Abstract
Background
Sleep dysfunctions impose a large burden on quality of life for patients with Parkinson’s disease (PD). Several studies on PD reported potential therapeutic effects of transcranial direct current stimulation (tDCS) on motor and non-motor functions, but not related to sleep quality. Therefore, the present study examined sleep quality, depression perception, and quality of life changes after bilateral anodal tDCS in patients with PD.
Material/Methods
Twenty-one patients (n=21) with PD underwent 10 sessions (20 min each, 5 per week) of bilateral anodal tDCS stimulation applied simultaneously over the left and right prefrontal and motor areas. The Pittsburgh Sleep Quality Index (PSQI) total score and sub-scores, Geriatric Depression Scale (GDS), and Health-related quality of life questionnaire (SF-36) were measured pre/post bilateral tDCS anodal stimulation.
Results
PSQI total score (P=0.045), sleep latency sub-score (P=0.02), and GDS total score (P=0.016) significantly decreased, and physical and mental components scores of SF-36 (P=0.018 and P=0.001, respectively) significantly increased after bilateral anodal tDCS stimulation. The GDS score decrease was directly correlated with decrease in PSQI total score (P=0.01), sleep latency sub-score (P=0.002), and sleep disturbance sub-score (P=0.003). In addition, the GDS score decrease was inversely correlated with increasing mental component score of SF-36 (P=0.001), which was directly correlated with an increase in sleep efficiency sub-score (P=0.03) and the physical component score of SF-36 (P=0.0001).
Conclusions
Bilateral anodal tDCS stimulation showed potential therapeutic effects in patients with PD in terms of sleep quality and depression level improvement, which together improved mental and physical quality of life in patients with PD.
MeSH Keywords: Depression, Electric Stimulation Therapy, Parkinson Disease, Quality of Life, Sleep
Background
Parkinson’s disease (PD) is a prevalent neurodegenerative disease associated with common motor and non-motor complications such as tremor, rigidity, bradykinesia, cognitive impairments, psychiatric, autonomic, and sensory dysfunctions [1,2]. Recently, studies have focused on sleep quality measures as an important non-motor symptom that might negatively impact individuals with PD. Community-based studies reported 40% of patients with PD suffer from sleep quality disturbances [3,4]. Sleep disturbances were found to contribute to a high level of disability and reduced quality of life. In addition, PD patients who suffer from sleep disturbances were found to be at high risk of suffering from neuropsychiatric complications such as depression. This was confirmed in previous cross-sectional studies showing that approximately 40% of patients with PD suffer from depression [5,6]. Despite the association between poor sleep quality in PD and compromised quality of life indices (including physical, social, and psychological functions), interventions that target improving sleep quality are rare. This is because previous studies on PD that reported correlation data and associations between sleep disturbances and depression [7], depression and mental health [6,7], and sleep, depression, and quality of life [8] were cross-sectional designs with no therapeutic intervention.
Recently, noninvasive brain stimulation techniques, especially transcranial direct current stimulation (tDCS), have been introduced as a safe and effective treatment option for PD, as it overcomes many complications associated with drug therapy and surgical intervention (e.g., dopamine-resistant symptoms, cognitive deficits, depression, dementia, and hallucinations) [9–11].
Previous tDCS studies in PD have traditionally focused their investigation on motor or cognitive functions [12–17], although many studies have examined the effect of tDCS on sleep quality measures [18–22], there were discrepancies in their findings and little attention has been given to investigating the effect of tDCS on sleep quality and depression, and its association with quality of life in patients with PD. Only 1 tDCS study examined the effects of unilateral anodal tDCS over the prefrontal cortex on sleep quality and depression in patients with PD [18]; however, it reported that both the left and right prefrontal, premotor area, and primary motor area are all involved in sleep regulation and depression pathophysiology [23–26]. The study used only daytime sleepiness as a measure of sleep quality and reported contradictory findings, and the researchers recommended further studies that measure more sleep domains, such as overnight sleep, sleep latency, and duration, as well as depression and its association with sleep [18]. To the best of our knowledge, no study has investigated the effect of bilateral anodal tDCS stimulation applied over the left and right prefrontal cortex, premotor, and primary motor areas on sleep quality, health-related quality of life, and depression measures and their potential association in patients with PD.
