Short abstract
Objective
Previous studies comparing surgical pleth index (SPI)-guided and conventional analgesia have shown differing results. Therefore, we compared the intraoperative opioid requirement, extubation time, postoperative pain scores, and perioperative adverse events between these two modalities.
Methods
A comprehensive literature search was conducted to identify randomized controlled trials comparing the intraoperative opioid requirement and other outcomes between the two modalities. The mean difference (MD) or the pooled risk ratio and corresponding 95% confidence interval (CI) were used for analysis. A heterogeneity (I2) assessment was performed.
Results
Six randomized controlled trials comparing 463 patients were included. Intraoperative opioid consumption was significantly lower in the SPI-guided than conventional analgesia group (standardized MD, −0.41; 95% CI, −0.70 to −0.11; I2 = 53%). No significant intergroup difference was observed in the pain score on the first postoperative day or the incidence of perioperative adverse events. The extubation time was considerably shorter in the SPI-guided than conventional analgesia group (MD, −1.91; 95% CI, −3.33 to −0.49; I2 = 67%).
Conclusions
Compared with conventional analgesia, SPI-guided analgesia can reduce intraoperative opioid consumption and facilitate extubation. Moreover, no intergroup difference was observed in the degree of postoperative pain or incidence of perioperative adverse events.
Keywords: Analgesia, analgesics, opioid, anesthesia, general, hemodynamics, photoplethysmography
Introduction
Individualized and situationally fitted intraoperative opioid administration by nociception–antinociception balance monitoring helps to avoid opioid overtreatment, opioid-induced hyperalgesia, and adverse effects and shortens the arousal time from anesthesia.1,2 However, a specialized monitoring device that depicts the nociception–antinociception balance during general anesthesia has not yet been developed. Various monitoring parameters and devices have been described, such as the analgesia nociception index (MDoloris Medical Systems, Loos, France), which is derived from electrocardiography-based heart rate variability; devices that detect skin conductance (Stress Detector; Med-Storm Innovation AS, Oslo, Norway) or reflex pupillary dilatation (AlgiScan; IDMED, Marseille, France); the surgical pleth index (SPI), which was previously termed the “surgical stress index”; the autonomic nervous system state; and the autonomic nervous system state index, which is derived from finger photoplethysmography results.2–12 Hamunen et al.8 reported that parameters derived from finger photoplethysmography appear to be suitable for monitoring autonomic nervous system activation. Among them, clinical application of the SPI has been more frequently reported than other devices.5–7,10,12,13
The SPI is used to monitor nociceptive stimuli and antinociceptive drug effects during surgery using the pulse photoplethysmographic amplitude and heart rate interval derived from pulse oximetry measurements.14 SPI-guided analgesia appears to effectively provide adequate analgesia during general anesthesia based on several studies that have shown a good response to the SPI following the administration of various opioids.1,3,15,16 A recent study showed that in critically ill patients with polytrauma, SPI monitoring added to entropy could decrease the incidence of anesthesia-related complications by reducing consumption of noradrenaline and opioids.17 Nevertheless, because the clinical utility of the systems used for monitoring the nociception–antinociception balance remains controversial,18 some unsolved and debated issues associated with several potential limiting factors or clinical situations are still problematic, such as the age of the patient population,16,19–21 type of anesthesia regimen, and concomitant use of catecholamines (such as atropine) or vasoactive drugs.22–24 Moreover, considering the differences in the reported intraoperative opioid requirement and the degree of postoperative pain between SPI-guided and conventional (standard clinical practice) analgesia as indicated by hemodynamic parameters,15,16,19,21,25,26 there is a need to investigate whether SPI-guided analgesia is more beneficial than conventional analgesia. Therefore, we sought to quantitate its effectiveness, summarize the available evidence, and provide a higher level of evidence through a meta-analysis of existing randomized controlled trials.
We aimed to compare the intraoperative opioid requirement (primary endpoint) and the degree of postoperative pain, extubation time, and perioperative adverse events such as postoperative nausea and vomiting (PONV) and intraoperative hemodynamic or somatic (body movement) events (secondary endpoints) between patients undergoing SPI-guided and conventional analgesia during surgery by general anesthesia. This was accomplished by performing a systematic review of randomized controlled trials comparing the intraoperative opioid requirement and the degree of postoperative pain after SPI-guided and conventional analgesia during surgery in patients who underwent surgery by general anesthesia. We hypothesized that intraoperative opioid requirement would be lower in those who received SPI-guided analgesia than in those who received conventional analgesia and the degree of postoperative pain would be comparable between the two analgesic methods.
Methods
This systematic review and meta-analysis was performed using existing literature and did not involve new human data. Thus, the study was exempt from institutional review board assessment. We searched multiple comprehensive databases for literature regarding the SPI and intraoperative opioid requirement. The study protocol was based on the Cochrane Review Methods.
