Abstract
Objectives:
This study aimed to determine the prevalence and progression of diabetic retinopathy (DR) and its risk factors in patients with diabetes attending primary care centers.
Methods:
This study was a cross-sectional chart review that was conducted in three randomly selected primary care centers. A total of 250 patients with diabetes had three consecutive annual screenings for DR from April 2014 to April 2017. At the initial visit, the ophthalmological findings were recorded. For three successive yearly screening, the screening results were assessed to estimate the changes that occurred in the prevalence, incidence, and progression of DR in addition to the degree of association with the most predictable risk factors.
Results:
The initial prevalence of DR was 15.2%. In this study, the findings over three consecutive screening intervals revealed that there was a steady increase in the prevalence of DR. The findings of this study showed that there was no significant association with DR and known risk factors including sex, type of diabetes mellitus (DM), obesity, and smoking. On the other hand, the duration of DM, hemoglobin A1c level, uncontrolled diabetes, hypertension, dyslipidemia, nephropathy, insulin treatment, and age were identified as strong predictors of DR among diabetics in this study.
Conclusion:
DR, a serious microvascular complication of DM, is an asymptomatic disease with a slow onset and gradual progression. Primary prevention is highly recommended to control the risk factors that will delay the onset and progression of DR.
Keywords: Diabetic retinopathy, prevalence, risk factors, screening interval
Background
Diabetes mellitus (DM) is one of the most prevalent diseases worldwide.[1] The Kingdom of Saudi Arabia has a high prevalence of DM at 23.7%,[2] and this rate is expected to increase to 44.1% in 2022.[3] DM is a well-known cause of microvascular and macrovascular complications. Patients with diabetes often develop eye diseases as a complication. However, the most common and number one risk factor among these complications is diabetic retinopathy (DR).[4] The onset and progression of DR is slow and gradual, advancing from mild non-proliferative DR (NPDR), indicated by the presence of at least one microaneurysm, to moderate NPDR, indicated by the presence of hemorrhages, microaneurysms, and hard exudates. The severe form is characterized by hemorrhages and microaneurysms in four quadrants, with venous beading in at least two quadrants and intraretinal microvascular abnormalities in at least one quadrant. Proliferative DR (PDR) is characterized by neovascularization, preretinal hemorrhages, hemorrhage into the vitreous, traction retinal detachments, or macular edema (ME).[5]
DR is considered a frequent and leading cause of blindness, especially among the most productive age group between 20 and 60 years of age, with approximate estimates for the prevalence of retinopathy and vision-threatening retinopathy at 40.3% and 8.2%, respectively.[6] Among the 37 million blind people reported worldwide in the year 2002, DR was responsible for 1.8 million (4.8%) cases.[6] DR is also considered as the leading cause of blindness in the Kingdom of Saudi Arabia.[6] There is a wide discrepancy among the reported prevalence of DR. The overall global prevalence of DR among patients with type 2 diabetes is 27.23%, whereas the prevalence of DR among different ethnic groups varies widely from 20.8% among Asians to 46.7% among Caucasians.[7] Moreover, the data collected from the Saudi National Diabetes Registry indicate that the overall prevalence of DR is 19.7%.[8] The risk factors are mainly related to age, the duration of diabetes, and glycemic control; other factors such as obesity, dyslipidemia, and nephropathy were variably associated with DR.[8,9] In Saudi Arabia, nephropathy, neuropathy, insulin use, poor glycemic control, hypertension, and male gender were found to be associated with significant increase in the risk for DR, whereas smoking, hyperlipidemia, and obesity were associated with significant reduction in the risk for DR among Saudi type 2 diabetics.[8] Regular screening is the best method for early detection of DR and is strongly recommended since early detection has the best chance of preventing retinal complications and blindness.[5,6,10,11] In the primary care setting, patients should be referred by a healthcare professional to an ophthalmologist or an optometrist who should perform a comprehensive eye examination including a dilated fundoscopy. Such an eye examination should be conducted annually and at regular intervals thereafter as recommended by the eye care professional.[12] Retinal photography can enhance the efficiency of screening and reduce costs.[13] With the rapid increase in the prevalence of diabetes, the burden and cost of annual screening are rising with no evidence of significant rewards in delaying the onset of DR, especially in newly diagnosed diabetics with controlled risk factors.[14] There is reported evidence that biennial screening is safe and cost-effective in patients with diabetes who have not yet developed retinopathy.[13] Screened patients who had no or non-proliferative retinopathy were found to have a very low risk of eventual blindness from diabetes.[15] In this study, we aimed to estimate the prevalence and progression of DR and to identify its risk factors in the primary care setting.