Therefore, the major aims of this study were: 1) to identify the potential therapeutic effects of bilateral anodal tDCS stimulation on sleep quality, depression, and quality of life measures in patients with PD; and 2) to identify the potential association between sleep quality, depression perception level, and quality of life changes after the stimulation in patients with PD, hypothesizing that there would be a therapeutic effect of bilateral anodal tDCS on these outcome measurements in patients with PD, and there would be a direct correlation between these outcome measurement changes.
Material and Methods
Study design
This was a feasibility study with pre/post-intervention design, examining the potential therapeutic effects of bilateral anodal tDCS stimulation on sleep quality, quality of life, and depression perception in patients with PD. All procedures performed involving human participants were in accordance with the ethical standards of the Institutional Research Committee (Jordan University of Science and Technology/Institutional Review Board (approval No. 2016/97/8) and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all patients before participation in the study.
Participants
During 1 year of recruitment, a convenience sampling of 21 subjects (mean age 62 years, range 43–76 years, 15 males and 6 females) with idiopathic PD participated in the study. All participants were in stages 1 to 5 (modified Hoehn and Yahr stage), at “on” stage of medication, without a history of deep brain stimulation, in a stable medical regimen for at least 3 weeks before entering the study, and had to remain clinically stable throughout the study. Patients with seizures and/or brain metal implants were excluded. For the duration of the study, patients had to maintain the same dose of medication, by which the assessments and therapeutic sessions were conducted during the peak effect of L-dopa medication (1 hour after medication intake) to avoid any problems relevant to “on–off” periods associated with PD. In addition, evaluation and assessment were carried out within 3 days before starting the tDCS therapy and after the completion of tDCS therapy.
Intervention protocol
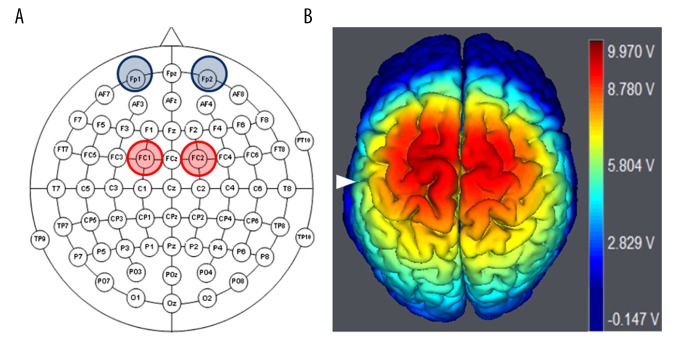
Previous unilateral anodal tDCS studies in a patient with PD used the 10–20 EEG system, where anode tDCS stimulation was applied over C3 as the excitation site for M1 or over F3 as the excitation site for DLPFC [10–16]. However, in this study, bilateral anodal tDCS stimulation used the 10–10 EEG system to place 2 anode electrodes over the left FC1 and right FC2, and 2 cathode electrodes were placed over the left and right supraorbital areas, respectively (Figure 1). FC1 and FC2 are just between and over the left and right M1 and DLPFC, respectively. Therefore, these anodal electrodes placement over FC1 and FC2 was chosen to stimulate the M1 and DLPFC of the right and left hemispheres simultaneously, and it has been confirmed in a previous study [27] that investigated the spatial distribution, magnitude, and direction of current density in the computer-aided spherical head model during tDCS, and reported the accurate excitation locations as well as the safety of the protocol.
Figure 1.
