Abstract
Background:
Walnut green husk (WGH) extract has been known as potential preventive and therapeutic antioxidants and antimicrobials due to its high polyphenol content. In this study, preparation of spray dried WGH extract-loaded microcapsules by maltodextrin and its blending with two other natural biodegradable polymers, pectin, or alginate were investigated.
Methods:
In this study, encapsulation efficiency (EE), total phenol content (Folin–Ciocalteu reagent method), antioxidant (DPPH scavenging assay) and antimicrobial activities (agar well diffusion method) structural (SEM and FTIR studies), and release properties of WGH extract-loaded microcapsules were investigated.
Results:
High retention of phenolic content in microcapsules indicated the successful encapsulation of WGH extract. Addition of biopolymers to maltodextrin matrix has a positive effect on EE and other properties of microcapsules. The microcapsules prepared with mixture of maltodextrin and pectin had higher EE (79.35 ± 0.87%) and total phenolic (TP) content (56.83 ± 1.04 mg gallic acid equivalents [GAE]/100 g) in comparison to maltodextrin and alginate mixture (EE: 75.21 ± 0.24%, TP content: 54.33 ± 1.53 mg GAE/100 g) and maltodextrin only matrix (EE: 72.50 ± 1.00%, TP content: 50.67 ± 1.35 mg GAE/100 g). Extract-loaded microcapsules also showed nearly spherical structure, good antioxidant (with the percentage DPPH inhibition ranged from 75.17 ± 1.42% to 80.87 ± 2.29%), and antimicrobial properties (with mean inhibition diameter zone ranged from 7.76 ± 0.86 mm to 11.53 ± 0.45 mm). Fourier transform infrared analyses suggested the presence of extract on microcapsules. The in vitro extract release from microcapsules followed an anomalous non-Fickian diffusion mechanism with almost complete release.
Conclusions:
WGH extract microcapsules can be used as novel and economic bioactive phytochemical and therapeutic agents to prevent oxidation and microbial activity.
Keywords: Antioxidants, Juglans, microencapsulation, polyphenols
Introduction
Development of alternative pharmaceuticals compounds without side effect is more necessary than before.[1] The use of extracts rich in naturally health promoting polyphenols compounds that are produced from different plants and biowastes is gaining increasing interest in the food industry. These compounds are effective in the treatment of diseases and have biological effects such as anti-ulcerogenic, cytotoxic, analgesic, antineoplastic, hypolipidemic, antidiabetic effects, and modulating immune activity.[2,3] The consumption of these plant-derived products has been related to a lower risk of diseases caused by oxidative stress due to their antioxidant properties.[4]
Walnut (Juglans regia L.) as an important crop is commonly cultivated worldwide for its valuable nutrition properties and consumer acceptability. Green husk of walnut is a by-product generated in the walnut harvest, which is considered as an economic source of phenolic compounds with antioxidant. In addition, it has shown an important preventive effect against different microorganisms.[5,6]
However, chemical stability of plant polyphenols is affected by a variety of factors such as temperature, light, and presence of oxygen and easily decomposes.[7]
An applied strategy to improve the stability and organoleptic properties of polyphenol-rich extracts is conversion of liquid extract into encapsulated powder by spray drying with suitable polymers. The spray-drying method is one of the most important strategies for the synthesis of spherical particles which involves solvent evaporation and self-assembly of matrix materials inside the droplet system. The selection of the appropriate coating agent is crucial for successful encapsulation in spray-drying process. One of the most commonly used biopolymers as carrier is maltodextrin.[8] It has number of advantages including low cost, low viscosity at high concentrations, flavor-binding ability, and reduction of oxygen permeability of coating matrix. However, the problem associated with the use of different types of maltodextrins as wall material is their low glass transition temperatures that lead to formation of crystal under increasing temperature conditions and consequently induce disruption of wall matrix integrity and develop agglomeration of encapsulated powder.[4,9,10] Blending with other biopolymers such as pectin, gum arabic, gelatin, and alginate is an alternative approach to improve functional and structural properties of maltodextrin matrixes. Pectin and alginate are of interest as biopolymeric coats due to their nontoxicity, biodegradability, and film-forming properties and their advantages as delivery system for sensitive compounds.[4,11]
This study aimed to develop walnut green husk (WGH) extract-loaded microcapsules by spray-drying technique. The effect of maltodextrin and its combination with alginate and pectin as coating material on encapsulation efficiency (EE), total phenol content, antioxidant, and antimicrobial activities of WGH extract-loaded microcapsules were investigated. The microcapsules were characterized by scanning electron microscope (SEM) and Fourier transform infrared (FTIR) studies. Moreover, the in vitro release behaviors of samples were studied.
