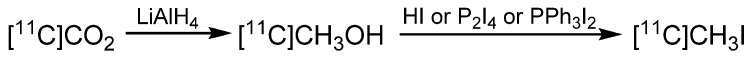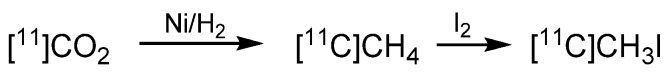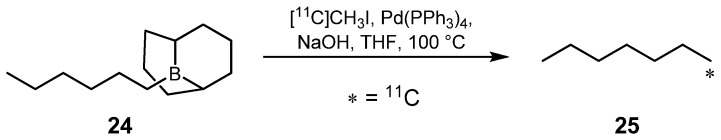Abstract
The increasing application of positron emission tomography (PET) in nuclear medicine has stimulated the extensive development of a multitude of new radiotracers and novel radiolabeling procedures with the most prominent short-lived positron emitters carbon-11 and fluorine-18. Radiolabeling with these radionuclides represents a remarkable challenge. Special attention has to be paid to synthesis time and specific labeling techniques due to the short physical half life of the respective radionuclides 11C (t1/2 = 20.4 min) and 18F (t1/2 = 109.8 min). In the past, numerous transition metal-catalyzed reactions were employed in organic chemistry, even though only a handful of these coupling reactions were adopted in radiochemical practice. Thus, the implementation of modern synthesis methods like cross-coupling reactions offers the possibility to develop a wide variety of novel radiotracers. The introduction of catalysts based on transition metal complexes bears a high potential for rapid, efficient, highly selective and functional group-tolerating incorporation of carbon-11 and fluorine-18 into target molecules. This review deals with design, application and improvement of transition metal-mediated carbon-carbon as well as carbon-heteroatom cross-coupling reactions as a labeling feature with the focus on the preparation of radiolabeled compounds for molecular imaging.
Keywords: cross-coupling, radiolabeling, carbon-11, fluorine-18
1. Introduction
Radiopharmaceutical chemistry deals with the design and synthesis of radiolabeled compounds, also referred to as radiotracers. These days, radiopharmaceutical chemistry has developed into a complex chemical science which combines progress in modern organic and inorganic chemistry with novel trends in molecular biology. Development of new radiotracers for molecular imaging has to account for special requirements of their preparation with respect to the choice of the appropriate radionuclide and the labeling position. Furthermore, the field of radiotracers ranges from small organic and bioactive molecules such as carbohydrates, amino acids or steroids to high molecular weight compounds like peptides, proteins or oligonucleotides. Therefore, special attention should be paid to the implementation of fast and highly selective reactions which tolerate other functional groups. As enzyme and transition metal-catalyzed reactions both comply with these requirements, they were introduced for the design and synthesis of a multitude of radiotracers labeled with carbon-11 (t1/2 = 20.4 min) and fluorine-18 (t1/2 = 109.8 min). The catalyst in cross-coupling reactions applied for radiolabeling purposes is used in large excess compared to the respective carbon-11 or fluorine-18- containing building block. Due to this unusual stochiometrical scale, the term "mediated" is rather appropriate than "catalyzed", as it is statistically unlikely for the metal to take part in another cycle. Therefore, the term “metal-mediated” is preferred in radiolabeling reactions.
Transition metal-catalyzed cross-coupling reactions have been used extensively in carbon-carbon as well as carbon-heteroatom bond-forming reactions [1]. The central importance of these reactions using cross-coupling methods in organic chemistry as well as life sciences was honored with the Nobel Price in Chemistry in the year 2010 [2]. In the majority of cases, this kind of reactions is adopted for the preparation of the precursor scaffold which is subsequently labeled directly or indirectly with radiolabeled building blocks. Accessorily, cross-coupling reactions can be applied for indirect labeling due to the mostly mild reaction conditions and the short reaction times which are of major importance in radiochemistry. Therefore, it is possible to use these reactions in rapid and facile labeling syntheses involving the introduction of short-lived nuclides carbon-11 and fluorine-18. Advantageous, these coupling reactions allow highly selective and functional group-tolerating syntheses of radiotracers bearing both radionuclides.
The present review summarizes recent developments in the field of labeling procedures using transition metal-mediated cross-coupling reactions for the introduction of the short-lived positron emitting radionuclides carbon-11 and fluorine-18 into organic molecules. Applications for radiolabeling using the Cu-mediated "click chemistry" have been reviewed in detail elsewhere [3,4] and will not be discussed here.
2. Carbon-11 Labeling
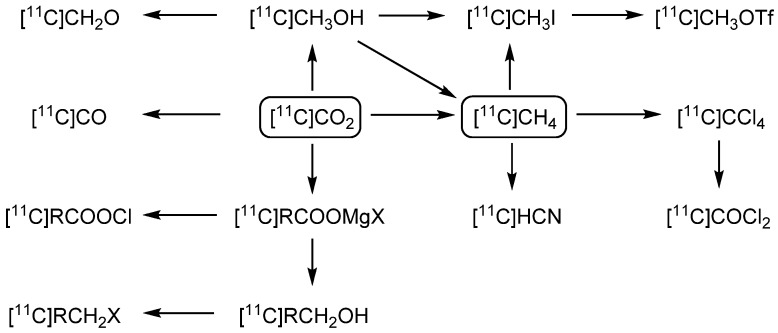
Carbon-11 is one of 13 known isotopes of the element carbon [5]. It is cyclotron-produced by a proton beam of about 10–17 MeV from a nitrogen-14 target which has previously been filled with a low concentration of oxygen or hydrogen. For this purpose, the 14N(p,α)11C nuclear reaction is exploited with a maximum theoretical specific activity of 3.4∙105 GBq·µmol−1 [6,7,8] which is never reached in practice. Carbon-11 is a suitable nuclide for PET imaging because of its high positron emission rate (99.8%) and its low energy positrons (1.0 MeV). Another advantage of carbon-11 is based on its chemical equivalence to carbon-12 and carbon-13, when incorporated into bioactive compounds for PET imaging purposes. Incorporation of carbon-11 results in equivalent, bioactive radiopharmaceuticals. In contrast, in particular fluorine-18 usually replaces hydrogen atoms or hydroxyl groups and might therefore be responsible for a different (bio-)chemical behavior of the resulting radiotracer [7,8]. Due to its short half-life of 20.4 min, radiosyntheses utilizing carbon-11 are generally performed within 60 min including purification of the crude radiolabeled product [9]. This fact led to the development of rapid 'on-line'-syntheses, by which the primary building block can be converted into the radiochemical compound, after the carbon-11 species has been transferred from the cyclotron directly to the hot cell. Carbon-11 usually arrives in the chemical form of [11C]CO2 or [11C]CH4 from the cyclotron, which represent the two most important 11C-labeled primary building blocks [10] from which nearly all other 11C-labeling units are produced (Scheme 1).
Scheme 1.
11C-labeled primary building blocks and resulting secondary 11C labeling units.
Two main methods for the production of [11C]methyl iodide as the most important and most widely used secondary [11C]building block are stated in the literature. One approach, referred to as "wet" chemistry method, deals with the reduction of [11C]CO2 to [11C]methanol utilizing LiAlH4 in THF or diethyl ether. The resulting [11C]methanol is then converted into [11C]methyl iodide by the use of hydroiodic acid, diphosphorus tetraiodide [9] or triphenylphosphane diiodide [10], respectively (Scheme 2). Afterwards, the resulting [11C]methyl iodide is dried and separated from by-products.
Scheme 2.
"Wet" chemistry route yielding [11C]methyl iodide.
The second approach, also known as "gas phase" method, deals with the conversion of [11C]methane into [11C]methyl iodide by radical iodination with elemental iodine at 700–750 °C (Scheme 3). Depending on the primary radiolabeled building block produced by the cyclotron, it might be necessary to convert [11C]CO2 into [11C]CH4 beforehand by the use of a nickel catalyst [11], otherwise [11C]CH4 is used directly [12].
Scheme 3.
"Gas phase" chemistry route to [11C]methyl iodide.
The "gas phase" method offers several advantages. Due to the direct synthesis of [11C]methyl iodide, highest specific activities of max. 4,700 GBq·μmol−1 can be achieved [13], compared to 74–370 GBq·μmol−1 by the "wet" method [14]. The low specific activity obtained by the "wet" method is a result of contamination from naturally occurring [12/13C]CO2, which originates from LiAlH4 of the reduction step. Carbon-12/13 contamination may also arise from solvents and chemical impurities.
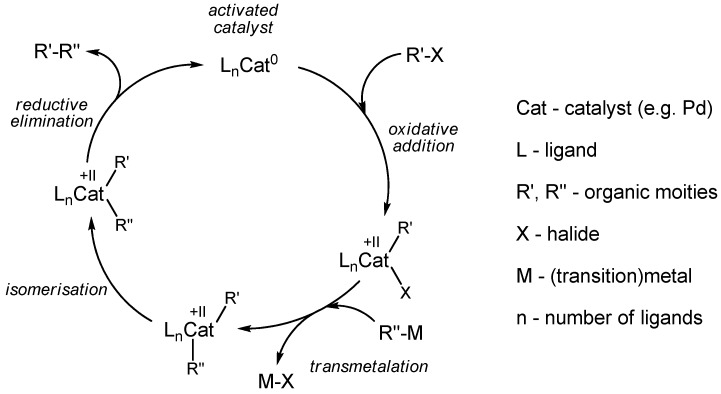
The most prominent and most commonly applied procedure for the radiolabeling with carbon-11 represents the nucleophilic [11C]methylation of alcohols, primary and secondary amines, thiols, carbanions or carboxylic acid derivatives with [11C]methyl iodide (or [11C]methyl triflate). Besides, transition metal-mediated cross-coupling reactions are gaining in importance for labeling reactions with carbon-11. Although the mechanism of cross-coupling is not fully understood, most intermediates have been identified, supporting the proposed classical mechanism depicted in Scheme 4. In most cases, the catalyst is a palladium-0 species. According to this theory, the reaction steps proceed as follows: An organic halide R'-X is coordinated to the Pd-catalyst by oxidative addition. A second (metal)organo compound R''-M reacts with the activated Pd species by transmetalation in the rate-determining step. After isomerization, the desired cross-coupled compound R'-R'' is formed via reductive elimination in the final step. The catalyst is released in the process, is thereby regenerated and available for the next catalysis cycle.
Scheme 4.
Proposed classical mechanism of cross-coupling.
2.1. Cross-coupling reactions with palladium
2.1.1. Stille reaction
One of the mildest and most applied cross-coupling methods is the Stille reaction [15,16]. Organotin compounds function as starting material and alkyl/aryl halogenides as coupling partners, which are cross-coupled by the use of Pd-catalyst and a phosphane-based supporting ligand (PR3). A wide variety of functional groups such as amino, hydroxyl, thiol or carboxylate is tolerated. Another benefit of the Stille reaction is the stability of the organotin compounds against Brønsted acids and bases. However, one major drawback of organotin reagents used for the transmetalation step is their inherent toxicity in contrast to other starting materials like boronic acids. This may limit the application of this kind of cross-coupling reactions especially when pharmaceuticals are prepared.
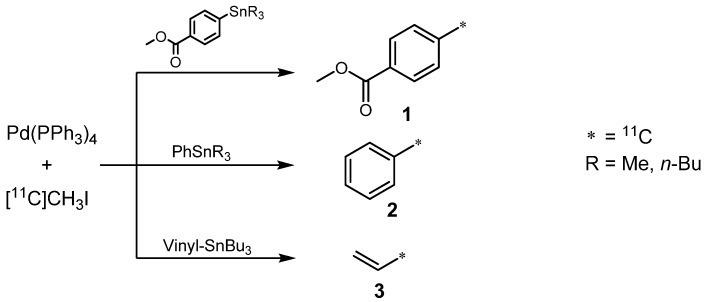
The first attempt for the introduction of carbon-11 in molecules by the Stille reaction was reported in a work of Andersson et al. in 1995. Several organotin sample molecules were used for the synthesis of 11C-labeled model arenes 1 and 2, as well as alkene 3 with radiochemical yields (RCYs) from 30 to 85% (Scheme 5) [17]. Optimizations to increase RCY and radiochemical purity were performed by exchanging solvents and stannane residues. Best results were found for aromatic trimethylstannyl compounds in DMF or DMSO. It was thereby demonstrated that the Stille reaction is a versatile tool for syntheses of 11C-labeled PET tracers as seen in Scheme 5.
Scheme 5.
First approach of 11C-labeling by the Stille reaction.
An attempt for a general radiolabeling strategy with carbon-11 using the Stille reaction was published years later [18]. Therefore, high numbers of aromatic model stannanes were labeled with [11C]methyl iodide under different conditions. Best results were obtained when Pd2(dba)3 was used with P(o-Tol)3 as co-ligand and CuCl/K2CO3 as additive in DMF.
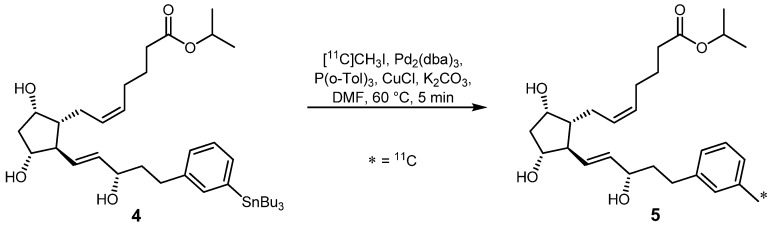
Based on these investigations, Björkman et al. presented the preparation of stannylated prostaglandin analogue 4 which was used as precursor for a 11C-labeling in an improved Stille reaction (Scheme 6).
Scheme 6.
Synthesis of 17-(3-[11C]methylphenyl)-18,19,20-trinor-PGF2α isopropyl ester (5).
The resulting radiotracer 5 is based on the known PGF2α drug Xatalan® and is applied for studies of prostaglandin (PG) receptors in the brain. Compound 5 was obtained in 34% RCY decay-corrected (d.c.) within 30 minutes after end of bombardment (EOB) with a specific activity of 100 GBq·µmol−1 and a radiochemical purity >95% [19].
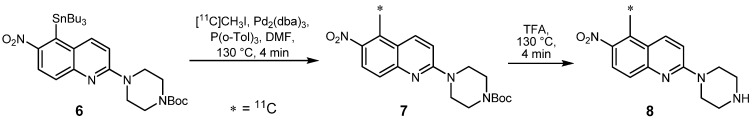
In 2002, Sandell et al. used similar conditions (Pd2(dba)3/P(o-Tol)3, DMF) for the preparation of [11C]5-methyl-6-nitroquipazine (8), an inhibitor for the serotonin (5-hydroxytryptamine) transporter (5-HTT) [20]. In contrast to the reaction conditions reported before [18,19], neither CuCl nor K2CO3 were added. In consequence of this omission, a higher reaction temperature (130 °C) and longer reaction time (~40 min after EOB) were required. However, compared to synthesis of 5, these reaction conditions delivered higher RCYs (60–80%) and radiochemical purities (>99%) of 8, but with a specific activity of only 19 GBq·µmol−1 after the two step synthesis depicted in Scheme 7. Upon radiosynthesis, compound 8 was tested as PET-tracer for the serotonin transporter 5-HTT in monkey brains. Unfortunately, it exhibited brain uptake kinetics which were too slow to justify further investigations.
Scheme 7.
Synthesis of 5-HTT inhibitor [11C]5-methyl-6-nitroquipazine (8).