Electrodes placement of bilateral anodal tDCS. (A) 10–10 EEG schematic view showing bilateral anodal tDCS electrodes placement, in which 2 anodal electrodes are placed over FC1 and FC2, and the cathode electrodes are placed over the left and right supraorbital area. (B) 3D brain image is structured by using software (Neuroelectric, NIC, Spain) to show the simulated electric field distribution in the bilateral DLPFC, supplementary motor, and M1 areas generated by the bilateral anodal tDCS stimulation, White triangle indicates central sulcus.
Each patient received 10 sessions (5 sessions per week for 2 weeks) of bilateral anodal stimulation of low-intensity direct electrical current (1 mA per each electrode) for 20 min per session. The anode and cathode electrodes were 25 cm2 surface area and the skin was moistened. The tDCS was mainly applied in the morning hours between 9.00 am and 12.00 pm. This was consistent between all subjects, and this stimulation dose and protocol exhibited no adverse or side effects on our patients’ health.
Outcome measures
Three main outcome measures were examined before and after the bilateral anodal tDCS therapy: (1) sleep quality, (2) quality of life, and (3) depression measures. In terms of sleep quality, quality of life, and depression measures, 3 scales were evaluated before and after the bilateral anodal stimulation: the Pittsburgh Sleep Quality Index (PSQI) for sleep quality measures, the short form health status questionnaire (SF-36) for health-related quality of life measures, and the Geriatric Depression Scale (GDS) for depression measures.
The PSQI is a well-known, validated, and reliable self-reported instrument used to measure the clinical construct of sleep quality. It consists of 18 questions scored from 0 (no difficulty) to 3 (severe difficulty) and ends with total score and sub-scores of 7 domains: 1) sleep quality, 2) sleep latency, 3) sleep duration, 4) sleep medication, 5) sleep disturbances, 6) daytime dysfunction, and 7) habitual sleep efficiency [28]. The SF-36 is a valid and reliable tool to evaluate the perceived health-related quality of life and level of participation. It is a self-reported questionnaire and consists of 36 questions measured as a total score of 2 domains (physical and mental components summary scores) and sub-scores of 8 domains: 1) physical functioning, 2) role physical, 3) vitality/energy, 4) bodily pain, 5) social functioning, 6) role emotional, 7) mental health, and 8) general health perceptions [29]. SF-36 items and scales are scored so that a higher score indicates a better health state. For example, functioning scales are scored so that a high score indicates better functioning and the pain scale is scored so that a high score indicates freedom from pain. After data entry, items and scales are scored in 3 steps: 1) item recoding, for the items that require recoding; 2) computing scale scores by summing across items in the same scale (raw scale scores); and then 3) transforming raw scale scores to a 0–100 scale (transformed scale scores) [30]. The GDS is a 30-item questionnaire and is a highly reliable and valid screening tool used to measure the depression level across different ages, genders, and ethnicities in community and social service settings [31].
Statistics
Paired t tests were used to compare the outcome measures between pre- and post-conditions. In addition, Pearson’s correlation test was used to identify if there was any correlation between the changes in outcome measurements after the bilateral anodal tDCS (i.e., if the change scores in depression, sleep, and quality of life outcomes correlate with each other). This correlational analysis was only exploratory and was performed to provide further insight into the obtained findings. Only the significant results are reported here.
Results
Twenty-one participants completed the study with no reported side effects. The average duration of PD diagnosis was 7.0±2.9 years, and the average modified Hoehn and Yahr stage in the “on” state was 3.0±0.8 (Table 1).
Table 1.
Demographic data.
| N=21 | Age (years) | Disease duration (years) | Hoehn and Yahr stage |
|---|---|---|---|
| Female (n=6) | 63.0±9.5 | 7.0±2.3 | 3.0±0.8 |
| Male (n=15) | 62.0±8.4 | 7.0±3.2 | 3.0±0.8 |
The table shows participants’ demographic data including gender, age, years of diagnosis, and their disease stage according to modified Hoehn and Yahr Scale. Data presented as mean ±SD.