Database and literature sources
We searched MEDLINE (1 February 2017), EMBASE (1 February 2017), the Cochrane Controlled Trials Register and Cochrane Database on Systematic Reviews (1 February, 2017), Web of Science (1 February 2017), and Scopus (1 February 2017) and databases of randomized controlled trials that compared SPI-guided and conventional analgesia in patients who underwent general anesthesia during surgery. The following keywords were used for the search of each database: “general anesthesia,” “plethysmography,” “analgesia,” “surgical pleth index,” and “surgical stress index.” The databases were also searched using the Medical Subject Heading terms “Anesthesia, General,” “Photoplethysmography,” “Plethysmography,” “Analgesics, Opioid,” and “Hemodynamics” as well as the free-text terms “anesthesia,” “surgical stress index,” “surgical pleth index,” “surgical plethysmographic index,” “SPI,” “SSI,” “photoplethysmography,” “plethysmography,” “opioid analgesics,” “opioid agonists,” “opioid,” and “hemodynamics,” After the initial electronic search, we evaluated the bibliographies from all identified studies and performed a manual search using Google Scholar. To identify unpublished or ongoing studies, we searched the World Health Organization International Clinical Trials Registry Platform and the ClinicalTrials.gov websites. The articles identified were assessed individually for inclusion in the analysis.
Study selection
A decision regarding the inclusion of studies in the analysis was made independently by two reviewers (BG Lim and YJ Won). Studies were selected after they were subjected to two levels of screening. At the first level, we screened the titles and abstracts of the identified studies. At the second level, we screened the full texts. Discrepancies between the reviewers were resolved by discussion. Studies meeting the following criteria were included in our meta-analysis: (1) studies involving patients who underwent general anesthesia during surgery, (2) studies comparing the surgical stress index or SPI-guided analgesia and conventional analgesia (standard clinical practice), and (3) studies involving assessment of the intraoperative opioid requirement and degree of postoperative pain as evaluated with the numerical rating scale (NRS) for pain, extubation time, and adverse events including hemodynamic or somatic events or PONV as the main outcomes.
Data extraction
The two reviewers independently extracted data from each study using a predefined data extraction form. Any disagreement unresolved by discussion was resolved in consultation with a third reviewer (H Kim).
The following variables were extracted from the studies: (1) mean and standard deviation of the intraoperative opioid requirement, the NRS scores (1–10) for pain and extubation time as continuous variables, and dichotomous variables including the incidence of adverse events in the surgical stress index or SPI-guided analgesia and conventional analgesia groups (for studies that reported the median and range, the mean was assumed to be equal to the range divided by 4); (2) demographic and clinical characteristics (e.g., age, sex, number of patients in the two different analgesic groups, and type of surgery); (3) type of opioid and intervention protocol; (4) first author, country, and year of publication; and (5) method of assessment. If the above variables were not found in the articles, we requested the data from the authors via email.
Assessment of methodological quality
The two reviewers independently assessed the methodological quality of each study using the Cochrane Collaboration’s tool for assessing the risk of bias. This tool is widely used to assess the methodological quality of randomized controlled trials and consists of the following six items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. The risk of bias was classified into three categories: high, low, or unclear. Any disagreements between the reviewers were resolved through discussion or by the third reviewer.
Statistical analysis
The primary outcome of the systematic review was the intraoperative opioid requirement in the SPI-guided and conventional analgesia groups. The secondary outcomes were the degree of postoperative pain measured using the NRS for pain, extubation time defined as the time period from termination of the anesthetic agent to extubation, and the incidences of perioperative adverse events including PONV and intraoperative unwanted somatic movement measured as the number of patients (PONV) or the number of adverse events occurring in the patients (body movement). As another secondary outcome, the incidence of intraoperative hemodynamic events such as hypertension (an increase in mean arterial pressure [MAP] to >120% of the initial value before anesthesia) and tachycardia (an increase in heart rate [HR] to >120% of the initial value before anesthesia) was measured as the number of events occurring among the total number of MAP or HR measurements during anesthesia in all patients.
