Abstract
Introduction:
The objective of this study was to classify dyspneic patients and to evaluate outcome variables on the basis of lung ultrasound (LUS) and arterial blood gas (ABG) findings.
Methods:
We performed a retrospective chart-based review in which we included patients with dyspnea admitted to our intensive care unit (ICU) between March 2015 and August 2016. On the basis of LUS (presence of A-lines/B-lines) and ABG (hypoxia/hypercarbia), patients were classified into six groups: (i) metabolic defect (dry lung, no hypoxia); (ii) perfusion defect (dry lung, hypoxia); (iii) ventilation defect (dry lung, hypoxia, and hypercarbia); (iv) ventilation and alveolar defect (wet lung, hypoxia, and hypercarbia); (v) alveolar defect-consolidation ([wet lung] hypoxia, no echocardiographic [ECG] abnormality); (vi) alveolar defect-pulmonary edema (wet lung [usually bilateral], hypoxia, ECG abnormality). The patient's demographic data, sequential organ failure assessment (SOFA) score, need for intubation, vasopressors, form of mechanical ventilation, ICU outcome, and length of stay were noted.
Results:
A total of 244 out of 435 patients were eligible for inclusion in the study. The median age was 56 years. 132 patients (54.1%) required mechanical ventilation, and median SOFA score was 7. Noninvasive ventilation was required in 87.5% of patients with ventilation defect as compared to 9.2% with alveolar defect-consolidation (P < 0.0001). We had 21.7% mortality in patients with alveolar defect-consolidation, 10.8% mortality in patients with metabolic defect, and 8.7% mortality in patients with alveolar defect-pulmonary edema (P < 0.0001).
Conclusion:
This classification gives an organized approach in managing patients with dyspnea. It predicts that patients with alveolar defect-consolidation are most sick of all the groups and need immediate intervention.
Keywords: A-lines, arterial blood gas analysis, B-lines, lung ultrasound
INTRODUCTION
Dyspnea is the most common presenting symptom among critically ill patients.[1] Various etiologic classifications of dyspnea focus on history, physical examination, chest radiography, or electrocardiography (ECG) to classify patients in cardiovascular, respiratory, or metabolic categories.[1,2] Such classifications are difficult to interpret in critically ill patients, due to overlapping symptoms and signs or lack of them in acutely developing conditions.[3] In the previous decade, elevated B-type natriuretic peptide was considered sensitive and was a specific marker to distinguish cardiovascular causes of acute dyspnea, but this biomarker is elevated in a variety of noncardiac critical conditions, including sepsis.[4,5] Further, etiologies of dyspnea are more diverse, and an objective, minimally-invasive, less expensive, and rapid assessment tool for the evaluation of dyspnea is required for critically ill patients.
Development and expansion of lung ultrasound (LUS) have helped in the classification of acute dyspnea.[6] This technique is rapid, is noninvasive, and has no additional expense in settings where a bedside ultrasound is available. It is an extension of clinical examination and can distinguish between a dry and a wet lung field. In combination with cardiac ultrasound, it was possible to distinguish pulmonary and cardiac causes of dyspnea in one study.[6] Arterial blood gas (ABG) analysis has been used in intensive care units (ICUs) to make treatment decisions.[7] While they have a limited utility in distinguishing different causes of dyspnea, they do provide valuable insights into pathophysiology of various pulmonary processes, such as ventilation, oxygenation, or perfusion defects. The ease with which ABG can be measured in critical care units with such a capability has made it mainstay in the evaluation of a breathless patient.[8]
We performed this study to evaluate if a combined LUS- and ABG-based algorithm can discriminate between different causes of dyspnea. Information from these two sequential modalities can be used to indicate dominant pathophysiology responsible for dyspnea, and together information from these modalities is available within 1st h of admission to a critical care facility.
METHODS
Design
We performed a retrospective chart-based review of all patients admitted to the ICU at a tertiary care institute in Central India. Institutional Human Ethics Committee approved the study and a waiver of consent from individual patients was sought.