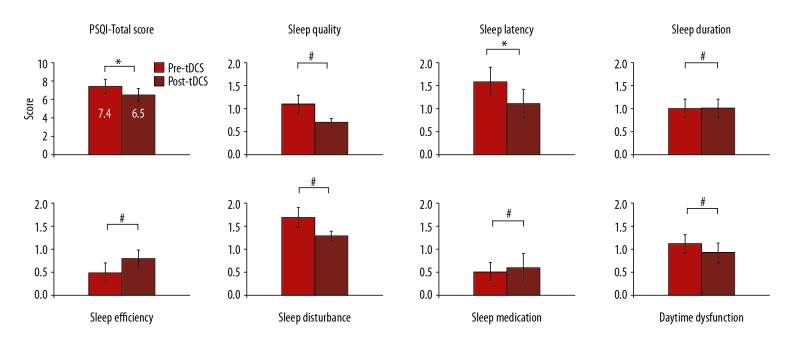
For the sleep quality measures, the results showed significant improvements in sleep quality. A paired t test showed a significant decrease in the mean total score of the PSQI (P=0.045) and its sleep latency domain sub-scores (P=0.02) after bilateral anodal tDCS stimulation. Sleep quality, sleep duration, sleep medication, sleep disturbances, daytime dysfunction, and habitual sleep efficiency domain sub-scores of the PSQI questionnaire were remarkably decreased after the bilateral anodal tDCS stimulation; however, the decrease in sub-scores was not statistically significant (P>0.05) (Figure 2).
Figure 2.
Sleep quality scores. Graph shows the comparison between the calculated PSQI total score and sub-scores before and after the bilateral anodal tDCS stimulation. * Indicates significant difference at P<0.05, and # indicates difference is not significant at P<0.05.
For the depression perceived level, the paired t test showed a significant decrease in the mean total score of GDS (P=0.016) after bilateral anodal tDCS stimulation compared with before tDCS stimulation (Figure 3).
Figure 3.
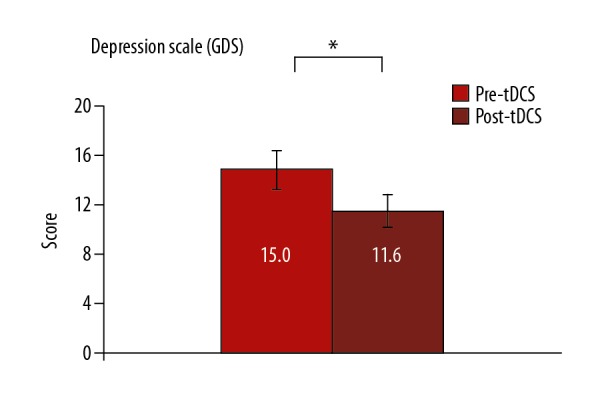
Depression perception level. Graph shows the comparison between the calculated GDS total score before and after the bilateral anodal tDCS stimulation. * Indicates significant decrease (P<0.05) in patient depression level after bilateral anodal tDCS stimulation.
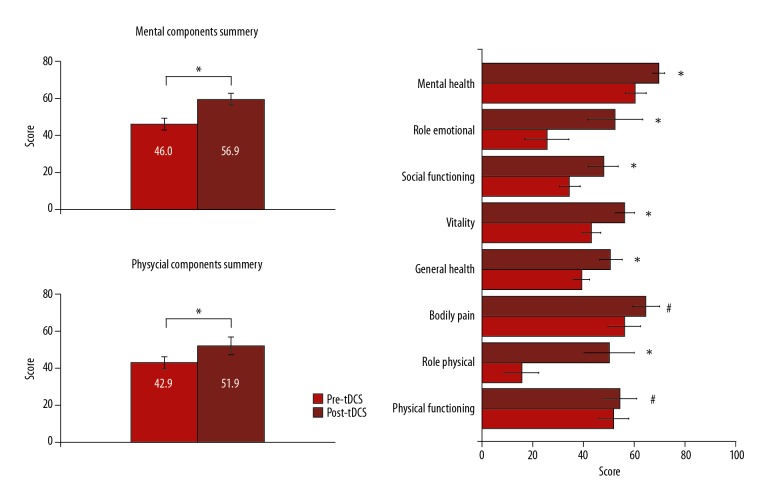
Health-related quality of life (SF-36 measures) data showed that there were significant improvements, as the paired t test showed significant increases in the mean total score of the physical and mental components summary score (P=0.018) and (P=0.001), respectively, after bilateral anodal tDCS stimulation. In addition, there were significant changes in the SF-36 domains related to role physical, vitality/energy, social functioning, role emotional, mental health, and general health perceptions’ sub-scores (P<0.05) after bilateral anodal tDCS stimulation compared with their scores before tDCS stimulation. Bodily pain and physical function domains of SF-36 sub-scores were remarkably decreased and increased, respectively, after the bilateral anodal tDCS stimulation; however, the mean changes were not statistically significant (P>0.05) (Figure 4).