We used RevMan version 5.3 (The Cochrane Collaboration, Oxford, UK) for the meta-analysis. Continuous variables including the intraoperative opioid requirement and NRS scores for pain and extubation time were analyzed using the mean difference (MD) with 95% confidence interval (CI). An MD with a 95% CI of <0 indicated that such values were lower in the assessed group than in another group. Dichotomous variables such as the incidence of adverse events were analyzed using the pooled risk ratio and 95% CI by the Mantel–Haenszel method. Fixed- and random-effects models were used with both types of variables to estimate the treatment effect. Each analysis was assessed for statistical heterogeneity using Cochran’s Q test and I2 statistics. For the I2 statistics, the proportion of between-study inconsistency due to true differences between the studies rather than differences due to random error or chance was determined; values of >50% were considered to have significant heterogeneity. A P-value of <0.1 for Cochran’s Q test was considered statistically significant. If the P-value was >0.1 and I2 was <50%, a fixed-effects model was used; otherwise, a random-effects model was used.27
Initially, subgroup analyses were pre-planned to compare the different types of anesthetic agents (inhalational vs. intravenous) or opioids (remifentanil vs. others) because several studies were identified for each subgroup analysis. Sensitivity analysis and publication bias (used for at least 10 studies) were not analyzed because of the small number of included studies.
Results
Identification of studies
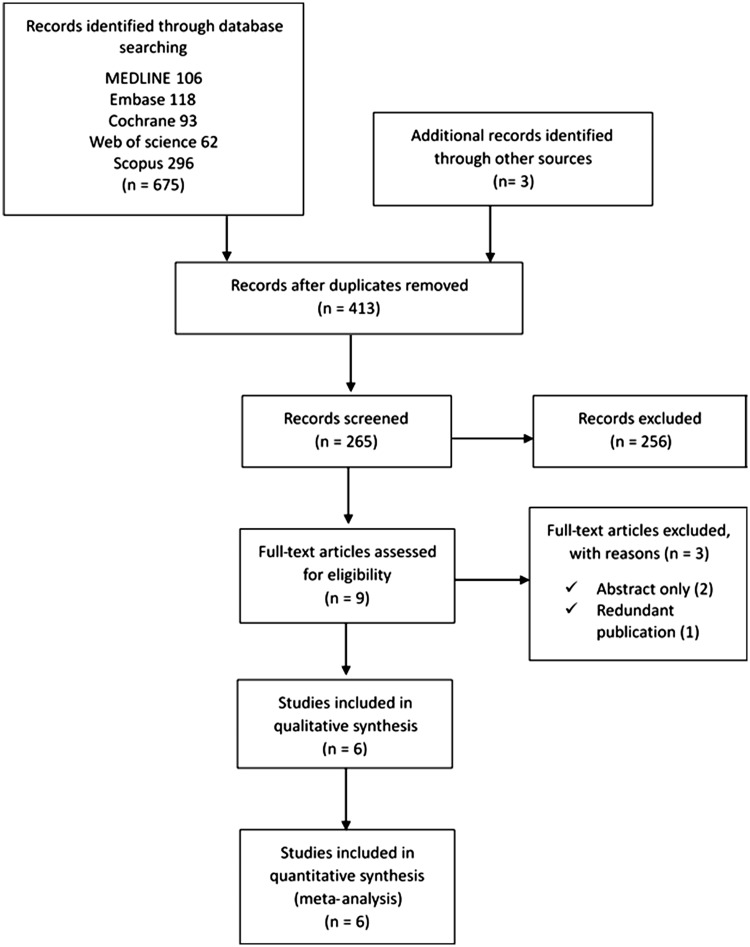
Searches of the databases yielded 678 articles (Figure 1). Of these, 669 publications were excluded because it was clear from the title and abstract that they did not fulfill the selection criteria. We obtained the full manuscripts of the remaining nine articles; these were scrutinized to identify six potentially relevant articles. Three articles were excluded for the following reasons: two were published abstracts only and one was a redundant publication. Therefore, six studies were included in the systematic review15,16,19,21,25,26 (Figure 1).
Figure 1.
Flow chart of the meta-analysis.
Study characteristics and patient populations
In these six studies, 463 patients (SPI-guided analgesia group, n = 232; conventional analgesia group, n = 231) were evaluated for opioid consumption during general anesthesia. Two studies were conducted in Asian countries and four were conducted in European countries. The characteristics of the selected studies are presented in Table 1. The trial sizes ranged from 45 to 151 patients. The patients underwent thyroidectomy (n = 1), orthopedic surgery (n = 1), laparoscopic cholecystectomy (n = 1), gynecological surgery (n = 1), and ear-nose-throat surgery (n = 2). One study included children aged 3 to 10 years. Three studies evaluated adult patients excluding older patients aged >65 years, and two studies evaluated adult patients including older patients aged ≤75 years. In three studies, general anesthesia was maintained with an inhaled anesthetic (sevoflurane), and in the other three studies, general anesthesia was maintained with an intravenous anesthetic (propofol). The opioids administered for intraoperative analgesia were intravenous remifentanil, which was continuously infused in three studies, and intravenous oxycodone, sufentanil, and fentanyl, which were administered by bolus in one study each. All studies reported the intraoperative opioid requirement. The SPI-guided analgesia group was defined in all studies as the group in which the opioid was administered when the SPI value was >50 during surgery. The control group was defined in five studies as the group in which the opioid was administered when the MAP was >120% of the baseline value (or 100 mmHg) or when the HR was >120% of the baseline value (or >90 beats/minute). In the study by Bergmann et al.,15 the opioid dose was adjusted according to clinical parameters in the control group. Of the secondary outcomes, postoperative pain as measured by the NRS score, PONV, and time to extubation was reported in four studies. Won et al.16 checked the incidence of PONV during the first postoperative hour in the recovery room, Park et al.19 checked it during the stay in the recovery room, Bergmann et al.15 checked it for the first 2 postoperative days, and Chen et al.25 checked it in the recovery room and on the first postoperative day. For postoperative pain as measured by the NRS, three studies checked the NRS score on a scale of 0 to 10, while one study25 used a scale of 0 to 100. Two studies checked the NRS score on the first operative day,15,25 and one study measured it 24 hours after surgery.26
Table 1.
