Abstract
Background
Radiotherapy is the most effective non-surgical modality in lung cancer treatment, and microRNAs (miRNAs) have been suggested as key regulators in radiosensitization. Herein, we explored the specific function of miR-339-5p in the radiosensitivity of lung cancer cells.
Material/Methods
Radiosensitivity was assessed by cell viability (CCK-8 assay), cell apoptosis, and cell cycle changes (flow cytometry). qRT-PCR and subsequent Western blot assays were used to determine the expression of miR-339-5p and other related proteins.
Results
We demonstrated that ionizing radiation (IR) exposure impaired lung cancer cell viability, and found that miR-339-5p is a novel IR-inducible miRNA. Overexpression of miR-339-5p enhanced radiosensitivity of A549 and H460 cells by inhibiting cell viability, increasing apoptosis, inducing cell cycle arrest, and suppressing cell proliferation. Further exploration validated that miR-339-5p can target phosphatases of regenerating liver-1 (PRL-1) in lung cancer cells. Restoration of PRL-1 partially reverses the enhanced radiosensitivity of lung cancer cells induced by miR-339-5p.
Conclusions
Our data support that miR-339-5p has potential therapeutic value by sensitizing lung cancer cells to radiation via targeting of PRL-1.
MeSH Keywords: Apoptosis, Lung Neoplasms, MicroRNAs, Radiation Tolerance
Background
Lung cancer remains the most frequent cause of cancer-related death worldwide; non-small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancer cases and has a 5-year overall survival rate of less than 15% [1–3]. Radiotherapy is the second-most effective modality for the treatment of lung cancer, while surgery is the most effective method [4]. However, radioresistance is the key factor limiting the effects of radiotherapy, which in turn induces local recurrence and inevitably leads to treatment failure [5,6]. In the future, research on therapy should mainly focus on developing radiosensitizers to treat this lethal disease, as well as improving survival of patients after standard first-line therapy.
MicroRNAs (miRNAs) are a subtype of short non-coding RNAs (with a length of 20–25 nucleotides) that function by regulating gene expression via targeting mRNA for degradation or inhibition of mRNA translation [7,8]. An increasing number of studies have demonstrated that miRNAs are potential oncogenes or oncosuppressor genes, according to the cell context and the targets they regulate [8]. Moreover, recent studies have suggested a link between altered expression of some miRNAs and radiotherapy, especially in lung cancer [9–11]. Because a single miRNA has many possible targets, efforts of identify critical miRNAs that guide radiation sensitivity may facilitate the development of miRNA-censored approaches to overcome radioresistance.
MiR-339-5p was originally reported in a serum miRNA analysis showing that it is detected at low levels in patients with lung adenocarcinoma [12], indicating its tumor-suppressing roles in lung cancer. Subsequent research demonstrated that miR-339-5p strongly repressed cell migration and invasion in vitro, and was associated with the tumor-node-metastasis (TNM) staging and lymph node metastasis (LNM) of NSCLC [13]. However, its role in the radiosensitivity of lung cancer cells remains elusive.
In the present study, we explored the effects of miR-339-5p in the in vitro radiosensitivity of lung cancer cells, and confirmed the targeting of phosphatases of regenerating liver-1 (PRL-1) by miR-339-5p in mediating radiosensitivity in lung cancer cells. Our data may provide a way to enhance the efficacy of radiation therapy in lung cancer treatment.
Material and Methods
Cell culture and ionizing radiation (IR)
Human lung cancer cells A549 and H460 cells were purchased from Cell Resource of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). A549 cells were maintained in DMEM medium (Gibco, USA) while H460 cells were maintained in RMPI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5% CO2. IR was achieved by using Linear accelerator (Siemens, Germany). A 200 cGy/min dose rate was used for indicated cells at room temperature to reach a required total dose and then collected at the indicated time points in each experiment.