Figure 4.
Physical and mental quality of life (SF-36). Graph shows the comparison between the calculated and transformed total score of mental and physical components of health-related quality of life and its sub-scores before and after the bilateral anodal tDCS stimulation. * Indicates significant difference at P<0.05, and # indicates difference is not significant at P<0.05. After data entry, items and scales were scored in 3 steps: 1) item recoding, for the items that require recoding; 2) computing scale scores by summing across items in the same scale (raw scale scores); and then 3) transforming raw scale scores to a 0–100 scale (transformed scale scores).
The correlation between the change score in outcome measurements showed significant relationships between improvements in sleep quality, health-related quality of life, and depression measures after bilateral anodal tDCS therapy. Specifically, Pearson’s correlation test showed significant correlation between a decrease in GDS (depression) score and decrease in sleep latency score (P=0.002), sleep disturbance score (P=0.003), and PSQI total score (P=0.01). A significant inverse correlation was found between the GDS score and the score for mental component summary of SF-36 (P=0.001). In addition, the mental component summary of SF-36 was directly correlated with an increase in sleep efficiency sub-score of PSQI (P=0.03) and the physical component summary of SF-36 (P=0.0001).
Discussion
This is the first study to investigate the effect of bilateral anodal tDCS stimulation on sleep quality, health-related quality of life, and depression measures, and their potential association in patients with PD. In summary, the additional advantages of this study over the previous tDCS studies in PD [12–17], or sleep-related tDCS studies [18–22] are: 1) its bilateral anodal tDCS stimulation montage and protocol applied over motor and prefrontal areas simultaneously; and 2) the sleep quality, health-related quality of life, and depression evaluation in patients with PD. Although many studies have examined the effect of tDCS on sleep quality measures, there were discrepancies in their findings [18–22]. Furthermore, only 1 study evaluated sleep quality in patients with PD after applying unilateral anodal tDCS stimulation over the left DLPFC, and it reported no significant sleep improvement in terms of daytime sleepiness [18]. Although a study by Frase et al. [20] showed that anodal tDCS over left and right DLPFC decreases the total sleep time in healthy participants, a study by Harvey et al. [21] that applied unilateral anodal tDCS over M1 reported no sleep quality improvement in terms of PSQI total score in healthy participants. Both Minichino et al. [19] and Acler et al. [22] reported sleep quality improvement in terms of PSQI score and sub-scores after applying anodal tDCS in patients with bipolar disorders and patients with post-polio syndrome, respectively.
This discrepancy is expected because there is no neuroimaging and clinical study showing preference for one brain hemisphere over the other in chronic PD cases; this is because not only the left DLPFC, SMA, premotor, and M1 areas are involved in PD deficits, but also because the right DLPFC, premotor, and M1 areas are all involved [32]. An fMRI and source modeling EEG have shown that sleep slow waves are primarily associated with activity in a core set of cortical areas that are mainly located in the left and right prefrontal cortex and DLPFC [23]. In addition, an EEG study identified the cortical topography of local sleep and showed that prefrontal cortex and DLPFC activities are associated with sleep functions, as are SMA, premotor, and primary motor (M1) areas [24]. Therefore, we assumed that this study’s bilateral anodal tDCS stimulation protocol over both left and right DLPFC, premotor, and M1 areas served as a comprehensive stimulation protocol assuring activation of the major cortical areas involved in sleep regulation. This, in turn, improved the sleep quality measures (PSQI total score) in our patients with PD.