Methods
Materials
Green walnuts were collected from Tarasht region (Tehran, Iran). Maltodextrin (DE = 18–22), sodium alginate (molecular mass of 197 kDa), and pectin with high methoxylation degree were purchased from Food Chem Co. (Japan), BDH Co. (Poole, UK), and Danisco Co. (Copenhagen, Denmark), respectively. All other reagents used were of analytical grade.
Preparation of walnut green husk extract
The WGH was placed on a metal tray in a dark place and dried by a household electric fan for 72 h. The dried husks were grounded three times and then sieved. The husk powder was stored in glass tubes at ambient temperature until use. The extract was obtained according to the procedure described previously.[5] The aqueous extract was then transferred into plastic plates and frozen at −80°C for 30 min and finally freeze-dried at −80°C and 0.001 mbar for 14 h (ALPHA 2-4; Christ, Harz, Germany).
Encapsulation of walnut green husk extract by spray drying
Microencapsulation process was done by spray-drying technique according to modified procedure.[4] Maltodextrin, maltodextrin–pectin (MD-pectin), and maltodextrin–alginate (MD-alginate) mixtures were used as carriers to encapsulate the extract.
Briefly, a maltodextrin solution (10% (w/v)) was prepared in distilled water under magnetic stirring for 1 h to complete hydration of maltodextrin. WGH powdered extract was added to maltodextrin solution to reach a 10:1 carrier:extract weight ratio. The mixture was stirred for another 20 min. Then, the solutions were spray-dried by a spray dryer (BÜCHI, Mini Spray Dryer B-290, Germany) under the following conditions:inlet temperature 120°C, outlet temperature 77–75°C, feed flow rate 6.6 mL/min, nozzle diameter 5–8 mm, dry air flow rate 42–45 L/h, aspirator 90%, and pump rate 20% (5.0 mL/min). Finally, microcapsules were collected and weighed.
For preparation of the MD-pectin and MD-alginate microcapsules containing the WGH extract, 2.0 g pectin or alginate was dissolved in 200 mL distilled water at room temperature. Then, 100 mL of the pectin or alginate solution was mixed with 100 mL of the 20% (w/v) maltodextrin solution to reach maltodextrin:each biopolymer ratio of 10:1 (w/w). The solutions were stirred for 30 min and then the extract was added (carrier-to-extract ratio of 10:1 w/w). The drying process was done according to the abovementioned conditions.
Determination of yield and encapsulation efficiency
The ratio of the amount of the produced microcapsules to the amount of the initial solid material of the feed solution is expressed as process yield.[7]
Microencapsulation efficiency was calculated according to the following equation:[12]
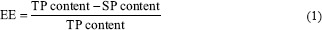
Where TP and SP contents are the total and surface phenolic content of microcapsules, respectively.
Total phenolic and surface phenolic content of microcapsules
The extraction of the surface and total phenolic (TP) compounds of microcapsules was done by the ethanol–methanol (1:1) and methanol–acetic acid–water (50:8:42, v/v/v) solvents, respectively, according to the method described in the literature.[13] The phenolic content of resulting solutions was determined by the Folin–Ciocalteu reagent method and the results expressed as milligram of gallic acid equivalents (GAE) per 100 g of dried extract or microcapsules.[14]
Determination of antioxidant activity
Antioxidant activity of samples was determined by DPPH (2,2-diphenyl-1-picrylhydrazine) scavenging assay and the radical scavenging activity of the samples was calculated according to the following equation:[15]
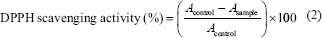
Where Acontrol and Asample are the absorbance of the control and sample, respectively.
