Abstract
Background:
The cartridge-based nucleic acid amplification test (CBNAAT) Xpert MTB/RIF is more sensitive than smear microscopy for the diagnosis of tuberculosis (TB). It is also more expensive, costing 1450 INR as compared to 10 INR per smear.
Objectives:
We conducted a prospective study to evaluate the impact of CBNAAT results on patient management in our low-resource, high-burden Indian rural setting.
Materials and Method:
Between February and July 2017, clinicians were asked to complete one questionnaire at the time of CBNAAT request and another when reviewing the result. The first questionnaire, “Form 1,” concerned pretest treatment status and asked clinicians to rate their confidence in the diagnosis. “Form 2” concerned postresult treatment and investigation plan.
Results:
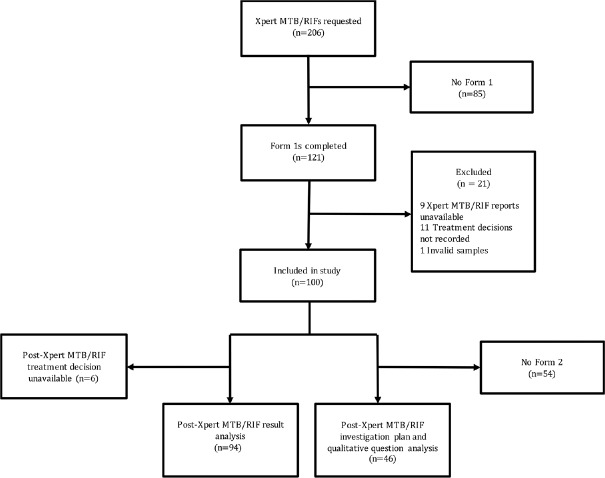
Over the study period, 206 CBNAATs were requested. Form 1 was not completed for 85 patients and 21 were excluded leaving 100 in the main analysis. MTB was detected (MTB-D) in 60 of 100 (60%) of samples tested. At the time of CBNAAT request, 56 of 100 (56%) of patients were already on treatment, this being empirical in 34 of 100 (34%). Despite this, 17 of 60 (28.3%) of MTB-D results occurred in patients not yet started on treatment. Postresult treatment status was available for 94 of 100 CBNAATs (55 MTB-D and 39 MTB-ND). Following an MTB-D result, all 17 patients not on treatment started and all 38 on so already continued. Following an MTB Not Detected (MTB-ND) result, 26 of 27 (96.3%) of patients not yet on treatment remained so, but only 2 of 12 (16.7%) already on treatment stopped. Even where the clinician's pretest confidence in TB was low, 9of 30 (30%) of CBNAAT results were MTB-D.
Conclusion:
In a low-resource high-burden setting, CBNAAT may have greatest impact where the clinician's pretest confidence in TB is low and empirical treatment has not been started. This is because MTB-D results will lead to appropriate initiation of treatment and MTB-ND results may enable clinicians to hold-off treatment.
Keywords: Cartridge-based nucleic acid amplification test (CBNAAT), CBNAAT MTB/RIF assay (geneCBNAAT), Mycobacterium tuberculosis, PCR, point of care testing
Introduction
The cartridge-based nucleic acid amplification test (CBNAAT) Xpert MTB/RIF (Cepheid, Sunnyvale, USA) has two key advantages over conventional smear microscopy (smear) for the diagnosis of tuberculosis (TB). First, it is more sensitive both for pulmonary[1] and extrapulmonary,[2] TB and second it can detect rpoB gene mutations that confer rifampicin-resistance (RR-TB). It is also considerably more expensive with each test costing approximately 1450 INR (22.50 USD), as compared to just 10 INR (0.15 USD) per smear. In low-resource settings, it is therefore imperative that CBNAAT is used in the manner that will have the greatest impact on patient care.
Our institution in central India serves a population of tribal and rural poor who often travel great distances to be seen in clinic. There is an emphasis on same-day diagnostics and treatment wherever possible and so all patients with suspected pulmonary TB receive same-day on-site smear, blood tests, and chest X-ray. Use of CBNAAT is more limited, and while they can be performed on site, most are referred to nearby testing centers.