In terms of applying tDCS many hours before the sleeping time instead of just before the sleeping time, it was reported that repeated or multi tDCS sessions lead to cumulative therapeutic effects more than that of a single tDCS session, when compared with baseline [33]. In addition, Frase et al. [20] examined the tDCS therapeutic effect on sleep by measuring the EEG polysomnography before tDCS (T0), immediately after a single tDCS session (T1 at 11 pm, while the patient is sleeping), and the next day morning (T2, at 7 am), and they reported a therapeutic effect of tDCS on sleep in T1/T2 – EEG records compared with T0 – EEG record, and there were no differences in EEG recording between T1 and T2 [20]. Therefore, this study examined the cumulative effects of 10 sessions of tDCS on the sleep domains instead of a single-session effect, and this comes with the previous tDCS protocol in previous tDCS studies on sleep [18,22].
In terms of tDCS and depression, several functional imaging, MRI, and actigraphy studies have sought to identify brain areas involved in depression, and they reported abnormally low levels of DLPFC activity in patients with depression [25], as well as the involvement of supplementary and primary motor areas in patients with major depressive disorders [26]. In addition, previous tDCS studies reported the antidepressant effect of tDCS in patients with PD [34] or patients with various neurological disorders [35]. On the other hand, previous clinical and EEG studies reported correlation data and association between sleep disturbances and latency, and depression in various neurological disorders [36,37], and in patients with PD [10]. Therefore, we assumed the significant decrease in depression level after bilateral anodal stimulation, over DLPFC, premotor, and M1 areas, was a result of the direct effect of tDCS stimulation and the indirect effect of sleep quality improvement.
In terms of tDCS and health-related quality of life (SF-36), previous studies on PD reported and provided confirmatory evidence showing that depression, night-time sleep disorders, and fatigue are the variables that most affect the health-related quality of life in patients with PD [38,39]. Nicolettiet et al. [40] reported a significant inverse correlation between the overall psychological well-being score and the depression level, and the extent of sleep disruption in patients with PD, and Caap-Ahlgren et al. [41] reported a significant correlation between sleep disturbance and depression with the decrease in patients’ quality of life (SF-36). In addition, our finding showed a significant inverse correlation between depression level and mental health component of the health-related quality of life (SF-36). Therefore, we assumed that improvement in sleep quality and a decrease in depression level improved the mental well-being of our subjects with PD and also improved their physical well-being.
Limitation
This study was a feasibility study with a few limitations that should be taken into consideration during interpretation of this study’s findings as well as for future studies. The study had no tDCS sham or control group, rather, it was a pre- and post-study design in which the baseline data served as a control group. The study was had a small sample size, and outcomes for sleep were based only on self-reported measures. Therefore, future studies with a control arm that use a large sample size and combine objective data measures beside the self-reported measures are needed. In addition, the intervention was short (2 weeks) with no longer periods of follow-up. Future work with longer periods of follow-up is warranted. It should be further noted that the data reported here should be treated with some caution because the statistical analysis did not account for the number of multiple comparisons. Overall, the feasibility data reported here warrant further randomized controlled trials in accordance with the new Medical Research Council guidelines [42].
Conclusions
In conclusion, bilateral anodal tDCS leads to improved sleep quality and quality of life, and decreased depression level, with no reported adverse effects. Furthermore, a direct correlation between the sleep quality improvement and decrease in depression perception level was found, with both variables showing a direct correlation with improvements in mental and physical quality of life. Therefore, improving sleep quality may have beneficial effects beyond improving sleep because it can reduce depression and improve the quality of life of patients with PD.