Materials and Methods
This study was a cross-sectional chart review study using patient medical records. Three governmental primary health care centers were randomly selected from 12 centers equipped with well-established diabetic care units located in the city of Jeddah, Saudi Arabia. The total number of registered diabetics was 896 from April 2014 to April 2017. Medical records were reviewed, and only the complete medical records containing all the variables to be studied were included in this study. These variables included the following: the sociodemographic data [age, gender, height, weight, body mass index (BMI), smoking status]; systolic and diastolic blood pressure measurements obtained by the unit nurse for each visit or noted as a hypertensive patient undergoing treatment; type, onset, and duration of DM; presence or absence of microvascular complications, such as retinopathy, neuropathy, or nephropathy; presence or absence of macrovascular complications, such as stroke and cardiovascular disease; type of treatments prescribed (oral hypoglycemic, insulin combined with oral hypoglycemic or insulin alone); the frequency of ophthalmological referral; the results of screening at the initial visit performed by ophthalmologists at the General Eye Hospital in Jeddah; the results of three consecutive screenings at regular 1-, 2-, and 3-year follow-up visits in the primary care center; and the measurement of hemoglobin A1c (HbA1C) and low-density lipoprotein (LDL) levels and the glomerular filtration rate (GFR). The level of HbA1C was measured using the method certified by the National Glycohemoglobin Standardization Program and standardized per the Diabetes Control and Complications Trial assay. The HbA1C level was categorized into three groups (less than 8, 8.1–10, and greater than 10). A value of less than 8 was considered controlled. The LDL level was used a marker for dyslipidemia, and a value greater than 100 mmol/L was considered high. The GFR was calculated using the National Kidney Foundation method of calculation, and a GFR less than 60 was considered to indicate nephropathy. The data were categorized as dependent variable, which was DR of any grade, and independent variables (age, sex, the duration of diabetes, BMI, HbA1C and LDL levels, and the GFR). The screening intervals were categorized into four groups: the initial visit findings, and the findings after the first, second, and third annual screening intervals. The incidence of DR of any grade and the percentage of progression occurring over the 3 years were determined. This study was ethically approved by the Directorate of Health Affairs, the Research and Studies Department, the Scientific and Biomedical Committee, Jeddah, Saudi Arabia (ethical approval no. H-02-J-002).
Statistical analysis
Statistical analysis was conducted using Statistical Package for Social Sciences (SPSS) version 20.0 software (IBM Corp., Armonk, NY, USA). Chi-square test (χ2) was used for categorical variables, whereas t-test was used for continuous variables. A P value of <0.05 was used as the level of significance. Independent t-tests were used to evaluate the changes that occurred during the three screening intervals. Multinomial logistic regression analyses were performed to determine the most significant risk factors associated with the changes in DR. Adjusted odds ratio (ORs), 95% confidence intervals (CIs), and a P value <0.05 as the level of significance were used to assess the risk factors most closely related to changes in the incidence and progression of DR during the 3 years.
