Abstract
Three different altitudes were selected to study the variability of terpenoid composition from leaves of female plants of Pistacia lentiscus L. throughout the elevation gradient. GC-MS analyses showed that terpenoid contents change with altitude. Forty nine compounds were identified with a high interpopulation variability for low- and midaltitude sites that also exhibited the same major components when data were expressed on dry weight basis. However, Two-Way-ANOVA followed by Tukey’s post hoc test showed that monoterpene hydrocarbons increased with elevation, giving values of 21.7, 37.5 and 221.5 µg g−1 dw for low- mid- and highlands, respectively. On the other hand, applying P.C.A. with data expressed in percentage of the chromatogram of the volatile extract led to the identification of three chemotypes associated with altitudinal levels. In highlands (Group I), the major compounds were β-caryophyllene (12%), δ-cadinene (9.3%) and α-pinene (6.3%) while in midlands (Group II), β-caryophyllene (11.5%), δ-cadinene (8.6%) and caryophyllene oxide (6.8%) were the main components. In lowlands (Group III) δ-cadinene (10.9%), cubebol (10.5%) and β-bisabolene (7.7%) were chiefly present. Hence, the involvement of genetic factors, temperature and drought in the chemical polymorphism of P. lentiscus associated with elevation is discussed in this report.
Keywords: Pistacia lentiscus, terpenes, altitude, Algeria, α-pinene, β-caryophyllene, caryophyllene oxide, cubebol, β-bisabolene, variability
1. Introduction
Biosynthesis of secondary metabolites is not only controlled genetically, but it is also strongly affected by different biotic and abiotic stresses [1]. Among plant secondary metabolites, terpenoids are the most abundant and structurally diverse group [2].
Altitude is one of the abiotic stresses associated with alterations in a number of environmental factors such as air temperature, precipitation, wind exposure, light intensity, UV-B radiation, ozone density and oxidizing air pollutants [3]. The effect of the altitudinal gradient on essential oil content from many species has been evaluated by several authors [4,5].
Pistacia lentiscus L. (Anacardiaceae) is a sclerophyllous dioecious shrub which forms bushes of up to 2 m height, sometimes attaining a tree growth form in more humid and protected sites [6]. It is a low altitude species [7], which has been found to be one of the more drought tolerant plants among other evergreen species [8] and to be very tolerant to salinity [9]. This species is very common in the Mediterranean Basin [10]. In Algeria, P. lentiscus is dispersed along the entire littoral [11] and grows in diverse habitats along a climatic gradient that varies in solar radiation, temperature, and precipitation.
P. lentiscus is extensively used in folk medicine [12], and the pharmaceutical and antimicrobial activity of this species has been reported by several authors [13,14]. The Anacardiaceae is a family that secrete substances containing terpenes and carbohydrates in ducts which are found in vascular rays, especially from the genus Pistacia [15]. P. lentiscus is recognized as a terpene-storing species which produces the largest number of individual terpenes [16].
Numerous essential oil studies have been conducted on P. lentiscus leaves from different provenances [17,18,19,20,21] but, neither the plant sex, which is an additional source of variability [22] nor the ecological conditions of plant growth were taken into account. Because of the differential volatility of terpenes, this prompted us to study the variability of terpenoid content of female P. lentiscus with increasing altitude. The result of this work will allow us to further understand the ecology of the plant in a constrained environment given its importance in reforestation programs in semi-arid Mediterranean Regions.