Characteristics of included studies.
| Study ID | Journal | Setting, country | ASA class | Age, years | Opioid | Postoperative analgesics | Anesthetic agent | Sex (M/F) |
Type of surgery | |
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||
| Won et al. 2016 | Medicine | Korea University Guro Hospital, South Korea | I–II | 20–65 | Oxycodone | Same as intraoperative analgesics | Sevoflurane | 4/19 | 4/18 | Elective thyroidectomy |
| Bergmann et al. 2013 | British Journal of Anaesthesia | University of Göttingen Medical School, Germany | I–III | 18–75 | Remifentanil | Intra-articular injection, 5 mg bupivacaine 0.5% + 4 mg dexamethasone | Propofol | 50/26 | 54/21 | Elective outpatient orthopedic surgery |
| Colombo et al. 2015 | Minerva Anesthesiologica | University of Milan, Italy | I–II | 18–50 | Remifentanil | Tramadol 100 mg + acetaminophen 1 g | Propofol | 14/16 | 13/17 | Elective laparoscopic cholecystectomy |
| Gruenewald et al. 2014 | British Journal of Anaesthesia | University Hospital Schleswig Holstein Campus Kiel, Germany | I–II | 18–65 | Sufentanil | 1000 mg metamizole or 1000 mg acetaminophen intravenously | Sevoflurane | 27/15 | 27/13 | Elective gynecological and orthopedic procedures |
| Chen et al. 2010 | Anesthesiology | University Hospital Schleswig Holstein Campus Kiel, Germany | I–II | 18–70 | Remifentanil | 0.1 mg/kg piritramide | Propofol | 13/27 | 21/19 | Elective ear-nose-throat surgery expected to last at least 1 h |
| Park et al. 2015 | Anesthesiology | Korea University Guro Hospital, South Korea | I | 3–10 | Fentanyl | Same as intraoperative analgesics | Sevoflurane | 12/9 | 11/13 | Elective adenotonsillectomy |
ASA: American Society of Anesthesiologists, M: male, F: female, Intervention: surgical pleth index-guided analgesia group, Control: conventional analgesia group
Quality of the included studies (risk-of-bias assessment)
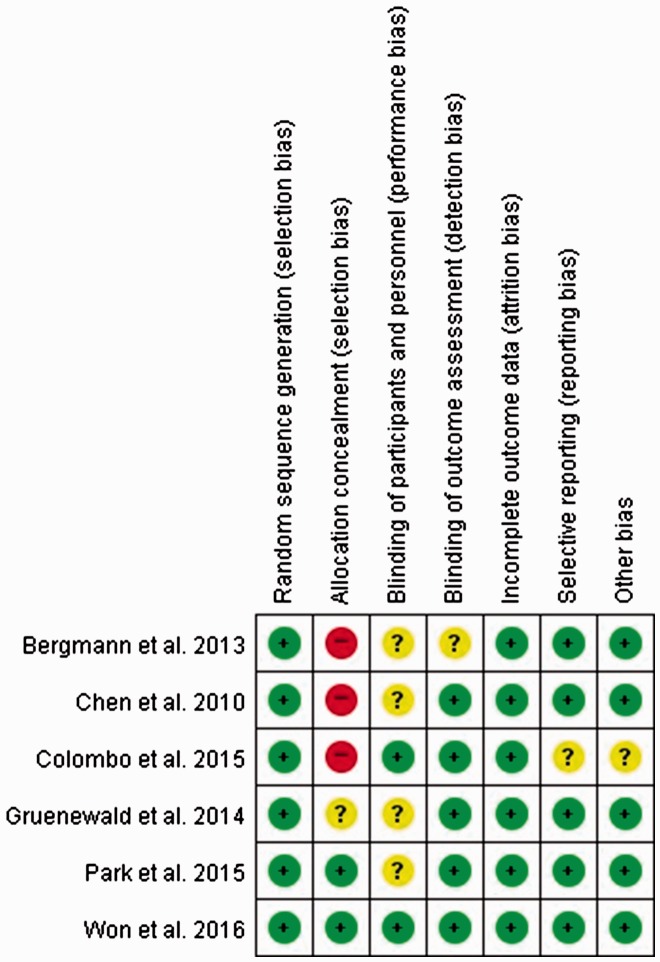
The risk of bias was assessed as shown in Figure 2.