High prevalence of TB and severe malnutrition in our population accompanied by intermittent patient encounters and delays in CBNAAT results mean that TB treatment is often started empirically (i.e., in the absence of a positive smear, culture, or CBNAAT). Even when subsequent investigations fail to isolate TB, treatment may be continued if the pretest probability of TB was deemed sufficiently great. This raises the following question: whether CBNAAT “MTB Detected” (MTB-D) results merely confirm TB in those already on treatment, and “MTB Not Detected” (MTB-ND) results are not relied upon to cease treatment, then is it cost-effective? We conducted a prospective study to explore how CBNAAT was being used at our institution and whether results were influencing patient management.
Materials and Method
This pragmatic, prospective study was conducted at our non-governmental institution Jan Swasthya Sahyog (JSS) Health Centre, central India.[3] At JSS, all patients with suspected pulmonary TB receive same-day on-site smear. Sputum samples are induced with 0.9% saline where expectoration is inadequate and smears prepared using Ziehl–Neelsen stain. Where a spot smear is negative, or a CBNAAT is requested, early morning sputum is collected. CBNAATs can be performed on site, but most are referred to nearby testing centers in Bilaspur and Jabalpur.
Over a 5-month period between February and July 2017, all clinicians requesting a CBNAAT were asked to complete two questionnaires. Nine clinicians took part in the study. Eight were Indian postgraduate specialists in pediatrics, internal medicine, or surgery. One was an American post-residency HEAL initiative fellow.[4]
The first questionnaire, Form 1, was completed at the time of a CBNAAT request. This collected demographic data and information regarding sample type, pretest treatment status of the patient, and whether CBNAAT was being requested before or after a smear result. Form 1 also asked clinicians whether they were “almost certain,” “confident,” or “not confident” in the diagnosis of TB (Appendix 1 – Form 1 and Form 2). It was important that this question be asked prior the CBNAAT result to avoid recall bias.
Form 2 was completed after the clinician had reviewed the CBNAAT result. It collected data on the postresult treatment and investigation plan and contained two qualitative questions exploring whether the clinician found CBNAAT useful (Appendix 1). The first question asked clinicians whether they agreed with the statement “Overall CBNAAT was useful in guiding the management of this patient” by selecting an option ranging from “highly agree” to “highly disagree.” The following question asked “Why was CBNAAT useful?” Clinicians had the option to either select an answer or write free text.
Where a Form 1 was completed, but no Form 2, medical records were retrospectively reviewed to determine post-CBNAAT treatment decision. Records were also reviewed where a CBNAAT was requested without a Form 1 to assess whether these patients differed from those included in the study.
All CBNAAT requests were included – pulmonary and extra-pulmonary, children and adults, and inpatient and outpatient. Spearman's rank correlation between pretest confidence and CBNAAT result for MTB was calculated by converting confidence to an ordinal scale where “Not Confident” = 1, “Confident” = 2, and “Almost Certain” = 3. Calculations were performed using Python and Microsoft Excel.
The length of the study was determined by practical considerations such as ability to oversee the project and sustain clinician involvement rather than targeting a specific sample size.
Ethics approval
Ethical approval was granted by the Institutional Scientific Committee of JSS.
Results
Over the study period, 206 CBNAATs were requested. Form 1 was not completed for 85 patients and a further 21 were excluded leaving 100 in the main analysis [Figure 1]. Post CBNAAT result investigation plan and qualitative feedback were only available for the 46 patients where a Form 2 was also completed [Figure 1].
Figure 1.
Patient exclusion flow diagram
No patient had more than one CBNAAT requested, so each result represents a unique patient. The median age of patients was 44 years [interquartile range (IQR) 31–59 years], and 58 of 100 (58%) were male. The median body mass index (BMI) was 16.5 kg/m2 (IQR 14.8–18.8 kg/m2). Two patients were HIV-positive and 14 had been treated for TB previously.
Most CBNAATs were referred to nearby centers for testing (61/100, 61%). The median duration from CBNAAT request to result was 5 days (IQR 0–15 days). The median duration for on-site CBNAATs was less than 1 day (IQR 0–2 days).
Breakdown of CBNAAT results by “sample type,” “prior treatment status,” and “pre-test confidence” is provided in Table 1a–c, respectively. MTB was detected in 60 of 100 (60%) of CBNAATs and rpoB mutations in 3 of 60 (5%) of MTB-D samples [Table 1a]. At the time of CBNAAT request, 56 of 100 (56%) of patients were already on treatment. In 34 of 100 (34%) of cases, this was empirical, with the remaining 22 of 100 (22%) being post positive smear. Despite this, 17 of 100 (28.3%) of MTB-D results occurred in patients not yet started on treatment.