2. Results and Discussion
The leaves of P. lentiscus contain 49 identified compounds, which are listed in Table 1. Among these, twelve are monoterpenes (eight hydrocarbons, two oxygenated and two derivatives) and thirty seven are sesquiterpenes (25 hydrocarbons and 12 oxygenated).
Table 1.
Concentration of terpenoids (µg g–1 dw) found in female Pistacia lentiscus L. leaves from low, mid and high altitude sites in Algeria.
| Compounds | Mean concentration ± standard deviation of terpenoid contents in leaves extracts of Pistacia lentiscus (µg.g dw−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | Low altitude | Mid altitude | High altitude | |||||||||||||
| L1(n = 5) | L2(n = 5) | L3(n = 5) | L4(n = 5) | L5(n = 5) | M1(n = 5) | M2(n = 5) | M3(n = 5) | M4(n = 5) | M5(n = 5) | H1(n = 5) | H2(n = 5) | H3(n = 5) | H4(n = 5) | H5(n = 5) | ||
| α-thujene | 926 | 4.8±1.6 | 3.8±1.3 | 1.1±0.9 | 1.4±0.9 | 4.6±1.5 | 8.2±1.1 | 8.1±0.4 | 4.5±0.9 | 3.4±0.5 | 6.5±1.2 | 13.6±0.7 | 15.2±0.7 | 12.5±1.5 | 15.1±0.7 | 16.9±0.5 |
| α-pinene | 933 | 8.1±3.3 | 7.6±3.0 | 2.9±1.1 | 2.7±0.9 | 5.5±1.2 | 2.2±0.2 | 5.7±0.9 | 5.2±0.8 | 1.9±0.3 | 13.5±2.0 | 102.9± 6.2 | 100.9± 3.6 | 76.3± 14.2 | 105.0± 4.5 | 98.0± 0.5 |
| camphene | 947 | 0.6±0.2 | 0.6±0.2 | 0.3±0.1 | 0.3±0.1 | 0.4±0.1 | 0.9±0.2 | 3.0±1.1 | 1.1±0.5 | 0.9±0.3 | 2.2±0.7 | 14.3±1.3 | 17.1±1.8 | 12.8±3.4 | 15.9±2.1 | 20.3±0.6 |
| β-pinene | 973 | 12.2±4.3 | 10.9±3.8 | 3.0±1.5 | 3.3±1.5 | 7.9±2.3 | 11.5±1.3 | 12.1±1.8 | 11.4±3.0 | 4.5±0.4 | 14.5±2.2 | 35.3±7.4 | 36.7±7.1 | 56.2±14.9 | 44.3±8.8 | 30.9±0.6 |
| α-terpinene | 1018 | 2.0±0.9 | 1.9±0.7 | 0.1±0.1 | 0.6±0.3 | 1.9±0.6 | 7.3±0.7 | 6.2±1.4 | 2.9±1.1 | 1.3±0.3 | 1.9±0.4 | 7.9±0.6 | 9.3±0.5 | 8.5±1.4 | 9.5±0.4 | 10.5±0.4 |
| p-cymene | 1027 | 0.9±0.4 | 0.9±0.4 | 0.4±0.1 | 0.3±0.1 | 0.7±0.1 | 1.9±0.4 | 3.5±0.9 | 1.4±0.5 | 0.7±0.3 | 1.6±0.6 | 15.7±0.7 | 17.7±0.5 | 21.3±4.8 | 18.5±0.5 | 18.8±0.4 |
| limonene | 1029 | 2.3±0.2 | 2.3±0.3 | 1.7±0.0 | 1.5±0.1 | 2.1±0.1 | 5.8±0.5 | 5.7±1.6 | 2.9±0.9 | 2.4±0.3 | 4.0±0.4 | 11.5±2.6 | 13.6±2.2 | 32.6±12.3 | 16.3±2.7 | 12.7±0.3 |
| γ-terpinene | 1062 | 2.1±0.6 | 1.7±0.6 | 0.5±0.3 | 0.8±0.4 | 2.0±0.6 | 4.7±0.4 | 5.6±1.0 | 2.5±0.8 | 1.4±0.3 | 2.4±0.4 | 7.2±0.6 | 8.9±0.7 | 7.7±0.9 | 8.8±0.7 | 10.5±0.4 |
| terpinen-4-ol | 1179 | 5.1±1.2 | 4.0±0.7 | 3.5±0.6 | 2.4±0.1 | 3.1±0.4 | 5.9±0.8 | 7.0±1.3 | 4.6±0.9 | 4.1±0.4 | 6.3±0.6 | 27.4±0.6 | 29.6±0.4 | 42.5±7.8 | 30.3±0.4 | 30.5±0.4 |
| α-terpineol | 1197 | 2.4±0.6 | 1.6±0.5 | 0.8±0.2 | 0.5±0.2 | 1.4±0.1 | 1.9±0.3 | 3.5±1.0 | 2.0±0.5 | 1.1±0.3 | 2.0±0.7 | 5.1±0.8 | 7.5±0.5 | 6.0±1.1 | 8.4±0.6 | 8.2±0.4 |
| borneol acetate | 1287 | 6.8±1.9 | 5.6±1.5 | 3.9±0.2 | 3.6±0.2 | 3.2±0.4 | 7.3±0.8 | 6.5±1.1 | 6.3±1.5 | 6.9±0.7 | 18.7±6.2 | 18.6±0.7 | 22.3±0.5 | 25.8±6.9 | 22.4±0.5 | 23.6±0.4 |
| α-terpineol acetate* | 1351 | 4.4±0.2 | 4.0±0.6 | 10.3±1.0 | 8.8±0.5 | 8.9±2.9 | 17.7±2.3 | 10.4±1.5 | 5.2±1.4 | 7.9±0.4 | 14.1±3.8 | 15.3±0.3 | 16.5±0.2 | 11.7±1.5 | 16.7±0.2 | 17.2±0.2 |
| δ-elemene* | 1338 | 0.5±0.1 | 0.5±0.1 | 0.6±0.1 | 0.4±0.0 | 0.0±0.0 | 1.2±0.3 | 2.8±0.9 | 0.7±0.4 | 1.7±0.3 | 7.0±3.1 | 0.4±0.2 | 2.6±0.5 | 1.4±0.9 | 2.7±0.4 | 3.9±0.4 |
| α-cubebene* | 1351 | 4.4±0.2 | 4.0±0.6 | 10.3±1.0 | 8.8±0.5 | 8.9±2.9 | 17.7±2.3 | 10.4±1.5 | 5.2±1.4 | 7.9±0.4 | 14.1±3.8 | 15.3±0.3 | 16.5±0.2 | 11.7±1.5 | 16.7±0.2 | 17.2±0.2 |