Figure 2.
Risk-of-bias summary: review of authors’ judgments about each risk-of-bias item for each included study. Green circle: low risk of bias; yellow circle: unclear risk of bias; red circle: high risk of bias.
Randomization and allocation
All six studies were randomized. However, only two studies revealed the methods used for allocation concealment.16,19
Blinding
In four studies, blinding of the study performers was not definitively described,15,19,21,25 and one study did not definitively describe the blinding of the outcome assessors.15 The other two studies reported the blinding of the study performers and outcome assessors.16,26
Incomplete outcome data, selective reporting, and other potential sources of bias
All studies except one26 were assessed as having a low risk of bias.
Result 1: primary outcome
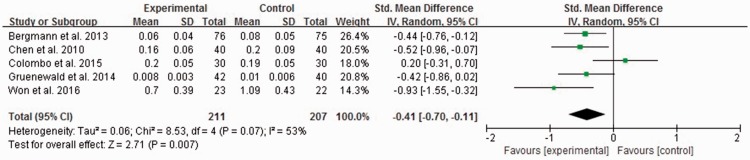
Intraoperative opioid consumption was significantly lower in the SPI-guided analgesia group than in the conventional analgesia group, but the heterogeneity was high (standardized MD, −0.41; 95% CI, −0.70 to −0.11; I2 = 53%) (Figure 3).
Figure 3.
Intraoperative opioid requirement in five study groups (unit: µg/kg/minute). The experimental group is the surgical pleth index (SPI)-guided analgesia group, and the control group is the conventional analgesia group. CI = confidence interval, IV = inverse variance, SD = standard deviation.
Result 2: secondary outcomes
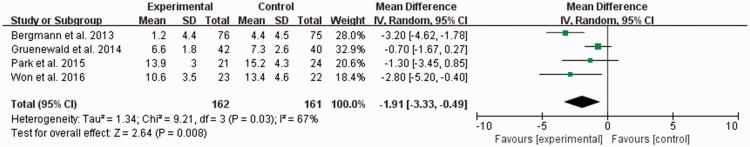
The extubation time was significantly lower in the SPI-guided analgesia group than in the conventional analgesia group, but the heterogeneity was relatively high (MD, −1.91; 95% CI, −3.33 to −0.49; I2 = 67%) (Figure 4).
Figure 4.
Extubation time (minutes). The experimental group is the surgical pleth index (SPI)-guided analgesia group, and the control group is the conventional analgesia group. CI = confidence interval, IV = inverse variance, SD = standard deviation.
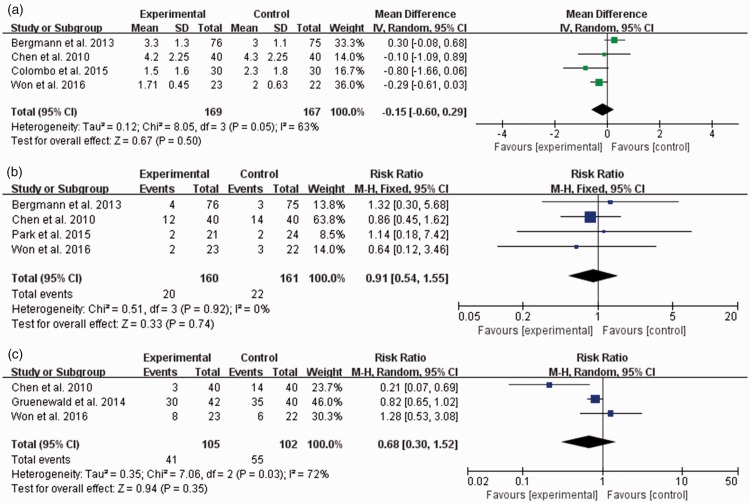
The NRS scores for pain were compared to assess the postoperative pain severity on the first postoperative day, and no significant intergroup difference was observed (MD, −0.15; 95% CI, −0.60 to 0.29; I2 = 63%) (Figure 5(a)).
Figure 5.