Table 1a.
CBNAAT result by sample type
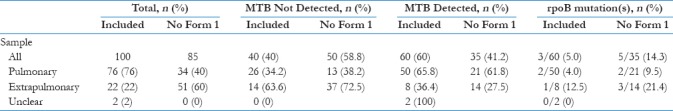
Table 1b.
CBNAAT result by prior treatment status
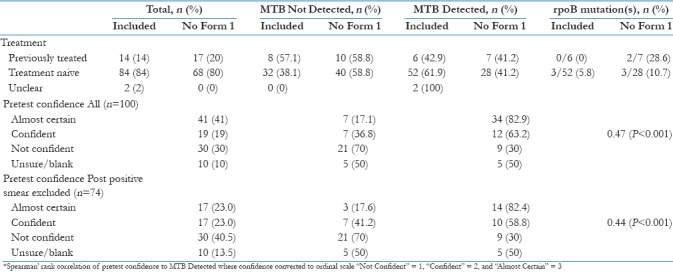
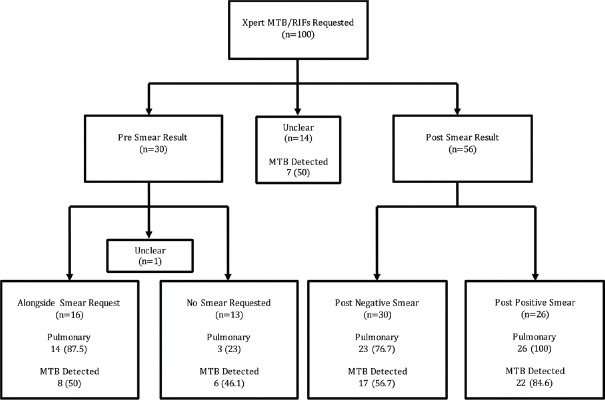
Figure 2 details whether CBNAATs were requested before or after a smear result. The 30 CBNAATs requested before a smear result are subdivided by whether a smear was also requested alongside the CBNAAT. The 56 CBNAATs requested after a smear result are subdivided by the smear result. “Unclear” denotes instances where the clinician either selected “unsure” or left blank the relevant question in Form 1.
Figure 2.
CBNAAT results by relationship to smear, n (%)
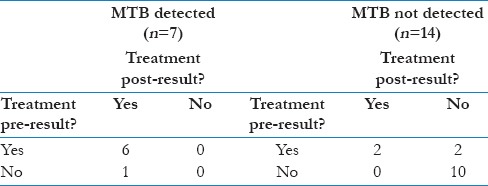
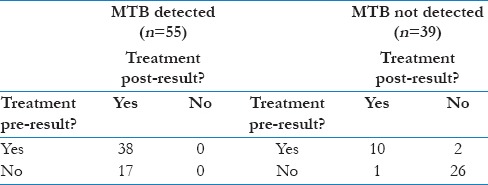
Table 2b and c describes CBNAAT influence on treatment decisions for the 94 patients where this information was available (55 MTB-D and 39 MTB-ND). Following an MTB-D result, all 17 patients not on treatment started and all 38 on so already continued. All three with rpoB mutations started RR-TB treatment. Following an MTB-ND result, 26 of 27 (96.3%) of patients not yet on treatment remained so, but only 2 of 12 (16.7%) already on treatment stopped.
Table 2b.
CBNAAT result impact on treatment status - Pulmonary (n=71)

Table 2c.
CBNAAT result impact on treatment status - Extra-Pulmonary (n=21)
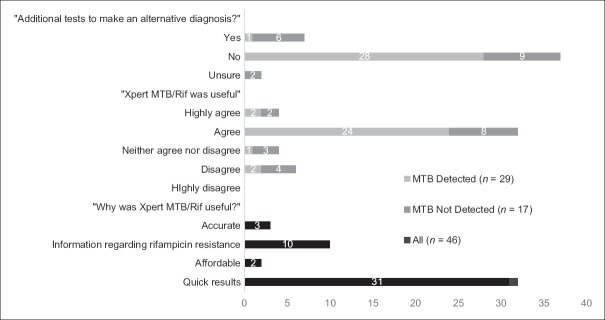
Clinicians were more likely to investigate for an alternative diagnosis where CBNAAT did not detect MTB (6/17, 35.3%) than where it did (1/29, 3.5%) [Figure 3]. Figure 3 also displays qualitative information regarding the clinician-perceived utility of CBNAAT. In 36 of 46 (78%) of encounters, the clinician either “Agreed” or “Highly Agreed”’ the result was useful. They were more likely to do so following an MTB-D result (26/29, 89.7%) than an MTB-ND one (10/17, 58.8%).