Setting
The study was conducted in a ten-bedded closed medical and surgical unit. It is equipped with bedside portable ultrasound machines (Sonosite) and blood gas analyzer (Roche-Cobas b 221). As per the ICU protocol, all patients brought to the ICU are immediately assessed for vitals (heart rate, respiratory rate [RR], noninvasive blood pressure, ECG, oxygen saturation), peripheral lines are secured, and an arterial blood sample is drawn. As immediate management is initiated, an LUS with ECG and inferior vena cava (IVC) assessment is performed by one of the three trained intensivists available in the unit. All investigation findings, treatment decisions, and diagnosis are recorded on the ICU treatment charts.
Inclusion and information sources
We enlisted all patients who were admitted to the ICU between March 2015 and August 2016 and obtained their ICU treatment charts. We sought to include patients who were admitted with dyspnea (defined as RR recorded as 20 bpm or more at the time of admission). Duration of ICU stay of 24 h or longer was required for inclusion, assuming that complete diagnostic information would have been received till this time. We excluded patients if information about on-admission LUS or echo or ABG report was not available from the treatment chart.
Study procedures
We collected information about patient demographics (age, gender), type of illness based on final diagnosis, severity of illness, length of stay, need for intubation, form of mechanical ventilation, central venous catheter (CVC), need of vasopressor, ICU outcome, and primary admission source. We collected information about the LUS patterns and ABG values to classify patients in different pathophysiological categories as described later.
Ultrasonography protocol
As per the standard ultrasonography (USG) protocol in the ICU, the following views are obtained as part of standard of care:
Lung views
The linear probe with frequency of 5–8 Hz was used for lung. As described in BLUE protocol, i.e., the upper BLUE-point, lower BLUE-point, and PLAPS-point were assessed.[9] Fundamental ultrasonographic signs were considered multiple B-lines, subpleural consolidations, air bronchograms, and lung sliding.
Cardiac views
A cardiac-phased array 2–4-MHz probe was used for the study of the heart through the subcostal, parasternal long–axis, and the apical four-chamber views. A subcostal four-chamber view was examined for fluid collection within the pericardial sac, right atrium/right ventricle (RV) diastolic collapse, left ventricle (LV) impaired function by visual estimation of gross wall contraction and wall thickening, or LV hyperkinesia with impaired filling, RV dilation, and visual estimation of impaired function. At least one of the other two cardiac views was used in case of doubtful diagnosis, difficult visualization, and confirmation of RV dilation. The parasternal long-axis view was examined for pericardial effusion, visual estimation of qualitative LV function, and signs of RV dilation (RV/LV end-diastolic diameter [0.7]). The apical four-chamber view was examined for pericardial effusion, qualitative LV function, and signs of RV dilation (RV/LV end-diastolic diameter).
Inferior vena cava view
The subcostal view was used for long-axis visualization of the proximal IVC to measure maximum diameter and estimate the percent of respiratory collapsibility in spontaneously breathing patients. The same probe used for cardiac views was used. All measurements were made no <2 cm caudal from the junction of the right atrium. In spontaneously breathing patients, 50% of collapsibility was indicative of hypovolemia.
Study definitions
We defined A-pattern (dry lung) if all of the scanned LUS areas showed A-lines. A-lines are repetitive horizontal artifacts arising from the pleural line generated by subpleural air. The absence of multiple B-lines with regular sliding is the “A pattern,” which is a sign of normally aerated or hyperinflated lungs and rules out pulmonary edema.
B-pattern (wet lung) was defined if two or more scanned LUS areas showed B-lines. The B-line is the name given to an artifact with seven features: a hydroaeric comet-tail artifact; arising from the pleural line; hyperechoic; well defined; spreading up indefinitely; erasing A-lines; and moving with lung sliding when lung sliding is present. It reflects the coexistence of elements with a major acoustic impedance gradient, such as fluid and air. Fluid at the subpleural interlobular septum surrounded by air-filled alveoli (i.e., septal edema) fulfills this condition. Three or more B-lines in a single view are called B-lines. B-lines indicate the subpleural part of interstitial syndrome.[9]
A patient was designated to have ECG abnormalities if dilatation of cardiac chambers, systolic dysfunction, regional wall motion abnormalities, concentric LV hypertrophy, severe valvular regurgitation, pericardial tamponade, or noncollapsible or dilated IVC were present. Hypoxia was defined as PaO2 <60 on room air. Hypercarbia was defined as PaCO2 >45 mmHg.