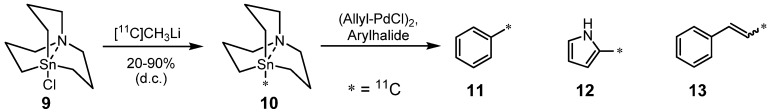
Due to the presence of other functional groups in the precursor like alcohols or amines, side reactions may occur [21]. Furthermore, preparation and purification of highly functionalized stannyl compounds can be difficult at times. In order to prevent such obstacles during the labeling process with [11C]methyl iodide and functionalized stannyl compounds, Forngren et al. presented the synthesis of the novel organotin compound 10. This molecule already contains the [11C]methyl group and is well suited for radiolabeling purposes under Stille conditions. In this case, various organohalides were labeled using the novel 11C-containing stannane 10 [22]. First, [11C]methyl iodide was converted into [11C]methyl lithium [23] and subsequently mixed with 5-chloro-1-aza-5-stanna-bicyclo-[3.3.3]-undecane (9) in order to form 5-[11C]methyl-1-aza-5-stannabicyclo[3.3.3]undecane (10) with RCYs ranging from 20 to 90% (d.c.) related to [11C]methyl iodide (Scheme 8). As stated by the authors, the wide range in yields observed when preparing 10 might to some extent be explained by the presence of proton sources in the reaction mixture, which results in the formation of [11C]methane as a side-product.
Scheme 8.
Examples of 11C-labeled products synthesized from 10.
The following cross-coupling reaction was arranged successfully in a 'one pot' procedure in which various functionalized organohalides were tested with regard to their applicability as precursor. Depending on reaction temperature and time as well as the employed arylhalide, maximum RCYs of 9% for 11, 47% for 12, and 90% for 13 were achieved with respect to [11C]stannane 10. Unfortunately, no labeling products and only moderate yields were obtained at ambient temperature and at 50–65 °C, respectively. Best results were achieved at 100 °C in DMF with allylpalladium chloride dimer as mediator, reaction conditions which starkly limit the number of potential biomolecule precursors.
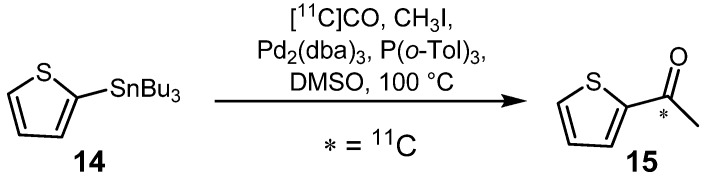
Automation of radiosyntheses is always desirable, which is also the case for the Stille reaction. In 2005, Karimi et al. demonstrated a straightforward procedure at which [11C]CO was used in the palladium-mediated carbonylative coupling of organic iodides with organostannanes to synthesize various unsymmetrical alkyl and aryl-[11C-carbonyl]ketones [24]. According to the authors, this method could be automated easily, although no further application has been reported to date. The syntheses carried out were fast and led to 20 different 11C-labeled molecules with RCYs ranging from 37 to 98% and specific activities of up to 300 GBq·µmol−1. Different reaction conditions were applied in order to scrutinize scope and limitations of the respective Pd-catalyst, the co-ligands, the temperature and the solvent. Highest RCYs were obtained in DMSO at 100 °C with an excess of P(o-Tol)3 relative to Pd-catalyst, which is depicted exemplarily for radiosynthesis of compound 15 (Scheme 9).
Scheme 9.
Synthesis of 15 by the Stille reaction.
Arai et al. improved the method established by Karimi et al. [24] (vide supra) which was applied as previously described by using a different co-ligand for the Pd-catalyst and [1-11C]acetyl chloride (16) instead of [11C]CO as depicted in Scheme 10 [25].
Scheme 10.
Improvement of Stille reaction using 16 as starting material and 18 as co-ligand.
Co-ligands PPh3 and P(o-Tol)3 did not support synthesis of the desired model substance [carbonyl-11C]acetophenone (19). In contrast, 2,8,9-trimethyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane hydrochloride (18) showed a significant improvement of the yield when used in a ratio of 1:0.5 (co-ligand 18 to Pd2(dba)3). Finally, it was possible to obtain similar RCYs (60%; d.c.), yet under milder reaction conditions (ambient temperature) than reported by Karimi et al. for the preparation of compound 19. The authors mentioned that chloride in the co-catalyst is critical in the mechanism, which still remains unclear, than the support of co-catalyst 18 without HCl. Furthermore, utilization of triaminophosphane hydrochloride 18 is advantageous in the regard that this compound is easy to handle and is stable against moisture and air [26].
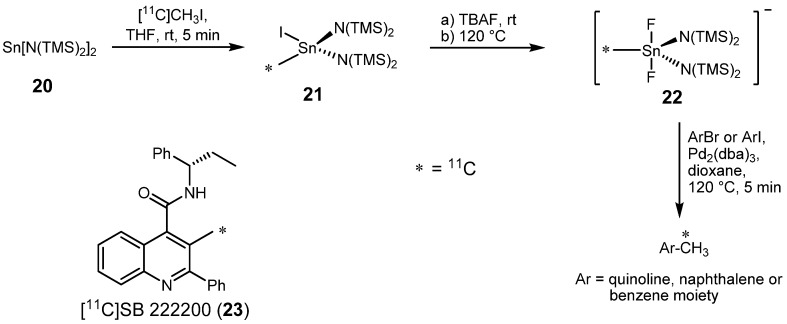
In 2006, Huiban et al. reported further improvements of the Stille reaction for radiochemical purposes. An application of less toxic mono-alkylstannanes instead of previously used di-, tri-, or tetra-alkylstannanes delivered higher radiochemical yields and allowed a facile removal of inorganic byproducts [27]. In a first step, [11C]methyl iodide was reacted with Lappert’s stannylene Sn[N(TMS)2]2 (20) [28] quantitatively (Scheme 11). Obtained mono[11C]methyl tin compound 21 was activated thereafter by addition of tetra-n-butylammonium fluoride at ambient temperature leading to stannate 22. The subsequent Pd-mediated Stille reaction of 22 with aryl bromides and iodides led to a wide variety of desired labeled heteroarenes with high RCYs (63 to 78%). Since no co-ligand like PPh3 was used, a formation of by-products (biaryl compounds), often formed by an aryl exchange reaction of such co-ligands with aryl substrates, was prevented. Based on this method, a new radiotracer [11C]SB 222200 (23) for the non-invasive study of functions and diseases involving cerebral neurokinin and opiate receptors, such as anxiety, depression, psychosis, schizophrenia and Parkinson’s disease [29], was prepared by the same working group [30]. However, no biological application has been reported to date.
Scheme 11.
Stille reaction with monomethyltin reagent 22.
Selected target molecules have been radiolabeled with carbon-11 via standard Stille reaction before, thus enabling a comparison of these methods. The results of this study confirmed the group’s previous findings, as similar or higher RCYs and specific activities, respectively, were obtained by using the improved reaction conditions. Thus, the authors postulate that their method, whenever applicable, is superior due to easier purification of 11C-labeled products, less toxicity of the used organotin compounds along with similar RCYs. A wide range of 11C-labeled radiotracers has been developed using conditions similar to the typical Stille reaction.
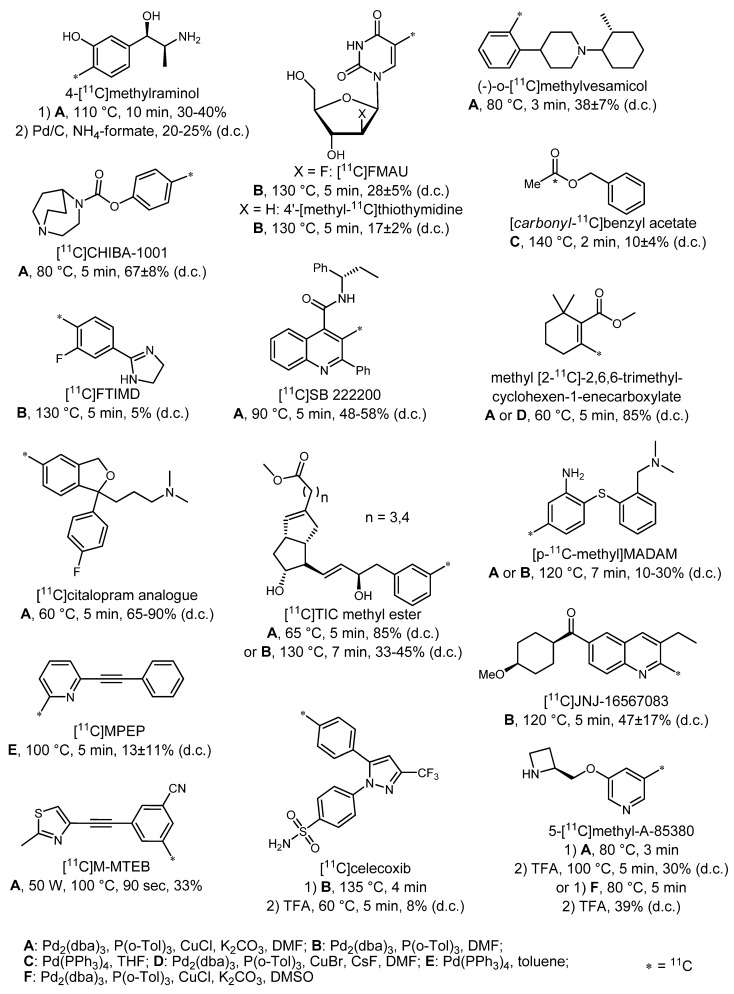
Figure 1 shows an overwiev, including 4-[11C]methylraminol, a tracer to assess myocardial sympathetic innervation [31] as well as nucleosides such as [11C]FMAU [32] and 4'-[methyl-11C]thiothymidine [33] which are adopted for cell proliferation imaging.
Figure 1.
Overview of [11C]radiotracers prepared by Stille reaction.
With (–)-o-[11C]methylvesamicol [34] and ([11C]CHIBA-1001) [35], tracers for vesicular acetylcholine transporters as well as α7 nicotinic receptors, respectively, were developed successfully. Furthermore, a tracer for imaging of glial metabolism ([carbonyl-11C]benzyl acetate) was prepared [36] as well as [2-11C]-2,6,6-trimethylcyclohexen-1-eneyl carboxylic acid methyl ester, a potential ligand for the nicotinic acetylcholine receptor (nAChR) [37], a ligand for I2-imidazoline receptors ([11C]FTIMD) [38], and [11C]SB 222200, an antagonist of the neurokinin-3 receptor [39]. In addition, tracers for the serotonin transporter like the [11C]citalopram analog [40] and [(p-11C)methyl]MADAM [41] as well as ligands for metabotropic glutamate subtype 5 receptor, [11C]MPEP [42] and [11C]M-MTEB [43], respectively, were prepared. Another example is a radiocarbonylated ligand for the glutamate 1 receptor, [11C]JNJ-16567083 [44]. The prostaglandins [11C]TIC methyl ester [45,46,47], cyclooxygenase-2 inhibitor [11C]celecoxib [48] and nicotinic acetylcholine receptor ligand 5-[11C]methyl-A-85380 [49,50] were also found in the literature.
2.1.2. Suzuki reaction
Cross-coupling reactions under Suzuki conditions with mostly nontoxic organoboron derivatives as transmetalation reagents are largely unaffected by the presence of water and tolerate a wide variety of functionalities [51,52,53]. The main advantage of this method is the ability for the cross-coupling of sp3-sp3 hybridized carbon species in contrast to other cross-coupling methods. Therefore, a wide range of alkyl, 1-alkenyl and arylboron reagents can undergo the palladium-catalyzed coupling reactions with alkyl, allyl, 1-alkenyl, 1-alkynyl and aryl substrates.
A method for the rapid incorporation of carbon-11 was presented by Andersson and co-workers in 1995 using the Suzuki cross-coupling [17]. For this purpose, 9-hexyl-9-BBN (24) was converted into 1-[11C]heptane (25) in a sample reaction with a RCY of 35% and a radiochemical purity >95% (Scheme 12). The labeling reaction was carried out using [11C]methyl iodide and Pd(PPh3)4 as catalyst with THF as solvent under basic conditions.
Scheme 12.
First radiolabeling with [11C]methyl iodide using the Suzuki cross-coupling.
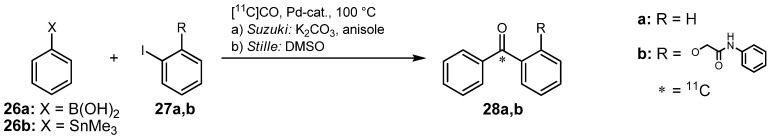
A novel approach for the preparation of no-carrier-added (n.c.a.) [11C]CO from [11C]CO2 was presented in 1997 using elemental molybdenum instead of typically employed zinc metal [7]. A first attempt to use [11C]CO in Suzuki cross-coupling reactions was made [54], and [11C]benzophenone (28a) was chosen as target compound (Scheme 13). For this purpose, phenylboronic acid (26a) and iodobenzene (27a) were converted into 28a in a Pd-mediated reaction using Pd(PPh3)2Cl2 as catalyst, K2CO3 as base and DMSO as solvent at 100 °C. After 16 min synthesis time, the desired product was obtained in 69% RCY (d.c.) with >99% radiochemical purity and a specific activity of 33 GBq·μmol−1.
Scheme 13.
Suzuki and Stille reaction with [11C]CO.
Another example of the use of the Suzuki reaction in comparison to the Stille reaction for labeling purposes with carbon-11 was presented in 2002 [55]. The structurally more complex, unsymmetrical benzophenone [carbonyl-11C]2-(2-benzoylphenoxy)-N-phenylacetamide (28b) was developed which functions as inhibitor of the reverse transcriptase of the human immunodeficiency virus type 1 (HIV 1), (Scheme 13). For this purpose, trapped [11C]CO gas was released into a vessel containing phenylboronic acid (26a), Pd(PPh3)2Cl2, K2CO3 in anisole and 2-iodophenylacetamide (27b). K2CO3 is used for the elimination of the dihydroxyboron residue in the transmetalation step. Unfavorably, K2CO3, which is insoluble in anisole, led to the adsorption of the carbon-11-labeled product 28b on the solid surface of K2CO3 and hence to a RCY of 20% only. Therefore, Stille reaction conditions were scrutinized by the authors as well, and yields and scope were compared to Suzuki conditions [55]. For this purpose, trimethyl(phenyl)stannane (26b) and 27b were reacted with Pd(PPh3)4 in DMSO under Stille conditions. As stated above, inhibitor 28b was obtained in 20% RCY in case of Suzuki conditions, whereas 71% RCY was achieved when Stille reaction conditions were applied. Obtained radiochemical purities >99% and specific activities >30 GBq·µmol−1 were similar with both synthesis routes. In this case, the Stille reaction is superior to the Suzuki reaction.
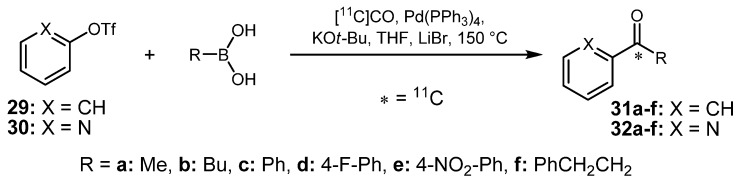
In 2004, Rahman and co-workers applied [11C]CO for a labeling technique allowing the synthesis of [11C-carbonyl]compounds in high radiochemical yields and with good specific activities in relatively short synthesis times [56]. Due to the low reactivity and solubility of [11C]CO in organic solvents, labeling reactions were carried out in a fully automated system [57]. Thus, the cyclotron-produced [11C]carbon monoxide was concentrated to a small volume, trapped on liquid nitrogen-cooled silica and transferred into a micro-autoclave that could be pressurized to 40 MPa. Different functionalized aromatic boronic acid derivatives and boronic esters were tested and a variation of applied additives and bases led to an increased RCY for 11C-labeled compounds (Scheme 14). Regarding the starting materials, it was pointed out in a previous publication that the use of aryl triflates represents a good alternative to aryl iodides and delivers higher RCYs of the respective labeled products [58]. A further improvement was achieved by the addition of bases such as tetra-butylammonium fluoride or potassium tert-butoxide which increases the RCY in most cases. These bases are believed to facilitate the formation of a boronate, which in turn supports the transmetalation step with palladium. Furthermore, lithium bromide was added to prevent catalyst decomposition or to promote the cross-coupling carbonylation reaction. The best results were obtained in a sample synthesis, in which pyridin-2-yl triflate (30) was reacted with phenylboronic acid (26a) and potassium tert-butoxide as base, leading to the formation of [carbonyl-11C]ketone 32c. The RCY was higher with KOt-Bu (78%) than without the use of a base (59%). It was discovered that in general, the RCY highly depends on the use of the respective boronic acid and the base: tetra-butylammonium fluoride delivered higher RCYs when used for cross-coupling purposes with alkyl boronic acids, whereas potassium tert-butoxide was more suitable when aromatic boronic acids were applied.