Determination of antimicrobial activity
For determination of antimicrobial activity of extract-loaded microcapsules, the lyophilized Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028, ATCC 2592 Staphylococcus aureus, and Bacillus cereus PTCC 1154 were provided from Iranian Research Organization for Science and Technology (Tehran, Iran). The bacterial cultures were then prepared from abovementioned bacteria under sterilized conditions and stored at −20°C until use.
The antimicrobial activity of the samples was evaluated by the agar well-diffusion method described previously.[16] The inhibition diameter zone was expressed as millimeter and was considered as an indicator of antimicrobial activity.
Scanning electron microscopy
The morphology of the microcapsules was evaluated using SEM (Oxford Instruments INCA Penta, FET× X3). Microcapsules were attached to the specimen aluminum holder and sputtered with gold for 5 min. SEM images were obtained at the required magnification at ambient temperature and an acceleration voltage of 26 kV.
Fourier transform-infrared spectroscopy
The FTIR spectra of the microcapsules were recorded at 400–4000 cm−1 by spectrophotometer (Perkin Elmer Spectrum RX I, USA). The dried samples were mixed with KBr, compressed to make pellets, and then scanned at a resolution of 4 cm−1.
Differential scanning calorimetry
The DSC thermograms of the microcapsules were obtained by a differential scanning calorimetry instrument (Shimadzu Co., Japan). For this purpose, 6–12 mg of the samples were attached on aluminum pans and heated at temperature range from 20 to 300°C under nitrogen atmosphere with flow rate of 30 mL/min and at scanning rate of 10°C/min.
In vitro release studies
In vitro release of WGH extract from microcapsules was measured by dispersing the microcapsules (500 mg) in 25 mL of distilled water at ambient temperature and stirring. At particular time intervals, 5 mL of the dispersion was taken and replaced with an equivalent volume of distilled water. The aliquots were centrifuged at 9000 rpm for 5 min. TP content of the supernatant was measured byFolin–Ciocalteu method. The cumulative percentage of extract released was plotted against time in minute.
To predict the release behavior of the extract from the biopolymeric matrices, the experimental release data were fitted to Korsmeyer and Peppas semi-empirical model:[16]

Where mt and m∞ are the amount of extract released at time t and equilibrium time; k is the release rate constant; and n is the release exponent that could be used to indicate the mechanism of release.
Statistical analysis
Statistical analysis of the obtained data was performed using SPSS software. Analysis of variance followed by the Bonferroni post hoc test was used to show significant differences between the samples at P < 0.05.
Results and Discussion
Microencapsulation process yield, total phenolic and surface phenolic content, and encapsulation efficiency
The process yield in all three samples was higher than 50% without any significant difference between them [Table 1]. It has been reported that recovery above 50% is considered as a criterion of an effective drying process.[17]
Table 1.
Encapsulation yield, encapsulation efficiency, surface phenolic content, total phenolic content, and 2,2-diphenyl-1-picrylhydrazine-scavenging activity of walnut green husk extract-loaded microcapsules
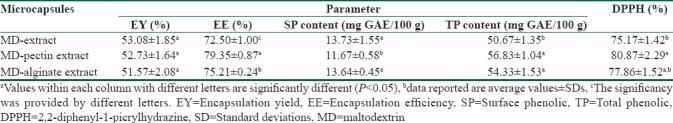
The TP content of powder extract-loaded microcapsules ranged from 50.67 ± 1.35 to 56.83 ± 1.04 mg GAE/100 g of dry powder [Table 1]. It was observed that the addition of pectin or alginate significantly increased the TP content (P < 0.05). The highest value was for MD-pectin microcapsules. Similar results were also observed by Dag et al.[12] who reported an increase in TP content of encapsulated golden berry juice powder with MD-pectin, MD-alginate, and MD-gum arabic in comparison to MD as control. In the case of SP content of microcapsules, it is important to reduce the phenolic content of surface to improve the stability, sensory, and release properties of polyphenol-rich extracts. The lowest content of SP was obtained for MD-pectin microcapsules (11.67 ± 0.57 mg GAE/100 g).