Cell counting kit (CCK)-8 assay
Cells were plated into 96-well plates at a density of 5×103 cells/well. Twenty-four hours later, cells were exposed to indicated doses of γ-irradiation and allowed to settle for another 12 h in the incubator at 37°C. Cell viability was measured using the cell counting kit (CCK)-8 assay according to the standard protocol. Relative cell viabilities of irradiated cells were calculated by normalizing the absorbance at 450 nm of irradiated cells to that of non-irradiated control cells.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNA of the cell samples was extracted using the mirVana RNA isolation kit (Ambion, USA) according to the standard protocol. After quality and quantity control using ND-100 (NanoDrop, USA), equal amounts of RNA were subjected to reverse transcription using the PrimeScript RT Master Mix and Mir-X miRNA First-Strand Synthesis kit (Takara, China). qRT-PCR was performed using the SYBR Premix Ex Taq II kit (Takara). U6 was used as the internal control. Each sample was prepared and assayed in triplicate. Data were calculated using 2−ΔΔCT method.
Transfection of oligonucleotides or plasmid
Mimics for miR-339-5p (sense 5′-UCCCUGUCCUC CAGGAGCUCACG-3′; antisense 5′-UGAGCUCCUGGAGGACAGG GAUU-3′) and negative control (NC) (sense 5′-UCCUCCGAACGU GUCACGUTT-3′; antisense 5′-ACGUGACACGUUCGGAGAATT-3′) were chemically synthesized by GenePharma (Shanghai, China). To construct the expression plasmid of pcDNA3.1-PRL-1, the completed sequence of human PRL-1 open-reading frame was synthesized and inserted into a pcDNA3.1 (+) vector. For miRNA mimics transfection, cells were plated to 50% confluency in a 6-well plate and transfected with 200 nM miRNA mimics using Lipofectamine RNAiMAx (Invitrogen, USA) following the manufacturer’s protocol. For plasmid transfection, cells were plated to 80% confluency in a 6-well plate and transfected with 2 μg plasmid using Lipofectamine 2000 (Invitrogen, USA) as per the standard method. Twenty-four hours later, cells were collected for RNA/protein extraction and other experiments.
Analysis of cell cycle and apoptosis
For cell cycle analysis, cells were collected and fixed in 70% ethanol at −20°C overnight. After washing twice in cold PBS, cells were incubated with 50 μg/ml PI for 30 min for further flow cytometry analysis. For apoptosis assay, the Annexin V-PE/7-AAD apoptosis detection kit (KeyGen, China) was used to quantify cell apoptosis, following the indicated protocol. Flow cytometry (BD Biosciences, USA) was used to visualize cell cycle and apoptosis of the above prepared cells, and results were analyzed with Modfit LT (Verity Software House, USA) or FlowJo (version 10; Tree Star, USA) software, respectively.
Luciferase reporter assay
The wild-type sequences of PRL-1 3′-untranslated region (3′-UTR; p-WT) and its deletion mutant (p-MT) without the miR-339-5p binding sites were inserted downstream of the firefly luciferase reporter gene in the pEZX-MT01 vectors (GeneCopoeia, China). The 2 reporter plasmids were used to transfect A549 or H1299 cells alone (Control) or with the NC and miR-339-5p mimics using Lipo2000 Transfection Reagent (Invitrogen, USA). The activities of firefly and Renilla luciferase activities were detected using the dual-luciferase reporter assay system (Promega, USA). The results were presented as the firefly luciferase activities normalized against those of Renilla.
Western blotting
Protein was isolated from cultured cells with RIPA lysis buffer supplemented with protease inhibitor (Beyotime, China) and protein concentration was determined by using the BCA quantification kit (Beyotime, China). Equal amounts of protein were separated in 10% SDS-PAGE, electrophoretically transferred to PVDF membranes (Millipore, USA), then detected with anti-human PRL-1 (Santa Cruz, USA), Bcl-2 (Cell Signaling Technology, USA), Bax (Cell Signaling Technology, USA), and β-actin (Santa Cruz, USA) antibodies. The proteins were visualized using the Image Quant software (Molecular Dynamics, USA).