Scheme 14.
Examples of the improved Suzuki reaction.
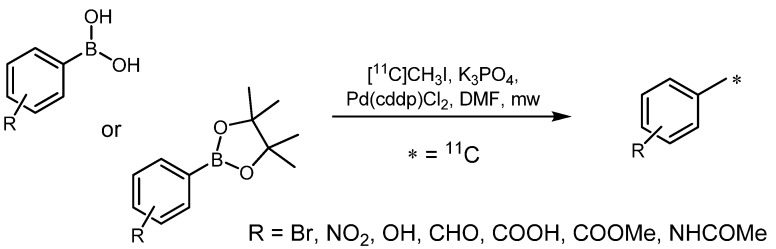
A further improvement of the Suzuki reaction for the preparation of carbon-11-labeled toluene derivatives was reported by Hostetler et al. in 2005. They found that the addition sequence of starting materials is crucial for the preparation of radiolabeled compounds in high RCYs [59]. The oxidative addition to the palladium complex has to be executed first when using trace amounts of alkyl halides, as it is the case in radiosyntheses. Therefore, first of all [11C]methyl iodide was distilled directly into the reaction vessel containing Pd(dppf)Cl2 dissolved in DMF (Scheme 15). Secondly, organoboron compound and base were added. Although the palladium catalyst is hardly soluble in DMF, this combination resulted in the highest RCYs, whereas the addition of typically used Pd(PPh3)4 catalyst led to palladium black as by-product along with lower RCYs. Another improvement was achieved by varying the used base. Aqueous KOAc and aqueous Cs2CO3 resulted in no product formation, whereas aqueous NaOH led to increased RCYs. However, aqueous K3PO4 was the mildest base and yielded the desired product in high RCYs. In general, a short treatment with microwaves (90 s) still raised the RCYs. In addition, application of different organoboron species was scrutinized, however, all investigated boron species showed similar RCYs. Acidic proton possessing moieties like 4-hydroxyphenylboronic acid pinacol ester as well as 4-carboxybenzeneboronic acid pinacol ester could be used in combination with the base K3PO4 without any difficulty. In these reactions a maximum RCY of 92% (d.c.) was reached for the preparation of p-[11C]cresol with >95% radiochemical purity in less than 20 minutes according to the release of [11C]methyl iodide.
Scheme 15.
Suzuki reaction with different organoboron derivatives.
After the Suzuki reaction was proven to be a versatile and mild cross-coupling method for the introduction of [11C]CO or [11C]methyl iodide with good RCYs, an application for the preparation of 11C-labeled PET-tracers using cross-coupling seems to be a reasonable approach. However, no further applications for this synthesis strategy were found in the literature to date.
2.1.3. Heck reaction
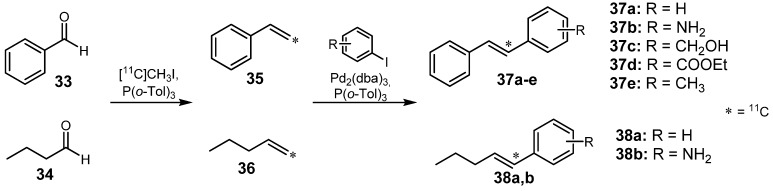
The Heck reaction does not follow the classical mechanism of a cross-coupling reaction, but also involves a palladium-catalyzed C-C-bond formation between olefins and aryl/vinyl halides [60]. Björkmann and Långström described a method in which 11C-labeled olefins 35 and 36 were prepared and subsequently cross-coupled using a Pd-mediated Heck reaction yielding (E)-[11C]stilbenes 37a-e and (E)-[1-11C]pent-1-enes 38a,b, respectively (Scheme 16) [61]. During this reaction sequence, [11C]methyl iodide was incorporated in a first step into benzaldehyde (33) and butyraldehyde (34) via Wittig olefination to form [11C]styrene (35) and [1-11C]pent-1-ene (36), respectively. Since traces of triphenylphosphane as co-ligand were able to perform an aryl exchange in the Pd-complex with the appropriate substituted aryl halide, [11C]stilbene was formed as by-product in all preparations. To circumvent this side reaction, the use of tri-o-tolylphosphane in the primary Wittig olefination as well as in the subsequent Heck reaction was essential to obtain the desired coupling products. Additionally, an excess of Pd species was necessary in the Heck reaction in order to transform the [11C]olefins 35 and 36 quantitatively. Of the model compounds depicted in Scheme 16, highest RCYs were obtained for 37b (40%) and 38b (54%) (d.c.) under optimized conditions (150 °C, 5 min). In both cases, the radiochemical purity achieved was greater than 95% after 40 min total synthesis time.
Scheme 16.
Heck reaction for [11C]-labeled olefins 37a-e and 38a,b.
2.1.4. Sonogashira reaction
Like the Heck reaction, the Sonogashira reaction belongs to cross-coupling reactions although it follows a different mechanism compared to classical cross-coupling methods like Suzuki or Stille. In case of the Sonogashira reaction, the difference to classical cross-coupling mechanisms is the formation of an intermediate organocopper species which interacts with the Pd-catalyst in a transmetalation step. Terminal alkynes, which are coupled with aromatic or vinylic halides, represent the principal ligands of the organocopper species [62].
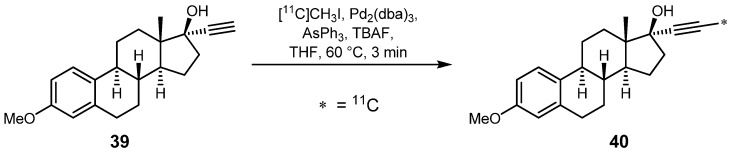
Regarding radiolabeling with carbon-11, the only work found in the literature dealing with the Sonogashira reaction was published in 2003. Wuest et al. reported the radiolabeling of model steroid 17α-(3'-[11C]prop-1-yn-1-yl)-3-methoxy-3,17ß-estradiol (40) by means of [11C]methyl iodide (Scheme 17) [63]. Classical Sonogashira reaction conditions with copper(I) salts and bases such as triethylamine were tested but resulted in very low RCYs (1-6%) due to the consumption of [11C]methyl iodide by the used amine base. However, the reaction conditions were modified which finally led to preparation of 11C-labeled steroid 40 at RCYs in the range of 49 to 64% (d.c.). Terminal alkyne 39 was used as precursor as well as Pd2(dba)3, AsPh3 and tetra-n-butyl-ammonium fluoride. After a total synthesis time of 21-27 min, a radiochemical purity >99% was achieved, unfavorably along with quite low specific activities (10-19 GBq·µmol−1). Functional groups like the hydroxyl group were not affected and made this procedure a valuable tool for 11C-radiolabeling.
Scheme 17.
Sonogashira-like reaction with terminal alkynyl steroid 39.
2.1.5. Miscellaneous cross-coupling reactions
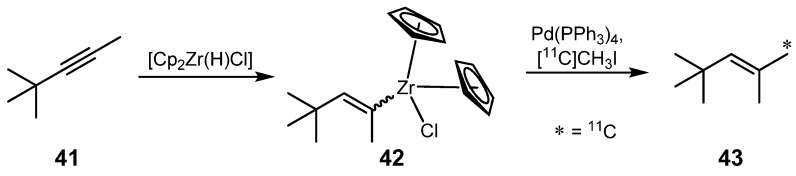
In 2006, Wuest and Berndt described a novel palladium-mediated cross-coupling strategy for 11C-C bond formations utilizing alkenylzirconocene complexes [64], which represent a valuable tool for the preparation of 11C-labeled compounds containing a prenyl group. The synthesis route of model compound 2,4,4-[11C]trimethyl-pent-2-ene (43) is depicted in Scheme 18. (4,4-Dimethylpent-2-en-2-yl)zirconocene complex (42) was applied as precursor in this reaction and was obtained by syn-addition of the Zr-H bond of Schwartz reagent [Cp2Zr(H)Cl] onto an internal C-C triple bond of 4,4-dimethylpent-2-yne (41) [65]. Treatment of 41 with an excess (2.5 equivalents) of Schwartz reagent was necessary in order to form the thermodynamically stable alkenylzirconocene 42 exclusively. The authors demonstrated that this complex undergoes transmetalation with metal complexes M(PPh3)4 (M = Pd, Pt, Ni), at which this reaction step with Pd is superior to other investigated transition metals such as Pt or Ni. A subsequent cross-coupling was initiated and, upon addition of [11C]alkyl halides and 5 mol% of Pd(PPh3)4, new 11C-C bonds were formed under retention of the double bond configuration yielding prenyl derivatives such as model compound 43. These reactions require an internal alkyne in order to present a good electrophile for the insertion step. In contrast, slightly reducible functions like esters or nitro groups do not yield the desired product due to the formation of alkenylzirconocenes followed by hydrozirconization. Various internal alkynes were applied, but the highest RCY of 75% based upon unreacted [11C]methyl iodide was obtained for compound 43.
Scheme 18.
Synthesis of model compound 2,4,4-[11C]trimethyl-pent-2-ene (43).
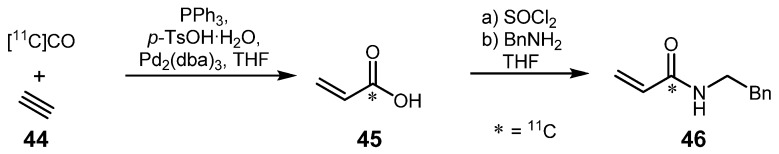
The acrylamide moiety is part of the structure of various bioactive compounds and could, after further chemical modifications, be used to design new PET tracers [66]. Previous methods for the synthesis of N-substituted [carbonyl-11C]acrylamides dealt with the use of [11C]carbon dioxide. In this case, [11C]CO2 is treated with the respective Grignard reagent to obtain the corresponding [1-11C]acrylic acid with a moderate specific radioactivity of 200–500 MBq·μmol−1 due to isotopic dilution originating from atmospheric carbon dioxide, which also reacted with the Grignard reagent [67]. To increase the specific radioactivity associated with an application of [11C]CO2, Eriksson et al. developed two novel procedures for the preparation of several [carbonyl-11C/13C]acrylamides [68]. The first method involved the palladium-catalyzed cross-coupling reaction between [11C/13C]CO and acetylene (44) to yield [1-11C]acrylic acid (45). This step was followed by chlorination with SOCl2 and subsequent treatment with benzylamine. The respective 11C-labeled acrylamide 46 was obtained in 51 ± 4% (d.c.) radiochemical yield based on [11C]carbon monoxide with a specific radioactivity of 330 ± 4 GBq·μmol−1 (Scheme 19). Co-labeling with [13C]carbon monoxide confirmed the labeling position via 13C-NMR spectroscopy.
Scheme 19.
Preparation of [1-11C]acrylamide (46).
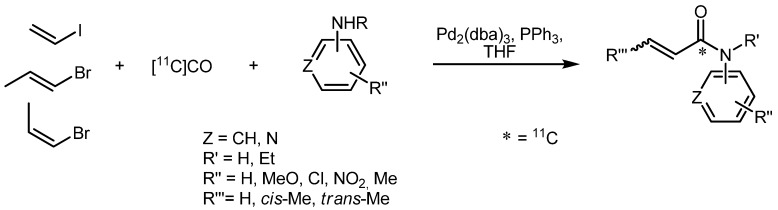
In the second approach by Eriksson et al., several other N-substituted [carbonyl-11C]acrylamides were prepared from vinyl halides and amines in a novel 'one pot' synthesis procedure as depicted in Scheme 20. For this purpose, a Pd(0) complex was formed using Pd2(dba)3, PPh3 and p-toluenesulfonic acid monohydrate in THF [69]. The subsequent addition of vinyl halide caused a color change indicating the oxidative addition to the Pd complex. Afterwards, the amine was added and the solution was transferred to a micro autoclave containing [11C]CO, where the mixture was heated to 110 °C for 4 min. With this method, acrylamide 46 was obtained in significantly higher isolated RCYs (81 ± 3%) as compared to the first approach. Based on these improved results, several substituted amines were scrutinized for their reactivity with (E) and (Z)-isomers of 1-bromo-propene. RCYs ranged from 46 to 81% (d.c.) with radiochemical purities >97%. Of note, (E) and (Z)-isomers reacted with retention of their configuration. Compared to the method mentioned above using Grignard reagents, the specific radioactivity of N-benzyl[carbonyl-11C]acrylamide (46) was very high (330 ± 4 GBq·μmol–1).
Scheme 20.
Examples of the 'one pot' synthesis yielding N-substituted [carbonyl-11C]acrylamides.
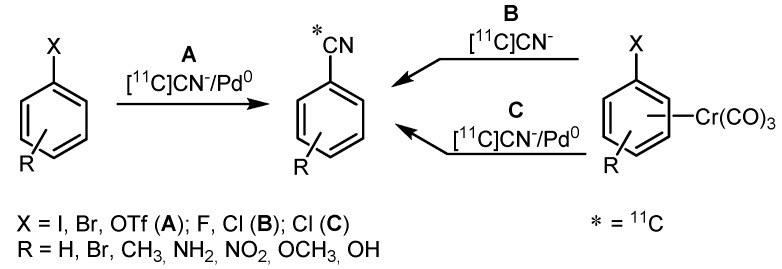
Since nitriles also comprise terminal triple bonds, the Pd-mediated cross-coupling with [11C]cyanide proceeds in a way similar to the Sonogashira reaction mentioned above. Andersson and Långström reported 11C-labeling of aryl halides and triflates by Pd-mediated [11C]cyanide cross-coupling [70]. This method included synthesis of several [11C]nitrile arenes and [11C]heteroarenes in a first approach, using aryl halogenides or aryl triflates as starting material and Pd(PPh3)4 as catalyst (Scheme 21, path A). Employment of iodide compounds resulted in the highest RCYs. The optimal solvent appeared to be THF (RCYs >99%) whereas acetonitrile or DMSO led to slightly lower RCYs (70–99%). In a second approach, the authors also investigated arene(tricarbony1)chromium complexes (Scheme 21, right). This approach (path B) entailed moderate to high RCYs (50–74%) in DMSO, which seemed to be the most suitable solvent under these conditions. Combination of both chromium and palladium-mediators (path C) resulted in a higher RCY (95%). Therefore, depending on the substrate, path A or C seem to be most suitable for this kind of reaction.
Scheme 21.
Pd/Cr-mediated cross-coupling with [11C]cyanide.
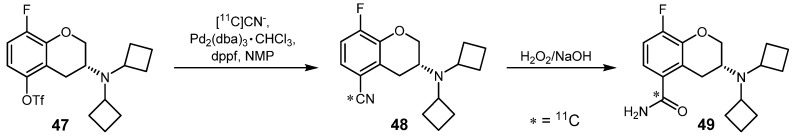
In 1999, Sandell et al. reported about the potential 5-HT1A receptor antagonist [11C]NAD-299 49, which was labeled with [11C]cyanide via a Pd-mediated cross-coupling [71]. It was mentioned that this PET tracer (Ki = 0.6 nM) should help finding serotonin transporter dysfunctions in the human brain. For that purpose, a straight forward method for the synthesis of compound 49 was developed. Reaction conditions evaluated by Andersson and Långström in 1995 [17] were applied and 1,1'-bis-(diphenyl-phosphino)ferrocene (dppf) was used as co-ligand and anhydrous N-methyl-2-pyrrolidinone (NMP) as assisting compound. Antagonist 49 was obtained in 20-40% RCY with a specific activity of 24 GBq·µmol−1 and a radiochemical purity >99% in a total synthesis time of 40-45 min. (Scheme 22) Biodistribution studies confirmed that [11C]NAD-299 (49) is a potential radiotracer for the 5-HT1A receptor due to its high affinity and selectivity in vivo.