| α-copaene | 1377 | 7.5±1.3 | 8.3±1.0 | 10.4±1.1 | 8.5±0.6 | 13.7±3.1 | 22.9±3.7 | 10.9±0.8 | 9.4±0.3 | 8.8±0.5 | 13.9±1.0 | 34.8±2.4 | 38.3±1.5 | 23.2±4.7 | 40.3±1.9 | 37.8±0.4 |
| β-bourbonene* | 1389 | 1.7±0.2 | 1.2±0.1 | 0.7±0.1 | 0.7±0.1 | 0.8±0.3 | 1.3±0.3 | 3.0±0.9 | 1.3±0.3 | 0.8±0.3 | 5.1±1.2 | 7.2±0.9 | 9.9±1.3 | 2.4±0.3 | 6.0±1.2 | 12.2±0.4 |
| β-cubebene* | 1391 | 3.9±0.5 | 3.3±0.5 | 14.2±1.0 | 13.8±1.3 | 32.5±8.7 | 21.0±3.6 | 10.4±2.0 | 4.5±1.1 | 11.0±0.9 | 5.7±1.1 | 13.2±0.6 | 16.4±0.5 | 9.6±2.0 | 16.7±0.4 | 17.7±0.4 |
| β-elemene* | 1393 | 34.9±0.9 | 34.4±0.4 | 3.1±0.4 | 2.5±0.3 | 3.4±0.5 | 4.4±0.7 | 5.5±0.5 | 3.9±0.6 | 3.5±0.3 | 7.3±0.6 | 4.5±1.2 | 6.9±0.8 | 6.7±1.8 | 8.2±1.0 | 7.2±0.4 |
| β-caryophyllene | 1422 | 92.7±18.5 | 78.4±16.4 | 71.4±6.8 | 54.3±2.7 | 57.2±4.7 | 95.5±6.6 | 71.4±2.9 | 80.3±14.9 | 82.8±9.0 | 146.8±10.8 | 178.2±14.9 | 186.3±11.7 | 156.4±30.1 | 198.5±14.3 | 175.6±0.4 |
| β-copaene* | 1431 | 6.6±0.6 | 6.2±0.5 | 6.0±0.5 | 5.0±0.2 | 17.3±11.6 | 10.4±1.5 | 7.6±0.8 | 5.9±0.6 | 7.1±1.0 | 9.4±1.0 | 14.7±4.5 | 17.2±3.9 | 13.2±2.4 | 21.6±4.9 | 14.3±0.4 |
| γ-elemene* | 1440 | 0.5±0.1 | 0.4±0.1 | 0.2±0.1 | 0.2±0.1 | 0.4±0.1 | 1.7±0.3 | 3.4±1.1 | 1.0±0.5 | 0.7±0.3 | 0.8±0.3 | 2.3±0.2 | 4.4±0.6 | 2.4±0.4 | 4.4±0.6 | 5.9±0.4 |
| trans-muurola-3,5-diene* | 1451 | 2.2±0.6 | 3.3±0.7 | 6.4±0.8 | 5.9±0.8 | 4.4±0.9 | 7.4±1.0 | 8.3±1.6 | 3.6±0.8 | 4.9±0.2 | 5.3±1.0 | 2.9±0.6 | 4.4±0.9 | 2.8±0.4 | 3.8±1.1 | 6.3±0.3 |
| α-humulene | 1457 | 20.9±2.6 | 19.3±3.0 | 20.5±1.7 | 16.3±0.7 | 19.4±1.8 | 35.6±4.9 | 19.1±0.7 | 18.6±2.1 | 23.9±1.8 | 30.8±1.6 | 28.4±6.6 | 33.3±7.6 | 29.0±5.6 | 26.2±9.3 | 42.0±0.4 |
| allo-aromadendrene* | 1462 | 6.4±0.5 | 5.6±0.7 | 7.6±0.7 | 6.1±0.3 | 8.0±0.9 | 17.5±2.6 | 9.2±1.2 | 5.9±0.8 | 7.5±0.4 | 9.3±0.9 | 15.8±3.2 | 18.7±2.6 | 10.2±2.2 | 21.8±3.3 | 17.1±0.4 |
| cis-muurola-4(14),5-diene* | 1465 | 3.8±0.3 | 3.6±0.3 | 3.7±0.3 | 3.5±0.7 | 3.9±0.6 | 5.9±0.9 | 5.3±0.7 | 3.5±0.3 | 4.6±0.4 | 5.4±0.6 | 7.0±2.4 | 9.7±1.8 | 6.6±1.1 | 12.0±2.3 | 8.8±0.4 |
| trans-cadina-1(6),4-diene* | 1476 | 2.7±0.3 | 2.8±0.5 | 6.8±0.5 | 5.0±0.3 | 5.5±0.6 | 3.8±0.7 | 5.9±1.3 | 3.2±0.7 | 5.0±0.6 | 3.2±0.7 | 12.4±1.2 | 15.4±0.6 | 5.7±1.3 | 16.4±0.7 | 15.9±0.4 |
| γ-muurolene* | 1479 | 32.5±1.8 | 29.7±3.1 | 37.3±1.9 | 30.8±1.0 | 34.7±4.0 | 62.5±6.7 | 36.7±1.9 | 28.6±2.4 | 29.9±2.9 | 37.7±2.4 | 59.7±5.4 | 62.6±4.4 | 40.7±5.9 | 67.3±5.4 | 58.9±0.2 |
| germacrene D* | 1484 | 23.5±3.3 | 21.5±2.0 | 16.4±0.8 | 13.9±0.5 | 14.1±0.8 | 19.0±1.1 | 19.3±2.4 | 19.0±2.4 | 24.3±6.8 | 37.6±5.0 | 33.7±14.7 | 36.9±13.9 | 44.6±9.3 | 51.4±17.1 | 24.0±0.4 |
| β-selinene* | 1489 | 7.5±0.8 | 7.1±0.5 | 5.7±0.7 | 5.4±0.2 | 8.4±0.8 | 10.7±1.2 | 8.9±0.7 | 6.1±0.6 | 6.0±0.3 | 9.7±1.1 | 8.7±2.6 | 11.3±2.1 | 10.0±1.6 | 13.8±2.6 | 10.2±0.4 |
| trans-muurola-1(14),5-diene* | 1494 | 5.5±0.4 | 5.8±0.9 | 13.6±0.8 | 10.6±0.5 | 13.1±0.9 | 14.7±2.9 | 6.8±0.5 | 4.7±0.2 | 10.5±0.8 | 7.6±1.0 | 15.3±1.4 | 18.3±0.8 | 9.7±2.1 | 19.5±0.9 | 18.6±0.4 |
| α-muurolene* | 1503 | 16.1±1.6 | 17.1±2.3 | 27.6±2.3 | 21.9±1.4 | 25.7±2.9 | 46.8±5.9 | 24.7±1.9 | 16.4±2.5 | 26.1±1.2 | 24.3±2.1 | 42.9±5.2 | 55.7±0.9 | 24.6±5.2 | 57.1±1.1 | 55.9±0.4 |
| β-bisabolene* | 1510 | 22.1±2.3 | 21.6±2.5 | 81.9±5.4 | 62.5±5.3 | 74.3±8.2 | 105.0±19.9 | 38.5±9.2 | 21.4±2.3 | 63.6±4.0 | 36.6±4.8 | 55.3±5.9 | 60.0±4.5 | 42.7±8.6 | 65.1±5.6 | 56.5±0.4 |
| γ-cadinene* | 1517 | 15.7±1.2 | 15.3±1.0 | 12.6±1.3 | 11.7±0.8 | 23.7±9.9 | 32.8±2.9 | 35.4±8.0 | 20.2±6.6 | 15.3±0.7 | 20.5±2.0 | 24.9±8.3 | 27.0±7.9 | 19.9±3.4 | 35.4±9.7 | 20.2±0.4 |