Statistical analysis
Experiments were all performed at least 3 times separately, each in triplicate. Data are expressed as means ±SD of 3 independent experiments. Statistical significance between 2 groups were calculated separately by t test. P values <0.05 were considered to be statistically significant.
Results
IR impairs cell viability and induces miR-339-5p expression in lung cancer cells
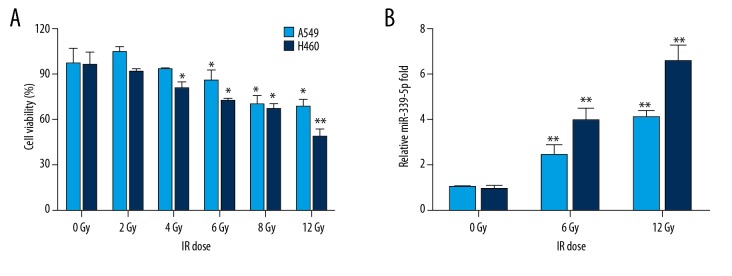
Following IR exposure (0, 2, 4, 6, 8, 12 Gy), CCK-8 assay showed that IR repressed cell viabilities of A549 and H460 cells in a dose-dependent manner, and high doses of IR (higher than 6 Gy) markedly decreased cell viability in both A549 and H460 cells (Figure 1A). To test whether miR-339-5p was a novel IR-responsive miRNA, we analyzed its expression in IR-exposed cell extracts. As shown in Figure 1B, the miR-339-5p level was evidently induced by IR exposure at 12 h after IR in both A549 and H460 cells (Figure 1B). Since there is no previous report on the role of miR-339-5p in radiosensitivity of lung cancer cells, we then investigated the function and target of miR-339-5p in the IR response of lung cancer cells.
Figure 1.
Upregulation of miR-339-5p following IR exposure in lung cancer cells. (A) Relative cellular viability of A549 and H460 cells after treatment with increasing doses of IR was determined by CCK-8 assay. (B) IR dose-dependent induction of miR-339-5p in A549 and H460 cells. miR-339-5p expression was analyzed by qRT-PCR. 5S rRNA was used as an internal control. Data are expressed as the mean ±SD (* P<0.05, ** P<0.01 vs. 0 Gy).