Based on above definitions, we a priori defined seven pathophysiological categories that may cause dyspnea [Figure 1]. These categories are (i) metabolic defect (dry lung, no hypoxia); (ii) perfusion defect (dry lung, hypoxia); (iii) ventilation defect (dry lung, hypoxia, and hypercarbia); iv) ventilation and alveolar defect (wet lung, hypoxia, and hypercarbia); (v) alveolar defect-consolidation (wet lung [usually localized], hypoxia, no ECG abnormality); (vi) alveolar defect-pulmonary edema (wet lung [usually bilateral], hypoxia, ECG abnormality); and (vii) metabolic and alveolar defect (wet lung, no hypoxia). Rationale for these groupings is that a patient who has a metabolic acidosis has dyspnea due to respiratory compensation and has low PaCO2 and usually no hypoxia. Lungs are expected to be dry unless there is some fluid overload. Patients with pulmonary embolism, pulmonary edema, or an air–space consolidation may all be hypoxic and can be distinguished based on dry lungs expected in perfusion defect, or bilateral wet lungs with cardiac abnormalities expected in pulmonary edema, or localized wet lung features in an alveolar defect-consolidation. Patients with alveolar defect-consolidations may have additional signs such as dynamic bronchograms (C-pattern). Patients with a ventilation defect have high PaCO2 levels along with hypoxia could have dry lungs or wet lungs in case of associated alveolar defect such as air–space consolidation.
Figure 1.
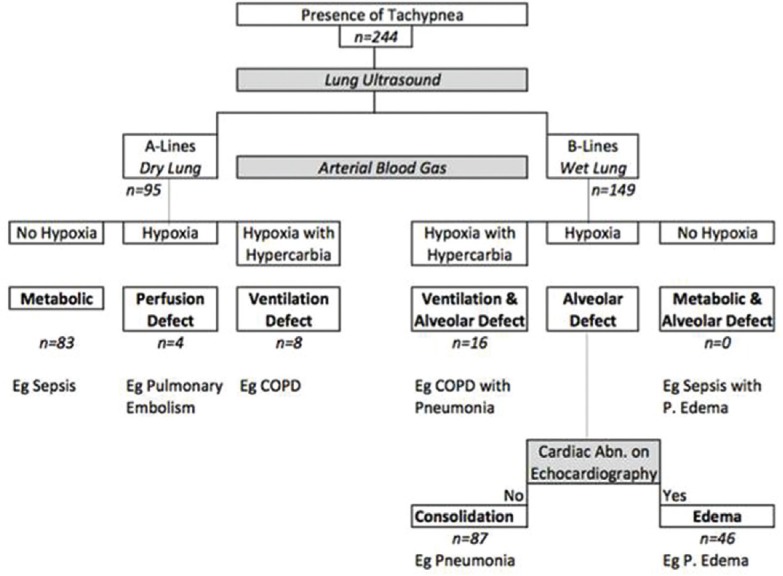
Classification on the basis of lung ultrasound and blood gas in dyspneic critically ill patients
Statistical analysis
We performed a descriptive statistical analysis of demographic characteristics, diagnostic categories, and severity characteristics across LUS-blood gas categories. We expressed measures of central tendency and dispersion for categorical variables as frequency and percentages and for continuous variables as means and standard deviation. We used Chi-square test for trend across the categories for categorical and Mann–Whitney for continuous variables. All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY: IBM Corp.). Kaplan–Meier analysis was done for different groups.
RESULTS
A total of 435 patients were admitted to the ICU between March 2015 and August 2016 (18 months). Of these, 244 (56.09%) were eligible for inclusion in the current study. Median age of the participants was 56 years (range 3–93 years) with median ICU stay of 3 days (range 1–83 days). Of 244 patients, 132 (54.1%) required mechanical ventilation, and median sequential organ failure assessment (SOFA) score of the participants was 7 (range 1–18). Overall ICU mortality in these patients was of 11.47%.