(a) Numerical rating scale (NRS) score for pain at postoperative 24 hours and incidences of (b) postoperative nausea and vomiting and (c) intraoperative unwanted somatic movement. The experimental group is the surgical pleth index (SPI)-guided analgesia group, and the control group is the conventional analgesia group. CI = confidence interval, IV = inverse variance, SD = standard deviation, M-H = Mantel–Haenszel method. Data of “Events” in panel (b) are given as the number of patients who had postoperative nausea and vomiting. Data of “Events” in panel (c) are given as the number of adverse events occurring in the patients in each group.
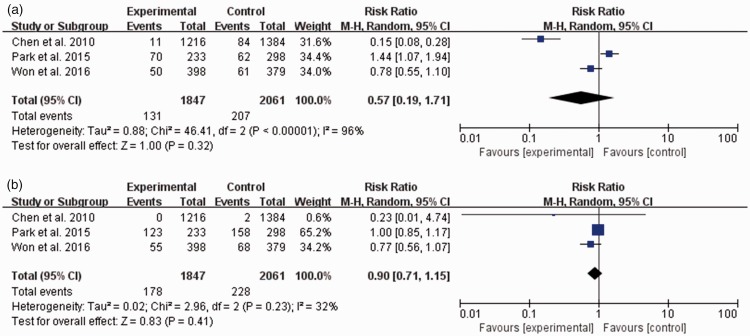
Among the perioperative adverse events, the incidences of PONV and unwanted somatic movement during surgery were not different between the two groups (PONV: pooled risk ratio, 0.91; 95% CI, 0.54–1.55; I2 = 0%; somatic movement: pooled risk ratio, 0.68; 95% CI, 0.30–1.52; I2 = 72%) (Figure 5(b) and (c)). The incidences of hemodynamic adverse events including hypertension or tachycardia during surgery were not significantly different between the two groups (hypertension: pooled risk ratio, 0.57; 95% CI, 0.19–1.71; I2 = 96%; tachycardia: pooled risk ratio, 0.90; 95% CI, 0.71–1.15; I2 = 32%) (Figure 6).
Figure 6.
Incidences of intraoperative hemodynamic adverse events. (a) Hypertension. (b) Tachycardia. The experimental group is the surgical pleth index (SPI)-guided analgesia group, and the control group is the conventional analgesia group. CI = confidence interval, M-H = Mantel–Haenszel method. Hypertension: an increase in arterial pressure to >120% of the initial value before anesthesia. Tachycardia: an increase in heart rate to >120% of the initial value before anesthesia. Data of “Events” in each figure are given as the number of events examined in all noninvasive blood pressure or heart rate measurements during anesthesia in the patients in each group.
Subgroup analysis
In this systematic review, we used the standardized MD for opioids to compare the intraoperative opioid requirement among studies that administered different kinds of opioids (fentanyl, remifentanil, sufentanil, or oxycodone) for intraoperative analgesia. Our analysis showed that the opioid consumption was significantly lower in the SPI-guided analgesia group than in the conventional analgesia group (standardized MD, −0.41; 95% CI, −0.70 to −0.11; I2 = 53%). Thus, we performed a subgroup analysis with the same kind of opioid administration. Three studies in which remifentanil was administered for analgesia were included in the subgroup analysis.15,25,26 Although no significant difference was observed in remifentanil consumption between the two analgesia groups, a tendency toward lower remifentanil consumption was noted in the SPI-guided analgesia group (MD, −0.02; 95% CI, −0.04 to 0.01; I2 = 64%). We also performed a subgroup analysis to compare the effect of different types of anesthetic agents (inhalational vs. intravenous). Three studies in which propofol was used as the main anesthetic agent were included in the above subgroup analysis with remifentanil;15,25,26 hence, the analysis with propofol showed the same result regarding opioid consumption. For the subgroup analysis performed with the two studies in which sevoflurane was used as the main anesthetic agent,16,21 a significant difference was observed in opioid consumption between the two analgesia groups, and lower opioid consumption was noted in the SPI-guided analgesia group (standardized MD, −0.61; 95% CI, −1.15 to −0.32; I2 = 52%).
Discussion
The main focus when assessing the superiority of SPI-guided analgesia should be whether it provides effective perioperative analgesia with less intraoperative hemodynamic complications and postoperative pain and reduced opioid-related adverse events, including delayed emergence or PONV, by reducing intraoperative opioid consumption. Although several previous studies have reported relevant results, they used various opioids (including remifentanil, fentanyl, sufentanil, or oxycodone) as analgesics for pain control during surgery. Moreover, these studies produced conflicting results regarding intraoperative opioid consumption,25,26 extubation time,16,21 and postoperative pain.19,25 Therefore, we aimed to provide a high level of evidence for the clinical effectiveness of SPI-guided analgesia by comparing the intraoperative opioid requirement as a primary endpoint and the postoperative pain score, extubation time, and perioperative adverse events such as intraoperative hemodynamic or somatic events or PONV as secondary endpoints between the SPI-guided and conventional analgesia groups undergoing surgery by general anesthesia in an systematic review with meta-analysis that summarized the results of carefully designed and relevant studies.
