Abstract
Fruits and vegetables are colorful pigment-containing food sources. Owing to their nutritional benefits and phytochemicals, they are considered as ‘functional food ingredients’. Carotenoids are some of the most vital colored phytochemicals, occurring as all-trans and cis-isomers, and accounting for the brilliant colors of a variety of fruits and vegetables. Carotenoids extensively studied in this regard include β-carotene, lycopene, lutein and zeaxanthin. Coloration of fruits and vegetables depends on their growth maturity, concentration of carotenoid isomers, and food processing methods. This article focuses more on several carotenoids and their isomers present in different fruits and vegetables along with their concentrations. Carotenoids and their geometric isomers also play an important role in protecting cells from oxidation and cellular damages.
Keywords: carotene, cryptoxanthin, fruit, lutein, lycopene, vegetables, zeaxanthin
1. Introduction
Increasing interest in nutrition, fitness and beauty consciousness has enhanced concerns over a healthy diet. Fruits and vegetables have assumed the status of ‘functional’ foods, capable of providing additional health benefits, like prevention or delaying onset of chronic diseases, as well as meeting basic nutritional requirements. Appropriate intake of a variety of fruits and vegetables ensures sufficient supply of nutrients and phytochemicals such as carotenoids. Low consumption of fruit and vegetable is among the top ten risk factors resulting in the global mortality. Annually, 2.7 million lives could be saved with sufficient consumption of various kinds of fruits and vegetables [1].
Nowadays, food scientists have collaborated with nutrition researchers to develop plant-based functional foods to promote healthy eating habits. In food research, carotenoids from fruits and vegetables have attracted a great deal of attention, mainly focused on the analysis of geometric carotenoid isomers. Carotenoids found in fruits and vegetables have also attracted great attention for their functional properties, health benefits and prevention of several major chronic diseases [2,3,4].
Carotenoids are synthesized in plants but not in animals. In nature, more than 600 types of carotenoid have been determined. Carotenoids are localized in subcellular organelles (plastids), i.e. chloroplasts and chromoplasts. In chloroplasts, the carotenoids are chiefly associated with proteins and serve as accessory pigments in photosynthesis, whereas in chromoplasts they are deposited in crystalline form or as oily droplets [5]. Some of the carotenoids such as the xanthophylls are involved in photosynthesis by participating in energy transfer in the presence of chlorophyll in plants [6].
Studies have shown that carotenoids contribute to the yellow color found in many fruits and vegetables [5,7]. The colors of fruits and vegetables depend on conjugated double bonds and the various functional groups contained in the carotenoid molecule [8]. A study also reported that the greater the number of conjugated double bonds, the higher the absorption maxima (λmax) [9]. As a result, the color ranges from yellow, red to orange in many fruits and vegetables [5,10]. Besides, esterification of carotenoids with fatty acids can also occur during fruit ripening, which may affect the color intensity [11].
Naturally, most of the carotenoids occur as trans-isomer in plants. However, cis-isomers may increase due to the isomerization of the trans-isomer of carotenoids during food processing [12]. Many studies have involved in the analysis of dietary carotenoids and their potential isomers [13,14,15], with much attention given to the geometric isomerization of carotenoids [16,17,18,19,20,21]. The investigation of carotenoid contents in fresh, frozen and canned foods has been carried out [22]. However, a recent review on contents of carotenoids and their isomers from diverse fruits and vegetables has not been made. The data collected from published literatures will be useful for food researchers, nutritionists and health practitioners in promoting right diets to minimize vitamin A deficiency and maintaining a healthy dietary practice.
2. Carotenoids and Their Isomers
There are many factors influencing the formation and isomerization of carotenoids. Heat, light, and structural differences are the prominent factors that affect the isomerization of carotenoids in foods [23,24,25]. Various processing methods, such as heating and drying also lead to the isomerization and even degradation of carotenoids [26,27]. De Rigal et al. [24] reported that isomerization of carotenoids in apricot purees was due to enzymatic browning. Oxidative degradation of carotenoids has also led to cis-trans isomerization and formation of carotenoid epoxides [28,29].