Figure 3.
Qualitative feedback on utility of CBNAAT (n=46)
Discussion
Impact of “MTB Detected” results
At the time of CBNAAT request, 56 of 100 (56%) of patients were already on treatment, this being empirical in 34 of 100 (34%). Despite this, 17 of 60 (28.3%) of MTB-D results occurred in patients not yet started on treatment. Following an MTB-D result, all 17 patients not on treatment started and all 38 on treatment continued. While this may suggest that MTB-D results increased true-positive treatment, these patients may have started treatment empirically anyway.
In TB-NEAT,[5] 1502 patients from healthcare facilities across southern Africa were randomized to either same-day smear or CBNAAT. While CBNAAT increased same-day diagnosis (24% vs 13%, P < 0.0001) and treatment (23% vs 15%, P = 0.0002), by day 56 treatment rates were similar (43% v. 42%, P = 0.6408). This was likely due to high levels of empirical treatment in the smear group (26%). Although CBNAAT reduced the time-to-treatment, no impact on morbidity was detected indicating that CBNAAT merely “replaced,” rather than “supplemented,” empirical treatment. Against this, however, is the fact that 7% more of those treated in the CBNAAT group were culture-positive.
XTEND[6] was a pragmatic trial examining the impact of CBNAAT's implementation across South Africa. In a cluster design, 20 laboratories were randomized to initial CBNAAT or smear. In agreement with TB-NEAT, CBNAAT increased bacteriological diagnosis (78.4% vs 65%, P = 0.07) but did not affect treatment rates or, in this case, mortality at 6 months.
Similar findings emerged from trials in South Africa[7,8] and Brazil,[9] but they may not translate to our high-burden, low-resource rural Indian setting. Our population is notable for lower rates of HIV (2% vs 60% in TB-NEAT[5]) and higher rates of malnutrition (BMI <18.5 kg/m2 in 85.4% vs 10.5% in XTEND[6]). Our patients’ have little physical reserve at presentation and so early treatment could have a greater impact.
A pre–post study evaluated CBNAATs’ introduction across 14 subdistrict level TB program units in India.[10] In agreement with prior studies, CBNAAT increased bacteriologically confirmed TB case notification [by 39%, 95% confidence interval (CI) 1%–64%]. There was also a 16% (95% CI 1%–33%) increase in overall case notification rate, but reported rates of empirical treatment were dubiously low (4.4% in smear group, 1.8% following CBNAAT). The authors observed that empirical treatment may not have been notified if it was started elsewhere after initial negative testing at the TB program unit.
Taken together, these trials suggest that where a clinician's pretest confidence in TB is sufficiently great, an MTB-D result may have little effect on patient outcome. This is because empirical treatment would have been started anyway and the reduced time-to-treatment with CBNAAT has not been shown to reduce morbidity or mortality. In our study, however, even where the clinician's pretest confidence in TB was low, 9 of 30 (30%) of CBNAATs detected MTB [Table 1c]. These patients may be less likely to receive empirical treatment. Furthermore, none of the three patients with rpoB mutations was already on RR-TB treatment and all were referred to the regional Drug Resistant TB Care Centre following the result.
Impact of “MTB Not Detected” results
Following an MTB-ND result, 26 of 27 (96.3%) of patients not yet on treatment remained so, but only 2 of 12 (16.7%) already on treatment stopped [Table 2a]. An MTB-D result was thus more likely to prompt treatment initiation than was an MTB-ND result treatment cessation (100% vs 16.7%).
Table 2a.