Most patients with dyspnea who required admission to ICU had wet lungs (149/244; 61.06%) and remaining 95 (38.14%) had dry lung as classified by LUS. Of the 149 patients with a wet lung, 133 (89.26%) had hypoxia and were classified as having an alveolar defect (either consolidation or pulmonary edema). Remaining 16 patients had hypoxia and hypercapnia and were classified as having combined ventilation and alveolar defect. Of the 95 patients with a dry lung, 83 (87.3%) were classified as having metabolic defect. The distribution of different pathophysiologic categories and their characteristics is shown in Table 1.
Table 1.
Characteristics of different pathophysiological categories

Different pathophysiologic categories were dissimilar in terms of interventions received in the ICU. The proportion of patients who received intubation, mechanical ventilation, central and arterial line insertion, and vasopressors was high in alveolar defect-consolidation (wet-lung hypoxia) and perfusion defect (dry-lung hypoxia) categories. These two patient groups also had a high SOFA score (median of 8 for alveolar and of 9 for perfusion defect) and a high mortality (50% for perfusion defect and 21.1% for alveolar defect-consolidation).
We compared discharge diagnosis of these patients with pathophysiologic categories. All patients with dry-lung hypercapnia or wet-lung hypercapnia had a ventilation disorder (mostly chronic obstructive pulmonary disease) as an underlying etiology. Similarly, there was homogeneity in discharge diagnosis in perfusion defect and alveolar defect-pulmonary edema categories. Diagnoses were most heterogeneous in metabolic (dry-lung nonhypoxia) category.
DISCUSSION
In this study, we report that it is possible to classify patients presenting to ICU with dyspnea in various pathophysiologic categories using a combination of LUS- and ABG-derived parameters. With round-the-clock availability of these techniques to the intensivists, such a categorization is also helpful in identification of sickest of all patients, as well as in prioritization of subsequent therapeutics.
Causes of dyspnea are diverse and vary from lung parenchyma disorders, ventilation disorders, pulmonary vascular disorders, cardiac disorders, and metabolic disorders. Each and every tachypneic patient does not need intubation. Furthermore, an ABG analysis is immediately done in ICU, by which we assess respiratory and metabolic parameters of the patient. However, ABG does not localize the cause of dyspnea and hence does not define definite treatment. We combined this parameter with LUS which is readily available as bedside tool in ICU, to localize the cause of dyspnea. Once the cause of dyspnea is localized, definite treatment could be initiated early. In our study, we enrolled tachypneic patients who underwent LUS along with ABG. We classified our patients into seven groups on the basis of LUS and ABG. We looked the characteristics of patients in each group and also assessed if this can have an influence on outcome.
The BLUE protocol by Lichtenstein and Mezière was the first of its kind in which the classification of dyspneic patients was done using LUS.[9] Disadvantage of only using LUS has been highlighted by previous authors in which use of Triple scan, i.e., lung along with IVC and cardiac, has been done.[6,10,11,12] The use of Triple scan gives us a holistic picture. Hence, we also used Triple scan in which lung along with cardiac along with IVC scan was done.
We had a group of tachypneic patients who had A-lines and were nonhypoxic on ABG, and they were classified as metabolic causes of dyspnea. These were patients with sepsis (with site other than lungs), chronic kidney disease, chronic liver disease, and diabetic ketoacidosis. The cause of dyspnea in such cases is basically the metabolic acidosis which leads to dyspnea. In all classifications, people have not mentioned metabolic causes of dyspnea; we found it as a large chunk where majority of patients were with sepsis leading to metabolic acidosis and hence dyspnea. We have included the metabolic causes, as the cause of dyspnea; to diagnose a metabolic problem, we need an ABG and hence it was included in our protocol.