Efficiency of encapsulation is an important parameter that would be indicated capability of carrier matrix to encapsulate active compounds. The EE of samples was significantly influenced by the composition of wall material used. The results showed that the addition of pectin or alginate at 1% in maltodextrin matrix significantly increases the EE of microcapsules (P < 0.05). EE increased from 72.5 ± 1.00% in maltodextrin microcapsules to 79.35 ± 0.87% and 75.21 ± 0.24% in MD-pectin and MD-alginate microcapsules, respectively. Higher EE with the mixture of pectin or alginate with maltodextrin was interpreted to be due to the effect that these biopolymers had in the stabilization of the maltodextrin matrix. This improvement in entrapment efficiency of active compounds in maltodextrin microcapsules with the addition of other biopolymers was also observed in previous studies.[4,12] In blending of pectin or alginate with maltodextrin, maltodextrins mainly act as a matrix forming and pectin or alginate serve as coating agents.
Antioxidant activity
The scavenging activity of all microcapsules against DPPH radical was higher than 75% [Table 1]. It was observed that the addition of pectin to maltodextrin led to significant increase in antioxidant activity of microcapsules compared the sample prepared with MD-alginate and maltodextrin only (80.87 ± 2.29%). The results also indicated that the microcapsules with stronger free radical-scavenging effect showed the highest TP content, being established a positive relation between the antioxidant activity and TP content.
It has been established that phenolic materials in polyphenol-rich extracts are responsible for the extracts' antioxidant activity. Green husk of walnut is rich in phenolics compounds which contribute to its antioxidant activity.[5] The results of TP content and DPPH values showed a similar trend in all treatments. These findings suggest that phenolic compounds have a major role to antioxidant capacity in the WGH extract. This relation between TP content and antioxidant ability has been reported.[12,14]
Antimicrobial activity
The microcapsules containing WGH extract were screened for their antimicrobial activities against S. aureus, B. cereus, E. coli, and S. typhimurium. Unloaded microcapsules were also evaluated separately and there was no inhibition zone around them for all of the tested microorganisms. In the case of loaded microcapsules, the response for each tested bacteria was different. The microcapsule prepared with maltodextrin only exhibited antimicrobial activities, with mean inhibition diameter zone of 9.17 ± 0.65 and 7.76 ± 0.86 mm against S. aureus, B. cereus, respectively, but could not inhibit E. coli and S. typhimurium. Addition of pectin or alginate to maltodextrin matrix resulted in an increase in inhibition diameter zone. MD-pectin microcapsules showed the largest inhibition diameter zones for S. aureus and B. cereus [Table 2]. On the other hand, gram-negative bacteria showed the highest resistance (no inhibition zone). Similarly, Oliveira et al. have reported that S. aureus was the most susceptible microorganism to walnut (J. regia L.) green husks aqueous extracts of five different cultivars.[5] Generally, the more sensitivity of the gram-positive to the gram-negative bacteria may be due to presence of only an outer peptidoglycan layer of gram-positive bacteria which is not an effective permeability barrier. The antimicrobial power of plant extracts, caused mainly by their phenolic components. Juglone has been introduced as the most important phenolic compound of WGH and has been known for its antimicrobial effect. In a recent study, antibacterial activity of Juglone against S. aureus was evaluated. The authors reported that juglone effectively enhanced the protein expression levels of oxidoreductase and created a peroxidative environment in the S. aureus cell, decreasing cell wall formation and increasing membrane permeability, remarkably.[18]
Table 2.