miR-339-5p enhances radiosensitivity of A549 and H460 cells
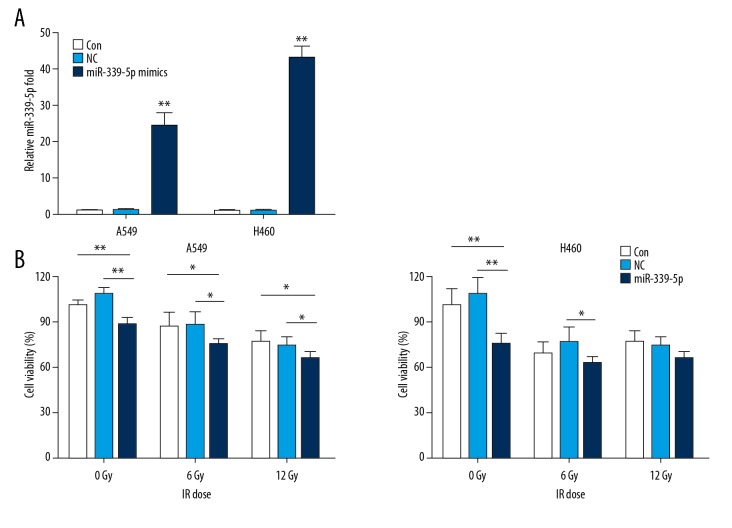
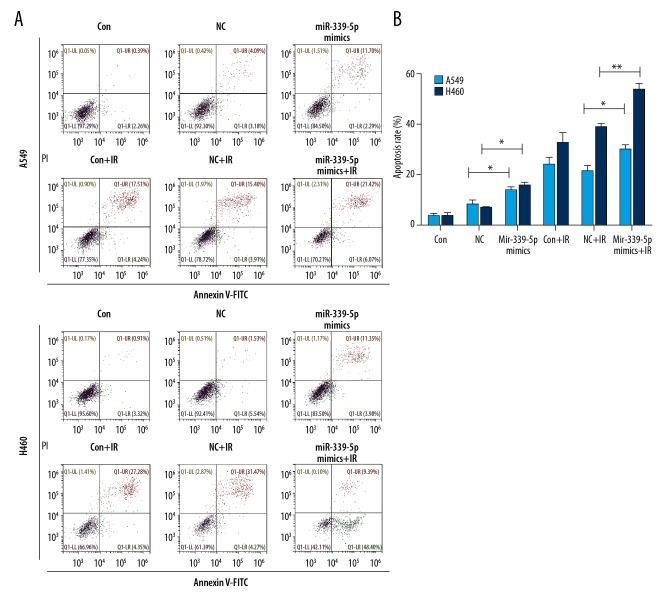
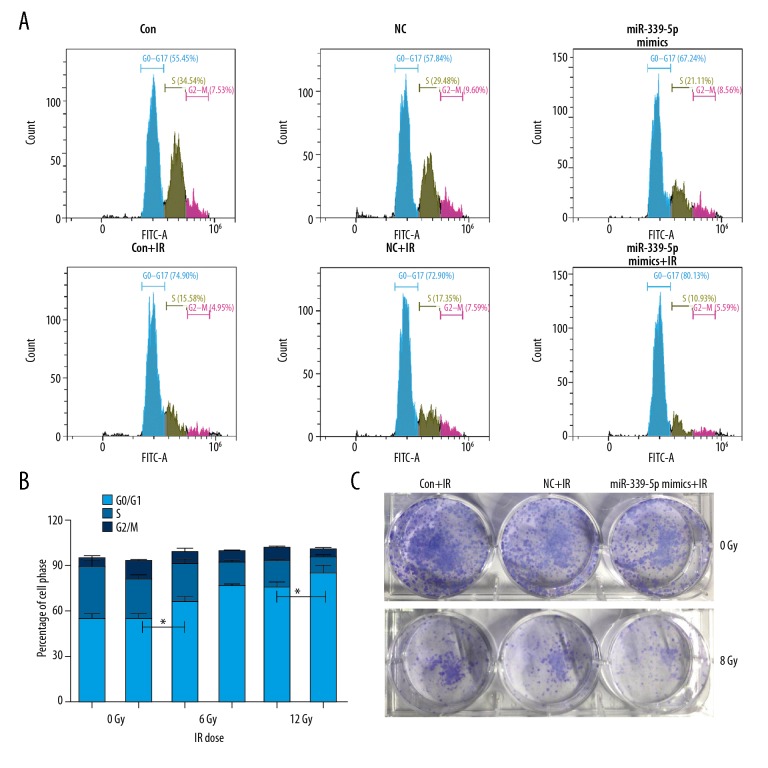
To examine the relationship between miR-339-5p and radiosensitivity, we irradiated NC or miR-339-5p mimics-transfected A549 and H460 cells, and then determined cell viability, apoptosis, and cell cycle distribution. The results of qRT-PCR indicated a significantly greater induction of miR-339-5p expression in miR-339-5p mimics-transfected A549 and H460 cells in comparison with the NC mimics-transfected cells (Figure 2A). Following IR exposure (0, 6, 12 Gy), miR-339-5p overexpression significantly reduced cell viability at each dose (0, 6, and 12 Gy) in A549 cells and only at lower doses (0 and 6 Gy) in H460 cells (Figure 2B) compared with controls (NC mimics-transfected cells). Alterations of apoptosis and cell cycle were then analyzed. We found that miR-339-5p overexpression strikingly elevated the apoptosis rates of A549 and H460 cells, both at 0 and 6 Gy of IR (Figure 3A, 3B). For H460 cell cycle, miR-339-5p mimics induced G0/G1 arrest at both 0 and 6 Gy of IR (Figure 4A, 4B). In addition, we tested the effect of miR-339-5p overexpression on proliferation of H460 cells and found that miR-339-5p obviously reduced clones compared with the Control and NC group (Figure 4C). These results suggest that upregulation of miR-339-5p inhibits cell viability, increases apoptosis, induces cell cycle arrest, suppresses cell proliferation, and enhances radiosensitivity in lung cancer cells.