We found that intraoperative opioid consumption was significantly lower in the SPI-guided analgesia group. However, a subgroup analysis including only the three studies that used remifentanil15,25,26 showed no significant difference in intraoperative remifentanil consumption between the two analgesia groups; it only showed a tendency for lower remifentanil consumption in the SPI-guided analgesia group. The subgroup analysis also failed to clearly reduce the heterogeneity caused by the use of various opioids. This contradictory result and unchanged heterogeneity in the subgroup analysis of the three studies were probably due to the small number of included studies and the differences in the types of surgeries performed (i.e., orthopedic surgery, laparoscopic cholecystectomy, and ear-nose-throat surgery) (Table 1). In other words, summation of the results from only three studies (including the positive results from two studies15,25 and the negative result from one study26) and the different degrees and sites of pain resulting from the different types of surgery could have led to the lack of a significant difference in remifentanil consumption between the two analgesia groups as well as the moderately high heterogeneity. Thus, further research on the types of opioids, surgeries, and patient populations similar to those of the previous studies is needed to confirm these results and reduce the heterogeneity.
Among the secondary endpoints, the extubation time was shorter in the SPI-guided analgesia than conventional analgesia group, and the postoperative pain score and incidence of perioperative adverse events were comparable between the two analgesia groups despite the fact that a lower amount of opioid was required in the SPI-guided analgesia group. Therefore, SPI-guided analgesia can be considered a more effective analgesic method during general anesthesia because it enables the use of a lower amount of opioid to induce a comparable analgesic effect and facilitates extubation.
This systematic review is limited by the moderately high heterogeneous data of several outcomes. This could have been caused by the differences in the types of surgery, anesthetics used, and postoperative analgesic protocols of the included studies. Therefore, further research needs to be performed, and future systematic reviews should aim to reduce the heterogeneity of the outcome data. Another limitation of this systematic review was that we could not assess the publication bias because the sample size was small (<10 studies).
In conclusion, SPI-guided analgesia can reduce intraoperative opioid consumption and shorten the extubation time when compared with conventional analgesia during surgery under general anesthesia and has no negative impact on the degree of postoperative pain and incidence of perioperative adverse events. We suggest that the use of SPI guidance is more useful and practical than conventional analgesia for analgesic titration during surgery under general anesthesia in various clinical circumstances.
Systematic review registration
This systematic review was registered in the PROSPERO registry (unique number: CRD42017042989; principal investigator’s name: Young Ju Won; date of registration: July 14, 2017).
Authors’ contributions
Study design/planning: YJW and BGLStudy conduct: YJW, BGL, ML, YSK, and HKData collection: YJW, BGL, ML, and YSKData analysis: YJW, BGL, and HKWriting of the first draft and revision of the paper: YJW and BGLConfirmation of the final paper: all authors
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by a Korea University Grant awarded to Dr. Byung Gun Lim from Korea University (Seoul, Republic of Korea).
References
- 1.Bonhomme V, Uutela K, Hans G, et al. Comparison of the surgical pleth index with haemodynamic variables to assess nociception-anti-nociception balance during general anaesthesia. Br J Anaesth 2011; 106: 101–111. [DOI] [PubMed] [Google Scholar]
- 2.Ledowski T, Burke J, Hruby J. Surgical pleth index: prediction of postoperative pain and influence of arousal. Br J Anaesth 2016; 117: 371–374. [DOI] [PubMed] [Google Scholar]
- 3.Struys MM, Vanpeteghem C, Huiku M, et al. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br J Anaesth 2007; 99: 359–367. [DOI] [PubMed] [Google Scholar]
- 4.Constant I, Sabourdin N. Monitoring depth of anesthesia: from consciousness to nociception. A window on subcortical brain activity. Paediatr Anaesth 2015; 25: 73–82. [DOI] [PubMed] [Google Scholar]
- 5.Edry R, Recea V, Dikust Y, et al. Preliminary intraoperative validation of the nociception level index: a noninvasive nociception monitor. Anesthesiology 2016; 125: 193–203. [DOI] [PubMed] [Google Scholar]
- 6.Gruenewald M, Herz J, Schoenherr T, et al. Measurement of the nociceptive balance by analgesia nociception index and surgical pleth index during sevoflurane-remifentanil anesthesia. Minerva Anestesiol 2015; 81: 480–489. [PubMed] [Google Scholar]