Results
A total of 250 medical records of those registered patients with diabetes who were in primary care centers during the period from April 2014 to April 2017 were complete and fulfilled the inclusion criteria. There were 120 (48.0%) males and 130 (52.0%) females. In the initial screening, there were 38 cases with DR. Each sex group (male and female) had the same number of DR cases, with 19 (50.0%) for each, whereas other eye diseases (cataract and refractive errors) were present in 40 (56.3%) females and 31 (43.7%) males. Normal eye examination results were observed in 141 diabetics, 71 (50.4%) were females and 70 (49.6%) were males. Among the 38 confirmed cases of DR, 4 (10.5%) had DM I, whereas majority (34; 89.5%) had DM II. With regard to duration of DM, 3 (7.9%) had DM for less than 10 years, 13 (34.2%) for 10–20 years, and 22 (57.9%) for more than 20 years. The majority of the patients (20; 52.6%) had a BMI of less than 30 and 18 had BMI of greater than 30. HbA1C level was also measured among diabetics with DR. Only 1 (2.6%) had a level of 8 or less, whereas 22 (57.9%) had levels of 8.1–10, and 15 (39.5%) had levels greater than 10. Among the 38 DR cases, 5 (13.2%) had controlled DM, whereas 33 (86.8%) had uncontrolled DM. Moreover, 30 (78.9%) had hypertension. All 38 patients were undergoing treatment. Four (10.5%) were on oral hypoglycemic drugs(OHD), 6 (15.8%) had insulin only, 5 (13.2%) had insulin and metformin, and 23 (60.5%) were on insulin and OHD. Three (7.9%) patients were smoking; 26 (68.4%); and 17 (44.7%) had nephropathy. Chi-square statistical test was performed to estimate the degree of association between DR and independent variables. There was no significant association with DR and known risk factors including sex (P = 0.668), type of DM (P = 0.080), obesity (P = 0.980), and smoking (P = 0.992). On the other hand, the duration of DM (P = 0.0005), HbA1C level (P = 0.0005), uncontrolled diabetes (P = 0.0005), hypertension (P = 0.0005), dyslipidemia (P = 0.002), nephropathy (P = 0.0005), and insulin treatment (P = 0.0005) were all significantly associated with DR [Table 1].
Table 1.
Association between sociodemographic characteristics and diabetic retinopathy and other eye disorders
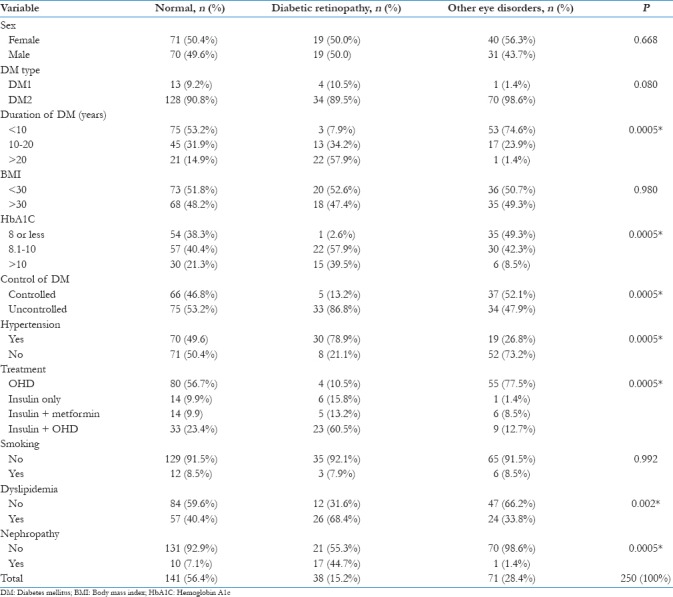
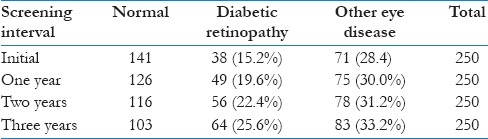
Table 2 shows the prevalence of DR every year of screening interval. Initially, the prevalence of DR of any grade was 15.2% (95% CI 10.8%–19.7%). The other eye diseases (refractive errors and cataract) were present in 71 cases with a prevalence of 28.4% (95% CI 22.8%–34.2%). The total number of DR cases increased to 49 cases at the first annual screening interval, with a prevalence of 19.6% (95% CI 14%–23.6%). At the second screening interval at 2 years, there were 56 cases of DR and the prevalence was 22.4% (95% CI 17.2%–27.6%). A total of 64 cases were detected in the third screening interval after 3 years with a prevalence of 25.6% (95% CI 20.6%–34.8%), as shown in Table 2.
Table 2.