Figure 2.
miR-339-5p increased the radiosensitivity of lung cancer cells. (A) Relative levels of miR-339-5p were examined by qRT-PCR at 48 h after transfection. 5S rRNA was used as an internal control. (B) After non-transfection (control) or transfection with NC or miR-339-5p mimics followed by exposure to increasing doses of IR, cellular viability of A549 and H460 cells was determined by CCK-8 assay. Data are expressed as the mean ±SD (* P<0.05, ** P<0.01).
Figure 3.
miR-339-5p promotes IR-induced apoptosis of lung cancer cells. (A) A549 and H460 cells were non-transfected (control) or transfected with NC or miR-339-5p mimics. Twenty-four hours later, cells were exposed to 12 Gy of IR or untreated for another 24 h. Cell apoptosis was detected by Annexin V/PI staining and flow cytometry. (B) Mean apoptosis rate assessed by flow cytometry in response to IR exposure as in (A). Mean ±SD of 3 independent experiments were performed (* P<0.05, ** P<0.01).
Figure 4.
miR-339-5p arrests cell cycle of lung cancer cells following IR exposure. (A) A549 and H460 cells were non-transfected (control) or transfected with NC or miR-339-5p mimics. Twenty-four hours later, cells were exposed by 12 Gy of IR or untreated for another 24 h. Cell cycle was analyzed by flow cytometry. MiR-339-5p caused a G0/G1 arrest. (B) Quantification for cell cycle attribution. The percentage of cell population at G0/G1, S, and G2/M phases are represented as mean ±SD (* P<0.05). (C) After transfection, the H460 cells were plated into 6-well plates. With/without radiation (12 Gy), cells clones were stained by crystal violet and were calculated 10 days later.
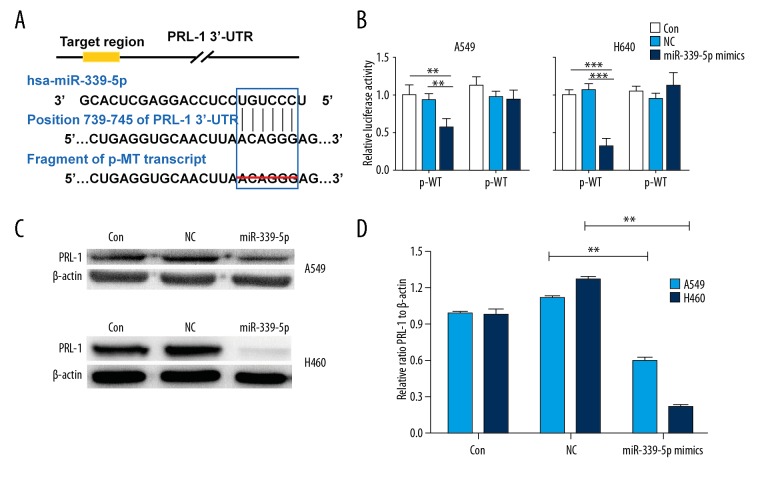
miR-339-5p directly targets PRL-1 in lung cancer cells
To further explore the underlying mechanism of miR-339-5p in lung cancer, we performed a bioinformatics (miRDB and TargetScan) search for predictive targets of miR-339-5p, and PRL-1 was selected as a candidate target (Figure 5A). Luciferase reporter assay revealed that miR-339-5p obviously suppressed the luciferase activity derived from the recombinant reporter plasmid containing wild-type 3′-UTR of PRL-1 (p-WT) in A549 and H460 cells (P<0.05~0.001; Figure 5A, 5B), whereas the mutant 3′-UTR without miR-339-5p binding sites (p-MT) failed to exert the same effect (Figure 5A, 5B). Western blotting was applied to determine if miR-339-5p could downregulate the expression of PRL-1 in A549 and H460 cells. We found that PRL-1 protein was decreased in the miR-339-5p mimics-transfected cells compared to the control groups (Figure 5C, 5D). Therefore, we confirm that PRL-1 is a target and is inversely regulated by miR-339-5p in lung cancer cells.