Scheme 22.
Synthesis of 5-HT1A receptor antagonist [11C]NAD-299 (49).
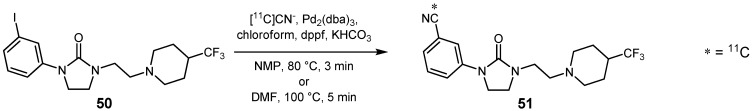
Another approach for the synthesis of dopamine receptor radiotracers was published in 2009. Bennacef et al. used the [11C]cyanide cross-coupling for the synthesis of the dopamine D3 receptor antagonist 51, which was applied as radiotracer for molecular imaging of Parkinson’s disease, schizophrenia or drug addiction [72]. A known potential structure (pKi = 9.1) was used as template and a straightforward labeling method was framed (Scheme 23).
Scheme 23.
Synthesis of the PET tracer 51 for the dopamine D3 receptor.
Antagonist 51 was prepared via a palladium(0)-catalyzed cross-coupling between precursor 50 and [11C]cyanide using Pd2(dba)3, CHCl3/dppf and KHCO3 as base in either NMP (80 °C, 3 min) or DMF (100 °C, 5 min). Afterwards, the crude product was purified by reverse-phase HPLC to yield 899 ± 326 MBq of 51 with a specific activity at end of synthesis (EOS) of 55.5 ± 25.9 GBq·μmol−1 and both chemical and radiochemical purities >99%. However, due to the fact that 51 has a too low pKi and due to the low density of D3 receptors in brain, no specific signal was found using this radiotracer in animal PET studies.
2.2. Cross-coupling reactions with cuprates
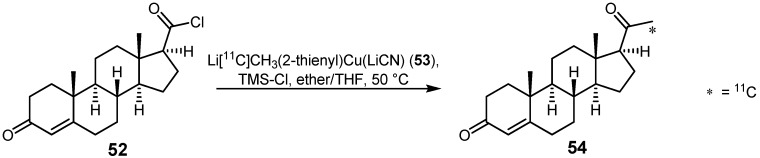
In 1997, Lidström and co-workers used the novel developed lithium [11C]methyl(2-thienyl)cuprate (53) in order to produce [21-11C]-labeled progesterone 54 for its application in in vitro and in vivo PET studies [73]. The required cuprate 53 was synthesized via an exchange reaction of n-BuLi with [11C]methyl iodide, followed by the addition of lithium(2-thienyl)cyanocuprate. Deoxycorticosterone in the form of acid chloride 52 was reacted with [11C]cuprate 53 to yield [21-11C]progesterone 54 using cross-coupling conditions as depicted in Scheme 24. Synthesized carbon-11 labeled hormone 54 was obtained with a specific activity of 14 GBq·µmol−1 and a decay-corrected radiochemical yield of 30-35% within 35 min.
Scheme 24.
Synthesis of [21-11C]progesterone 54.
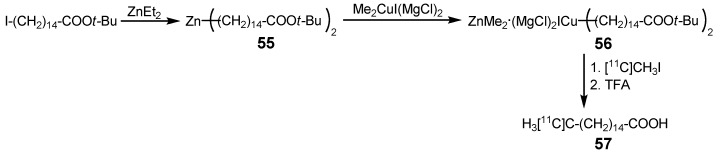
Amongst others, the fatty acid metabolism plays an important role as main energy source for the myocardium [74]. An accumulation of toxic metabolites in the mitochondria of these cells takes place when enzymatic dysfunctions interrupt the metabolism. Those toxic enrichments can cause sudden cardiac arrest. Therefore, fatty acids labeled with positron-emitting nuclides represent a promising approach to find toxic enrichments and thus probable cardiac issues. In 2000, Wuest et al. used the cross-coupling method developed by Tucker et al. [75] and produced the highly reactive dialkylzinc copper precursor 56 in order to synthesize radiolabeled fatty acids such as [11C]palmitic acid 57 with several [11C]alkyl iodides (Scheme 25) [76]. Cross-coupling reactions between functionalized dialkyl zinc compounds (ester, cyano groups, alkyl chains) and functionalized alkyl halides are highly chemoselective and result in good yields if certain cuprates (e.g. Me2Cu(CN)(MgCl)2) are used [75]. Interestingly, Wuest et al. recognized that the use of Me2CuI(MgCl)2 was essential for the reactivity of the copper-zinc species, when reacted with other halides. If Me2Cu(CN)(MgCl)2 or Me2CuI(MgBr)2 are used instead, only the cross-coupling with [11C]methyl iodide is successful. Furthermore, the authors of this study investigated different [11C]alkyl iodides (methyl, ethyl, n-butyl) as precursors and reacted them with the appropriate fatty acid moiety to obtain [ω, ω-1, ω-3-11C]palmitic acid, respectively. The utilization of [11C]methyl iodide as starting labeling material resulted in the highest decay-corrected RCY (16%) after 25-35 min in comparison to the other two [11C]alkyl halides (6% and 10%), most likely due to its higher reactivity.
Scheme 25.
Synthesis of 11C-labeled palmitic acid 57.
2.3. Cross-coupling reactions with rhodium
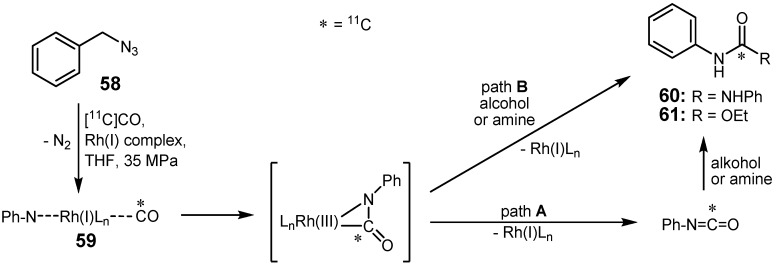
The use of various Rh(I)-complexes was proven to be milder and less aggressive to thermosensitive molecules in contrast to other transition metal-catalyzed reactions [77]. Doi et al. used a rhodium-promoted carbonylation of phenyl azide (58) with [11C]CO and [11C]CN- to synthesize N,N'-diphenyl[11C]urea (60) and ethyl phenyl[11C]carbamate (61), respectively [78]. The presumed reaction mechanism is shown in Scheme 26. The azide moiety binds to the rhodium complex, which has to be used in catalytic amounts of 0.01 molar related to phenyl azide, by abstracting nitrogen. Otherwise, the stoichiometric use of rhodium-complexes would involve the formation of a stable [11C]CO-coordinated Rh complex which would then not yield any 11C-labeled product. The authors pointed out that a strong nucleophile, such as a small amine, is needed to react with the [11C]isocyanate-coordinated Rh-complex 59.
Scheme 26.
Presumed cross-coupling paths A and B for the formation of 60 and 61.
Carbon-11-labeled urea derivative 60 was obtained in 84% RCY, whereas a RCY of 70% was achieved for the formation of [11C]carbamate 61, most likely due to the lower nucleophilicity of ethanol compared to aniline. The best catalytic system for [11C]CO trapping efficiency and product yield appeared to be [RhCl(cod)]2 (cod = 1,5-cyclooctadiene) with two equivalents of 1,2-bis-(diphenylphosphino)ethane (dppe) as co-ligand.
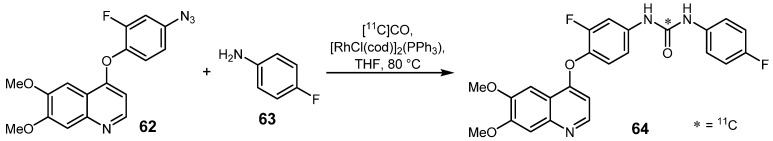
Ilovich and co-workers adapted a method [79] which was originally elaborated by La Monica et al. [80]. They designed the 11C-labeled urea derivative 64 which is a promising VEGFR-2/PDGFR-ß inhibitor and was applied as PET tracer in vivo in order to image tumor-induced vascularization. The cross-coupling of azide 62 and amine 63 with [11C]CO was performed with a catalytic amount of [RhCl(cod)]2 with PPh3 or dppe as co-ligand (Scheme 27). Surprisingly, the use of PPh3 significantly decreased the required reaction temperature from 140 °C down to 80 °C and moreover increased the RCY to 81% in contrast to 44% RCY yielded with dppe as co-ligand. The authors postulated that azide 62 probably forms a nitrene-metal complex under cleavage of N2. In the next step, [11C]CO presumably reacts with the coordinated nitrene under formation of a 11C-N bond. The presence of an amine in the initial solution is essential for a one-step reaction, since the amine can react either directly with the metal-carbonyl complex or with the in situ produced isocyanate to obtain the urea. An employment of small amines like 63 is essential to achieve high radiochemical yields and to avoid side products due to less sterical hindrance and the amine's higher solubility, compared to other primary amines. Compound 64 was obtained in 81% RCY (d.c.) at a specific activity of 92 ± 4 GBq·µmol−1 45 min after EOB.
Scheme 27.
Rhodium-mediated cross-coupling of azide 62 with amine 63.
3. Fluorine-18 Labeling
Fluorine-18, one of 17 known radioactive isotopes of fluorine [5], is currently the positron-emitting radioisotope of choice for the development of PET radiopharmaceuticals. The high interest for fluorine-18 applied in nuclear medicine is related to its favorable physical and nuclear properties: With a half-life of 109.8 min, a high positron decay ratio of 97% and a low positron energy (635 keV maximum; 2.3 mm range in matter), the application of this radionuclide is particularly advantageous in terms of resolution and dosimetry [81].
Fluorine-18 can be prepared in two chemical forms with different chemical behavior. On the one hand, [18F]F2 is provided in an extremely reactive electrophilic form from the nuclear reaction 20Ne(d,α)18F. Essentially, fluorine-19 has to be added as carrier gas to the target which decreases the specific activity of [18F]F2 (<750 MBq·μmol−1). On the other hand, the nuclear reaction 18O(p,n)18F is widely used for the preparation of large amounts of nucleophilic [18F]fluoride with high specific activities (up to 185 GBq·μmol−1). Highly enriched oxygen-18 water with an isotopic abundance of 99.9% is used as target and in this case addition of a fluorine-19 species is not necessary. Fluorine-18 is obtained as [18F]fluoride (in form of K[18F]F as a result of K2CO3 addition) in aqueous solution and is subsequently transformed into a more nucleophilic species by water elimination using a azeotropic co-distillation procedure with acetonitrile. Accessorily, a nitrogen-containing cryptand (in most cases Kryptofix K 222) is added in order to reinforce the nucleophilicity of 'naked' fluoride [82]. The drying stage can be avoided by using an ionic liquid as reactive fluorination medium [83]. Due to the higher specific activity and the better handling, most of the radiotracers were prepared using [18F]fluoride.
Simple organic bioactive molecules or complex macromolecules of biological interest such as peptides, proteins or oligonucleotides have been labeled with fluorine-18 successfully [84]. A direct labeling proceeds by the displacement of good leaving groups with fluorine-18 at the appropriate precursor. Sensitive organic structures and macromolecules cannot be labeled directly due to the harsh reaction conditions during the introduction of radiofluorine. Therefore, different approaches have been attempted. Typically, a fluorine-18-containing building block is prepared primarily which is subsequently coupled to the rest of the molecule under mild reaction conditions.
Examples for radiolabeling reactions with the most widely used PET radionuclide fluorine-18 using transition metal cross-couplings were rarely found in the literature [85]. A SciFinder-based research (June 2010) lists only eleven examples compared to numerous examples of carbon-11 radiotracers prepared using Pd-mediated cross-coupling reactions. As the 4-fluorophenyl group is frequently encountered in molecules of biological or medicinal interest, most radiofluorination procedures comprise the introduction of 18F-labeled aryl halides using carbon-carbon cross-couplings like Suzuki, Stille or Sonogashira. These methods can be regarded as mild and efficient procedures for the insertion of a 4-[18F]fluorophenyl moiety into various small organic molecules. Alongside, labeling procedures under Buchwald-Hartwig conditions as well as under conditions of the Ullmann type condensation led to radiofluorinations via carbon-heteroatom cross-coupling.
3.1. 18F-labeled compounds prepared via carbon-carbon cross-coupling reactions
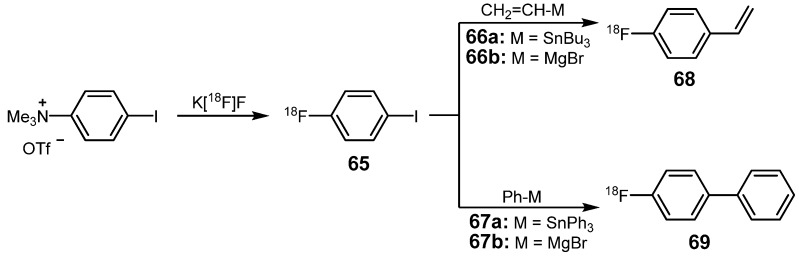
The 4-fluorophenyl group as the most prominent structural motif is frequently found in biologically active molecules and fluorinated drugs [86,87]. Thus, syntheses based on transition metal catalysis or mediation for the incorporation of this functional group were explored in the past. First examples were described in 1995 when the syntheses of two model compounds 4-[18F]fluorophenylethene (68) and 4-[18F]fluorobiphenyl (69) were reported (Scheme 28) [88,89].
Scheme 28.
Fluorine-18-labeled styrene 68 and biphenyl derivative 69.
Two synthesis routes were presented for the evaluation of this metal-mediated approach. The first procedure included the Pd-mediated cross-coupling of organotin compounds 66a and 67a (Stille cross-coupling), whereas the second approach exploited the application of Grignard-reagents 66b and 67b (Kumada cross-coupling). In both cases, fluorine-18 was introduced by the use of 1-[18F]fluoro-4-iodobenzene (65) which was prepared from the appropriate trimethylammonium precursor with [18F]fluoride via nucleophilic aromatic substitution. The trimethylammonium precursor was obtained in high yield via methylation of 4-iodo-N,N-dimethylaniline using methyl triflate [90].
Optimization of the conditions for the radiolabeling reaction of 65 with 67a revealed that the highest RCY (86%) of 69 can be obtained when hexamethylphosphoramide is used as solvent and Pd(PPh3)4 as catalyst. In general, application of organostannanes 66a and 67a delivered higher yields (15–80%) compared to the respective Grignard reagents 66b and 67b (20–53%).
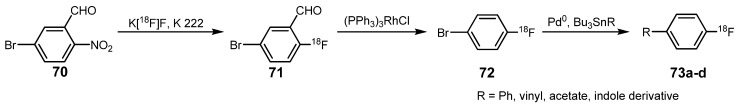
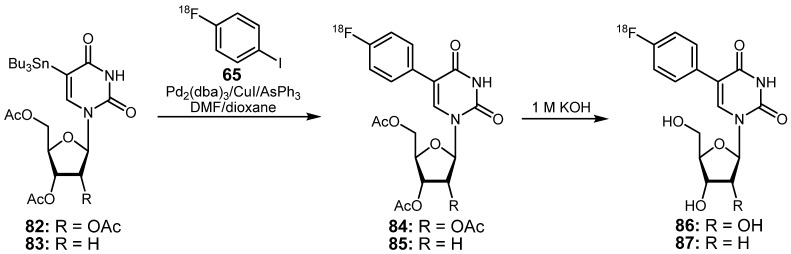
Fluvastatin is a prominent example of a bioactive compound containing the 4-fluorophenyl structural element. For this reason, a labeling method was investigated using palladium-promoted cross-coupling reactions of organotin compounds and aryl halides (Stille cross-coupling) for the introduction of fluorine-18 [91]. 5-Bromo-2-[18F]fluorobenzaldehyde (71) was prepared from the respective nitro-precursor 70 followed by subsequent decarbonylation with Wilkinson's catalyst [92] which afforded the actual labeling agent 1-bromo-4-[18F]fluorobenzene (72) in approx. 70% RCY (Scheme 29). In order to simplify the synthesis route, a 'one pot' procedure for the preparation of 72 was developed. Optimal reaction conditions [93] were elaborated for a subsequent radiolabeling of organostannanes with 72. Best radiochemical yields were obtained utilizing Pd2dba3/AsPh3 as mediator in a DMF:dioxane mixture (1:1) at 115–120 °C. It was shown that the purification of 72 is important and increased the RCY of the coupling product from 20% to 90% with respect to the preparation of 4-[18F]fluorobiphenyl (73a). In order to label fluvastatin, 1-isopropyl-2-methylindolyl(tributyl)tin was prepared as synthon for the introduction of the 4-[18F]fluorophenyl group into the indole moiety (Table 1), however, no preparation of fluorine-18-labeled fluvastatin has been reported to date.