| δ-cadinene* | 1528 | 60.3±5.5 | 59.0±9.3 | 112.5±9.5 | 83.3±3.8 | 98.4±9.1 | 176.2±19.6 | 88.4±8.5 | 59.7±10.7 | 98.6±4.8 | 83.9±8.0 | 143.6±19.0 | 153.9±16.3 | 87.4±18.2 | 170.7±20.0 | 138.6±0.4 |
| cadina-1,4-diene* | 1535 | 7.1±0.8 | 5.4±0.6 | 6.3±0.5 | 6.1±0.6 | 9.8±1.2 | 11.2±0.5 | 10.4±2.0 | 5.2±1.6 | 5.0±0.5 | 3.9±2.3 | 7.1±0.5 | 8.4±0.4 | 7.1±0.5 | 8.8±0.3 | 9.5±0.4 |
| α-cadinene* | 1541 | 4.9±0.4 | 4.1±0.3 | 14.1±0.4 | 13.1±0.1 | 5.1±0.5 | 10.4±1.7 | 14.6±4.0 | 7.0±3.0 | 5.1±0.5 | 5.4±0.8 | 6.6±1.6 | 8.2±1.3 | 5.0±0.8 | 10.0±1.6 | 8.0±0.4 |
| α-calacorene* | 1546 | 1.2±0.1 | 1.2±0.0 | 1.2±0.1 | 1.3±0.0 | 1.3±0.1 | 7.9±0.5 | 8.2±0.4 | 8.7±0.8 | 3.5±0.4 | 4.1±0.2 | 1.7±0.2 | 1.3±0.0 | 1.2±0.1 | 1.3±0.0 | 1.3±0.1 |
| cubebol* | 1520 | 15.8±3.5 | 15.5±3.0 | 112.9±12.0 | 75.5±20.6 | 108.9±16.4 | 117.6±21.7 | 60.3±27.8 | 17.9±4.9 | 71.4±5.0 | 36.6±7.8 | 57.8±2.5 | 62.3±1.6 | 42.4±9.3 | 61.3±1.8 | 64.8±0.4 |
| elemol* | 1553 | 8.7±2.0 | 8.4±1.4 | 7.5±1.2 | 6.3±1.0 | 9.4±1.8 | 6.7±0.7 | 10.3±1.5 | 5.9±1.2 | 7.8±0.3 | 11.5±0.8 | 9.6±1.5 | 13.1±0.7 | 9.6±1.9 | 14.3±0.9 | 13.5±0.4 |
| germacrene D-4-ol* | 1584 | 1.2±0.0 | 1.2±0.0 | 1.1±0.1 | 1.2±0.1 | 1.1±0.1 | 1.8±0.3 | 3.6±1.0 | 1.6±0.4 | 1.1±0.1 | 1.0±0.3 | 1.1±0.0 | 1.1±0.1 | 1.2±0.0 | 1.1±0.1 | 1.6±0.1 |
| spathulenol* | 1584 | 13.0±2.8 | 11.8±2.2 | 9.7±1.1 | 11.9±0.5 | 18.5±4.4 | 10.1±0.9 | 13.2±1.6 | 10.8±2.6 | 7.6±0.7 | 40.6±18.0 | 13.1±1.2 | 26.7±11.0 | 22.4±3.6 | 28.0±10.7 | 38.2±13.5 |
| caryophyllene oxide | 1587 | 56.0±18.3 | 52.0±15.9 | 53.0±8.6 | 45.6±6.4 | 67.7±11.6 | 54.8±2.2 | 53.3±2.1 | 55.1±14.3 | 48.1±1.7 | 71.8±6.6 | 58.6±2.6 | 70.4±10.2 | 49.1±9.0 | 67.9±11.0 | 83.6±10.9 |
| gleenol* | 1588 | 1.2±0.0 | 1.2±0.0 | 1.1±0.1 | 1.2±0.1 | 1.1±0.1 | 9.8±0.3 | 11.7±1.0 | 3.2±2.0 | 1.1±0.1 | 11.2±4.1 | 1.1±0.0 | 1.1±0.1 | 1.2±0.0 | 1.1±0.1 | 1.6±0.1 |
| humulene oxide II | 1613 | 12.3±2.0 | 11.5±1.7 | 16.8±1.9 | 15.2±1.5 | 24.3±3.0 | 22.6±3.2 | 13.5±1.2 | 11.1±1.5 | 16.0±1.2 | 14.7±1.5 | 17.6±2.4 | 21.3±2.3 | 12.7±2.6 | 23.7±2.2 | 22.4±2.1 |
| 1-epi-cubenol* | 1632 | 6.0±3.3 | 5.9±2.9 | 16.3±1.3 | 12.3±1.3 | 10.4±0.9 | 14.1±2.2 | 10.9±1.7 | 6.8±2.4 | 12.5±1.8 | 11.6±2.5 | 9.9±0.8 | 12.4±0.5 | 4.0±1.1 | 13.0±0.4 | 13.4±0.5 |
| α-muurolol* | 1648 | 11.5±2.3 | 11.7±2.1 | 26.9±1.5 | 23.0±1.6 | 20.3±5.1 | 19.0±2.5 | 14.8±1.2 | 10.4±2.1 | 21.6±1.2 | 12.3±2.4 | 15.9±3.6 | 21.3±3.8 | 13.8±2.8 | 24.7±3.8 | 22.2±3.8 |
| α-cadinol* | 1661 | 15.1±2.8 | 17.4±1.9 | 22.7±0.8 | 21.4±1.0 | 24.6±1.3 | 21.0±1.4 | 29.6±0.9 | 18.2±3.2 | 24.8±0.7 | 19.5±2.8 | 17.9±5.6 | 24.0±5.7 | 18.6±2.3 | 29.4±5.9 | 23.9±5.0 |
| α-bisabolol* | 1688 | 9.9±0.6 | 9.8±0.5 | 10.7±0.5 | 10.3±0.4 | 10.3±0.5 | 32.8±0.9 | 34.0±2.0 | 15.5±5.8 | 27.3±0.8 | 15.1±6.9 | 4.4±0.3 | 5.8±0.4 | 5.8±0.5 | 7.6±0.6 | 7.2±0.2 |
| eudesma-4(15),7-dien-1-beta-ol* | 1696 | 10.5±2.9 | 6.9±0.7 | 22.8±2.6 | 19.5±1.5 | 27.5±3.4 | 26.0±0.5 | 35.3±2.5 | 21.3±3.1 | 11.3±3.4 | 27.5±2.1 | 8.8±1.0 | 11.9±1.1 | 7.5±1.1 | 11.4±1.2 | 13.8±0.5 |
| Total monoterpenes | 39± 3.9 | 65± 5.0 | 299± 6.8 | |||||||||||||
| Total monoterpene hydrocarbons | 21.7± 3.4 | 37.5±3.2 | 221.5±5.3 | |||||||||||||
| Total oxygenated monoterpenes | 16.8±1.9 | 27.8±2.4 | 77.1±2.5 | |||||||||||||
| Total sesquiterpenes | 799±38.0 | 956±48.6 | 1181±55.0 | |||||||||||||
| Total sesquiterpene hydrocarbons | 422.18±18.0 | 509.1±32.4 | 762.5±37.1 | |||||||||||||
| Total oxygenated sesquiterpenes | 377.2±22.7 | 446.9±20.7 | 418.9±23.3 | |||||||||||||
| Total terpenes | 838±39.0 | 1021±51.6 | 1480±57.7 | |||||||||||||
RI: Retention Index; NI: Non Identified. ; * : tentatively identified.