Previous studies have shown that cis-isomer of carotenoids can be identified based on the absorption spectrum characteristics, Q ratios, and the relative intensity of the cis peak [8,30]. The UV spectrum of cis carotenoids is characterized with their λmax between 330–350 nm, which has greatest intensity when the double bond is located near or at the center of the chromophore [31]. On the other hand, a hypsochromic shift in the λmax and smaller extinction coefficient is observed. Thus, cis-trans isomerization of carotenoids leads to a decrease of color intensity [12].
Carotenoids that contain more than seven conjugated double bonds were reported to have stronger antioxidant capacity and protection against photo-bleaching of chlorophyll [32]. Di Mascio et al. [33] also reported 1O2 quenching capability of carotenoids is based on the number of conjugated double bonds and not the ionone ring of β-carotene. As the geometric isomers of carotenoids make great contribution to antioxidant activities and health improvement, analyses of carotenoids and their isomers in fruits and vegetables are needed.
Liquid chromatography (LC) enables separation and identification of individual carotenoids. Identification of carotenoid isomers can be achieved by high performance liquid chromatography (HPLC). The separation of carotenoid isomers can be done using either polymeric C30 or ODS-2 silica columns [34]. However, the identification of carotenoid isomers seemed to be ambiguous. In this review, the analyses of carotenoid geometric isomers and their levels are listed in Table 1, which also should enable researchers to understand the various carotenoid isomers present in different fruits and vegetables.
Table 1.
Analyses of carotenoid isomers in fruits and vegetables.
| Fruit/ vegetable | Analytical method | Carotenoid and its isomer | Ref. | |
|---|---|---|---|---|
| Bambangan (lyophilized pulp) [ Mangifera pajang Kosterm.] | HPLC: Polymeric C30 column (150 mm × 4.6 mm i.d., 3 μm particle) | cryptoxanthin (mg/100 g): 1.18α-carotene (mg/100 g): all- trans (7.96)β-carotene (mg/100 g): all-trans (20.04); 9-cis (2,72); cis-isomers (3.04–3.07) | [35] | |
| Loquat (fresh)[ Eriobotrya japonica (Thunb.) Lindl.] | HPLC-PDA-MS/MS: HPLC-MS: YMC C30 column (250 × 4.6 mm i.d., 5 μm particle) | β-cryptoxanthin (μg/100 g): all- trans (54.8–715.2); 9- or 90-cis (0.8); 13-or 130-cis (4.0–20.1); cis-5,6:50,60-diepoxy (1.8–3.5); 5,6:50,60-diepoxy (35.0–339.5); 5,8:50,60- or 5,6:50,80-diepoxy (1.8–34.8); cis-5,8:50,60- or 5,6:50,80-diepoxy (1.1–10.9); cis-5,6:50,60-diepoxy (1.9-12.1); 50,60-epoxy (11.5–109.4); 5,6-epoxy (19.0–213.9); 5,8-Epoxy (1.6–15.3) β-carotene (μg/100 g): all-trans (38.1–1441.5); 9-cis (1.6–18.0); 13-cis (5.0–45.9); 15-cis (0.7–4.8) | [36] | |