Figure 5.
PRL-1 is a target of miR-339-5p in lung cancer cells. (A) Predicted miR-339-5p binding sites in the 3′-UTR of PRL-1 by TargetScan and the designed mutant 3′-UTR in which miR-339-5p binding sites were deleted. (B) Luciferase reporter assays in A549 and H460 cells transfected with p-WT or p-MT alone (Mock) and cotransfected with p-WT or p-MT and NC or miR-339-5p mimics; p-WT=the reporter plasmid of wild-type PRL-1 3′-UTR, p-MT=the reporter plasmid of mutant-type PRL-1 3′-UTR. Data are normalized according to the ratio of Firefly luciferase activity to Renilla luciferase activity. (C) The expression levels of PRL-1 were detected by Western blotting in A549 and H460 cells after indicated transfections. β-actin was used as a loading control. (D) Quantification of Western blotting results as in (A). Data are expressed as the mean ±SD (** P<0.01, *** P<0.001).
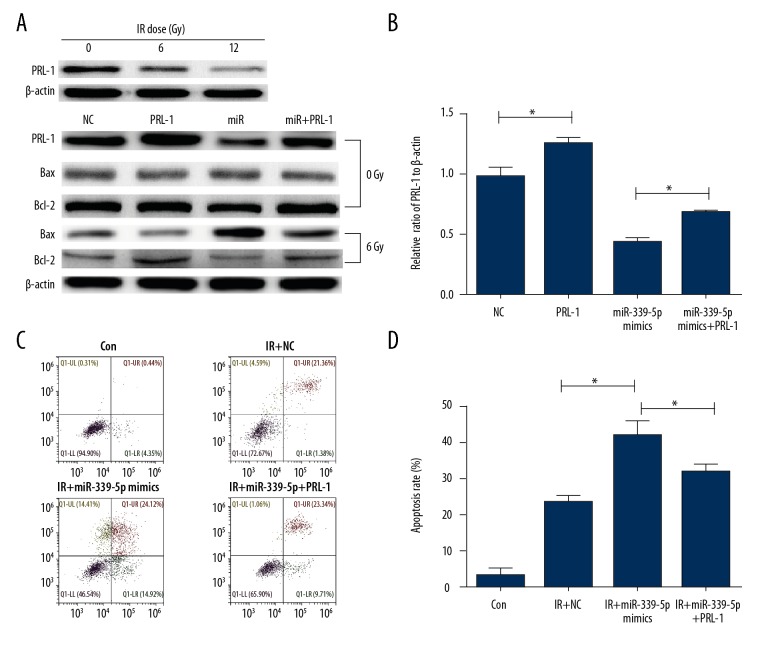
Restoration of PRL-1 relieves the enhanced radiosensitivity caused by miR-339-5p in lung cancer cells
To investigate whether miR-339-5p mediates radiosensitivity via targeting PRL-1, PRL-1 rescue experiments were done in H460 cells and subsequent experiments were performed to analyze cell apoptosis, since apoptosis of cancer cells is closely related to radiosensitivity. Notably, under different IR doses (0, 6, and 12 Gy), the expression of PRL-1 was decreased gradiently, which was consistent with our previous result (Figure 1B) that IR induced miR-339-5p upregulation, which in turn downregulated PRL-1. As shown in Figure 6A and 6B, PRL-1 plasmid was used to overexpress its protein in H460 cells, and overexpression of PRL-1 reversed the downregulation of PRL-1 protein by miR-339-5p mimics in H460 cells. Consistently, miR-339-5p mimics markedly elevated the expression of Bax proteins and reduced Bcl-2 level under 6Gy of radiation, but overexpression of PRL-1 reversed the stimulation of the mitochondrial apoptosis pathway. Importantly, co-transfection of PRL-1 plasmid and miR-339-5p mimics partially relieved apoptosis induction in miR-339-5p mimics-transfected H460 cells at 6 Gy of IR (Figure 6C, 6D). To conclude, miR-339-5p enhances radiosensitivity of lung cancer cells by targeting PRL-1, at least partially.