For a given altitude level five populations were selected, each of them exhibiting a low intra-populational variability regarding the individual components identified. However for low and mid-altitudes inter-population variability increases. For instance β-elemene content in lowlands plants ranged from 2.5–34.9 µg g−1 dw. This raises the complexity of the identification of molecular markers that discriminate ecological gradients. Taking into account such variability the main compounds found in low altitude sites were: δ-cadinene (50–112 µg g−1 dw), β-caryophyllene (54–92 µg g-1 dw) and cubebol (15–112 µg g−1 dw). Mid-altitude site samples contained similar main components: δ-cadinene (59–176 µg g−1 dw), β-caryophyllene (71–146 µg g−1 dw) and cubebol (17–117 µg g−1 dw). However, in high altitude site, β-caryophyllene (156–186 µg g−1 dw), δ-cadinene (83–153 µg g−1 dw) and α-pinene (76–105 µg g−1 dw) were the dominant components. Hence, the three altitudinal levels were qualitatively similar except for the high content of α-pinene found in highlands specimens. When considering the amount of the different families of terpenic constituents, Two-Way-ANOVA showed that there is no significant effect of the interaction between site and station for monoterpene hydrocarbons (Table 2). The subsequent One-Way-ANOVA carried out followed by a Tukey’s post hoc test showed that monoterpene hydrocarbons found in low (21.7 µg g−1 dw), mid- (37.5 µg g−1 dw) and highlands (221.5 µg g−1 dw) were significantly different (F = 742.22; p < 0.001). Thus, we may conclude that monoterpene hydrocarbons significantly increase with altitude. The high richness of terpenes content found in leaves of P. lentiscus of the high elevated site is in accordance with several authors who have stated that altitude is a factor influencing plant chemistry [23,24]. Enhanced UV-B radiation and lower temperatures at high altitudes have been exhaustively discussed as having an impact on flavonoid and monoterpene hydrocarbon contents [25].
Table 2.
Variance analysis (Two-Way Anovatest, 5%) of monoterpenes (hydrocarbons and oxygenated), sesquiterpenes (hydrocarbons and oxygenated) and total terpenes between sites, stations and interaction site vs station.
| Terpenoids | factors | df | SS | MS | F | P |
|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | Site | 2 | 617137 | 308569 | 479.144 | < 0.001 |
| Site:Station | 12 | 7725 | 644 | 1.754 | 0.08 | |
| Residuals | 60 | 22007 | 367 | |||
| Oxygenated monoterpenes | Site | 2 | 51551 | 25775.3 | 108.802 | < 0.001 |
| Site:Station | 12 | 2843 | 236.9 | 2.823 | 0.004 | |
| Residuals | 60 | 5037 | 83.9 | |||
| Sesquiterpene hydrocarbons | Site | 2 | 1563493 | 781746 | 11.700 | 0.001 |
| Site:Station | 12 | 801748 | 66812 | 4.718 | < 0.001 | |
| Residuals | 60 | 849581 | 14160 | |||
| Oxygenated sesquiterpenes | Site | 2 | 61397 | 30698 | 0.688 | 0.52 |
| Site:Station | 12 | 535103 | 44592 | 7.484 | < 0.001 | |
| Residuals | 60 | 357500 | 5958 | |||
| Total terpenes | Site | 2 | 5470660 | 2735330 | 13.782 | < 0.001 |
| Site:Station | 12 | 2381658 | 1984472 | 5.601 | < 0.001 | |
| Residuals | 60 | 2126026 | 35434 |
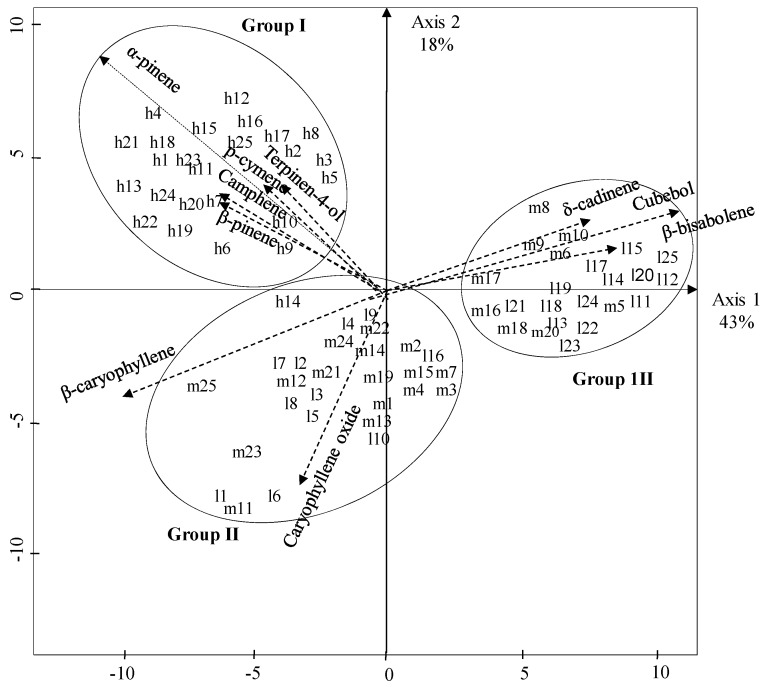
Because the individual oil components, expressed on dw basis (w/w) could not clearly discriminate between the three altitude levels, data were expressed in terms of percentage of the chromatogram prior to Principal Component Analysis (PCA). Figure 1 represents two-dimensional mapping of the PCA, Axis 1 and Axis 2 represent 43% and 18% of the information, respectively.