People have tried B type Natriuretic Peptide (BNP) to differentiate cardiac cause of dyspnea from other causes.[13] In critical care, BNP is raised in conditions such as sepsis, renal failure, and conditions where RV strain is there such as Acute Respiratory Distress Syndrome (ARDS) and also on mechanical ventilation. ABG is a simple bedside test which is done in each and every dyspneic patient; hence, it was included in our case. The advantage of ABG is that raised CO2 invariably points toward ventilation diseases; hence, in conditions where we are getting B-lines with raised CO2, it points toward alveolar and ventilation disease. In a study by Xirouchaki et al., they assessed the impact of LUS on clinical decision-making in critically ill patients; in 189 patients, LUS was done if the patient had deterioration of ABG or suspected pathological entity, i.e., alveolar defect-consolidation, pneumothorax, atelectasis, and pulmonary edema.[14] In this study, 42.7% of patients had LUS for deterioration of ABG; hence, we incorporated LUS with ABG in our study. Hence, we used a new parameter which people have not integrated with these algorithms is ABG; ABG will tell us whether it a metabolic/respiratory problem.
Specifically, it has been shown that LUS has high diagnostic accuracy in identifying pneumothorax, consolidation/atelectasis, interstitial syndromes (i.e., pulmonary edema of cardiogenic or noncardiogenic origin), and pleural effusion.[15] LUS is better than chest X-ray (CXR) which has been studied extensively in emergency and critical care settings.[16,17,18,19,20,21,22,23,24] In an Italian study by Xirouchaki et al., who looked after 84 hemithoraces, they have shown that sensitivity and specificity of LUS for each of the diagnosis, i.e., consolidation, interstitial syndrome, and pleural effusion, is much higher as compared to CXR.[15] LUS is a simple, radiation-free bedside investigation which can be repeated many times a day in contrast to CXR which exposes radiation hazard and is cumbersome. For Acute decompensated heart failure-LUS is gold standard whereas it overdiagnosis the consolidation. It has low sensitivity and specificity as compared to chest X ray in cases of pneumothorax.[15,25,26] No X-rays were routinely performed in our study. In patients with nonresolving alveolar defect-consolidations, we got a computed tomography (CT); in few suspected causes of pneumothorax, we also got CXR done. Since the diagnosis of alveolar defect-consolidation in elderly multimorbid individuals is often challenging, LUS could be introduced in various settings of geriatric care and integrated with other screening tools for alveolar defect-consolidation detection.[27] More specifically, bedside LUS could be recommended in those older patients with poor functional performance and high clinical suspicion of alveolar defect-consolidation, in the presence of a negative chest radiograph. In this situation, the ultrasound results could help clarify the diagnosis and avoid chest CT prescription. However, we acknowledge that a wide application of this algorithm will need confirmation of our findings in larger studies, comparing the LUS results also with CT in all participants. CXR will remain the standard first-level diagnostic test until that. Moreover, ultrasonographic skills are not uniform among specialists across different hospitals and countries. This represents a further limitation to the applicability of the algorithm proposed earlier although LUS is a relatively simple technique that can be effectively learned by training physicians. Randomized, blinded well-controlled studies that test the abilities of ultrasound to detect and differentiate chest disease are the need of the hour. We and others have clearly shown the superiority of LUS over bedside chest radiography in identifying these specific pathological entities. In these studies, thoracic CT was the gold standard imaging technique evaluated.
The median age in our study was 55 years (6–93), the median age in perfusion group was 40 years, whereas in metabolic, it was 51 years. Gender distribution was similar across all groups. Out of the 244 tachypneic patients admitted to our ICU, we had 221 medical patients (90.57%) as compared to 23 surgical patients. Regarding site of admission, most of our admissions were from wards followed by the outpatient department, emergency unit, and operation theater. The median SOFA score in our study was 7; patients with ventilation disorder had low SOFA score as compared to others which led to statistical significance. SOFA comprises six factors; hence, patient with ventilation disorder had problem with respiratory system and hence had lower SOFA scores.
The need of intubation was highest in group with B-lines, i.e., interstitial syndromes. These interstitial syndromes could be seen in cases of alveolar defect-consolidation, alveolar defect-pulmonary edema, and ventilation with alveolar disorder. The presence of B-lines indicates alveolar disease and hypercarbia indicates ventilation problem. The presence of both (B-lines with hypercarbia) indicates that the patient has ventilation problem with alveolar disease while the presence of hypercarbia with A-lines indicates ventilation defect. It is that this group of patients with ventilation and alveolar disease will eventually need intubation. As we can see in Table 1, we had 24 patients with ventilation and alveolar disorder of which 56.1% needed intubation as compared to ventilation disorders in which 12.5% needed intubation. Hence, as compared to other algorithms, this algorithm applied on admission will help in predicting need of intubation in cases of patient with ventilation problem.