Scheme 29.
Syntheses of fluorine-18-labeled model compounds 73a-d.
Table 1.
Selected conditions and RCYs of the Pd-mediated cross-coupling with organostannanes.
| Reagent | T / °C | t / min | Product | RCY / % |
|---|---|---|---|---|
| Bu3SnPh | 115 | 15 | 4-[18F]fluorobiphenyl 73a | >90 |
| Bu3SnCH=CH2 | 115 | 30 | 4-[18F]fluorostyrole 73b | >80 |
| Bu3SnOOCCH3 | 120 | 5 | 4-[18F]fluorophenyl acetate 73c | 78 |
| 1-isopropyl-2-methyl-3-(tributylstannyl)indole | 115 | 15 | 1-isopropyl-2-methyl-3-(4-[18F]fluorophenyl)indole 73d | 15 |
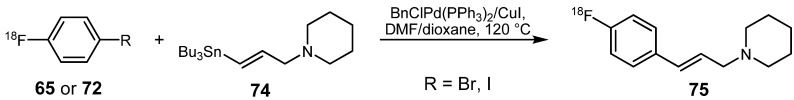
For the preparation of model compound 75, functionalized stannane 74 was reacted with 1-[18F]fluoro-4-iodobenzene 65 or 1-bromo-4-[18F]fluorobenzene 72 as labeling agents using the Pd-mediated Stille cross-coupling [89]. The best reaction conditions were found to be a DMF:dioxane mixture (1:1) as solvent and BnClPd(PPh3)2:CuI (ratio 1:1) as catalyst at 120 °C. The regio and stereoselectivity of the coupling reaction were also investigated. It was observed that only the E-isomer of 4-[18F]fluorophenylallylpiperidine (75) was obtained (Scheme 30) when 65 or 72 were employed as starting material. A RCY of 90% was achieved when 65 was used compared to only 41% when 72 was used.
Scheme 30.
Preparation of model compound 75 utilizing 65 or 72.
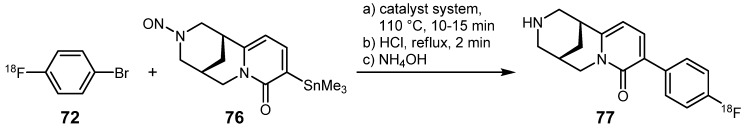
Another application based on the previous work was published in 2000 for the synthesis of a cytisine analog [94]. This compound was prepared for in vivo studies of the α4β2 nicotinic acetylcholine receptor [95] using PET. The incorporation of fluorine-18 was put into practice by exploitation of the rapid and efficient Stille cross-coupling. As depicted in Scheme 31, 1-bromo-4-[18F]fluorobenzene (72) was prepared and reacted with 9-trimethylstannylcytisine 76. Radiotracer 77 was received after a subsequent denitrosation. The highest radiochemical yields of the radiofluorinated cytisine derivative 77 were obtained using either a DMF/dioxane mixture and Pd(PPh3)4 (68%) or dioxane solely together with PdCl(PPh3)2 (56–74%) after 150 min total synthesis time starting from K[18F]F. The results were in good agreement with those obtained using the respective fluorine-19 compound but within a much shorter reaction time.
Scheme 31.
Preparation of radiofluorinated cytisine analog 77.
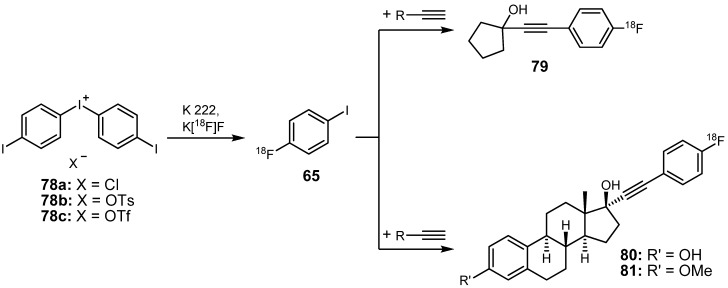
In 2003, Wuest et al. demonstrated the applicability of 4-[18F]fluoro-1-iodobenzene (65) for radiolabeling purposes in Sonogashira cross-coupling reactions [96]. The final aim of that work was the synthesis of novel fluorine-18 labeled steroids. On that account, a new synthesis procedure for the preparation of 65 was established using the respective iodonium salts 78a-c which were treated with [18F]fluoride in a nucleophilic reaction as depicted in Scheme 32. To optimize the RCY of 65, different counter ions (chloride, tosylate, triflate) of the respective iodonium salt as labeling precursor as well as several solvents and temperatures were tested. Irrespective of other reaction conditions, utilization of DMF as solvent always resulted in higher yields compared to acetonitrile or DMSO. Moreover, high reaction temperatures (>120 °C) seemed to be essential for an efficient thermal cleavage of 78a-c by [18F]fluoride. No product formation was observed at a reaction temperature of 60 °C. Highest RCYs (up to 70%; determined by radio-TLC) of compound 65 were obtained when iodonium chloride 78a was used in DMF under microwave conditions (5 min/120 watt) for the radiosynthesis of compound 65. Upon completion of radiosynthesis, 65 was purified via solid phase extraction (SPE) followed by removal of the solvent. A comparison of the most commonly used methods for the preparation of 65 and 72 can be found in the literature [90].
Scheme 32.
Preparation of 65 from iodonium salts and subsequent radiolabeling to obtain 79 as well as steroids 80 and 81.
Three precursors were radiolabeled with 4-[18F]fluoro-1-iodobenzene (65) using the Sonogashira cross-coupling. The reaction was performed in a sealed tube using THF as solvent and Et3N as base at 110 °C for 20 min. Upon addition of the terminal alkyne in the final reaction step, 85% of compound 65 were converted into the cyclopentyl carbinol 79. Furthermore, radiolabeling of steroid precursors led to 65–88% and 34–64% conversion into steroids 80 and 81, respectively.
Radiolabeling of nucleosides has become a highly attractive method over the past years due to their central role in biological systems. Several attempts were made to label nucleosides with the PET nuclides 11C, 18F, 76Br as well as 124I [97,98,99]. In the case of fluorine-18, two main strategies are described in most publications. The radionuclide is either introduced directly into the carbohydrate scaffold of a nucleoside possessing a good leaving group, or it is used to label a sugar which is subsequently linked to the respective nucleobase.
Utilizing 4-[18F]fluoro-1-iodobenzene (65) as actual labeling agent, two novel 18F-labeled nucleosides 86 and 87 were prepared under mild conditions of the Stille cross-coupling (Scheme 33) [100]. For this purpose, a tributylstannyl group was incorporated into the pyrimidine moiety yielding uridine 82 and deoxyuridine 83, respectively. The highest radiochemical yield (69%) of the reaction of 82 with 65 (which was synthesized from the respective iodonium salts [96]; vide supra) was obtained when the coupling reaction was carried out at 65 °C for 20 min in a DMF/dioxane (1:1) or THF/dioxane (1:1) mixture using Pd2(dba)3/CuI/AsPh3 as catalyst system. The radiofluorinated nucleoside 85 was synthesized in the same manner with a maximum RCY of 61% starting from the stannyl precursor 83 which was reacted with 65. In both cases, the last step involved the cleavage of the acetyl protecting groups from the carbohydrate moiety of nucleosides 84 and 85, which were removed quantitatively using a 1 M KOH solution at 65 °C for 10 min, yielding the final radiotracers 86 or 87 with 61% and 69% RCY (related to 65), respectively, with a radiochemical purity of ≥95%.
Scheme 33.
Preparation of uridine-based fluorine-18-labeled nucleosides.
The appropriate non-radioactive reference compounds were obtained in a similar way by a Stille cross-coupling using Pd(PPh3)2Cl2/CuI as catalyst in dioxane at 100 °C for 24 h followed by deprotection of the hydroxyl groups.
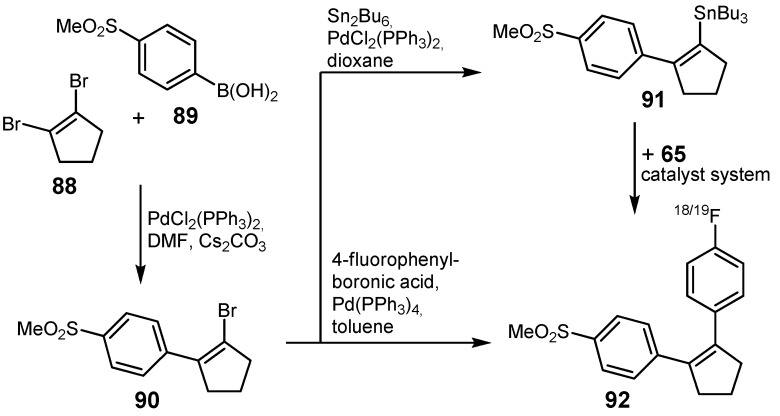
Cyclooxygenase (COX) enzymes play an important role in inflammatory and pain causing processes in general. Celecoxib and Rofecoxib represent two selective COX-2 inhibitors and are among the most widely used therapeutics for the treatment of pain and inflammation. Moreover, the COX-2 enzyme is overexpressed in many human cancer entities [101]. The group of Wuest et al. presented another approach using the Stille cross-coupling for radiofluorination purposes [102]. In this connection, two novel fluorine-18-labeled cyclooxygenase-2 inhibitors based on Rofecoxib were prepared with a slightly different core structure: One of these radiotracers comprehended a cyclopentene ring whereas the other contained a 3,4-substituted furanone unit. Different synthesis routes were investigated for the preparation of the labeled radiotracer 92 and the non-labeled analog based on the cyclopentene core as depicted in Scheme 34. Preparation of the second radiolabeled COX-2 inhibitor 94 as well as its non-labeled reference compound is depicted in Scheme 35.
Scheme 34.
Preparation of the radiofluorinated COX-2 inhibitor 92 and the non-radioactive analog.
Scheme 35.
Preparation of the second COX-2 inhibitor 94 and its non-radiolabeled reference.
Composition of the non-radioactive COX-2 inhibitor and its radiofluorinated counterpart 92 commenced with the preparation of methylsulfonylphenyl-substituted bromocyclopentene 90 which was synthesized from dibromocyclopentene 88 and boronic acid 89.
Synthesis of 90 was modified as compared to the literature procedure and it was found that 90 could be prepared in a single step [103]. Improvements involved the utilization of DMF which appeared to be superior to the previously reported solvent system toluene/EtOH/2 M Na2CO3. Furthermore, a twofold excess of 1,2-dibromocyclopentene 88 compared to 89 was necessary to obtain 90 in a single step in 45% yield. Subsequent cross-coupling of 90 with 4-fluorophenyl boronic acid yielded the reference substance directly, whereas the preparation of fluorine-18-labeled compound 92 implied a two-step process: Initial reaction of 90 with Sn2Bu6 yielded 91, which was subsequently converted into the desired radiotracer 92 utilizing 4-[18F]fluoro-1-iodobenzene (65). The radiolabeled COX-2 inhibitor 92 was obtained in 68% RCY relative to compound 65.
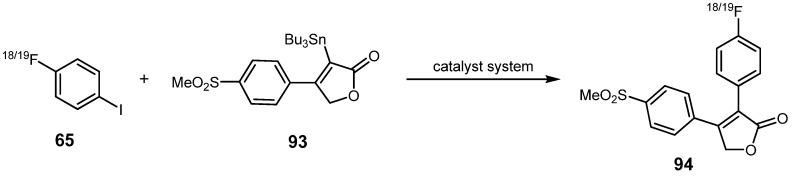
Regarding the second COX-2 inhibitor, both 94 as well as the non-radiolabeled reference were prepared using the stannylated organotin compound 93 as depicted in Scheme 36. Optimized reaction conditions were applied for the synthesis of the 18F-labeled inhibitor 94 (Pd2(dba)3/P(o-Tol)3/CuI, DMF/toluene (1:1), 65 °C, 20 min) with 68% RCY based upon 4-[18F]fluoro-1-iodobenzene (65). The analogous non-radioactive compound was synthesized in 69% yield using the Pd(PPh3)2Cl2/CuI catalyst system in a toluene/ethanol (1:1) mixture at 50 °C overnight.
Scheme 36.
Model Suzuki labeling reactions.
A further application using transition metal-mediated cross-coupling reactions for labeling purposes with fluorine-18 was demonstrated in 2006 with the implementation of the Suzuki cross-coupling [104]. Through this technique, a multitude of organoboron compounds were labeled with 4-[18F]fluoro-1-iodobenzene (65) to yield the respective biphenyl compounds in RCYs from 30 to 90% as demonstrated in Scheme 36.
A wide variety of functional groups like esters, ethers, thioethers, halogens, alcohols and nitro derivatives were shown to be tolerable and compatible with the Suzuki cross-coupling. Excellent radiochemical yields greater than 80% within a synthesis time of just 5 min were achieved for most of the tested organoboron derivatives (except for: R = Br, Cl, COOH) with the Suzuki coupling of 65 using Pd2(dba)3 as mediator, Cs2CO3 as base and acetonitrile as solvent at 60 °C.
3.2. 18F-labeled compounds prepared via carbon-heteroatom cross-coupling reactions
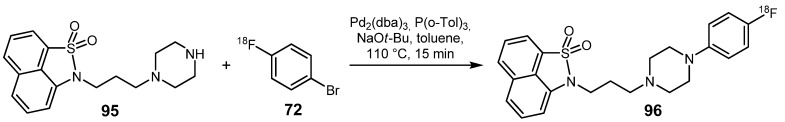
Pd-mediated cross-couplings were also used for radiolabeling purposes which comprise the formation of carbon-heteroatom bonds. Marrière et al. were the first to use the synthetic approach [105] based on Buchwald-Hartwig conditions for the preparation of fanserin [18F]RP 62203, a 5-HT2A serotonin receptor antagonist, as depicted in Scheme 37 [106]. Reaction of piperazine 95 with 1-bromo-4-[18F]fluorobenzene (72) in the presence of the catalyst system Pd2dba3/P(o-Tol)3 in toluene at 110 °C yielded radiolabeled fanserin 96 with 60% RCY after 15 min. Other methods the authors had attempted previously were either time consuming [107], too difficult to automate [108] or did not deliver sufficient specific activities [109].
Scheme 37.
Radiolabeling with 1-bromo-4[18F]fluorobenzene 72 under Buchwald-Hartwig conditions.