- 7.Gruenewald M, Ilies C, Herz J, et al. Influence of nociceptive stimulation on analgesia nociception index (ANI) during propofol-remifentanil anaesthesia. Br J Anaesth 2013; 110: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 8.Hamunen K, Kontinen V, Hakala E, et al. Effect of pain on autonomic nervous system indices derived from photoplethysmography in healthy volunteers. Br J Anaesth 2012; 108: 838–844. [DOI] [PubMed] [Google Scholar]
- 9.Paloheimo MP, Sahanne S, Uutela KH. Autonomic nervous system state: the effect of general anaesthesia and bilateral tonsillectomy after unilateral infiltration of lidocaine. Br J Anaesth 2010; 104: 587–595. [DOI] [PubMed] [Google Scholar]
- 10.Funcke S, Sauerlaender S, Pinnschmidt HO, et al. Validation of innovative techniques for monitoring nociception during general anesthesia: a clinical study using tetanic and intracutaneous electrical stimulation. Anesthesiology 2017; 127: 272–283. [DOI] [PubMed] [Google Scholar]
- 11.Ledowski T, Ang B, Schmarbeck T, et al. Monitoring of sympathetic tone to assess postoperative pain: skin conductance vs surgical stress index. Anaesthesia 2009; 64: 727–731. [DOI] [PubMed] [Google Scholar]
- 12.Sabourdin N, Barrois J, Louvet N, et al. Pupillometry-guided intraoperative remifentanil administration versus standard practice influences opioid use: a randomized study. Anesthesiology 2017; 127: 284–292. [DOI] [PubMed] [Google Scholar]
- 13.Thee C, Ilies C, Gruenewald M, et al. Reliability of the surgical Pleth index for assessment of postoperative pain: a pilot study. Eur J Anaesthesiol 2015; 32: 44–48. [DOI] [PubMed] [Google Scholar]
- 14.Huiku M, Uutela K, van Gils M, et al. Assessment of surgical stress during general anaesthesia. Br J Anaesth 2007; 98: 447–455. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann I, Gohner A, Crozier TA, et al. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br J Anaesth 2013; 110: 622–628. [DOI] [PubMed] [Google Scholar]
- 16.Won YJ, Lim BG, Lee SH, et al. Comparison of relative oxycodone consumption in surgical pleth index-guided analgesia versus conventional analgesia during sevoflurane anesthesia: a randomized controlled trial. Medicine (Baltimore) 2016; 95: e4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogobete AF, Sandesc D, Cradigati CA, et al. Implications of entropy and surgical pleth index-guided general anaesthesia on clinical outcomes in critically ill polytrauma patients. A prospective observational non-randomized single centre study. J Clin Monit Comput 2018; 32: 771–778. [DOI] [PubMed] [Google Scholar]
- 18.Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, et al. Monitoring of nociception, reality or fiction? Rev Esp Anestesiol Reanim 2017; 64: 406–414. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Lim BG, Kim H, et al. Comparison of surgical pleth index-guided analgesia with conventional analgesia practices in children: a randomized controlled trial. Anesthesiology 2015; 122: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 20.Harju J, Kalliomaki ML, Leppikangas H, et al. Surgical pleth index in children younger than 24 months of age: a randomized double-blinded trial. Br J Anaesth 2016; 117: 358–364. [DOI] [PubMed] [Google Scholar]
- 21.Gruenewald M, Willms S, Broch O, et al. Sufentanil administration guided by surgical pleth index vs standard practice during sevoflurane anaesthesia: a randomized controlled pilot study. Br J Anaesth 2014; 112: 898–905. [DOI] [PubMed] [Google Scholar]
- 22.Ahonen J, Jokela R, Uutela K, et al. Surgical stress index reflects surgical stress in gynaecological laparoscopic day-case surgery. Br J Anaesth 2007; 98: 456–461. [DOI] [PubMed] [Google Scholar]
- 23.Won YJ, Lim BG, Yeo GE, et al. The effect of nicardipine on the surgical pleth index during thyroidectomy under general anesthesia: a prospective double-blind randomized controlled trial. Medicine (Baltimore) 2017; 96: e6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hocker J, Broch O, Grasner JT, et al. Surgical stress index in response to pacemaker stimulation or atropine. Br J Anaesth 2010; 105: 150–154. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Thee C, Gruenewald M, et al. Comparison of surgical stress index-guided analgesia with standard clinical practice during routine general anesthesia: a pilot study. Anesthesiology 2010; 112: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 26.Colombo R, Raimondi F, Rech R, et al. Surgical pleth index guided analgesia blunts the intraoperative sympathetic response to laparoscopic cholecystectomy. Minerva Anestesiol 2015; 81: 837–845. [PubMed] [Google Scholar]
- 27.Higgins JPT, Green S. and Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Oxford: Cochrane Collaboration, 2011. [Google Scholar]