The results of agar well diffusion antimicrobial test of walnut green husk extract-loaded microcapsules

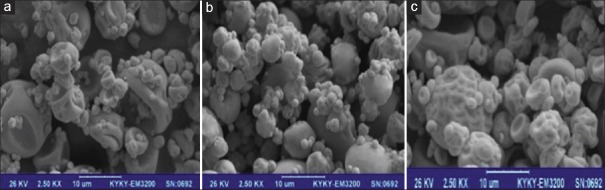
Morphology
The surface morphology of the extract-loaded microcapsules was studied by SEM [Figure 1]. All the samples [Figure 1a–c] showed nearly spherical structures without any crystal on the surface. Absence of crystal or aggregate could be due to solubilization of WGH extract in the polymeric matrix.[7] Incorporation of pectin to maltodextrin matrix resulted in smoother surface [Figure 1b]. This is probably due to formation of pectin film on the surface of microcapsules which caused a reinforcement of the maltodextrin matrix. A similar finding was observed by Sansone et al. who proposed the two-sided MD-pectin as more suitable carrier for the extracts compared to maltodextrin alone with a physical protection for polyphenols, in which pectin and MD act as coating and matrix-forming materials, respectively.[4]
Figure 1.
Scanning electron micrograph of walnut green husk extract-loaded maltodextrin microcapsules (a), extract-loaded maltodextrin–pectin microcapsules (b), and extract-loaded maltodextrin–alginate microcapsules (c)
Fourier transform infrared analyses
The FTIR of unloaded maltodextrin microcapsules [Figure 2] exhibited characteristic peaks at 3393 cm−1 (O–H stretching vibrations), 2928 cm−1 (C–H stretching vibrations), 1647 cm−1 (C=O stretching vibrations), 1416 cm−1 (–CH2 stretching vibrations), 1369 cm−1 (O–H bending vibrations), 1154 and 1022 cm−1 (C–O stretching vibration). This spectra pattern of MD has been reported previously.[19] The addition of pectin and alginate to MD formulation caused a decrease in the wave number of hydroxyl group from 3393 cm−1 to 3369 and 3368 cm−1 in MD-pectin and MD-alginate microcapsules, respectively. These changes could be attributed to an increase in the hydrogen bonding between biopolymers. In spectrums of WGH extract loaded-microcapsules, there were almost no changes compared to unloaded samples. This might be suggesting that no new chemical bonds are formed between the extract and the matrix, and the extract is just physically entrapped in the biopolymeric network. On the other hand, because of low ratio of the extract to wall materials, the weak absorbance shifts might be covered by the strong peaks of the carrier materials.[19]
Figure 2.
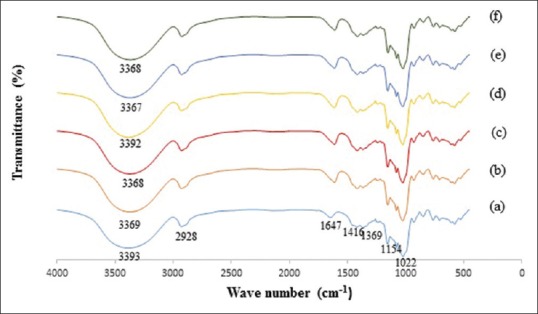
Fourier transform infrared spectra of maltodextrin (a), maltodextrin–pectin (b), maltodextrin–alginate (c), walnut green husk extract loaded-maltodextrin microcapsules (d), extract-loaded maltodextrin–pectin microcapsules (e), and extract-loaded maltodextrin–alginate microcapsules (f)
Release studies
The release studies of the extract from polymeric matrixes were carried out to observe differences in the release profile of microcapsules [Figure 3]. The release of extract was started at the beginning of test and reached to steady state after 50 min with complete dissolution of microcapsules. The total content of extract almost completely released from all the microcapsules, suggesting no interaction between the carriers and extract. The microcapsules composed of maltodextrin and pectin permitted a relatively low and sustained release of extract, compared to two other microcapsules, although MD-alginate microcapsules also showed similar trend to MD-pectin with higher release rate. It could be concluded that the presence of pectin or alginate in maltodextrin matrix reduced the release of extract.