CBNAAT result impact on treatment status - All samples (n=94)
Continuing treatment despite an MTB-ND result is not unreasonable in a high-burden setting where the pretest probability of TB is high. Even for pulmonary TB, CBNAAT is only 89% (95% CI 85%–92%) sensitive as compared with sputum culture,[1] and sputum culture itself (the “gold standard”) is not 100% sensitive.[11]
In a Ugandan study of 90 patients with HIV, 22 (25%) started treatment despite a negative CBNAAT result and 50 (56%) started without a CBNAAT request despite this being available.[12] In this setting, the pretest probability was often so high that an MTB-ND result would not have reduced the posttest probability below the clinician's treatment threshold. This being so, not even requesting a CBNAAT could be justified.[13]
Interestingly, in our study, clinicians were more likely to “Agree“/”Highly Agree” that CBNAAT was useful after it gave an MTB-D result than an MTB-ND one (26/29, 89.7% vs 10/17, 58.8%; [Figure 3]). This may reflect the greater impact MTB-D results had on their treatment decisions. Overall, clinician satisfaction with the test appeared comparable to previous studies.[14] But while empirical treatment can often be justified, in the face of an MTB-ND result, the harms of treatment in the absence of a bacteriologically confirmed diagnosis must be considered. This includes not just the direct toxicity of medications, but also the stigma and economic burden that a diagnosis of TB can bring.[15]
The median time from CBNAAT request to result at our institution was 5 days (IQR 0–15 days). This may have made it difficult for clinicians to stop empirical therapy if there had been a clinical response in the interim. Furthermore, it is significant that 96.3% of treatment-naive patients remained so following an MTB-ND result. A new version of GeneXpert (GeneXpert Omni) is smaller, battery-operated, and expected to be cheaper than the existing model which could make it viable for rural settings.[16] It may be that same-day MTB-ND results at presentation have greater impact on reducing initiation of empirical treatment (which is different than stopping once already begun). Alternatively, future roll out of GeneXpert Ultra (which has greater sensitivity over the existing model) may provide clinicians with greater confidence in an MTB-ND result.
How is CBNAAT best used?
(a) By pretest probability
Our results suggest that in a low-resource, high-burden setting where CBNAAT use must be rationalized, it may be better to target it at those cases where the clinician is not confident in the diagnosis of TB. CBNAAT still detected a significant number of cases in this group (9/30, 30%; [Table 1c]) and MTB-ND results may enable the clinician to hold-off treatment. In contrast, where the pretest probability is deemed high, the patient will probably be treated whatever the result. There are problems with this approach. First, MTB-ND results may still trigger clinicians to search for an alternative diagnosis – 35.3% in our study [Figure 3]. Second, even where a patient is already on treatment, an MTB-D result may detect an rpoB mutation conferring RR (5% of MTB-D results in our study). Finally, an MTB-D result may offer additional benefits such as motivating infection control measures, contact tracing, or patient engagement with treatment. It can also ensure new clinicians have confidence in the diagnosis, where a patient moves between health providers.[17]
(b) By relation to smear
In our study, 30 of 100 (30%) of CBNAATs were used as an initial diagnostic test [Figure 2]. World Health Organization currently recommends that CBNAAT may replace smear in this way in any patient and should do so for those with suspected multidrug resistant (MDR)-TB or HIV-associated TB.[2]
CBNAAT detected MTB in 17 of 30 (56.7%) of patients when using post negative smear [Figure 2] and 6 of 17 (35.3%) of these were not already on treatment. Our results align with a Cochrane review that found CBNAAT has excellent yield when used as an “add-on-test” following a negative smear – 67% sensitivity (95% CI 60%–74%).[1] This approach also aligns with Revised National Tuberculosis Control Programme (RNTCP) guidelines that advocate CBNAAT should be used post negative smear where clinical suspicion of TB remains high.[18]
Surprisingly, 26 of 100 (26%) of our CBNAATs were requested post positive smear. All 26 were sputum samples, none had HIV, and only one had been previously treated so none had conventional risk factors for drug resistance. This may not represent the best use of CBNAAT in a low-resource setting where the pretest probability of TB is high, unless there are risk factors for RR-TB or suspicion of a non-tubercular mycobacterium. Most of these patients will be treated based on the smear so the result is unlikely to affect patient management.
In a recent study spanning four Indian cities, clinicians were interviewed to explore their perspectives on the utility of CBNAAT in children with presumptive TB.[17] Interestingly, despite CBNAAT being made available free of cost, clinicians diverged in various ways from RNTCP guidance that recommends its use “upfront” as a replacement for smear in children. Instead, some clinicians reported using CBNAAT only after Mantoux and chest X-ray were suggestive; others used it post-smear and in some cases CBNAAT was used only after a trial of empirical treatment. This study highlights that cost and availability are not the only barriers to effective implementation of CBNAAT. If CBNAAT is to have its greatest impact, clinician understanding and confidence in the test are paramount.