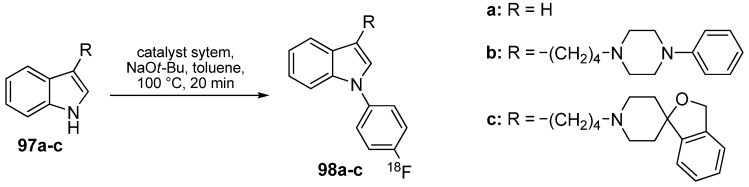
The N-arylindole moiety represents a central structural motif found in pharmacologically important compounds such as angiotensin II-1 antagonists [110], melatonin receptor MT1 agonists [111], antipsychotic drugs [112] or selective σ2 receptor ligands [113,114]. Two examples (98b,c) of high affinity σ2 receptor ligands were successfully labeled using 4-[18F]fluoro-1-iodobenzene (65) [115]. For this purpose, 65 was prepared from the respective iodonium salts and reacted in a model reaction with 1H-indole (97a). Best labeling conditions were carried out using Pd2(dba)3 and 2-(dicyclohexyl-phosphino)-20-(N,N-dimethylamino)biphenyl as co-ligand in toluene and with NaOt-Bu as base to yield the radiofluorinated indole 98a (70%) after 30 min synthesis time. Based on these results, two other inhibitors, as seen in Scheme 38, were prepared with RCYs of 91% for 98b and 84% for 98c (d.c.), respectively.
Scheme 38.
Preparation of model indole 98a and σ2 receptor ligands 98b,c.
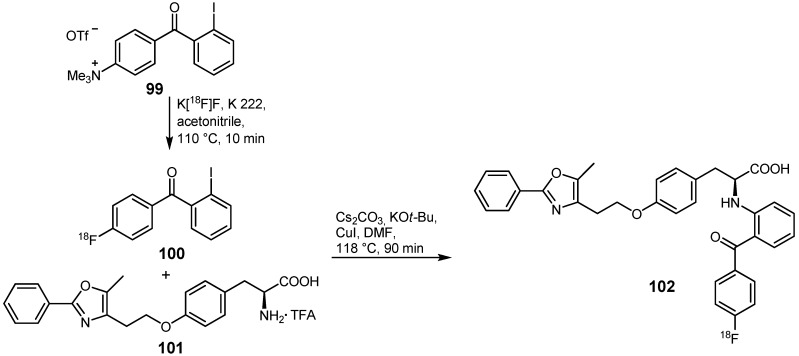
Another example using the cross-coupling of nitrogen with carbon was demonstrated in 2009 [116] with the preparation of radiofluorinated farglitazar 102, a compound for the imaging of peroxisome proliferator-activated receptor-γ ligands (PRARγ) [117,118]. Two synthesis routes for radiofluorination purposes were exemplified: On the one hand, direct labeling of the target molecule with Cs[18F]F was reported, whereas indirect labeling under Ullmann conditions was demonstrated on the other hand. 2-Iodo-4'-[18F]fluorobenzophenone (100) was prepared by means of the latter method with RCYs >97% by nucleophilic aromatic displacement of the trimethylammonium leaving group of precursor 99 (Scheme 39). The direct conversion of 100 with 101 via Ullmann condensation was unsuccessful, therefore the inorganic salts had to be removed prior to further reaction. After separation from by-products, CuI was added to 100. In a second reaction vessel, 101 was dissolved in dry DMF and treated with a Cs2CO3/KOt-Bu mixture for 5 min. Afterwards, both mixtures were combined and heated to 118 °C for 90 min. The resulting green solution was purified via an HPLC system. The combination of Cs2CO3 and KOt-Bu instead of K2CO3 as base always achieved higher yields. In addition, the ratio between the starting material and the bases also influenced the yield. The optimal ratio between 99 + 101 and the base mixture was scrutinized to be 3:1, as stated by the authors.
Scheme 39.
Preparation of 102 using Ullmann-type conditions.
A different approach for the synthesis of novel functionalized phosphanes was described in 2010 concerning the Pd-mediated phosphorus-carbon cross-coupling [119,120]. These phosphanes were introduced as starting material in labeling reactions using the traceless Staudinger ligation. For this purpose, ω-functionalized 2-iodophenyl carboxylic esters were prepared and labeled with fluorine-18 successfully (Scheme 40). Starting from 2-iodophenyl 4-iodobutanoate (103), tosylated compound 104 as well as the non-radioactive fluorine-19 compound 105 were prepared in high yields using the respective silver salts in acetonitrile at ambient temperature. Toslyate 104 served as starting material for the preparation of the radiolabeled fluorine-18 building block 105. Optimal labeling conditions were evaluated by systematic variation of temperature and reaction time of which the latter seemed to have only little influence on the RCY of 105. No radiofluorination product was obtained using DMF or DMSO and only low-yield conversion to 105 was observed when acetonitrile was used as solvent. An application of n-Bu4NOH instead of K2CO3 as base was important for a high radiochemical yield. Best conditions were obtained using a mixture of acetonitrile and t-BuOH (1:4) at 100 °C and a reaction time of 10 min with 58% conversion into the desired fluorine-18-containing 2-iodophenyl ester 105. Unfortunately, the subsequent cross-coupling did not lead to the desired radiofluorinated phosphane 106 (Scheme 39), whereas the coupling reaction with the non-radiolabeled analog yielded the 19F-fluorinated phosphane 106 in 51% yield after 3 h.
Scheme 40.
Synthetic approach to 18F-labeled phosphanes as precursors for Staudinger ligations.
4. Conclusions
Positron emission tomography (PET) is a sophisticated method for quantitative and noninvasive imaging of biological functions by monitoring the distribution of tracers labeled with positron emitters. A wide variety of labeled compounds has been developed as molecular imaging probes for the scrutiny of biochemical and physiological parameters.
The present review summarized the implementation of transition metal cross-coupling reactions for labeling purposes with carbon-11 and fluorine-18 which has gained great interest for the preparation of radiotracers and radiopharmaceuticals for molecular imaging. Because of its functional group-tolerating character, mostly mild reaction conditions and short synthesis times, this kind of coupling reaction represents a valuable and multifunctional tool not only regarding the preparation of the precursor core. Especially in carbon-11 chemistry, time is an important criterion due to the short half- life of this isotope. As pointed out in this review, a multitude of cross-coupling reactions have been developed for the introduction of carbon-11, mostly in the form of [11C]methyl iodide or [11C]carbon monoxide, with high radiochemical yields and within short synthesis times. Advantageously, no secondary labeling building blocks have to be synthesized. Another benefit is that cross-coupling reactions normally yield by-products in very small amounts only; therefore side reactions can usually be neglected. However, in the case of labeling reactions, the carbon-11 species is only available in the nanomolar range. Thus, the prevention of side reactions as well as the purification of the labeled product become important to obtain the radiotracer in high radiochemical yields and with high specific activities.
Due to the non-physiological conditions (e.g. organic solvents, high temperatures) of most of the cross-coupling reactions, only small organic bioactive molecules could be labeled to date. No example of a labeled high molecular weight compound (peptide, protein or antibody) was found in the literature. However, due to the wide applicability, cross-coupling reactions have proven to be remarkable and valuable tools for the preparation of novel fluorine-18 and carbon-11-containing radiotracers based on small organic molecules. Consequently, recent trends in the evolution of metal-mediated cross coupling reactions for radiolabeling purposes will stimulate the positron emission tomography as powerful imaging technique with respect to clinical applications as well as drug research and development.
Cross-coupling reactions applied for radiofluorination purposes are of less importance due to the necessity to prepare primary radiofluorinated building blocks like the 4-[18F]fluorophenyl moiety. This represents an additional synthesis step in contrast to direct electrophilic and nucleophilic labeling procedures with fluorine-18. Thus, cross-coupling reactions are mostly applied for the preparation of the precursor core. In addition, special attention has to be paid to an application of cross-coupling reactions in the routinely large scale preparation of radiopharmaceuticals regarding GMP and GLP regulations. This will be a huge challenge in the future.
Acknowledgements
The authors thank the Fonds der Chemischen Industrie (FCI) for financial support.
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Diederich F., Stang P.J. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim, Germany: 2001. [Google Scholar]
- 2.Wu X.-F., Anbarasan P., Neumann H. Beller., M. Vom Edelmetall zum Nobelpreis: Palladiumkatalysierte Kupplungen als Schlüsselmethode in der organischen Chemie. Angew. Chem. 2010;122:9231–9234. [Google Scholar]
- 3.Mamat C., Ramenda T., Wuest F.R. Recent Applications of Click Chemistry for the Synthesis of Radiotracers for Molecular Imaging. Mini-Rev. Org. Chem. 2009;6:21–34. doi: 10.2174/157019309787316148. [DOI] [Google Scholar]
- 4.Glaser M., Robins E.G. Click labelling’ in PET radiochemistry. J. Labelled Compd. Radiopharm. 2009;52:407–414. doi: 10.1002/jlcr.1656. [DOI] [Google Scholar]
- 5.Pfennig G., Klewe-Nebenius H., Seelmann-Eggebert W. Karlsruher Nuklidkarte. Haberbeck; Lage, Germany: 2009. [Google Scholar]
- 6.Antoni G., Kihlberg T., Långström B. In: Handbook of Radiopharmaceuticals, Radiochemistry and Applications. Welch M.J., Redvanly C.S., editors. John Wiley & Sons; Chichester, UK: 2003. pp. 141–195. Chapter 5. [Google Scholar]
- 7.Scott P.J.H. Methoden für den Einbau von Kohlenstoff-11 zur Erzeugung von Radiopharmaka für die Positronenemissionstomographie. Angew. Chem. 2009;121:6115–6118. doi: 10.1002/ange.200901481. [DOI] [Google Scholar]
- 8.Allard M., Fouquet E., James D., Szlosek-Pinaud M. State of the Art in 11C Labelled Radiotracers Synthesis. Curr. Med. Chem. 2008;15:235–277. doi: 10.2174/092986708783497292. [DOI] [PubMed] [Google Scholar]
- 9.Oberdorfer F., Hanisch M., Helus F., Maier-Borst W. A New Procedure for the Preparation of 11C-Labelled Methyl Iodide. Int. J. Appl. Radiat. Isot. 1985;36:435–438. doi: 10.1016/0020-708X(85)90205-4. [DOI] [Google Scholar]
- 10.Holschbach M., Schüller M. A New and Simple On-line Method for the Preparation of n.c.a. [11C]Methyl Iodide. Appl. Radiat. Isot. 1993;44:779–780. doi: 10.1016/0969-8043(93)90149-5. [DOI] [Google Scholar]
- 11.Larsen P., Ulin J., Dahlstrom K., Jensen M. Synthesis of [11C]Iodomethane by of [11C]Methane Iodination. Appl. Radiat. Isot. 1997;48:153–157. doi: 10.1016/S0969-8043(96)00177-7. [DOI] [Google Scholar]
- 12.Buckley K.R., Jivan S., Ruth T.J. Improved yields for the in situ production of [11C]CH4 using a niobium target chamber. Nucl. Med. Biol. 2004;31:825–827. doi: 10.1016/j.nucmedbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M.-R., Suzuki K. Sources of carbon which decrease the specific activity of [11C]CH3I synthesized by the single pass I2 method. Appl. Radiat. Isot. 2005;62:447–450. doi: 10.1016/j.apradiso.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Matarrese M., Soloviev D., Todde S., Neutro F., Petta P., Carpinelli A., Brüssermann M., Kienle M.G., Fazio F. Preparation of [11C] radioligands with high specific radioactivity on a commercial PET tracer synthesizer. Nucl. Med. Biol. 2003;30:79–83. doi: 10.1016/S0969-8051(02)00353-0. [DOI] [PubMed] [Google Scholar]
- 15.Stille J.K. Palladium-katalysierte Kupplungsreaktionen organischer Elektrophile mit Organo-zinn-Verbindungen. Angew. Chem. 1986;98:504–519. doi: 10.1002/ange.19860980605. [DOI] [Google Scholar]
- 16.Mitchell T.N. Palladium-Catalysed Reactions of Organotin Compounds. Synthesis. 1992:803–815. doi: 10.1055/s-1992-26230. [DOI] [Google Scholar]
- 17.Andersson Yvonne, Cheng Aiping, Långström Bengt. Palladium-promoted Coupling Reactions of [11C]Methyl Iodide with Organotin and Organoboron Compounds. Acta. Chem. Scand. 1995;49:683–688. doi: 10.3891/acta.chem.scand.49-0683. [DOI] [Google Scholar]
- 18.Suzuki M., Doi H., Björkman M., Andersson Y., Långström B., Watanabe Y., Noyori R. Rapid Coupling of Methyl Iodide with Aryltributylstannanes Mediated by Palladium(0) Complexes: A General Protocol for the Synthesis of 11CH3-Labeled PET Tracers. Chem. Eur. J. 1997;3:2039–2042. doi: 10.1002/chem.19970031219. [DOI] [Google Scholar]
- 19.Björkman M., Doi H., Resul B., Suzuki M., Noyori R., Watanabe Y., Långström B. F2α Analogue Using An Improved Method For Stille Reactions With [11C]Methyl Iodide. J. Labelled Compd. Radiopharm. 2000;43:1327–1334. doi: 10.1002/1099-1344(200012)43:14<1327::AID-JLCR419>3.0.CO;2-D. [DOI] [Google Scholar]
- 20.Sandell J., Halldin C., Sovago J., Chou Y.-H., Gulyás B., Yu M., Emond P., Någren K., Guilloteau D., Farde L. PET examination of [11C]5-methyl-6-nitroquipazine, a radioligand for visualization of the serotonin transporter. Nucl. Med. Biol. 2002;29:651–656. doi: 10.1016/S0969-8051(02)00318-9. [DOI] [PubMed] [Google Scholar]
- 21.Langer O., Forngren T., Sandell J., Dollé F., Långström B., Någren K., Halldin C. Preparation of 4-[11C]methylmetaraminol, a potential PET tracer for assessment of myocardial sympathetic innervation. J. Labelled Compd. Radiopharm. 2003;46:55–65. doi: 10.1002/jlcr.642. [DOI] [Google Scholar]
- 22.Forngren T., Samuelsson L., Långström B. A 11C-Methyl stannane (5-[11C]methyl-1-aza-5-stannabicyclo[3.3.3]undecane) for use in palladium-mediated [11C]C–C bond forming reactions with organohalides. J. Labelled Compd. Radiopharm. 2004;47:71–78. doi: 10.1002/jlcr.798. [DOI] [Google Scholar]
- 23.Reiffers S., Vaalburg W., Wiegman T., Wynberg H., Woldring M.G. Carbon-11 labelled methyllithium as methyl donating agent: The addition to 17-Keto steroids. Int. J. Appl. Radiat. Isot. 1980;31:535–539. doi: 10.1016/0020-708X(80)90093-9. [DOI] [Google Scholar]