Prevalence of diabetic retinopathy cases at each screening interval
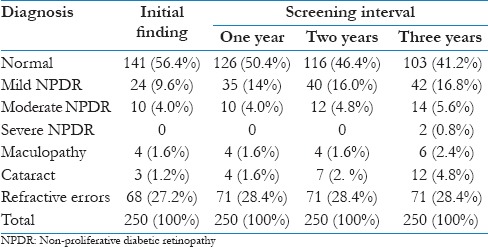
The frequency and percentage of the different grades and diagnoses at the three screening intervals were also determined [Table 3]. The initial findings showed that 141 (56.4%) patients with diabetes had normal eye conditions, whereas patients who had mild NPDR, moderate NPDR, maculopathy, cataract, and refractive errors were 24 (9.6%), 10 (4.0%), 4 (1.6%), 3 (1.2%), and 68 (27.2%), respectively. During the 3-year screening interval, it was notable that diabetics with mild NPDR increased to 35 (14%) in the first year, to 40 (16.0%) in the second year, and to 42 (16.8%) in the third year. Patients with moderate NPDR also increased to 12 (4.8%) in the second year, and to 14 (5.6%) in the third year. During the third year of screening interval, two patients reported having severe NPDR. Patients with diabetes with maculopathy increased to 6 (2.4%) in the third year of screening. Those with cataract condition also increased to 4 (1.6%) in the first year, 7 (2.8%) in the second year, and 12 (4.8%) in the third year. Patients with refractive errors increased to 71 (28.4%) in the first year and remained the same in the succeeding years of screening.
Table 3.
Frequency and percentage of the different diagnoses at the three screening intervals
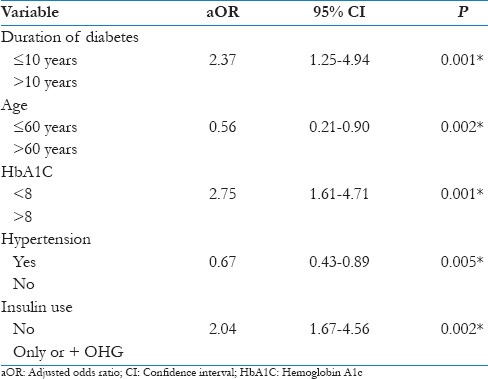
A multinominal logistic regression with calculation of adjusted OR and 95% CI revealed a significant association of these changes with DR and risk factors such as age >60 years (P = 0.002), duration of diabetes of >10 years (P = 0.001), an HbA1C level >8 (P = 0.001), hypertension (P = 0.005), and insulin therapy (P = 0.002). Therefore, these factors were identified as strong predictors of the incidence and progression of DR [Table 4].
Table 4.
Logistic regression analysis for the risk factors that might be associated with the main changes
Discussion
DR is one of the major microvascular complications of DM. DR can be a threat to the eyesight and even progress to blindness.[16,17] In general, this study aimed to estimate the prevalence and progression of DR. Several national and international studies that have been conducted to determine the prevalence of DR have reported values that range from 15% in some studies to 36% in others.[18,19,20,21,22] Studies from Saudi Arabia's southern region reported a prevalence of 36.4%, which is much higher than the previously reported prevalence of 11.3% in the same region by Ahmed et al.[23] The last study using the Saudi National Diabetes Registry found that the overall prevalence of DR in Saudi Arabia is 19.7%,[8] which was fairly similar to the initial findings of this study with 15.3% confirmed cases of DR.[24] In this study, the findings over three consecutive screening intervals revealed that there was a steady increase in the prevalence of DR. At the first annual screening interval, there was an increase from the initial prevalence of 15.2%–19.6%, and then to 22.4% at the second screening interval. The prevalence reached 25.6% at the third screening interval. During the 3-year screening interval, it was notable that diabetics with mild NPDR, moderate NPDR, and severe NPDR have increased in number, which means that there is progression in the severity of DR every year of screening interval. The screening program for DR in primary care centers follows the Saudi National Diabetic Guideline for Primary Care, which recommends an annual referral to ophthalmologists or optometrist to perform a comprehensive ophthalmological examination for early detection of DR, and the findings and recommendations of these professionals are sent back to the referring center. DR has a delayed onset and a slow, gradual progression over many years and can even regress when the disease is in an early mild form.[25]
This study also aimed to identify the risk factors of DR in the primary care setting. The findings of this study showed that there was no significant association with DR and known risk factors including sex, type of DM, obesity, and smoking. Some studies including one in China[26] and Saudi Arabia[27] have presented a predominant rate in the prevalence of DR among male patients with diabetes, compared to females. However, no known study has showed a clear explanation on this correlation. Although it was found to be statistically insignificant, DR occurrence in DM type 2 was relatively higher (89.5%) than that of DM type 1 (10.5%). In contrast to this, there were higher rates of DR in DM type 1 than in DM type 2, as exhibited by studies of Konstantinidis et al.