| Mango (dried pulp)[ Mangifera indica L.] | HPLC: Polymeric C30 column (250 mm × 4.6 mm i.d., 5 μm particle) | Neoxanthin (μg/g): all- trans (0.44–0.71); cis-isomers (0.19–0.57)Violaxanthin (μg/g): all-trans (0.16–0.32); cis-isomers (0.10–4.70)Zeaxanthin (μg/g): all-trans (0.89–1.33); cis-isomers (0.72–0.96)Lutein (μg/g): 9- or 9’-cis (0.53–0.78)β-carotene (μg/g): all-trans (9.32–29.34); 13- or 13’-cis (0.78–3.79); 15- or 15’-cis (0.98–7.20); cis-isomers (0.35–0.70) | [37, 38] | |
| Peach (fresh)[ Prunus persica(L.) Batsch] | HPLC: Polymeric C30 column (250 mm × 4.6 mm i.d., 5 μm particle) | β-cryptoxanthin (μg/g): all- trans (0.3); 13/13’-cis (0.1); 15-cis (0.1) β-carotene (μg/g): all-trans (2.2); 9-cis (0.3); 13-cis (0.5); 15-cis (trace) | [39] | |
| Tree tomato (yellow) [ Solanum betaceum Cav.] | HPLC-MS: YMC C30 column (250 × 4.6 mm i.d., 5 μm particle) | β-carotene (% residual carotenoid): all- trans (61.1–85.5); 13-cis (284.2–518.6)ζ-carotene (% residual carotenoid): cis-isomer (46.5–83.9) | [13] | |
| Broccoli (fresh)[ Brassica oleracea var. Italica] | HPLC: Polymeric C30 column (250 mm × 4.6 mm i.d., 5 μm particle) | β-carotene (μg/g): all- trans (29.2); 9-cis (5.0); 13-cis (3.3), 15-cis (1.9); cis-isomers (2.0) | [39] | |
| Maize (mutant, fresh)[ Zea mays L.] | HPLC: Spherisorb ODS-2 silica column (250 × 3.2 mm i.d., 5 μm particle) | ζ-carotene: di- cis (55.8) tri-cis (17.6–46.3) | [40] | |
| Maize (kernel) - 13 varieties [ Zea mays L.] | HPLC: Vydac218TP53 column (250 × 3.2 mm i.d.) | β-carotene (μg/100 g): all- trans (37–879); cis-isomers (<0.1–301) | [41] | |
| Pumpkin (fresh)[ Curcurbita moschata var. Orange] | HPLC: Polymeric C30 column (250 mm × 4.6 mm i.d., 5 μm particle) | β-carotene (μg/g): all- trans (61.6); 9-cis (2.5); 13-cis (2.7) | [15] | |
| Spinach (fresh)[ Spinacia oleracea L.] | HPLC: Polymeric C30 column (250 mm × 4.6 mm i.d., 5 μm particle) | β-carotene (μg/g): all- trans (311.9); 9-cis (38.6); 13-cis (24.5), 15-cis (trace); cis-isomers (22.5) | [39] | |
| Tomato (fresh)[ Solanum lycopersicum L.] | HPLC: Polymeric C30 column (250 mm × 4.6 mm i.d., 5 μm particle) | β-carotene (μg/g): all- trans (71.0); 9-cis (4.8); 13-cis (5.8) | [39] | |
a Ref.: References.
2.1. Carotenes
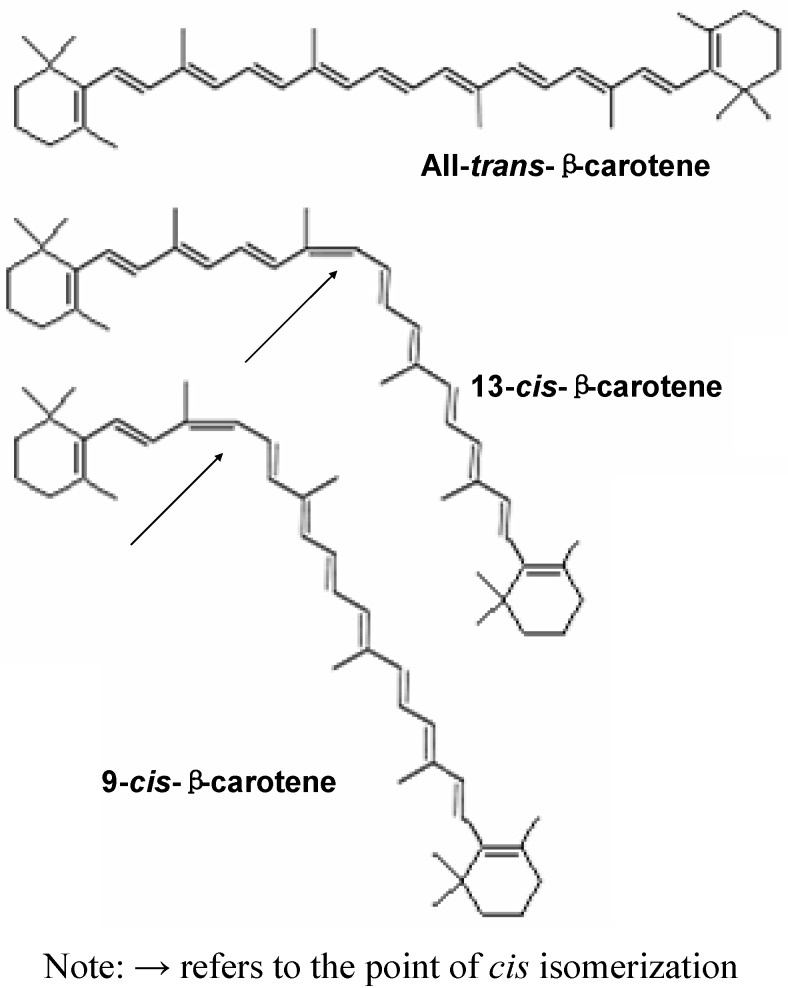
Carotenes include several related compounds having the general formula C40H56. They are a simple type of carotenoid and occur in several isomeric forms, such as alpha (α), beta (β), gamma (γ), delta (δ), epsilon (ε), and zeta (ζ) [42]. Among the various carotenoids, α- and β-carotene are the two primary forms of carotenes. In human body, β-carotene is broken down by β-carotene dioxygenase in the mucosa of small intestine into two retinyl molecules, which is later reduced to vitamin A (retinol) [43]. Carotenes can be found in many dark green and yellow leafy vegetables and appear as fat soluble pigments, while β-carotene can be found in yellow, orange and red colored fruits and vegetables [44]. Naturally, β-carotene is mostly found as all-trans isomers and lesser as cis-isomers (Figure 1), with the relative abundances in the following order: all-trans > 9-cis > 13-cis > 15-cis [45].