Figure 1.
Tow-dimensional mapping of the P.C.A. analysis of Pistacia lentiscus L. individual distribution from low, mid and high altitude sites in Algeria (l: low; m: medium and h: high altitude sites).
The distribution of individual points in plan 1-2 based on cluster analysis (Ward’s technique) divided the 75 accessions into three main groups according to their content (expressed in % because of maximum of variability) in α-pinene, caryophyllene oxide and cubebol. The group I (33.3% of total samples) includes individuals harvested from the high elevated sites which are characterized by a high content in β-caryophyllene (12.0%), δ-cadinene (9.3%) and α-pinene (6.8%). Among monoterpenes which characterize this group, camphene, p-cymene, β-pinene, terpinen-4-ol are also the most discriminating compounds. The groups II and III are dominated by individuals harvested from low and mid-altitude sites, respectively. The chemical composition of group II (37.3% of samples) was characterized by high contents of β-caryophyllene (11.5%), δ-cadinene (8.6%) and caryophyllene oxide (6.8%). The last group (26.4% of samples) contains mainly individuals of low altitude sites with δ-cadinene (10.9%), cubebol (10.5%) and β-bisabolene (7.7%) as discriminating components. Samples of Groups I and II were similar to Sardinian (Costa Rey) chemotype where β-caryophyllene (31.38%), germacrene D (12.08%) and δ-cadinene (6.48%) were identified as the dominant compounds from supercritical CO2 leaves extracts [26]. However the composition of the hydrodistillates were totally different. The chemical variability of P. lentiscus essential oil obtained through hydrodistillation from samples harvested at Tigzirt (Algeria) was reported as α-pinene (22–29%), myrcene (1.4–23%) and sabinene (8–11.7%) were the major constituents [27]. In our samples from Tigzirt (see Table 1: L3) myrcene and sabinene were not detected. This disagreement with our data is probably the result of thermal hydrolysis that occurs during hydrodistillation, as suggested earlier [26]. Consequently, it is likely that cold extraction leads to the recovery of the true components in plants. That said, axis 1 clearly separates Groups I and III while axis 2 distinguishes Group I from Group II. This raises the question of the characterization of the ecological factors involved. A careful analysis of our sampling sites (Table 3) revealed that lowlands habitats were highly heterogeneous in terms of specific composition. All the highland habitats were composed by Olea europea plant association. Taking into account the other ecological data collected, the mean maximum temperature in highlands (about 27 °C) was lower that that of the lowlands (31 °C) suggesting that, axis 1 determines temperatures decrease. Because most of lowlands were located near the sea, it is not excluded that other factors such as substrate type and soil salinity may be particularly discriminating factors. On the other hand axis 2 can be interpreted as the result of abiotic factors associated with drought since midlands exhibited a lower mean coefficient of Emberger (Q2 = 85) compared to that of highlands (Q2 = 154).
Table 3.
Ecological factors of Pistacia lentiscus collection sites.
| Sites | Stations | Alt.(m) | Geographical coordinates | Habitat | P (mm) | T0 Max (C0) | Q2 |
|---|---|---|---|---|---|---|---|
| Low-altitude | Cherchel (L1) | 15 | 36°36’N/2°11’E | Phillyrea latifolia | 502.24±25.08 | 31.75±0.42 | 73.54 |
| Azzefoun (L2) | 14 | 36°53’N/4°25’E | Phillyrea latifolia | 775.78±50.04 | 31.50±0.48 | 122.06 | |
| Zemmouri Bahri (L5) | 9 | 36°48’N/3°35’E | Erica arborea | 741.14±45.34 | 29.10±0.51 | 125.85 | |
| Tigzirt (L3) | 6 | 36°53’N/4°08’E | Arbutus unedo | 923.09±67.98 | 30.30±0.44 | 150.77 | |
| Ziama Mansouriah (L4) | 8 | 36°39’N/5°28’E | Erica arborea | 968.11±60.82 | 30.80±0.38 | 148.24 | |
| Mid-altitude | Thenia (M1) | 168 | 36°43’N/3°32’E | Eucalyptus radiata | 728.53±58.43 | 35.00±0.37 | 88.61 |
| Beni Amrane (M4) | 121 | 36°39’N/5°14’E | Erica arborea | 729.58±55.22 | 35.50±0.38 | 87.50 | |
| Tizi Ouzou (M5) | 182 | 36°43’N/4°03’E | Pinus halepensis | 731.92±57.42 | 36.10±0.41 | 83.68 | |
| El Kseur (M3) | 167 | 36°41’N/4°51’E | Olea europea | 758.39±59.33 | 30.80±0.35 | 112.12 | |
| Lakhdaria (M2) | 307 | 36°34’N/3°35’E | Olea europea | 513.85±54.14 | 35.60±0.39 | 55.25 | |
| High- altitude | Ait Khelifa (H3) | 1042 | 36°31’N/4°20’E | Olea europea | 1039.30±56.57 | 23.20±0.32 | 168.95 |
| Tala Moumene (H4) | 1008 | 36°33’N/4°48’E | Olea europea | 1041.27±67.45 | 24.46±0.35 | 190.18 | |
| Beni Mendes (H1) | 809 | 36°30’N/3°59’E | Olea europea | 924.56±64.54 | 31.75±0.38 | 112.74 | |
| Aourir N’Athjelil (H2) | 868 | 36°34’N/4°48’E | Olea europea | 1019.62±69.33 | 25.44±0.31 | 182.15 | |
| Tizi-Oumalou (H5) | 942 | 36°30’N/4°20’E | Olea europea | 939.06±78.75 | 30.82±0.36 | 116.11 |
Data sources: Office Nationale de la Météorologie d’Alger (O.N.M.) and Agence Nationale des Ressources Hydriques d’Alger (A.N.R.H.). Period from 1997–2007; Specimens were deposited at the herbarium of the University of Provence (Marseille) and referred as PL1-MAR -2010, PL2-MAR-2010, PL3-MAR-2010 for Algerian low, mid and high altitude sites, respectively.