The need of mechanical ventilation, i.e., use on invasive ventilation/noninvasive ventilation (NIV) has also influence on outcome. The invasive ventilation is associated with its complications such as both mechanical and infectious complications such as ventilator-associated pneumonia. As we can see in patients with ventilation problem, 87.5% were managed with NIV as compared to 37.5% in patients with ventilation and alveolar disorder.
The remaining two differentials of B-lines were pulmonary edema and alveolar defect-consolidation. The presence of diffuse B-lines with sliding sign with poor cardiac contractility and dilated IVC indicated toward pulmonary edema. In contrast, the presence of focal B-lines along with the presence of Shrek sign and dynamic bronchograms pointed toward alveolar defect-consolidation. This differentiation played an important role as shown in Table 1; the need of intubation was 60.9% in alveolar defect-consolidation as compared to 21.7% in patients with pulmonary edema. Other characterstic feature of this differentiation is need of NIV was 9.2% in patients with alveolar defect-Consolidation as compared to 43.5% in patients with alveolar defect-Edema. This classification as compared to other classification helps us in organized approach in managing patients with dyspnea.
Previous studies have supported the value of LUS in the diagnosis of respiratory system diseases and indirectly on the patient's management. In a study by Yu et al., in all 41 patients included in the study, 19 of whom were managed in an ICU performed LUS in cases where portable radiographs were difficult to interpret.[28] LUS was helpful in diagnosing (in 66% of cases) and treatment planning (in 41% of patients).[28] Lichtenstein and Mezière, in an observational study, performed LUS on consecutive patients admitted in ICU with acute respiratory failure;[9] they compared the LUS results on initial presentation with the final diagnosis by the ICU team and demonstrated that LUS findings immediately provided a diagnosis of acute respiratory failure in 90.5% of cases. A study by Xirouchaki et al. showed that in a large population of mechanically ventilated critically ill patients, whose clinical status necessitated a diagnostic procedure and therapeutic intervention, LUS information provided to the primary physician changed the patients' management in almost half of the cases.[14] Furthermore, in one-fifth of the studies, LUS revealed findings compatible with specific diagnoses not suspected by the primary physician. It follows that in the process of addressing specific questions, the LUS has significant impact on decision-making.
The patient in metabolic group which mostly comprised of septic patients had hypotension for which CVC was inserted in 62.7% of the patients; similarly, in patients with alveolar defect-consolidation, CVC was inserted in 73.6% of the patients. In patients with ventilation, disorder rate of CVC insertion was low as these patients were already stable. CVC insertion and need of vasopressors were directly correlated with ICU stay, need of vasopressors, and severity of illness. To identify the source of sepsis – cultures were sent and abdomen scan along with LUS was done. Any area of consolidation as evidenced by Shrek sign and dynamic bronchograms was suggestive of alveolar defect-consolidation. For urosepsis – kidney-ureter-bladder USG was good enough to tell about hydronephrosis and pyelonephritis. Similarly, scan of liver was able to suggest chronic liver disease along with any abscess (one of our patients had liver abscess). Scan of spleen was able to tell about any splenic hematoma and abscess (one patient had splenic hematoma). Pelvic ultrasound was done by our radiologist. In two of our septic patients with pyrexia of unknown origin, we did CT scan to localize the source of sepsis.
Notwithstanding the lack of randomized, controlled trials on point-of-care USG (POC-US) use in the specific setting of sepsis, increasing evidence suggests that it has tremendous potential in managing critical patients with infectious diseases. POC-US can be of great aid in the diagnosis of infectious diseases and generically in the detection of septic foci in febrile states. It has a significant impact on differentiating different types of shock.