[28] and Nentwich and Ulbig.[29] The prevalence was found to vary over time, which was likely because of the better quality healthcare for DM over time and the prevalence studies can stipulate better understanding into these sequential variations.[30] Although the influence of obesity on DR had shown inconsistent results,[31,32,33] more recent reports showed positive correlation of increased BMI and waist-to-hip ratio with increased risk of DR.[34,35,36] Similar to this study findings, smoking history in another study was not identified as a risk factor to DR because of its very low occurrence in patients with diabetes.[37] On the other hand, the duration of DM, HbA1C level, uncontrolled diabetes, hypertension, dyslipidemia, nephropathy, insulin treatment, and age were identified as risk factors of DR among diabetics in this study. A significant association was shown between DR and the duration of diabetes, similar to the results reported in other studies.[37,38,39,40,41] Some studies even reported that the duration of diabetes seemed to be the strongest factor associated with the development of DR.[42,43,44] Similar to the identified association between DR and HbA1C level, a study conducted in the Netherlands helped to identify patterns of DR progression, showing that especially in a small group of people with higher HbA1Clevels, fast progression from NPDR to PDR may occur.[45] Therefore, a close examination of patients with DR and poor glycemic control is suitable.[25] A high rate of uncontrolled diabetes was also found to be a significant risk factor in this study. Similar finding was revealed in a Korean study wherein it was stated that proper glycemic control can reduce the possibility of DR development, even in patients with a long duration of diabetes.[46] Several studies have suggested that diabetes duration is one of the strongest and non-modifiable risk factors for DR.[47] Therefore, achieving proper glycemic control might help reduce the risk of DR in patients who have a long diabetes duration.[46] Conflicting results have been reported about the effect of HbA1C variability on diabetic microvascular complications.[48,49,50] In spite of several epidemiologic studies that did not find blood pressure to be a consistent risk factor for DR incidence and progression,[51,52,53] multiple randomized controlled trials have established the benefits of tight blood pressure control as a major modifiable factor for DR incidence and progression, which was also found in this study findings.[30] Similar to the results of this study, there have been numerous clinical trials and observational studies on proving the association between dyslipidemia and DR.[40,54,55,56] However, it remains uncertain whether dyslipidemia is related to the incidence and progression of DR.[46] In contrast to this study, a recent study conducted in Oman[57] did not detect any association between DR and nephropathy. Only 13.7% of all patients with diabetic nephropathy have reported concomitant DR, which is less than our results of 44.7%. Few studies also reported low prevalence of DR among nephropathic patients.[38,39] DR and other microvascular complications such as neuropathy proceed the onset of nephropathy and are expected to have strong association between them. Although the difference in method and accuracy of diagnosing DR may result in low prevalence.[57] There was also a strong association between insulin therapy and DR found in this study. Similar findings have been reported in studies from China, Finland, Jordan, and Saudi Arabia.[8,20,23,26,58] Finally, it was showed in this study that there is a significant association between DR and the age of the patient. In a study conducted in mainland China,[26] an older age seemed to be a protective variable for DR but was instead found to be a variable for sight-threatening DR, especially in patients with diabetes older than 60 years. Even though older age was associated with a lower incidence of DR in their study, it was associated with a greater threat to vision. They explained this phenomenon by a higher mortality risk in older DR populations.[26]
Conclusion
In view of these findings, it can be concluded that the duration of DM, HbA1C level, uncontrolled diabetes, hypertension, dyslipidemia, nephropathy, insulin treatment, and age are strong predictors or risk factors of DR, having an increasing prevalence and progression in severity in the 3-year screening interval done in this study. Therefore, primary prevention is highly recommended to control the risk factors that will delay the onset and progression of DR.