Among the identified components, α-pinene, camphene, β-pinene and p-cymene are the most discriminating monoterpene hydrocarbons of individuals from high altitude sites. The increase of these compounds with altitude regardless to the specific composition of the habitats may be explained by the involvement of genetic and/or abiotic factors. For instance camphene is found to be cytoprotective by decreasing lipid peroxidation and inhibiting NO release and ROS generation [28] whereas, p-cymene was reported to be positively correlated with aridity index and altitude from Thymus piperella [29].
3. Experimental
3.1. Plant material
Seventy five sample of Pistacia lentiscus were collected in September 2008 in three Algerian sites which are located at different altitudes. Five stations were chosen per site with five female individuals per station. The first site includes individuals harvested from low altitude, the second one includes individuals located at mid-altitude and finally, we have collected individuals at the high altitude site in Kabylia. Ecological factors of the sampling sites are described in Table 3.
Sampling was carried out during fructification stage in order to take into account the phenological shift due to local climatic conditions. Twenty five female individuals were chosen per site. Mature and sun exposed leaves were harvested, dried in dark at ambient air temperature (25 °C) conditions until constant weight then, 100 g per tree were grounded and stored until use.
3.2. Terpenoids extraction
The extraction method used consisted of suspending leaf dry matter in dichloromethane according to a ratio of 1:2 (w/v), for 30 min, under constant shaking at room temperature. Dodecane (50 µL, 5 mg mL−1) were added as internal standard for quantification.
3.3. Quantitative and qualitative analysis of terpenoids
Extracts were filtered on an Regenerated Cellulose syringe filter (RC, 0.45 µm, 25 mm, Phenomenex) then analyzed with a gas chromatograph (Hewlett Packard® GC 6890) coupled to a mass selective detector (5973 Network). The system was fitted with an HP-5MS capillary column (30 m, 0.25 mm, 0.25 µm). Extract (2 µL) was injected through an automatic injector (ALS 7683) in splitless mode. Purge was set at 50 min mL−1 after 1 min. Injection temperature was maintained at 250 °C. Helium was used as carrier gas. A constant flow rate of 1 mL min-1 was set throughout the run. The oven temperature initially set at 40 °C was increased to 270 °C at a rate of 4 °C min−1 and remained constant for 5 min. The MSD transfer line heater was maintained at 280 °C. Terpenes were identified by comparison of their retention index (RI) and mass spectra (NIST, 2008) with those obtained from authentic samples and literature [30].
3.4. Statistical analyses
The data were analyzed by a two-way ANOVA model. Turkey’s HSD (Honestly Significant Differences) procedure was used to test for significant differences in monoterpenes (hydrocarbons and oxygenated), sesquiterpenes (hydrocarbons and oxygenated) and total terpene concentrations between stations and sites. In order to evaluate the qualitative distribution of major chemical compounds (expressed in % of the chromatogram) in our 75 samples, Principal Component Analysis (PCA) was carried out. The statistical analyses were performed using the R statistical software [31], packages "ade4" [32] and “agricolae” [33].
4. Conclusions
Our investigation revealed a chemical diversity of P. lentiscus associated with the geographic location. Three chemotypes were identified according to the different elevation levels. This variability may be interpreted as the result of biotic and abiotic factors, as well as genetic variability. The main discriminating environmental factors identified were temperature and drought. Consequently our finding should be further verified through analysis of terpenoids in seedlings of multiple provenance held under identical growth conditions.
Acknowledgements
The authors gratefully acknowledges the French and Algerian Inter-University Cooperation that has funded this work.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Naghdi Badi H., Yazdani D., Mohammad A.S., Nazari F. Effects of spacing and harvesting on herbage yield and quality/quantity of oil in thyme, Thymus vulgaris L. Ind. Crop. Prod. 2004;19:231–236. doi: 10.1016/j.indcrop.2003.10.005. [DOI] [Google Scholar]
- 2.Yu F.N.A., Utsumi R. Diversity, regulation, and genetic manipulation of plant mono-and sesquiterpenoid biosynthesis. Cell. Mol. Life Sci. 2009;66:3043–3052. doi: 10.1007/s00018-009-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kofidis G., Bosabalidis A., Moustakas M. Chemical composition of essential oils from leaves and twigs of Pistacia lentiscus, Pistacia lentiscus var. chia, and Pistacia terebinthus from Turkey. Pharm. Biol. 2003;42:360–366. [Google Scholar]
- 4.Tkachev A.V., Korolyuk E.A., Letchamo W. Chemical screening of volatile oil- bearing flora of Siberia IX. Variations in chemical composition of the essential oil of Heteropappus altaicus Willd. (Novopokr.) growing wild at different altitudes of Altai Region, Russia. J. Essent. Oil Res. 2006;18:149–151. doi: 10.1080/10412905.2006.9699048. [DOI] [Google Scholar]
- 5.Agnihotri V.K., Lattoo S.K., Thappa R.K., Kaul P., Qazi G.N., Dhar A.K., Saraf A., Kapahi B.K., Saxena R.K., Agarwal S.G. Chemical variability in the essential oil components of Achillea millefolium Agg. from different Himalayan habitats (India) Planta Med. 2005;71:280–283. doi: 10.1055/s-2005-837828. [DOI] [PubMed] [Google Scholar]