Figure 6.
miR-339-5p sensitizes lung cancer cells to IR by targeting PRL-1. (A) The expression levels of PRL-1, Bax, and Bcl-2 under 0Gy/8Gy were detected by Western blotting in H460 cells after indicated transfections. β-actin was used as a loading control. (B) Quantification of Western blotting results as in (A). (C) Cell apoptosis was detected by annexin V/PI staining and flow cytometry in indicated cells. (D) Mean apoptosis rate assessed by flow cytometry in response to IR exposure as in (C). Data are expressed as the mean ±SD (* P<0.05).
Discussion
Although there has been great technological progress in radiotherapy over the last 2 decades on curing a wide range of human cancers, academic studies in radiation biology have mainly concentrated on elevating the therapeutic effect of radiotherapy. To attain this goal, much work is still needed to better understand the cellular response to IR exposure and to overcome radioresistance in cancer patients. According to recent studies, miRNAs play key roles in regulating radiosensitivity by interacting with the downstream targets [14,15].
One previous study concluded that miR-339-5p, a tumor suppressing miRNA in other malignancies [16,17] represses cell migration and invasion potential in NSCLC cancer [13]. Therefore, the possible therapeutic significance of miR-339-5p prompted us to investigate its role in lung cancer radiosensitivity. Herein, we found that miR-339-5p could be upregulated by IR exposure. In vitro experiments demonstrated that miR-339-5p elevated radiosensitivity of A549 and H460 cells by suppressing cell viability, increasing apoptosis, and inducing G0/G1 arrest. Further investigation suggested that miR-339-5p might be a radiosensitizer by targeting PRL-1 in lung cancer cells.
Because a single miRNA might concurrently target many mRNAs, which may differ in different cellular contexts, identifying these targets is important to interpret the functional mechanism of miRNAs. Previous studies showed that miR-339-5p has multiple biological targets [18–20]. By combining predictions made using 2 major tools (miRDB and TargetScan), we validated PRL-1 as a functional downstream target of miR-339-5p. In fact, Zhou et al. found that miR-339-5p could target PRL-1 and dramatically inhibit colorectal cancer cell growth and metastasis in vitro [20]. PRL-1 belongs to the PRL subfamily of protein tyrosine phosphatases that is implicated in oncogenic and metastatic phenotypes [21,22]. However, the role of PRL-1 in lung cancer progression remains unknown. From the perspective of radiosensitivity, our data suggest a tumor-promoting role of PRL-1 in lung cancer. Future research should focus on validation of the observations in tumor growth in vivo and further investigation of the mechanism of PRL-1 in mediating the radiosensitivity of miR-339-5p.
Conclusions
Our study verifies that IR-induced miR-339-5p enhanced radiosensitivity of lung cancer cells in vitro, at least by targeting PRL-1. The miR-339-5p/PRL-1 pathway may be a potential therapeutic approach to radiosensitization for lung cancer, but further studies are required to reveal the exact mechanism.