Limitations
The limitations of this study are the small sample size and the variation in the initial presentations and in the pre-registration history of diabetes and its complications in patients who were included in the study. This study was performed in a primary care setting, which is different from a hospital-based setting that may affect the outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, Al-Harthi SS, Arafah MR, Khalil MZ, et al. Diabetes mellitus in Saudi Arabia. Saudi Med J. 2004;25:1603–10. [PubMed] [Google Scholar]
- 3.Al-Quwaidhi AJ, Pearce MS, Sobngwi E, Critchley JA, O’Flaherty M. Comparison of type 2 diabetes prevalence estimates in Saudi Arabia from a validated Markov model against the International Diabetes Federation and other modelling studies. Diabetes Res Clin Pract. 2014;103:496–503. doi: 10.1016/j.diabres.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai X, McGinnis JF. Diabetic retinopathy: Animal models, therapies, and perspectives. J Diabetes Res 2016. 2016:3789217. doi: 10.1155/2016/3789217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Bab MF, Shawky N, Al-Sisi A, Akhtar M. Retinopathy and risk factors in diabetic patients from Al-Madinah Al-Munawarah in the Kingdom of Saudi Arabia. Clin Ophthalmol. 2012;6:269–76. doi: 10.2147/OPTH.S27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Turki YA. Blood sugar control, ophthalmology referral and creatinine level among adult diabetic patients in primary health care, Riyadh, Saudi Arabia. Saudi Med J. 2002;23:1332–4. [PubMed] [Google Scholar]
- 7.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Rubeaan K, Abu El-Asrar AM, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, et al. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes epidemic: A Saudi National Diabetes Registry-based study. Acta Ophthalmol. 2015;93:e140–7. doi: 10.1111/aos.12532. [DOI] [PubMed] [Google Scholar]
- 9.Elwali ES, Almobarak AO, Hassan MA, Mahmooud AA, Awadalla H, Ahmed MH. Frequency of diabetic retinopathy and associated risk factors in Khartoum, Sudan: Population based study. Int J Ophthalmol. 2017;10:948–54. doi: 10.18240/ijo.2017.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiello LP. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:17–23. doi: 10.2337/dc13-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Maskari F, El-Sadig M. Prevalence of diabetic retinopathy in the United Arab Emirates: A cross-sectional survey. BMC Ophthalmol. 2007;7:11. doi: 10.1186/1471-2415-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looker HC, Nyangoma SO, Cromie DT, Olson JA, Leese GP, Philip S, et al. Predicted impact of extending the screening interval for diabetic retinopathy: The Scottish Diabetic Retinopathy Screening programme. Diabetologia. 2013;56:1716–25. doi: 10.1007/s00125-013-2928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalk D, Pitt M, Vaidya B, Stein K. Can the retinal screening interval be safely increased to 2 years for type 2 diabetic patients without retinopathy? Diabetes Care. 2012;35:1663–8. doi: 10.2337/dc11-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatziralli I, Sergentanis TN, Crosby-Nwaobi R, Winkley K, Eleftheriadis H, Ismail K, et al. Model for risk-based screening of diabetic retinopathy in people with newly-diagnosed type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2017;58:BIO99–105. doi: 10.1167/iovs.17-21713. [DOI] [PubMed] [Google Scholar]
- 15.Hughes D, Nair S, Harvey JN. Determining diabetic retinopathy screening interval based on time from no retinopathy to laser therapy. J Med Screen. 2017;24:170–5. doi: 10.1177/0969141316672687. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso CRL, Leite NC, Dib E, Salles GF. Predictors of development and progression of retinopathy in patients with type 2 diabetes: Importance of blood pressure parameters. Sci Rep. 2017;7:4867. doi: 10.1038/s41598-017-05159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willis JR, Doan QV, Gleeson M, Haskova Z, Ramulu P, Morse L, et al. Vision- related functional burden of diabetic retinopathy across severity levels in the United States. JAMA Ophthalmol. 2017;135:926–32. doi: 10.1001/jamaophthalmol.2017.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raman R, Ganesan S, Pal SS, Gella L, Kulothungan V, Sharma T. Incidence and progression of diabetic retinopathy in urban India: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS II), Report 1. Ophthalmic Epidemiol. 2017;24:294–302. doi: 10.1080/09286586.2017.1290257. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G, Chen H, Chen W, Zhang M. Prevalence and risk factors for diabetic retinopathy in China: A multi-hospital-based cross-sectional study. Br J Ophthalmol. 2017;101:1591–5. doi: 10.1136/bjophthalmol-2017-310316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabiu MM, Al Bdour MD, Abu Ameerh MA, Jadoon MZ. Prevalence of blindness and diabetic retinopathy in northern Jordan. Eur J Ophthalmol. 2015;25:320–7. doi: 10.5301/ejo.5000557. [DOI] [PubMed] [Google Scholar]