- 6.Munne-Bosch S., Penuelas J. Photo- and antioxidative protection during summer leaf senescence in Pistacia lentiscus L. grown under Mediterranean field conditions. Ann. Bot. 2003;92:385–391. doi: 10.1093/aob/mcg152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro-Diez P., Villar-Salvador P., Perez-Rontome C., Maestro-Martinez M., Montserrat-Marti G. Leaf morphology, leaf chemical composition and stem xylem characteristics in two Pistacia (Anacardiaceae) species along a climatic gradient. Flora. 1998;193:195–202. [Google Scholar]
- 8.Ozturk M., Dogan Y., Sakcali M.S., Doulis A., Karam F. Ecophysiological responses of some maquis (Ceratonia siliqua L., Olea oleaster Hoffm. & Link, Pistacia lentiscus and Quercus coccifera L.) plant species to drought in the east Mediterranean ecosystem. J. Environ. Biol. 2010;31:233–245. [PubMed] [Google Scholar]
- 9.Barazani O., Golan-Goldhirsh A. Salt-driven interactions between Pistacia lentiscus and Salsola inermis. Environ. Sci. Poll. Res. 2009;16:855–861. doi: 10.1007/s11356-009-0231-4. [DOI] [PubMed] [Google Scholar]
- 10.Verdu M., Garcia-Fayos P. Reproductive ecology of Pistacia lentiscus L. (Anacardiaceae): an evolutionary anachronism in the Mediterranean shrubland. Rev. Chil. Hist. Nat. 2002;75:57–65. [Google Scholar]
- 11.Charef M., Yousfi M., Saidi M., Stocker P. Determination of the fatty acid composition of acorn (Quercus), Pistacia lentiscus seeds growing in Algeria. J. Am. Oil Chem. Soc. 2008;85:921–924. doi: 10.1007/s11746-008-1283-1. [DOI] [Google Scholar]
- 12.Janakat S., Al-Merie H. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. J. Ethnopharmacol. 2002;83:135–138. doi: 10.1016/S0378-8741(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 13.Benhammou N., Bekkara F.A., Panovska T.K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr. J. Pharm. Pharmacol. 2008;2:022–028. [Google Scholar]
- 14.Atmani D., Chaher N., Berboucha M., Ayouni K., Lounis H., Boudaoud H., Debbache N. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009;112:303–309. doi: 10.1016/j.foodchem.2008.05.077. [DOI] [Google Scholar]
- 15.Fahn A. Secretory Tissues in Plants. Academic Press; London, UK: 1979. [Google Scholar]
- 16.Llusià J., Peñuelas J. Changes in terpene content and emission in potted Mediterranean woody plants under severe drought. Can. J. Bot. Rev. Can. Bot. 1998;76:1366–1373. [Google Scholar]
- 17.Zrira S., Elamrani A., Benjilali B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco - a seasonal variation. Flavour Fragr. J. 2003;18:475–480. doi: 10.1002/ffj.1221. [DOI] [Google Scholar]
- 18.Duru M.E., Cakir A., Kordali S., Zengin H., Harmandar M., Izumi S., Hirata T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 2003;74:170–176. doi: 10.1016/S0367-326X(02)00318-0. [DOI] [PubMed] [Google Scholar]
- 19.Barra A., Coroneo V., Dessi S., Cabras P., Angioni A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. J. Agr. Food Chem. 2007;55:7093–7098. doi: 10.1021/jf071129w. [DOI] [PubMed] [Google Scholar]
- 20.Gardeli C., Vassiliki P., Athanasios M., Kibouris T., Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107:1120–1130. doi: 10.1016/j.foodchem.2007.09.036. [DOI] [Google Scholar]
- 21.Fernandez A., Camacho A., Fernandez C., Altarejos A., Perez P. Composition of the essential oils from galls and aerial parts of Pistacia lentiscus L. J. Essent. Oil Res. 2000;12:19–23. doi: 10.1080/10412905.2000.9712031. [DOI] [Google Scholar]
- 22.Tzakou O., Bazos I., Yannitsaros A. Volatile metabolites of Pistacia atlantica Desf. from Greece. Flavour Fragr. J. 2007;22:358–362. doi: 10.1002/ffj.1805. [DOI] [Google Scholar]
- 23.Haider F., Kumar N., Banerjee S., Naqvi A.A., Bagchi G.D. Effect of Altitude on the essential Oil Constituents of Artemisia roxburghiana Besser var. purpurascens (Jacq.) Hook. J. Essent. Oil Res. 2009;21:303–304. doi: 10.1080/10412905.2009.9700177. [DOI] [Google Scholar]
- 24.Vokou D., Kokkini S., Bessiere J.M. Geographic-Variation of Greek Oregano (Origanum-Vulgare ssp Hirtum) Essential Oils. Biochem. Syst. Ecol. 1993;21:287–295. doi: 10.1016/0305-1978(93)90047-U. [DOI] [Google Scholar]
- 25.Bilger W., Rolland M., Nybakken L. UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem. Photobiol. Sci. 2007;6:190–195. doi: 10.1039/b609820g. [DOI] [PubMed] [Google Scholar]
- 26.Congiu R., Falconieri D., Marongiu B., Piras A., Porcedda S. Extraction and isolation of Pistacia lentiscus L. essential oil by supercritical CO2. Flavour Fragr. J. 2002;17:239–244. doi: 10.1002/ffj.1095. [DOI] [Google Scholar]
- 27.Mecherara-Idjeri S., Hassani A., Castola V., Casanova J. Composition and Chemical Variability of the Essential oil from Pistacia lentiscus L. Growing Wild in Algeria Part: Leaf Oil. J. Essent. Oil Res. 2008;20:32–38. doi: 10.1080/10412905.2008.9699415. [DOI] [Google Scholar]
- 28.Tiwari M., Kakkar P. Plant derived antioxidants - Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. In Vitro. 2009;23:295–301. doi: 10.1016/j.tiv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Hudaib M., Aburjai T. Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour Fragr. J. 2007;22:322–327. doi: 10.1002/ffj.1800. [DOI] [Google Scholar]
- 30.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 31.R Development Core Team (2009) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. Available online: http://www.R-project.org. ISNB 3-900051-07-0. [Google Scholar]
- 32.Dray S., Dufour A.B., Chessel D. The ade4 package-II: Two-table and K-table methods. R News. 2007;7:47–52. [Google Scholar]
- 33.De Mendiburu F. Agricolae: Statistical Procedures for Agricultural Research, R package version 1.0-9. [on 24 February 2010]. Available online: http://CRAN.R-project.org/package=agricolae,accessed.