- 24.Karimi F., Barletta J., Långström B. Palladium-Mediated 11C-Carbonylative Cross-Coupling of Alkyl/Aryl Iodides with Organostannanes: An Efficient Synthesis of Unsymmetrical Alkyl/Aryl [11C-carbonyl]Ketones. Eur. J. Org. Chem. 2005:2374–2378. [Google Scholar]
- 25.Arai T., Kato K., Zhang M.R. Synthesis of [carbonyl-11C]acetophenone via the Stille cross-coupling reaction of [1-11C]acetyl chloride with tributylphenylstannane mediated by Pd2(dba)3/P(MeNCH2CH2)3N·HCl. Tetrahedron Lett. 2009;50:4788–4791. doi: 10.1016/j.tetlet.2009.06.043. [DOI] [Google Scholar]
- 26.Liu X., Verkade J.G. Free and Polymer-Bound Tricyclic Azaphosphatranes HP(RNCH2CH2)3N+: Procatalysts in Dehydrohalogenations and Debrominations with NaH. J. Org. Chem. 1999;64:4840–4843. doi: 10.1021/jo990217f. [DOI] [PubMed] [Google Scholar]
- 27.Huiban M., Huet A., Barré L., Sobrio F., Fouquet E., Perrio C. Methyl transfer reaction from monomethyltin reagent under palladium(0) catalysis: a versatile method for labelling with carbon-11. Chem. Commun. 2006:97–99. doi: 10.1039/b510286c. [DOI] [PubMed] [Google Scholar]
- 28.Harris D.H., Lappert M.F.J. Monomeric, volatile bivalent amides of group IVB elements, M(NR12)2 and M(NR1R2)2(M=Ge, Sn, or Pb; R1=Me3Si, R2=Me3C. J. Chem. Soc. Chem. Commun. 1974:895–896. [Google Scholar]
- 29.Giardina G.A.M., Sarau H.M., Farina C., Medhurst A.D., Grugni M., Foley J.J., Raveglia L.F., Schmidt D.B., Rigolio R., Vassallo M., Vecchietti V., Hay D.W.P. 2-Phenyl-4-quinolinecarboxamides: A Novel Class of Potent and Selective Non-Peptide Competitive Antagonists for the Human Neurokinin-3 Receptor. J. Med. Chem. 1996;39:2281–2284. doi: 10.1021/jm9602423. [DOI] [PubMed] [Google Scholar]
- 30.Bourdier T., Huiban M., Huet A., Sobrio F., Fouquet E., Perrio C., Barré L. Tetra- and Monoorganotin Reagents in Palladium-Mediated Cross-Coupling Reactions for the Labeling with Carbon-11 of PET Tracers. Synthesis. 2008:978–984. [Google Scholar]
- 31.Langer O., Forngren T., Sandell J., Dollé F., Långström B., Någren K., Halldin C. Preparation of 4-[11C]methylmetaraminol, a potential PET tracer for assessment of myocardial sympathetic innervation. J. Labelled Compd. Radiopharm. 2003;46:55–65. doi: 10.1002/jlcr.642. [DOI] [Google Scholar]
- 32.Samuelsson L., Långström B. Synthesis of 1-(2'-deoxy-2'-fluoro-ß-d-arabinofuranosyl)-[Methyl-11C]thymine ([11C]FMAU) via a Stille cross-coupling reaction with [11C]methyl iodide. J. Labelled Compd. Radiopharm. 2003;46:263–272. doi: 10.1002/jlcr.668. [DOI] [Google Scholar]
- 33.Toyohara J., Okada M., Toramatsu C., Suzuki K., Irie T. Feasibility studies of 4'-[methyl-11C]thiothymidine as a tumor proliferation imaging agent in mice. Nucl. Med. Biol. 2008;35:67–74. doi: 10.1016/j.nucmedbio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Kawamura K., Shi K., Tsukada H., Nishiyama S., Mori H., Ishiwata K. Synthesis and evaluation of vesamicol analog (–)-o-[11C]methylvesamicol as a PET ligand for vesicular acetylcholine transporter. Ann. Nucl. Med. 2006;20:417–424. doi: 10.1007/BF03027377. [DOI] [PubMed] [Google Scholar]
- 35.Toyohara J., Sakata M., Wu J., Ishikawa M., Oda K., Ishii K., Iyo M., Hashimoto K., Ishiwata K. Preclinical and the first clinical studies on [11C]CHIBA-1001 for mapping α7 nicotinic receptors by positron emission tomography. Ann. Nucl. Med. 2009;23:301–309. doi: 10.1007/s12149-009-0240-x. [DOI] [PubMed] [Google Scholar]
- 36.Lu S., Hong J., Itoh T., Fujita M., Inoue O., Innis R.B., Pike V.W. [carbonyl-11C]Benzyl acetate: Automated radiosynthesis via Pd-mediated [11C]carbon monoxide chemistry and PET measurement of brain uptake in monkey. J. Labelled Compd. Radiopharm. 2010;53:548–551. doi: 10.1002/jlcr.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosoya T., Sumi K., Doi H., Wakao M., Suzuki M. Rapid methylation on carbon frameworks useful for the synthesis of 11CH3-incorporated PET tracers: Pd(0)-mediated rapid coupling of methyl iodide with an alkenyltributylstannane leading to a 1-methylalkene. Org. Biomol. Chem. 2006;4:410–415. doi: 10.1039/b515215a. [DOI] [PubMed] [Google Scholar]
- 38.Kawamura K., Naganawa M., Konno F., Yui J., Wakizaka H., Yamasaki T., Yanamoto K., Hatori A., Takei M., Yoshida Y., Sakaguchi K., Fukumura T., Kimura Y., Zhang M.-R. Imaging of I2-imidazoline receptors by small-animal PET using 2-(3-fluoro-[4-11C]tolyl)-4,5-dihydro-1H-imidazole ([11C]FTIMD) Nucl. Med. Biol. 2010;37:625–635. doi: 10.1016/j.nucmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Bennacef I., Perrio C., Lasne M.-C., Barré L. Functionalization through Lithiation of (S)-N-(1-Phenylpropyl)-2-phenylquinoline-4-carboxamide. Application to the Labeling with Carbon-11 of NK-3 Receptor Antagonist SB 222200. J. Org. Chem. 2007;72:2161–2165. doi: 10.1021/jo062285p. [DOI] [PubMed] [Google Scholar]
- 40.Madsen J., Merachtsaki P., Davoodpour P., Bergström M., Långström B., Andersen K., Thomsen C., Martiny L., Knudsen G. M. Synthesis and Biological Evaluation of Novel Carbon-11-Labelled Analogues of Citalopram as Potential Radioligands for the Serotonin Transporter. Bioorg. Med. Chem. 2003;11:3447–3456. doi: 10.1016/s0968-0896(03)00307-9. [DOI] [PubMed] [Google Scholar]
- 41.Tarkiainen J., Vercouillie J., Emond P., Sandell J., Hiltunen J., Frangin Y., Guilloteau D., Halldin C. Carbon-11 labelling of MADAM in two different positions: a highly selective PET radioligand for the serotonin transporter. J. Labelled Compd. Radiopharm. 2001;44:1013–1023. doi: 10.1002/jlcr.523. [DOI] [Google Scholar]
- 42.Yu M., Tueckmantel W., Wang X., Zhu A., Kozikowski A.P., Brownell A.-L. Methoxyphenylethynyl, methoxypyridylethynyl and phenylethynyl derivatives of pyridine: synthesis, radiolabeling and evaluation of new PET ligands for metabotropic glutamate subtype 5 receptors. Nucl. Med. Biol. 2005;32:631–640. doi: 10.1016/j.nucmedbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Hamill T.G., Krause S., Ryan C., Bonnefous C., Govek S., Seiders T.J., Cosford N.D.P., Roppe J., Kamenecka T., Patel S., Gibson R.E., Sanabria S., Riffel K., Eng W., King C., Yang X., Green M.D., O’Malley S.S., Hargreaves R., Burns H.D. Synthesis, Characterization, and First Successful Monkey Imaging Studies of Metabotropic Glutamate Receptor Subtype 5 (mGluR5) PET Radiotracers. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Narendran R., Bischoff F., Guo N., Zhu Z., Bae S., Lesage A.S., Laruelle M. A Positron Emission Tomography Radioligand for the in Vivo Labeling of Metabotropic Glutamate 1 Receptor: (3-Ethyl-2-[11C]methyl-6-quinolinyl)(cis-4-methoxycyclohexyl)methanone. J. Med. Chem. 2005;48:5096–5099. doi: 10.1021/jm050263+. [DOI] [PubMed] [Google Scholar]
- 45.Björkman M., Andersson Y., Doi H., Kato K., Suzuki M., Noyori R., Watanabe Y., Långström B. Synthesis of 11C/13C-Labelled Prostacyclins. Acta Chem. Scand. 1998;52:635–640. doi: 10.3891/acta.chem.scand.52-0635. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M., Doi H., Kato K., Björkman M., Långström B., Watanabe Y., Noyori R. Rapid Methylation for the Synthesis of a 11C-Labeled Tolylisocarbacyclin Imaging the IP2 Receptor in a Living Human Brain. Tetrahedron. 2000;56:8263–8273. [Google Scholar]
- 47.Suzuki M., Doi H., Hosoya T., Långström B., Watanabe Y. Rapid methylation on carbon frameworks leading to the synthesis of a PET tracer capable of imaging a novel CNS-type prostacyclin receptor in living human brain. Trends Anal. Chem. 2004;23:595–607. [Google Scholar]
- 48.Prabhakaran J., Majo V.J., Simpson N.R., Van Heertum R.L., Mann J.J., Kumar J.S.D. Synthesis of [11C]celecoxib: A potential PET probe for imaging COX-2 expression. J. Labelled Compd. Radiopharm. 2005;48:887–895. doi: 10.1002/jlcr.1002. [DOI] [Google Scholar]
- 49.Karimi F., Långström B. Synthesis of 3-[(2S)-azetidin-2-ylmethoxy]-5-[11C]-methylpyridine, an analogue of A-85380, via a Stille coupling. J. Labelled Compd. Radiopharm. 2002;45:423–434. doi: 10.1002/jlcr.569. [DOI] [Google Scholar]
- 50.Iida Y., Ogawa M., Ueda M., Tominaga A., Kawashima H., Magata Y., Nishiyama S., Tsukada H., Mukai T., Saji H. Evaluation of 5-11C-Methyl-A-85380 as an Imaging Agent for PET Investigations of Brain Nicotinic Acetylcholine Receptors. J. Nucl. Med. 2004;45:878–884. [PubMed] [Google Scholar]
- 51.Suzuki A. Synthetic studies via the cross-coupling reaction of organoboron derivatives with organic halides. Pure and Appl. Chem. 1991;63:419–422. doi: 10.1351/pac199163030419. [DOI] [Google Scholar]
- 52.Ishiyama T., Kizaki H., Miyaura N., Suzuki A. Synthesis of unsymmetrical biaryl ketones via palladiumcatalyzed cross-coupling reaction of aryboronic acids with iodoarenes. Tetrahedron Lett. 1993;34:7595–7598. doi: 10.1016/S0040-4039(00)60409-4. [DOI] [Google Scholar]
- 53.Miyaura N., Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 54.Zeisler S.K., Nader M., Theobald A., Oberdorfer F. Conversion of No-carrier-added [11C]carbon dioxide to [11C]carbon monoxide on molybdenum for the synthesis of 11C-labelled aromatic ketones. Appl. Radiat. Isot. 1997;48:1091–1095. doi: 10.1016/S0969-8043(97)00109-7. [DOI] [Google Scholar]
- 55.Nader M.W., Oberdorfer F. Syntheses of [carbonyl-11C]2-(2-benzoylphenoxy)-N-phenylacet-amide from [11C]carbon monoxide by the Suzuki and the Stille reactions. Appl. Radiat. Isot. 2002;57:681–685. doi: 10.1016/S0969-8043(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 56.Rahman O., Llop J., Långström B. Organic Bases as Additives to Improve the Radiochemical Yields of [11C]Ketones Prepared by the Suzuki Coupling Reaction. Eur. J. Org. Chem. 2004:2674–2678. [Google Scholar]
- 57.Colquhoun H.M., Thompson D.J., Twigg M.V. Carbonylation. Plenum Press; New York, NY, USA: 1991. [Google Scholar]
- 58.Rahman O., Kihlberg T., Långström B. Synthesis of 11C-/13C-Ketones by Suzuki Coupling. Eur. J. Org. Chem. 2004:474–478. [Google Scholar]
- 59.Hostetler E.D., Terry G.E., Burns H.D. An improved synthesis of substituted [11C]toluenes via Suzuki coupling with [11C]methyl iodide. J. Labelled Compd. Radiopharm. 2005;48:629–634. doi: 10.1002/jlcr.953. [DOI] [Google Scholar]
- 60.De Meijere A., Meyer F.E. Kleider machen Leute: Heck-Reaktion im neuen Gewand. Angew. Chem. 1994;106:2473–2506. doi: 10.1002/ange.19941062307. [DOI] [Google Scholar]
- 61.Björkman M., Långström B. Functionalisation of 11C-labelled olefins via a Heck coupling reac-tion. J. Chem. Soc. Perkin Trans. 1. 2000:3031–3034. [Google Scholar]
- 62.Chinchilla R., Nájera C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007;107:874–922. doi: 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]
- 63.Wuest F., Zessin J., Johannsen B. A new approach for 11C–C bond formation: synthesis of 17α-(3'-[11C]prop-1-yn-1-yl)-3-methoxy-3,17ß-estradiol. J. Labelled Compd. Radiopharm. 2003;46:333–342. doi: 10.1002/jlcr.674. [DOI] [Google Scholar]
- 64.Wuest F., Berndt M. 11C–C bond formation by palladium-mediated cross-coupling of alkenylzirconocenes with [11C]methyl iodide. J. Labelled Compd. Radiopharm. 2006;49:91–100. doi: 10.1002/jlcr.1044. [DOI] [Google Scholar]
- 65.Hart D.W., Schwartz J. Hydrozirconation. Organic Synthesis via organozirconium intermediates. Synthesis and rearrangement of alkylzirconium(IV) complexes and their reaction with electrophiles. J. Am. Chem. Soc. 1974;96:8115–8116. doi: 10.1021/ja00833a048. [DOI] [Google Scholar]
- 66.Tarkiainen J., Vercouillie J., Emond P., Sandell J., Hiltunen J., Frangin Y., Guilloteau D., Halldin C. Carbon-11 labelling of MADAM in two different positions: a highly selective PET radioligand for the serotonin transporter. J. Label. Compd. Radiopharm. 2001;44:1013–1023. doi: 10.1002/jlcr.523. [DOI] [Google Scholar]
- 67.Lasne M.C., Pike V.W., Turton D,R. The radiosynthesis of [-methyl-11C]-sertraline. Int. J. Radiat. Appl. Instrum., Part A. 1989;40:147–151. doi: 10.1016/0883-2889(89)90190-1. [DOI] [PubMed] [Google Scholar]
- 68.Eriksson J., Åberg O., Långström B. Synthesis of [11C]/[13C]Acrylamides by Palladium-Media-ted Carbonylation. Eur. J. Org. Chem. 2007:455–461. [Google Scholar]
- 69.Amatore C., Jutand A. Role of dba in the reactivity of palladium(0) complexes generated in situ from mixtures of Pd(dba)2 and phosphines. Coord. Chem. Rev. 1998;178-180:511–528. [Google Scholar]
- 70.Andersson Y., Långström B. Transition Metal-mediated Reactions using [11C]Cyanide in Synthesis of 11C-labelled Aromatic Compounds. J. Chem. Soc. Perkin Trans. 1. 1994:1395–1400. doi: 10.1039/p19940001395. [DOI] [Google Scholar]
- 71.Sandell J., Halldin C., Hall H., Thorberg S.-O., Werner T., Sohn D., Sedvall G., Farde L. Radiosynthesis and Autoradiographic Evaluation of [11C]NAD-299, a Radioligand for Visualization of the 5-HT1A Receptor. Nucl. Med. Biol. 1999;26:159–164. doi: 10.1016/S0969-8051(98)00091-2. [DOI] [PubMed] [Google Scholar]
- 72.Bennacef I., Salinas C.A., Bonasera T.A., Gunn R.N., Audrain H., Jakobsen S., Nabulsi N., Weinzimmer D., Carson R.E., Huang Y., Holmes I., Micheli F., Heidbreder C., Gentile G., Rossi T., Laruelle M. Dopamine D3 receptor antagonists: The quest for a potentially selective PET ligand. Part 3: Radiosynthesis and in vivo studies. Bioorg. Med. Chem. Lett. 2009;19:5056–5059. doi: 10.1016/j.bmcl.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 73.Lidström P., Neu H., Långström B. Syntheses of [21-11C] and (21-13C) progesterone. J. Labelled Compd. Radiopharm. 1997;40:695–704. J. Labelled Compd. Radiopharm.1997, 40, 783-784. [Google Scholar]