Regarding ICU outcome, we had maximum mortality in patients with perfusion defect (50%); though number are small (i.e., only four patients) followed by alveolar defect-consolidation (21.7%) followed by metabolic group (10.8%), followed by alveolar defect-pulmonary edema (8.7%). Patients with ventilatory disorders had nil mortality. The same is depicted in Kaplan–Meier survival analysis [Figure 2]. This classification enables us to determine that patients with LUS pattern of consolidation and perfusion defect are sickest of all and needs immediate and early intervention. This classification also enables us to determine of all the seven groups which patient would benefit from NIV (Alveolar defect-edema) and intubation (Alveolar defect-consolidation).
Figure 2.
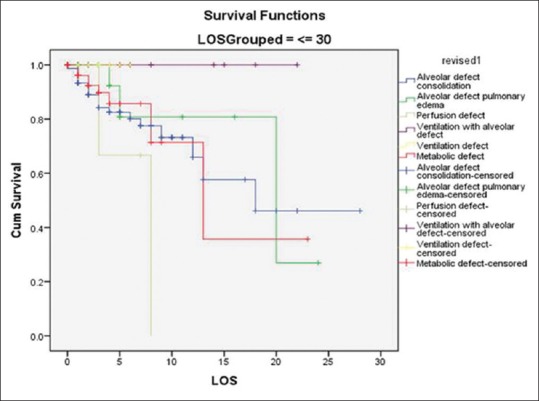
Kaplan–Meier survival curve of different pathophysiological groups
We included all consecutive patients with dyspnea in our study and attempted to classify patients based on LUS- and ABG-based pathophysiology. Our study has certain limitations. First, this being a retrospective chart review has potential for information bias. While information for most patients was available from the ICU charts, yet it is likely that some of the information was not strictly collected on admission. Second, LUS was performed and interpreted in light of usual clinical information that is available at the time of admission. Hence, performance of this test is not blinded to and independent of other available clinical variables. Third, different operators performed LUS and it is likely that their interpretation could be different. However, this limitation is likely of any diagnostic technique that has some subjectivity during interpretation. Finally, sometimes, more than one pathophysiology may exist in a critically ill patient with dyspnea, while we classified patients into a dominant pathophysiology and compared our findings with a diagnosis on discharge. Despite these limitations, our classification scheme is biologically plausible, and LUS is a more informative extension of clinical examination in patients with dyspnea. We believe that to be useful, this classification scheme needs to be prospectively validated.
CONCLUSION
The LUS along with ABG is a bedside investigational tool which helps us in classifying dyspneic critically ill patient. This tool apart from classifying these patients also predicts the need of intervention such as NIV or invasive ventilation in the groups. In today's world, combining LUS with ABG we feel will help in better managing our critically ill patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Collins S, Storrow AB, Kirk JD, Pang PS, Diercks DB, Gheorghiade M, et al. Beyond pulmonary edema: Diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department. Ann Emerg Med. 2008;51:45–57. doi: 10.1016/j.annemergmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Ray P, Delerme S, Jourdain P, Chenevier-Gobeaux C. Differential diagnosis of acute dyspnea: The value of B natriuretic peptides in the emergency department. QJM. 2008;101:831–43. doi: 10.1093/qjmed/hcn080. [DOI] [PubMed] [Google Scholar]
- 3.Peacock WF. Using the emergency department clinical decision unit for acute decompensated heart failure. Cardiol Clin. 2005;23:569–88. doi: 10.1016/j.ccl.2005.08.014. viii. [DOI] [PubMed] [Google Scholar]
- 4.Logeart D, Saudubray C, Beyne P, Thabut G, Ennezat PV, Chavelas C, et al. Comparative value of Doppler echocardiography and B-type natriuretic peptide assay in the etiologic diagnosis of acute dyspnea. J Am Coll Cardiol. 2002;40:1794–800. doi: 10.1016/s0735-1097(02)02482-8. [DOI] [PubMed] [Google Scholar]