(c) In certain populations
RNTCP advocates CBNAAT use in children, HIV-associated TB, and extrapulmonary TB because of its increased sensitivity over smear.[18,17] While our study only included three CBNAATs performed on children and two with HIV, 22 of 100 (22%) were performed on extrapulmonary samples. This figure rises to 73 of 185 (39.5%) if requests missing a Form 1 are included [Table 1a].
Extrapulmonary CBNAATs were less likely to detect MTB than pulmonary (36.4% vs 65.8%; Table 1a) and most extrapulmonary MTB-D results occurred in patients already on treatment (6/7, 85.7%; Table 2c). This was despite rates of pre-CBNAAT treatment being similar between pulmonary and extrapulmonary patients (54.9% vs 47.6%; Table 2b and c). While this might suggest extrapulmonary CBNAATs had less impact, numbers are very small. Furthermore, extrapulmonary MTB-D results could have greater impact than in pulmonary TB on the rationalization of treatments and investigations for alternative diagnosis.
RNTCP guidelines advocate CBNAAT use for the detection of drug resistance in those at risk, such as previously treated patients. CBNAATs were requested on 14 such patients in our study, 6 of whom had MTB-detected, but none had rpoB mutations [Table 1b]. The three patients with rpoB mutation did not have traditional risk factors for drug resistance which raises the question: what prevalence of RR-TB should trigger more liberal use of CBNAAT? In India, the estimated prevalence of MDR/RR-TB is 2.8% of new cases and 12% in those previously treated so targeting CBNAAT at those previously treated seems reasonable.[19] With “No Form 1” patients included, RR-TB represented 7.5% of new cases and 15.4% of those previously treated suggesting our rates might converge toward the national average with greater numbers. In the pre–post Indian CBNAAT study,[10] rpoB mutations were more common in those previously treated (24.1% vs 5.8%), but 30.4% of RR-TB cases occurred among new cases overall.
As a rapid point of care test, CBNAAT can identify RR-TB much quicker than conventional drug-susceptibility testing enabling prompt treatment and infection control measures. This benefit may be diminished if testing becomes centralized, as is increasingly common in India.
Limitations
Our study's greatest limitation is that 85of 206 (41.3%) of CBNAATs requested within the study period were not included in the main analysis because no Form 1 was completed [Figure 1]. This leaves the possibility of a selection bias whereby certain clinicians were more likely to fill out the forms. This may have the strongest influence on the qualitative questions and judgments about pretest confidence and postresult management. Patients missing a Form 1 were of a similar age and BMI to those included (median age 40 vs 44 years, median BMI 16.5 vs 16.7 kg/m2). The results from “No Form 1” patients are included in Table 1 for comparison. The most striking difference is the higher proportion of extrapulmonary samples (60% vs 22%) with a resultant lower proportion of MTB-D results overall (35% vs 60%). MTB-D results were less likely to prompt treatment initiation in extrapulmonary samples, so the test's overall impact on patient care may have been overestimated.
Asking clinicians to rate their pretest confidence in TB is a subjective endeavour. Furthermore, while our questionnaire asked questions of an individual, actual management decisions are often made in consultation with others. A future study might find it more insightful to ask a team how likely they are to treat for TB in the event of an MTB-ND result. If they are very likely to treat anyway, one might question the cost-effectiveness of the test.[13] If they would hold-off treatment, and the result is subsequently MTB-D, this will identify patients where CBNAAT prompted treatment. Alternatively, one could attempt an objective assessment of the pretest probability of TB rather than gage clinicians’ subjective belief in the diagnosis. However, given that it is ultimately the clinician's confidence in the diagnosis that influences their treatment decisions, this may not be more insightful.
Our patients were notable for low rates of HIV (2%), low BMIs (median 16.5 kg/m2), and a high proportion of MTB-D results (60%). Our findings may not translate populations that differ from these characteristics. In particular, they are unlikely to be relevant to a low-burden high-income setting.