Figure 3.
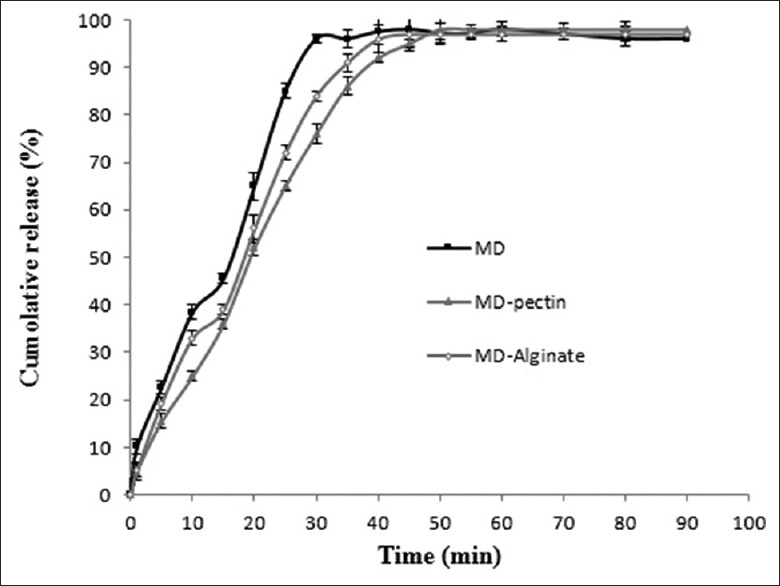
In vitro release profile walnut green husk extract from microcapsules. Error bars represent the standard deviation (n = 3)
In order to find out the mechanism of WGH extract release, the cumulative release data were fitted to the Korsmeyer and Peppas model. The model exhibited acceptable correlations with experimental results (R2 > 92%). Among samples, MD-pectin microcapsules showed the lowest value of release rate constant (0.818 s−1) and microcapsules prepared with maltodextrin only showed the highest release rate (1.052 s−1). In addition, all three microcapsules had the diffusion release index between 0.43 and 0.85 (0.544, 0.676, and 0.716 for maltodextrin, MD-alginate, and MD-pectin microcapsules, respectively), which implied the samples followed an anomalous non-Fickian diffusion release mechanism, with a swelling effect.[20] The observations indicated that the release of WGH from microcapsules occurred in a controlled manner. Therefore, these polymeric systems are suitable as a controlled release carrier, and combining it with WGH extract can be used as antimicrobial and antioxidant agent in pharmaceutical industry and also could improve shelf life of food products.
Conclusions
WGH extract-loaded microcapsules were successfully prepared by spray-drying technique. Maltodextrin and its blending with pectin and alginate were used as carrier agents. The characteristics of the microcapsules were affected by type of coating materials. An increase in EE and TP content of microcapsules was achieved by addition of pectin and alginate to maltodectrin matrix. Extract-loaded microcapsules also showed potential antioxidant and antimicrobial activities. Characterization of the powders by SEM and FTIR revealed the loading of extract to microcapsules. The experiment results of release kinetics showed an anomalous non-Fickian diffusion behavior for all samples. In addition, fortified yogurt with extract-loaded microcapsules showed sensory properties similar to control. These results prove the possibility of using of WGH extract-loaded microcapsules as natural antioxidant and antimicrobial agents in food and pharmaceutical products.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This article was a part of MS dissertation at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Hosseini MS, Shahbazizadeh S, Khosravi-Darani K, Mozafari MR. Spirulina paltensis: Food and function. Curr Nutr Food Sci. 2013;9:189–93. [Google Scholar]
- 2.Ganeshpurkar A, Saluja AK. Protective effect of catechin on humoral and cell mediated immunity in rat model. Int Immunopharmacol. 2018;54:261–6. doi: 10.1016/j.intimp.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Ganeshpurkar A, Saluja AK. Protective effect of rutin on humoral and cell mediated immunity in rat model. Chem Biol Interact. 2017;273:154–9. doi: 10.1016/j.cbi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Sansone F, Mencherini T, Picerno P, d'Amore M, Aquino RP, Lauro MR. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J Food Eng. 2011;105:468–76. [Google Scholar]
- 5.Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L, Pereira JA, et al. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46:2326–31. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Agullóa A, Pereirab E, Freire MS, Valentão P, Andrade PB, González-Álvarez J, et al. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod. 2013;42:126–32. [Google Scholar]