- 74.Madsen J., Merachtsaki P., Davoodpour P., Bergström M., Långström B., Andersen K., Thomsen C., Martiny L., Knudsen G.M. Synthesis and Biological Evaluation of Novel Carbon-11-Labelled Analogues of Citalopram as Potential Radioligands for the Serotonin Transporter. Bioorg. Med. Chem. 2003;11:3447–3456. doi: 10.1016/S0968-0896(03)00307-9. [DOI] [PubMed] [Google Scholar]
- 75.Tucker C.E., Knochel P. Cross-Coupling between Functionalized Alkylcopper Reagents and Functionalized Alkyl Halides. J. Org. Chem. 1993;58:4781–4782. doi: 10.1021/jo00070a003. [DOI] [Google Scholar]
- 76.Wuest F., Dence C.S., McCarthy T.J., Welch M.J. A New Approach for the Synthesis of [11C]-Labeled Fatty Acids. J. Labelled Compd. Radiopharm. 2000;43:1289–1300. doi: 10.1002/1099-1344(200011)43:13<1289::AID-JLCR420>3.0.CO;2-Q. [DOI] [Google Scholar]
- 77.Cenini S., Pizzotti M., Porta F., La Monica G. Isocyanate, urea and amide complexes from the reactions of organic azides with low oxidation state complexes of transition metals. J. Organomet. Chem. 1975;88:237–248. [Google Scholar]
- 78.Doi H., Barletta J., Suzuki M., Noyori R., Watanabe Y., Långström B. Synthesis of 11C-labelled N,N'-diphenylurea and ethyl phenylcarbamate by a rhodium-promoted carbonylation via [11C]isocyanatobenzene using phenyl azide and [11C]carbon monoxide. Org. Biomol. Chem. 2004;2:3063–3066. doi: 10.1039/b409294e. [DOI] [PubMed] [Google Scholar]
- 79.Ilovich O., Åberg O., Långström B., Mishania E. Rhodium-mediated [11C]Carbonylation: a li-brary of N-phenyl-N'-{4-(4-quinolyloxy)-phenyl}-[11C]-urea derivatives as potential PET angio-genic probes. J. Labelled Compd. Radiopharm. 2008;52:151–157. [Google Scholar]
- 80.La Monica G., Ardizzoia G. Five-membered Heterocycles by the Rh(I)-Catalysed Carbonylation of Aromatic ortho-Substituted Azides. J. Mol. Catal. 1986;38:327–330. doi: 10.1016/0304-5102(86)85040-4. [DOI] [Google Scholar]
- 81.Vallabhajosula S. Molecular Imaging. Springer; Berlin, Heidelberg, Germany: 2008. pp. 151–164. [Google Scholar]
- 82.Miller P.W., Long N.J., Vilar R., Gee A.D. Synthese von 11C-, 18F-, 15O- und 13N-Radiotracern für die Positronenemissionstomographie. Angew. Chem. 2008;120:9136–9172. [Google Scholar]
- 83.Kima D.W., Choeb Y.S., Chi D.Y. A new nucleophilic fluorine-18 labeling method for aliphatic mesylates: reaction in ionic liquids shows tolerance for water. Nucl. Med. Biol. 2003;30:345–350. doi: 10.1016/S0969-8051(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 84.Le Bars D. Fluorine-18 and medical imaging: Radiopharmaceuticals for positron emission tomography. J. Fluorine Chem. 2006;127:1488–1493. doi: 10.1016/j.jfluchem.2006.09.015. [DOI] [Google Scholar]
- 85.Wuest F., Berndt M., Kniess T. In: Recent Advances of Bioconjugation Chemistry in Molecular Imaging. Chen X., editor. Research Signpost; Kerala, India: 2008. pp. 155–173. [Google Scholar]
- 86.Piarraud A., Lasne M.C., Barré L., Vaugeois J.M., Lancelot J.C. Synthesis of no carrier added [18f] Gbr 12936 via a wittig reaction for use in a the dopamine reuptake site study. J. Labelled Compd. Radiopharm. 1993;32:253–254. [Google Scholar]
- 87.Hantraye P., Brownell A.-L., Elmaleh D., Spealman R.D., Wüllner U., Brownell G.L., Madras B.K., Isacson O. Dopamine fiber detection by [11C]-CFT and PET in a primate model of parkinsonism. Neuroreport. 1992;3:265–268. doi: 10.1097/00001756-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Allain-Barbier L., Lasne M.C., Huard C., Barré L. Palladium Mediated Synthesis of [18F]Fluorophenyl Alkenes or Arenes. J. Labelled Compd. Radiopharm. 1995;37:572–574. [Google Scholar]
- 89.Allain-Barbier L., Lasne M.C., Perrio-Huard C., Moreau B., Barré L. Synthesis of [18F]Fluorophenyl Alkenes or Arenes via Palladium-Catalyzed Coupling of 4-[18F]Fluoroiodo-benzene with Vinyl and Aryl Tin Reagents. Acta Chem. Scand. 1998;52:480–489. doi: 10.3891/acta.chem.scand.52-0480. [DOI] [Google Scholar]
- 90.Gail R., Coenen H.H. A one step preparation of the n.c.a. fluorine-18 labelled synthons: 4-Fluorobromobenzene and 4-fluoroiodobenzene. Appl. Radiat. Isot. 1994;45:105–111. doi: 10.1016/0969-8043(94)90155-4. [DOI] [Google Scholar]
- 91.Forngren T., Andersson Y., Lamm B., Långström B. Palladium-Mediated Coupling Reactions of 18F-Labelled Arylhalides with Organotin Compounds. J. Labelled Compd. Radiopharm. 1995;37:595–596. [Google Scholar]
- 92.Plenevaux A., Lemaire C., Palmer A.J., Damhaut P., Comar D. Synthesis of non-activated 18F-fluorinated aromatic compounds through nucleophilic substitution and decarboxylation reactions. Int. J. Radiat. Appl. Instrument. Part A; Appl. Radiat. Isot. 1992;43:1035–1040. doi: 10.1016/0883-2889(92)90223-2. [DOI] [Google Scholar]
- 93.Forngren T., Andersson Y., Lamm B., Långström B. Synthesis of [4-18F]-1-Bromo-4-fluorobenzene and its Use in Palladium-Promoted Cross-Coupling Reactions with Organostannanes. Acta Chem. Scand. 1998;52:475–479. doi: 10.3891/acta.chem.scand.52-0475. [DOI] [Google Scholar]
- 94.Marrière E., Rouden J., Tadino V., Lasne M.C. Synthesis of Analogues of (−)-Cytisine for in Vivo Studies of Nicotinic Receptors Using Positron Emission Tomography. Org. Lett. 2000;2:1121–1124. doi: 10.1021/ol005685m. [DOI] [PubMed] [Google Scholar]
- 95.Holladay M.W., Dart M.J., Lynch J.K. Neuronal Nicotinic Acetylcholine Receptors as Targets for Drug Discovery. J. Med. Chem. 1997;40:4169–4194. doi: 10.1021/jm970377o. [DOI] [PubMed] [Google Scholar]
- 96.Wüst F.R., Kniess T. Synthesis of 4-[18F]fluoroiodobenzene and its application in sonogashira cross-coupling reactions. J. Labelled Compd. Radiopharm. 2003;46:699–713. doi: 10.1002/jlcr.709. [DOI] [Google Scholar]
- 97.Mangner T.J., Klecker R.W., Anderson L., Shields A.F. Synthesis of 2′-deoxy-2′-[18F]fluoro-β-D-arabinofuranosyl nucleosides, [18F]FAU, [18F]FMAU, [18F]FBAU and [18F]FIAU, as potential PET agents for imaging cellular proliferation: Synthesis of [18F]labelled FAU, FMAU, FBAU. Nucl. Med. Biol. 2003;30:215–224. doi: 10.1016/S0969-8051(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 98.Toyohara J., Fujibayashi Y. Trends in nucleoside tracers for PET imaging of cell proliferation. Nucl. Med. Biol. 2003;30:681–685. doi: 10.1016/S0969-8051(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 99.Brust P., Haubner R., Friedrich A., Scheunemann M., Anton M., Koufaki O.-N., Hauses M., Noll S., Noll B., Haberkorn U., Schackert G., Schackert H.K., Avril N., Johannsen B. Comparison of [18F]FHPG and [124/125I]FIAU for imaging herpes simplex virus type 1 thymidine kinase gene expression. Eur. J. Nucl. Med. Mol. Imaging. 2001;28:721–729. doi: 10.1007/s002590100526. [DOI] [PubMed] [Google Scholar]
- 100.Wüst F.R., Kniess T. No-carrier added synthesis of 18F-labelled nucleosides using Stille cross-coupling reactions with 4-[18F]fluoroiodobenzene. J. Labelled Compd. Radiopharm. 2004;47:457–468. doi: 10.1002/jlcr.834. [DOI] [Google Scholar]
- 101.Marnett L.J. Cyclooxygenase mechanisms. Curr. Opin. Chem. Biol. 2000;4:545–552. doi: 10.1016/S1367-5931(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 102.Wüst F.R., Höhne A., Metz P. Synthesis of 18F-labelled cyclooxygenase-2 (COX-2) inhibitors via Stille reaction with 4-[18F]fluoroiodobenzene as radiotracers for positron emission tomography (PET) Org. Biomol. Chem. 2005;3:503–507. doi: 10.1039/b412871k. [DOI] [PubMed] [Google Scholar]
- 103.Li J.J., Anderson G.D., Burton E.G., Cogburn J.N., Collins J.T., Garland J.D., Gregory S.A., Huang H., Isakson P.C., Koboldt C.M., Logusch E.W., Norton M.B., Perkins W.E., Reinhard E.J., Seibert K., Veenhuizen A.W., Zhang Y., Reitz D.B. 1,2-Diarylcyclopentenes as Selective Cyclooxygenase-2 Inhibitors and Orally Active Anti-inflammatory Agents. J. Med. Chem. 1995;38:4570–4578. doi: 10.1021/jm00022a023. [DOI] [PubMed] [Google Scholar]
- 104.Steininger B., Wuest F.R. Synthesis of 18F-labelled biphenyls via SUZUKI cross-coupling with 4-[18F]fluoroiodobenzene. J. Labelled Compd. Radiopharm. 2006;49:817–827. doi: 10.1002/jlcr.1099. [DOI] [Google Scholar]
- 105.Marrière E., Chazalviel L., Dhilly M., Toutain J., Perrio C., Dauphin F., Lasne M.C. Synthesis of [18F]RP 62203, a potent and selective Serotonin 5-HT2A Receptor Antagonist and Biological Evaluation with ex vivo Autoradiography. J. Labelled Compd. Radiopharm. 1999;42:S69–S71. [Google Scholar]
- 106.Malleron J.L., Comte M.T., Gueremy C., Peyronel J.F., Truchon A., Blanchard J.C., Doble A., Piot O., Zundel J.L. Naphthosultam Derivatives: A New Class of Potent and Selective 5-HT2 Antagonists. J. Med. Chem. 1991;34:2477–2483. doi: 10.1021/jm00112a025. [DOI] [PubMed] [Google Scholar]
- 107.Das M.K., Mukherjee J. [18F]RP 62203: A High Affinity and Selective Radioligand as a Potential PET Tracer for Serotonin 5HT2 Receptor. J. Labelled Compd. Radiopharm. 1994;35:497–499. [Google Scholar]
- 108.Collins M., Lasne M.-C., Barré L. Rapid synthesis of N,N′-disubstituted piperazines. Application to the preparation of No carrier added 1-(4-[18F]fluorophenyl)piperazine and of an [18F]-selective ligand of serotoninergic receptors (5HT2 antagonist) J. Chem. Soc. Perkin Trans. 1. 1992;23:3185–3188. [Google Scholar]
- 109.Lasne M.C., Le Secq B., Barre L., Collins M., Huard C., Baron J.C. Synthesis of [18F]RP 62203, a Selective Antagonist of 5HT2 Receptors for Positron Emission Tomography Studies. J. Labelled Compd. Radiopharm. 1994;35:441–443. [Google Scholar]
- 110.Stabler S. R., Jahangir. Preparation of N-Arylated Heterocycles by Nucleophilic Aromatic Substitution. Synth. Commun. 1994;24:123–129. doi: 10.1080/00397919408012635. [DOI] [Google Scholar]
- 111.Mor M., Plazzi P.V., Rivara S. 2-[N-Acylamino(C1−C3)alkyl]indoles as MT1 Melatonin Receptor Partial Agonists, Antagonists, and Putative Inverse Agonists. J. Med. Chem. 1998;41:3624–3634. doi: 10.1021/jm970721h. [DOI] [PubMed] [Google Scholar]
- 112.Andersen K., Liljefors T., Hyttel J., Perregaard J. Serotonin 5-HT2 Receptor, Dopamine D2 Receptor, and α1 Adrenoceptor Antagonist. Conformationally Flexible Analogues of the Atypical Antipsychotic Sertindole. J. Med. Chem. 1996;39:3723–3738. doi: 10.1021/jm960159f. [DOI] [PubMed] [Google Scholar]
- 113.Perregaard J., Moltzen E.K., Meier E., Sanchez C. σ Ligands with Subnanomolar Affinity and Preference for the σ2 Binding Site. 1. 3-(ω-Aminoalkyl)-1H-indoles. J. Med. Chem. 1995;38:1998–2008. doi: 10.1021/jm00011a019. [DOI] [PubMed] [Google Scholar]
- 114.Sánchez C., Arnt J., Costall B., Kelly M.E., Meier E., Naylor R.J., Perregaard J. The Selective σ2-Ligand Lu 28-179 Has Potent Anxiolytic-Like Effects in Rodents. J. Pharmacol. Exp. Ther. 1997;283:1333–1341. [PubMed] [Google Scholar]
- 115.Wüst F.R., Kniess T. N-Arylation of indoles with 4-[18F]fluoroiodobenzene: synthesis of 18F-labelled σ2 receptor ligands for positron emission tomography (PET) J. Labelled Compd. Radiopharm. 2005;48:31–43. doi: 10.1002/jlcr.893. [DOI] [Google Scholar]
- 116.Lee B.-C., Dence C.S., Zhou H., Parent E.E., Welch M.J., Katzenellenbogen J.A. Fluorine-18 labeling and biodistribution studies on peroxisome proliferator-activated receptor-γ ligands: potential positron emission tomography imaging agents. Nucl. Med. Biol. 2009;36:147–153. doi: 10.1016/j.nucmedbio.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henke B.R., Blanchard S.G., Brackeen M.F., Brown K.K., Cobb J.E., Collins J.L., Harrington W.W., Jr., Hashim M.A., Hull-Ryde E.A., Kaldor I., Kliewer S.A., Lake D.H., Leesnitzer L.M., Lehmann J.M., Lenhard J.M., Orband-Miller L.A., Miller J.F., Mook R.A., Jr., Noble S.A., Oliver W., Jr., Parks D.J., Plunket K.D:, Szewczyk J.R:, Willson T.M. N-(2-Benzoylphenyl)-l-tyrosine PPARγ agonists: 1. Discovery of a Novel Series of Potent Antihyperglycemic and Antihyperlipidemic Agents. J. Med. Chem. 1998;41:5020–5036. doi: 10.1021/jm9804127. [DOI] [PubMed] [Google Scholar]
- 118.Young P.W., Buckle D.R., Cantello B.C.C., Chapman H., Clapham J.C., Coyle P.J., Haigh D., Hindley R.M., Holder J.C., Kallender H., Latter A.J., Lawrie K.W.M., Mossakowska D., Murphy G.J., Cox L.R., Smith S.A. Identification of High-Affinity Binding Sites for the Insulin Sensitizer Rosiglitazone (BRL-49653) in Rodent and Human Adipocytes Using a Radioiodinated Ligand for Peroxisomal Proliferator-Activated Receptor γ. J. Pharmacol. Exp. Ther. 1998;284:751–759. [PubMed] [Google Scholar]
- 119.Pretze M., Flemming A., Köckerling M., Mamat C. Synthesis and Radiofluorination of Iodophenyl Esters as Tool for the Traceless Staudinger Ligation. Z. Naturforsch. 2010;65b:1128–1136. [Google Scholar]
- 120.Pretze M., Wuest F., Peppel T., Köckerling M., Mamat C. The traceless Staudinger ligation with fluorine-18: A novel and versatile labeling technique for the synthesis of PET-radiotracers. Tetrahedron Lett. 2010;51:6410–6414. [Google Scholar]