Conclusion
Assuming drug resistance is not a concern, in a low-resource, high-burden setting, CBNAAT may have greatest impact where the clinician's pretest confidence in TB is low and empirical treatment has not been started. This is because MTB-D results will lead to appropriate initiation of treatment and MTB-ND results are more likely to be relied upon to hold-off treatment than where it has already been begun.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Yogesh Jain had the idea to explore utility of CBNAAT use at our institution and oversaw various drafts of the piece. Sushil Patil helped design and implement the questionnaires as well as collected data. Jonathan Youngs was responsible for overall study design, data analysis, and writing of the piece. We would also like to thank Benjamin Marshall, Nick Russo, Timothy Laux, and Naman Shah for their contributions.
Appendix 1 – Form 1 and Form 2
Forms 1 and 2 were embedded into the Electronic Medical Record (EMR) system of our institution - Jan Swasthya Sahyog (JSS) Health Centre.
The below screenshots are provided to give an idea of the format.
FORM 1
-
Testing Centre:
- JSS
- Bilaspur
- Jabalpur
- Unsure
-
Sample:
- Sputum
- Lymph Node FNA
- Pleural Fluid
- Ascitic Fluid
- CSF
- Tissue
- Other (please specify)
- Unsure
-
HIV Status:
- HIV +ve
- HIV –ve
- Unsure
-
Diabetes Status:
- Diabetic
- Not Diabetic
- Unsure
-
Has your patient previously been treated for TB?
- Yes
- No
- Unsure
- Other (please specify)
-
When are you requesting this CBNAAT?
- As an initial test before any smear-microscopy result
- After a POSITIVE smear-microscopy result
- After a NEGATIVE smear-microscopy result
- Other (please specify)
- Unsure
-
Are you also requesting smear-microscopy as well as this CBNAAT?
- Yes
- No
- Other (please specify)
- Unsure
-
I am using this CBNAAT to:
- To detect MTB
- Primarily to detect MTB but also to detect rifampicin resistance
- To detect MTB and rifampicin resistance
- Primarily to detect rifampicin resistance but also to detect MTB
- To detect Rifampicin resistance
-
Are you?
- Almost certain your patient has active MTB (for example, they already have a positive smear)
- Confident that your patient has active MTB (for example, symptoms, signs, radiology or histology make MTB your primary diagnosis)
- Not confident that your patient has MTB (for example, symptoms, signs, radiology or histology make you consider MTB, but it is not necessarily your primary diagnosis)
- Unsure
-
Do you consider your patient:
- At high-risk of rifampicin resistant MTB (for example, previous treatment failure or known MDR-TB contact)
- At normal-risk of rifampicin resistant MTB (for example, new presentation with possible TB and no risk factors for MDR-TB)
- Unsure
-
Is your patient:
- Already taking, or about to start taking, first line ATT (Rifampicin, Isoniazid, Ethambutol and Pyrazinamide)
- Already taking, or about to start taking, second line ATT (use of any agent other than Rifampicin, Isoniazid, Ethambutol and Pyrazinamide)
- Not taking, or about to start taking, ATT
- Unsure
When was the sample for this CBNAAT taken?
When did this patient present to JSS with symptoms of possible TB?
FORM 2
-
Was CBNAAT:
- Positive for MTB
- Negative for MTB
- Indeterminate for MTB
-
Was CBNAAT Result:
- Positive for Rifampicin resistance
- Negative for Rifampicin resistance
- Indeterminate for Rifampicin resistance
-
Following this CBNNAT result will you:
- Start first line ATT (they were not already on any ATT)
- Continue first line ATT (they were already on first line ATT)
- Step-down to first line ATT (they were taking second line ATT)
- Start second line ATT (they were not already on any ATT)
- Step-up to second line ATT (they were taking first line ATT)
- Continue second line ATT (they were already on second line ATT)
- Stop ATT altogether
- Remain off ATT (they were not already on any ATT)
- Other (please specify)
- Unsure
-
Following this CBNNAT result will your patient:
- Remain in the same inpatient bed they are in now
- Be stepped-up to greater respiratory isolation (For example, to the TB ward from a standard ward, or from the TB ward to a side room)
- Be stepped-down to lesser respiratory isolation (For example, to the TB ward from a side room, or from the TB ward to a standard ward)
- Remain an outpatient
- Unsure
- Other (please specify)
-
Are you now:
- Almost certain your patient has active MTB
- Confident that your patient has active MTB
- Not confident that your patient has MTB
- Unsure
-
Are you currently planning to request additional tests to diagnose TB:
- Yes
- No
- Unsure
-
Are you currently planning to request additional tests to make an alternative diagnosis to TB:
- Yes
- No
- Unsure
-
Overall CBNAAT was useful in guiding the management of this patient:
- Highly agree
- Agree
- Neither agree nor disagree
- Disagree
- Highly Disagree
-
Why was CBNAAT useful? (please select all options that you feel apply – i.e. you can select more than one):
- Affordable
- Quick Results
- Accurate Results
- Information regarding rifampicin resistance
- Easily Available
- Unsure
- Other (please specify)
When did you see this CBNAAT result?