- 5.Papanikolaou J, Makris D, Mpaka M, Palli E, Zygoulis P, Zakynthinos E, et al. New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients. Crit Care. 2014;18:R94. doi: 10.1186/cc13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajimoto K, Madeen K, Nakayama T, Tsudo H, Kuroda T, Abe T, et al. Rapid evaluation by lung-cardiac-inferior vena cava (LCI) integrated ultrasound for differentiating heart failure from pulmonary disease as the cause of acute dyspnea in the emergency setting. Cardiovasc Ultrasound. 2012;10:49. doi: 10.1186/1476-7120-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burri E, Potocki M, Drexler B, Schuetz P, Mebazaa A, Ahlfeld U, et al. Value of arterial blood gas analysis in patients with acute dyspnea: An observational study. Crit Care. 2011;15:R145. doi: 10.1186/cc10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junker C, Gutierrez G. Are arterial blood gases necessary in the evaluation of acutely dyspneic patients? Crit Care. 2011;15:176. doi: 10.1186/cc10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest. 2008;134:117–25. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17:46–53. doi: 10.5811/westjem.2015.11.28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31:1208–14. doi: 10.1016/j.ajem.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Russell FM, Ehrman RR, Cosby K, Ansari A, Tseeng S, Christain E, et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: A lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med. 2015;22:182–91. doi: 10.1111/acem.12570. [DOI] [PubMed] [Google Scholar]
- 13.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–54. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
- 14.Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40:57–65. doi: 10.1007/s00134-013-3133-3. [DOI] [PubMed] [Google Scholar]
- 15.Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A, et al. Lung ultrasound in critically ill patients: Comparison with bedside chest radiography. Intensive Care Med. 2011;37:1488–93. doi: 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein DA. Ultrasound examination of the lungs in the Intensive Care Unit. Pediatr Crit Care Med. 2009;10:693–8. doi: 10.1097/PCC.0b013e3181b7f637. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein DA, Lascols N, Mezière G, Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30:276–81. doi: 10.1007/s00134-003-2075-6. [DOI] [PubMed] [Google Scholar]
- 19.Remérand F, Dellamonica J, Mao Z, Ferrari F, Bouhemad B, Jianxin Y, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36:656–64. doi: 10.1007/s00134-010-1769-9. [DOI] [PubMed] [Google Scholar]
- 20.Volpicelli G, Caramello V, Cardinale L, Cravino M. Diagnosis of radio-occult pulmonary conditions by real-time chest ultrasonography in patients with pleuritic pain. Ultrasound Med Biol. 2008;34:1717–23. doi: 10.1016/j.ultrasmedbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Parlamento S, Copetti R, Di Bartolomeo S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2009;27:379–84. doi: 10.1016/j.ajem.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Cortellaro F, Colombo S, Coen D, Duca PG. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J. 2012;29:19–23. doi: 10.1136/emj.2010.101584. [DOI] [PubMed] [Google Scholar]
- 23.Gallard E, Redonnet JP, Bourcier JE, Deshaies D, Largeteau N, Amalric JM, et al. Diagnostic performance of cardiopulmonary ultrasound performed by the emergency physician in the management of acute dyspnea. Am J Emerg Med. 2015;33:352–8. doi: 10.1016/j.ajem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Bourcier JE, Paquet J, Seinger M, Gallard E, Redonnet JP, Cheddadi F, et al. Performance comparison of lung ultrasound and chest X-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32:115–8. doi: 10.1016/j.ajem.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Recommendations on pre-hospital and amp; early hospital management of acute heart failure: A consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544–58. doi: 10.1002/ejhf.289. [DOI] [PubMed] [Google Scholar]
- 26.Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: A systematic review and meta-analysis. Acad Emerg Med. 2014;21:843–52. doi: 10.1111/acem.12435. [DOI] [PubMed] [Google Scholar]
- 27.Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, et al. Lung ultrasound and chest x-ray for detecting pneumonia in an acute geriatric ward. Medicine (Baltimore) 2016;95:e4153. doi: 10.1097/MD.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu CJ, Yang PC, Chang DB, Luh KT. Diagnostic and therapeutic use of chest sonography: Value in critically ill patients. AJR Am J Roentgenol. 1992;159:695–701. doi: 10.2214/ajr.159.4.1529829. [DOI] [PubMed] [Google Scholar]