- 7.Klein T, Longhini R, Bruschi ML, de Mello JC. Microparticles containing guaraná extract obtained by spray-drying technique: Development and characterization. Rev Bras Farmacogn. 2015;25:292–300. [Google Scholar]
- 8.Ravichandran K, Palaniraj R, Saw NM, Gabr AM, Ahmed AR, Knorr D, et al. Effects of different encapsulation agents and drying process on stability of betalains extract. J Food Sci Technol. 2014;51:2216–21. doi: 10.1007/s13197-012-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villacrez JL, Carriazo JG, Osorio C. Microencapsulation of andes berry (Rubus glaucus Benth.) aqueous extract by spray drying. Food Bioprocess Technol. 2014;7:1445–56. [Google Scholar]
- 10.Bae EK, Lee SJ. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J Microencapsul. 2008;25:549–60. doi: 10.1080/02652040802075682. [DOI] [PubMed] [Google Scholar]
- 11.Maisuthisakul P, Gordon MH. Influence of polysaccharides and storage during processing on the properties of mango seed kernel extract (microencapsulation) Food Chem. 2012;134:1453–60. doi: 10.1016/j.foodchem.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Dag D, Kilercioglu M, Oztop MH. Physical and chemical characteristics of encapsulated goldenberry (Physalis peruviana L.) juice powder. LWT Food Sci Technol. 2017;83:86–94. [Google Scholar]
- 13.Robert P, Gorena T, Romero N, Sepulveda E, Chavez J, Saenz C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int J Food Sci Technol. 2010;45:1386–94. [Google Scholar]
- 14.Shojaee-Aliabadi S, Hosseini SM, Tiwari B, Hashemi M, Fadavi G, Khaksar R. Polyphenols content and antioxidant activity of Ghure (unripe grape) marc extract: Influence of extraction time, temperature and solvent type. Int J Food Sci Technol. 2013;48:412–8. [Google Scholar]
- 15.Delfan-Hosseini S, Nayebzadeh K, Mirmoghtadaie L, Kavosi M, Hosseini SM. Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chem. 2017;222:61–6. doi: 10.1016/j.foodchem.2016.11.150. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini SM, Hosseini H, Mohammadifar MA, Mortazavian AM, Mohammadi A, Khosravi-Darani K, et al. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int J Biol Macromol. 2013;62:582–8. doi: 10.1016/j.ijbiomac.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari BR, Datta N, Howes T. Problems associated with spray drying of sugar-rich foods. Drying Technol. 1997;15:671–84. [Google Scholar]
- 18.Wang J, Cheng Y, Wu R, Jiang D, Bai B, Tan D, et al. Antibacterial activity of juglone against staphylococcus aureus: From apparent to proteomic. Int J Mol Sci. 2016;17:965. doi: 10.3390/ijms17060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian G, Li Y, Yuan Q, Cheng L, Kuang P, Tang P, et al. The stability and degradation kinetics of sulforaphene in microcapsules based on several biopolymers via spray drying. Carbohydr Polym. 2015;122:5–10. doi: 10.1016/j.carbpol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Serrano-Cruz MR, Villanueva-Carvajal A, Rosales EJ, Dávila JF, Dominguez-Lopez A. Controlled release and antioxidant activity of roselle (Hibiscus sabdariffa L.) extract encapsulated in mixtures of carboxymethyl cellulose, whey protein, and pectin. LWT Food Sci Technol. 2013;50:554–61. [Google Scholar]