Footnotes
Source of support: This work is supported by the Deanship of Research at Jordan University of Science and Technology (grant number 335/2015)
Conflict of Interest
None.
References
- 1.Alrefai A, Habahbih M, Alkhawajah M, et al. Prevalence of Parkinson’s disease in Northern Jordan. Clin Neurol Neurosurg. 2009;11:812–15. doi: 10.1016/j.clineuro.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Maidan I, Nieuwhof F, Bernad-Elazari H, et al. The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: An fNIRS study. Neurorehabil Neural Repair. 2016;30:963–71. doi: 10.1177/1545968316650426. [DOI] [PubMed] [Google Scholar]
- 3.Gjerstad MD, Wentzel-Larsen T, Aarsland D, Larsen JP. Insomnia in Parkinson’s disease: Frequency and progression over time. J Neurol Neurosurg Psychiatry. 2007;78:476–79. doi: 10.1136/jnnp.2006.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandberg E, Larsen JP, Karlsen K. A community based study of sleep disorders in patients with Parkinson’s disease. Mov Disord. 1998;13:895–99. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 5.McDonald WM, Richard IH, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry. 2003;54:363–75. doi: 10.1016/s0006-3223(03)00530-4. [DOI] [PubMed] [Google Scholar]
- 6.Wiesli D, Meyer A, Fuhr P, Gschwandtner U. Influence of mild cognitive impairment, depression, and anxiety on the quality of life of patients with Parkinson disease. Dement Geriatr Cogn Disord. 2017;7:297–308. doi: 10.1159/000478849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowding CH, Shenton CL, Salek SS. A review of the health-related quality of life and economic impact of Parkinson’s disease. Drugs Aging. 2006;23:693–721. doi: 10.2165/00002512-200623090-00001. [DOI] [PubMed] [Google Scholar]
- 8.Nicoletti A, Mostile G, Stocchi F, et al. Factors influencing psychological well-being in patients with Parkinson’s disease. PLoS One. 2017;12:e0189682. doi: 10.1371/journal.pone.0189682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weintraub D, Moberg PJ, Duda JE, et al. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52:784–88. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 10.Dowding CH, Shenton CL, Salek SS. A review of the health-related quality of life and economic impact of Parkinson’s disease. Drugs Aging. 2006;23:693–721. doi: 10.2165/00002512-200623090-00001. [DOI] [PubMed] [Google Scholar]
- 11.Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson’s disease: A systematic review. Parkinsonism Relat Disord. 2013;19:783–88. doi: 10.1016/j.parkreldis.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fregni F, Boggio PS, Nitsche M, Pascual-Leone A. Transcranial direct current stimulation. Br J Psychiatry. 2005;186:446–47. doi: 10.1192/bjp.186.5.446. [DOI] [PubMed] [Google Scholar]
- 13.Brunoni AR, Amadera J, Berbel B, et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14:1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 14.Nitsche MA, Paulus W. Transcranial direct current stimulation – update 2011. Restor Neurol Neurosci. 2011;29:463–92. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 15.Fregni F, Boggio PS, Santos MC, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21:1693–702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- 16.Doruk D, Gray Z, Bravo GL, et al. Effects of tDCS on executive function in Parkinson’s disease. Neurosci Lett. 2014;582:27–31. doi: 10.1016/j.neulet.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Lattari E, Costa SS, Campos C, et al. Can transcranial direct current stimulation on the dorsolateral prefrontal cortex improves balance and functional mobility in Parkinson’s disease? Neurosci Lett. 2017;636:165–69. doi: 10.1016/j.neulet.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Forogh B, Rafiei M, Arbabi A, et al. Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson’s disease. Neurol Sci. 2017;38:249–54. doi: 10.1007/s10072-016-2748-x. [DOI] [PubMed] [Google Scholar]
