ, Lip GY, De Caterina R, et al. An update of the 2010 ESC Guidelines for the management of atrial fibrillation Developed with the special contribution of the European Heart Rhythm Association. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J 2012; 33: 2719–2747. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130: e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3: 32–38. [DOI] [PubMed] [Google Scholar]
- 4.Haeusler KG, Koch L, Herm J, et al. 3 Tesla MRI-detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. J Cardiovasc Electrophysiol 2013; 24: 14–21. [DOI] [PubMed] [Google Scholar]
- 5.Müller P, Halbfass P, Szöllösi A, et al. Impact of periprocedural anticoagulation strategy on the incidence of new-onset silent cerebral events after radiofrequency catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2016; 46: 203–211. [DOI] [PubMed] [Google Scholar]
- 6.von Barry C, Deneke T, Arentz T, et al. Silent serebral events as a result of left atrial catheter ablation do not cause neuropsychological sequelae—a MRI-controlled multicenter study. J Interv Card Electrophysiol 2015; 43: 217–226. [DOI] [PubMed] [Google Scholar]
- 7.Müller P, Maier J, Dietrich JW, et al. Association between left atrial low-voltage area, serum apoptosis, and fibrosis biomarkers and incidence of silent cerebral events after catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2015: 44; 55–62. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K, Naito S, Sasaki T, et al. Silent Cerebral Ischemic Lesions After Catheter Ablation of Atrial Fibrillation in Patients on 5 Types of Periprocedural Oral Anticoagulation - Predictors of Diffusion-Weighted Imaging-Positive Lesions and Follow-up Magnetic Resonance Imaging. Circ J 2016; 80: 870–877. [DOI] [PubMed] [Google Scholar]
- 9.Sramko M, Peichl P, Wichterle D, et al. A novel biomarker-based approach for the detection of asymptomatic brain injury during catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2014; 25: 349–354. [DOI] [PubMed] [Google Scholar]
- 10.Wunderlich MT, Ebert AD, Kratz T, et al. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke 1999; 30: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 11.Rech TH, Vieira SR, Nagel F, et al. Serum neuron-specific enolase as early predictor of outcome after in-hospital cardiac arrest: a cohort study. Crit Care 2006; 10: R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann M, Ebert AD, Galazky I, et al. Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke 2000; 31: 645–650. [DOI] [PubMed] [Google Scholar]
- 13.González-García S, González-Quevedo A, Fernández-Concepción O, et al. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem 2012; 45: 1302–1307. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad O, Wardlaw J, Whiteley WN. Correlation of levels of neuronal and glial markers with radiological measures of infarct volume in ischemic stroke: a systematic review. Cerebrovasc Dis 2012; 33: 47–54. [DOI] [PubMed] [Google Scholar]
- 15.Baranyi A, Rothenhäusler HB. The impact of S100b and persistent high levels of neuron-specific enolase on cognitive performance in elderly patients after cardiopulmonary bypass. Brain Inj 2013; 27: 417–424. [DOI] [PubMed] [Google Scholar]
- 16.Natale A, Raviele A, Arentz T, et al. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol 2007; 18: 560–580. [DOI] [PubMed] [Google Scholar]
- 17.Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014; 129: 2638–2644. [DOI] [PubMed] [Google Scholar]
- 18.Scaglione M, Blandino A, Raimondo C, et al. Impact of ablation catheter irrigation design on silent cerebral embolism after radiofrequency catheter ablation of atrial fibrillation: results from a pilot study. J Cardiovasc Electrophysiol 2012; 23: 801–805. [DOI] [PubMed] [Google Scholar]
- 19.Martinek M, Sigmund E, Lemes C, et al. Asymptomatic cerebral lesions during pulmonary vein isolation under uninterrupted oral anticoagulation. Europace 2013; 15: 325–331. [DOI] [PubMed] [Google Scholar]
- 20.Kuwahara T, Takahashi A, Takahashi Y, et al. Prevention of periprocedural ischemic stroke and management of hemorrhagic complications in atrial fibrillation ablation under continuous warfarin administration. J Cardiovasc Electrophysiol 2013; 24: 510–515. [DOI] [PubMed] [Google Scholar]
- 21.Gaita F, Caponi D, Pianelli M, et al. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation 2010; 122: 1667–1673. [DOI] [PubMed] [Google Scholar]
- 22.Wissner E, Metzner A, Neuzil P, et al. Asymptomatic brain lesions following laserballoon-based pulmonary vein isolation. Europace 2014; 16: 214–219. [DOI] [PubMed] [Google Scholar]
- 23.Lickfett L, Hackenbroch M, Lewalter T, et al. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation. J Cardiovasc Electrophysiol 2006; 17: 1–7. [DOI] [PubMed] [Google Scholar]