- 21.Sasongko MB, Widyaputri F, Agni AN, Wardhana FS, Kotha S, Gupta P, et al. Prevalence of diabetic retinopathy and blindness in Indonesian adults with type 2 diabetes. Am J Ophthalmol. 2017;181:79–87. doi: 10.1016/j.ajo.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Sunita M, Singh AK, Rogye A, Sonawane M, Gaonkar R, Srinivasan R, et al. Prevalence of diabetic retinopathy in urban slums: The Aditya Jyot Diabetic Retinopathy in Urban Mumbai Slums Study – Report 2. Ophthalmic Epidemiol. 2017;24:303–10. doi: 10.1080/09286586.2017.1290258. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed RA, Khalil SN, Al-Qahtani MA. Diabetic retinopathy and the associated risk factors in diabetes type 2 patients in Abha, Saudi Arabia. J Family Community Med. 2016;23:18–24. doi: 10.4103/2230-8229.172225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marahrens L, Kern R, Ziemssen T, Fritsche A, Martus P, Ziemssen F, et al. Patients’ preferences for involvement in the decision-making process for treating diabetic retinopathy. BMC Ophthalmol. 2017;17:139. doi: 10.1186/s12886-017-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voigt M, Schmidt S, Lehmann T, Köhler B, Kloos C, Voigt U, et al. Prevalence and progression rate of diabetic retinopathy in type 2 diabetes patients in correlation with the duration of diabetes. Exp Clin Endocrinol Diabetes. 2017 doi: 10.1055/s-0043-120570. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Yang J, Tao L, Lv H, Jiang X, Zhang M, et al. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: A cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ Open. 2017;7:e016280. doi: 10.1136/bmjopen-2017-016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Kangave D, Moharram OA. Risk factors for diabetic retinopathy among Saudi diabetics. Int Ophthalmol. 1998;22:155–61. doi: 10.1023/a:1006240928938. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinidis L, Carron T, de Ancos E, Chinet L, Hagon-Traub I, Zuercher E, et al. Awareness and practices regarding eye diseases among patients with diabetes: A cross sectional analysis of the CoDiab-VD cohort. BMC Endocr Disord. 2017;17:56. doi: 10.1186/s12902-017-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nentwich MM, Ulbig MW. Diabetic retinopathy – Ocular complications of diabetes mellitus. World J Diabetes. 2015;6:489–99. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin Exp Ophthalmol. 2016;44:260–77. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Moss SE. Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 1997;157:650–6. [PubMed] [Google Scholar]
- 32.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than years. Arch Ophthalmol. 1984;102:520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 33.Lim LS, Tai ES, Mitchell P, Wang JJ, Tay WT, Lamoureux E, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:4458–63. doi: 10.1167/iovs.09-4939. [DOI] [PubMed] [Google Scholar]
- 34.Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ, Fuller JH, Songini M, et al. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care. 2001;24:284–9. doi: 10.2337/diacare.24.2.284. [DOI] [PubMed] [Google Scholar]
- 35.Henricsson M, Nystrom L, Blohme G, Ostman J, Kullberg C, Svensson M, et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: Results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26:349–54. doi: 10.2337/diacare.26.2.349. [DOI] [PubMed] [Google Scholar]
- 36.van Hecke MV, Dekker JM, Stehouwer CD, Polak BC, Fuller JH, Sjolie AK, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: The EURODIAB prospective complications study. Diabetes Care. 2005;28:1383–9. doi: 10.2337/diacare.28.6.1383. [DOI] [PubMed] [Google Scholar]
- 37.Abougalambou SS, Abougalambou AS. Risk factors associated with diabetic retinopathy among type 2 diabetes patients at teaching hospital in Malaysia. Diabetes Metab Syndr. 2015;9:98–103. doi: 10.1016/j.dsx.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Keen H, Lee ET, Russell D, Miki E, Bennett PH, Lu M. The appearance of retinopathy and progression to proliferative retinopathy: The WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S22–30. doi: 10.1007/pl00002935. [DOI] [PubMed] [Google Scholar]