- 19.Minichino A, Bersani FS, Spagnoli F, et al. Prefronto-cerebellar transcranial direct current stimulation improves sleep quality in euthymic bipolar patients: A brief report. Behav Neurol. 2014;2014 doi: 10.1155/2014/876521. 876521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frase L, Piosczyk H, Zittel S, et al. Modulation of total sleep time by transcranial direct current stimulation (tDCS) Neuropsychopharmacology. 2016;41:2577. doi: 10.1038/npp.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey MP, Lorrain D, Martel M, et al. Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? – results from a randomized controlled pilot study. Clin Interv Aging. 2017;12:937. doi: 10.2147/CIA.S133423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acler M, Bocci T, Valenti D, et al. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor Neurol Neurosci. 2013;31:661–68. doi: 10.3233/RNN-130321. [DOI] [PubMed] [Google Scholar]
- 23.Murphy M, Riedner BA, Huber R, et al. Source modeling sleep slow waves. Proc Natl Acad Sci. 2009;106:1608–13. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy M, Huber R, Esser S, et al. The cortical topography of local sleep. Curr Top Med Chem. 2011;11:2438–46. doi: 10.2174/156802611797470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bracht T, Federspiel A, Schnell S, et al. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One. 2012;7:e52238. doi: 10.1371/journal.pone.0052238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117:1623–29. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Jomeen J, Martin CR. Assessment and relationship of sleep quality to depression in early pregnancy. J Reprod Infant Psychol. 2007;25:87–99. [Google Scholar]
- 29.Hee HT, Whitecloud TS, Myers L, et al. SF-36 health status of workers compensation cases with spinal disorders. Spine J. 2001;1:176–82. doi: 10.1016/s1529-9430(01)00080-8. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE., Jr . SF-36 health survey The use of psychological testing for treatment planning and outcomes assessment. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 1999. pp. 1227–46. [Google Scholar]
- 31.Wrobel NH, Farrag MF. A preliminary report on the validation of the Geriatric Depression Scale in Arabic. Clin Gerontol. 2006;29:33–46. [Google Scholar]
- 32.Claassen DO, McDonell KE, Donahue M, et al. Cortical asymmetry in Parkinson’s disease: Early susceptibility of the left hemisphere. Brain Behav. 2016;6:12. doi: 10.1002/brb3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori F, Codecà C, Kusayanagi H, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010;11:436–42. doi: 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Manenti R, Brambilla M, Benussi A, et al. Mild cognitive impairment in Parkinson’s disease is improved by transcranial direct current stimulation combined with physical therapy. Mov Disord. 2016;31:715–24. doi: 10.1002/mds.26561. [DOI] [PubMed] [Google Scholar]
- 35.Brunoni AR, Ferrucci R, Fregni F, et al. Transcranial direct current stimulation for the treatment of major depressive disorder: a summary of preclinical, clinical and translational findings. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:9–16. doi: 10.1016/j.pnpbp.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt K, Holsboer-Trachsler E, Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Ann Med. 2016;48:42–51. doi: 10.3109/07853890.2015.1131327. [DOI] [PubMed] [Google Scholar]
- 37.Bunney BG, Bunney WE. Rapid-acting antidepressant strategies: Mechanisms of action. Int J Neuropsychopharmacol. 2012;15:695–713. doi: 10.1017/S1461145711000927. [DOI] [PubMed] [Google Scholar]
- 38.Gómez-Esteban JC, Tijero B, Somme J, et al. Impact of psychiatric symptoms and sleep disorders on the quality of life of patients with Parkinson’s disease. J Neurol. 2011;258:494–99. doi: 10.1007/s00415-010-5786-y. [DOI] [PubMed] [Google Scholar]
- 39.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69:308–12. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoletti A, Mostile G, Stocchi F, et al. Factors influencing psychological well-being in patients with Parkinson’s disease. PLoS One. 2017;12:e0189682. doi: 10.1371/journal.pone.0189682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caap-Ahlgren M, Dehlin O. Insomnia and depressive symptoms in patients with Parkinson’s disease: Relationship to health-related quality of life. An interview study of patients living at home. Arch Gerontol Geriatr. 2001;32(1):23–33. doi: 10.1016/s0167-4943(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 42.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]