Any other comments?
References
- 1.Steingart K, Schiller I, Horne D, Pai M, Boehme, CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults (Review) Cochrane Database Syst Rev. 2014;1:CD 009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Automated Real. Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy update. World Heal Organ. 2013:1–79. [PubMed] [Google Scholar]
- 3.Jan Swasthya Sahyog (JSS) Health Centre. [Last accessed on 2018 Feb 17]. Available from: www.jssbilaspur.org .
- 4.HEAL initiative. [Last accessed on 2018 Feb 22]. Available from: www.healinitiative.org .
- 5.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: A multicentre, randomised, controlled trial. Lancet. 2014;383:424–35. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 6.Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: A cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3:e450–7. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 7.Cox HS, Mbhele S, Mohess N, Whitelaw A, Muller O, Zemanay W, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: A pragmatic randomised trial. PLoS Med. 2014;11:1–12. doi: 10.1371/journal.pmed.1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermans S, Caldwell J, Kaplan R, Wood R. The impact of the roll-out of rapid molecular diagnostic testing for tuberculosis on empirical treatment in Cape Town, South Africa. Bull World Heal Organ. 2017;95:554–63. doi: 10.2471/BLT.16.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durovni B, Saraceni V, van den Hof S, Trajman A, Cordeiro-Santos M, Cavalcante S, et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: A stepped-wedge cluster-randomized trial. PLoS Med. 2014:11. doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdeva KS, Raizada N, Sreenivas A, Van’t Hoog AH, van den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachh A, Gupta R, Haq I, Varudkar H. Diagnosing sputum/smear-negative pulmonary tuberculosis: Does fibre-optic bronchoscopy play a significant role? Lung India. 2010;27:58. doi: 10.4103/0970-2113.63607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decroo T, Henríquez-Trujillo AR, De Weggheleire A, Lynen L. Rational use of Xpert testing in patients with presumptive TB: Clinicians should be encouraged to use the test-treat threshold. BMC Infect Dis. 2017;17:1–6. doi: 10.1186/s12879-017-2798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyles TH. Why do clinical trials of Xpert W MTB/RIF fail to show an effect on patient relevant outcomes? Int J Tuberc Lung Dis. 2017;21:249–50. doi: 10.5588/ijtld.16.0801. [DOI] [PubMed] [Google Scholar]
- 14.Davids M, Dheda K, Pai NP, Cogill D, Pai M, Engel N. A survey on use of rapid tests and tuberculosis diagnostic practices by primary health care providers in South Africa: Implications for the development of new point-of-care tests. PLoS One. 2015;10:1–14. doi: 10.1371/journal.pone.0141453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14:527–32. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran R, Muniyandi M. Rapid molecular diagnostics for multi-drug resistant tuberculosis in India. Expert Rev Anti Infect Ther. 2018;16:197–204. doi: 10.1080/14787210.2018.1438262. [DOI] [PubMed] [Google Scholar]
- 17.McDowell A, Raizada N, Khaparde SD, Rao R, Sarin S, Kalra A, et al. “Before Xpert I only had my expertise”: A qualitative study on the utilization and effects of Xpert technology among pediatricians in 4 Indian cities. PLoS One. 2018;13:1–17. doi: 10.1371/journal.pone.0193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Central TB Division; Government of India. Revised National TB Control Programme: Tecnical and Operational Guidelines for Tuberculosis Control in India. Chapter 3: Case finding & diagnosis strategy. Minist Heal Fam Welfare, Govt. India. 2016:14. [Google Scholar]
- 19.WHO. Global Tuberculosis Report 2017. World Heal Organ. 2017:45. [Google Scholar]