Abbreviations
- miRNA
microRNA
- PRL-1
phosphatases of regenerating liver-1
- IR
ionizing radiation
- PI
propidium iodide
- BCA
bicinchoninic acid
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PVDF
polyvinylidene difluoride
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Fidias P, Novello S. Strategies for prolonged therapy in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(34):5116–23. doi: 10.1200/JCO.2010.30.7074. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhang W, Guo N, et al. Expression of molecular markers in primary sites and metastatic lymph nodes of lung cancer patients. Med Sci Monit. 2017;23:513–20. doi: 10.12659/MSM.898688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: Recent developments. Lancet. 2013;382(9893):709–19. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 5.Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: A reassessment based on new data. Oncologist. 2011;16(5):672–81. doi: 10.1634/theoncologist.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Provencio M, Sánchez A, Garrido P, Valcárcel F. New molecular targeted therapies integrated with radiation therapy in lung cancer. Clin Lung Cancer. 2010;11(2):91–97. doi: 10.3816/CLC.2010.n.012. [DOI] [PubMed] [Google Scholar]
- 7.Rana TM. Illuminating the silence: Understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 8.Iorio MV, Croce CM. MicroRNAs in cancer: Small molecules with a huge impact. J Clin Oncol. 2009;27(34):5848–56. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, Chen X, Wei K, et al. Identification of key transcription factors associated with lung squamous cell carcinoma. Med Sci Monit. 2017;23:172–206. doi: 10.12659/MSM.898297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salim H, Akbar NS, Zong D, et al. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br J Cancer. 2012;107(8):1361–73. doi: 10.1038/bjc.2012.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan D, Ng WL, Zhang X, et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2010;5(7):e11397. doi: 10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rani S, Gately K, Crown J, et al. Global analysis of serum microRNAs as potential biomarkers for lung adenocarcinoma. Cancer Biol Ther. 2013;14(12):1104–12. doi: 10.4161/cbt.26370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Zhao W, Bao P, et al. miR-339-5p inhibits cell migration and invasion in vitro and may be associated with the tumor-node-metastasis staging and lymph node metastasis of non-small cell lung cancer. Oncol Lett. 2014;8(2):719–25. doi: 10.3892/ol.2014.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad P, Sana J, Slavik M, et al. MicroRNAs involvement in radioresistance of head and neck cancer. Dis Markers. 2017;2017 doi: 10.1155/2017/8245345. 8245345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller AK, Lindner K, Hummel R, et al. MicroRNAs and their impact on radiotherapy for cancer. Radiat Res. 2016;185(6):668–77. doi: 10.1667/RR14370.1. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Lu Y, Li X. miR-339-3p inhibits proliferation and metastasis of colorectal cancer. Oncol Lett. 2015;10(5):2842–48. doi: 10.3892/ol.2015.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YL, Chen CM, Wang XM, Wang L. Effects of miR-339-5p on invasion and prognosis of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40(1):51–56. doi: 10.1016/j.clinre.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Shan W, Li J, Bai Y, Lu X. miR-339-5p inhibits migration and invasion in ovarian cancer cell lines by targeting NACC1 and BCL6. Tumour Biol. 2016;37(4):5203–11. doi: 10.1007/s13277-015-4390-2. [DOI] [PubMed] [Google Scholar]
- 19.Long JM, Ray B, Lahiri DK. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem. 2014;289(8):5184–98. doi: 10.1074/jbc.M113.518241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou C, Liu G, Wang L, et al. MiR-339-5p regulates the growth, colony formation and metastasis of colorectal cancer cells by targeting PRL-1. PLoS One. 2013;8(5):e63142. doi: 10.1371/journal.pone.0063142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond RH, Cressman DE, Laz TM, et al. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994;14(6):3752–62. doi: 10.1128/mcb.14.6.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achiwa H, Lazo JS. PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells. Cancer Res. 2007;67(2):643–50. doi: 10.1158/0008-5472.CAN-06-2436. [DOI] [PubMed] [Google Scholar]