- 39.Looker HC, Krakoff J, Knowler WC, Bennett PH, Klein R, Hanson RL. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in pima indians. Diabetes Care. 2003;26:320–6. doi: 10.2337/diacare.26.2.320. [DOI] [PubMed] [Google Scholar]
- 40.Tapp RJ, Shaw JE, Harper CA, de Courten MP, Balkau B, McCarty DJ, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26:1731–7. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 41.Tung TH, Liu JH, Lee FL, Chen SJ, Li AF, Chou P. Population-based study of nonproliferative diabetic retinopathy among type 2 diabetic patients in Kinmen, Taiwan. Jpn J Ophthalmol. 2006;50:44–52. doi: 10.1007/s10384-005-0269-x. [DOI] [PubMed] [Google Scholar]
- 42.Maberley DAL, King W, Cruess AF, Koushik A. Risk factors for diabetic retinopathy in the Cree of James Bay. Ophthalmic Epidemiol. 2002;9:153–67. doi: 10.1076/opep.9.3.153.1515. [DOI] [PubMed] [Google Scholar]
- 43.Okada S, Ichiki K, Tanokuchi S, Hamada H, Matsuo N, Ota Z. Factors related to the development and progression of diabetic retinopathy in patients with type 2 diabetes. J Int Med Res. 1996;24:214–20. doi: 10.1177/030006059602400206. [DOI] [PubMed] [Google Scholar]
- 44.Souza E, Esteves J, Broilo V, Domingues C, Lavinsky J. Retinopatia diabética não proliferativa. In: Abujamra S, Avila M, Barsante C, Farah ME, Gonçalves JOR, Lavinsky J, editors. Retina e vítreo: Clinica e cirurgia. São Paulo: Rocca; 2000. pp. 485–99. [Google Scholar]
- 45.Zavrelova H, Hoekstra T, Alssema M, Welschen LM, Nijpels G, Moll AC, et al. Progression and regression: Distinct developmental patterns of diabetic retinopathy in patients with type 2 diabetes treated in the diabetes care system west-friesland, the Netherlands. Diabetes Care. 2011;34:867–72. doi: 10.2337/dc10-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun JS, Lim TS, Cha SA, Ahn YB, Song KH, Choi JA, et al. Clinical course and risk factors of diabetic retinopathy in patients with type 2 diabetes mellitus in Korea. Diabetes Metab J. 2016;40:482–93. doi: 10.4093/dmj.2016.40.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawasaki R, Tanaka S, Abe S, Sone H, Yokote K, Ishibashi S, et al. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: The Japan Diabetes Complications Study. Ophthalmology. 2013;120:574–82. doi: 10.1016/j.ophtha.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care. 2014;37:2359–65. doi: 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 49.Luk AO, Ma RC, Lau ES, Yang X, Lau WW, Yu LW, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: Prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013;29:384–90. doi: 10.1002/dmrr.2404. [DOI] [PubMed] [Google Scholar]
- 50.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: The Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013;36:2301–10. doi: 10.2337/dc12-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy? Arch Intern Med. 1989;149:2427–32. [PubMed] [Google Scholar]
- 52.Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96:1501–10. doi: 10.1016/s0161-6420(89)32699-6. [DOI] [PubMed] [Google Scholar]
- 53.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–35. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 54.Klein BE, Klein R, Moss SE. Is serum cholesterol associated with progression of diabetic retinopathy or macular edema in persons with younger-onset diabetes of long duration? Am J Ophthalmol. 1999;128:652–4. doi: 10.1016/s0002-9394(99)00222-6. [DOI] [PubMed] [Google Scholar]
- 55.Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: The atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225–34. doi: 10.1016/s0161-6420(02)01074-6. [DOI] [PubMed] [Google Scholar]
- 56.Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians – The Chennai Urban Rural Epidemiology Study (CURES) Eye Study – 2. Diabet Med. 2006;23:1029–36. doi: 10.1111/j.1464-5491.2006.01890.x. [DOI] [PubMed] [Google Scholar]
- 57.Alrawahi AH, Rizvi SG, Al-Riyami D, Al-Anqoodi Z. Prevalence and risk factors of diabetic nephropathy in omani type 2 diabetics in Al-dakhiliyah region. Oman Med J. 2012;27:212–16. doi: 10.5001/omj.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naidoo K, Gichuhi S, Basanez MG, Flaxman SR, Jonas JB, Keeffe J, et al. Prevalence and causes of vision loss in sub-Saharan Africa: 1990-2010. Br J Ophthalmol. 2014;98:612–8. doi: 10.1136/bjophthalmol-2013-304081. [DOI] [PubMed] [Google Scholar]