Figure 1.
The structure of all-trans-β-carotene and its two geometric isomers [35,46].
All-trans-β-carotene is very unstable and can be easily isomerized into cis-isomers, when exposed to heat and light. Isomerization energy is involved in relocation of the single or double bond of one form of carotenoid into another [46,47]. A study has been carried out to determine the isomerization energy of carotenoids, especially neurosporene, spheroidene and spirilloxanthin [16], but the excited energy stages are not well understood. Besides, processing of fruit could result in significant cis-trans isomerization of β-carotene which was shown by the formation of 13-cis-β-carotene [48].
In regard to the effect of processing and isomerization of carotenoids in fruits and vegetables, 13-cis-β-carotene is the main product of geometric isomerization [49], 9-cis-β-carotene is formed when exposure to light [12,49], while 13-cis-α- and β-carotene isomers are formed during storage [50]. A study on the effect of β-carotene isomerization due to reflux heating has exhibited that degradation occurs to all-trans-β-carotene, with a significant increase in 13-cis-β-carotene [51]. Based on the structures of all-trans-β-carotenes, the double bonds can be relocated during heating and form several isomers (Figure 1) [46]. Marx et al. [52] have revealed that in pasteurized and sterilized samples, 13-cis-β-carotene was the only isomer formed during pasteurization and sterilization of carrot juice, while 9-cis-β-carotene was probably formed during blanching of sterilized carrot juice. Moreover, 9-cis- and 13-cis-β-carotenes were thought to originate independently from cis precursors by non-enzymatic isomerization of all-trans forms [53].
On the other hand, cis-β-carotene has been shown to isomerize into all-trans-isomer when heated and exposed to air [17]. It shows that isomerization of β-carotene occurs instead of degradation. The isomerization process was also known to occur when a crystaline β-carotene is heated at 90 °C and 140 °C in a nitrogen environment, which might be due to the partially melted β-carotene that has increased the probability of cis- to all-trans-β-carotene isomerization [17]. Carotene in all-trans form has higher bioavailability than its cis counterpart, while β-carotene and β-apo-12’-carotenal have the highest bioconversion rate at 100% and 120% (on a weight basis), respectively [54].
2.2. Lycopene
Lycopene is an unsaturated acyclic carotenoid with open straight chain hydrocarbon consisting of 11 conjugated and two unconjugated double bonds. Lycopene has no provitamin A activity due to the lack of terminal β-ionic ring as the basic structure for vitamin A [55]. Most of the lycopene occurs naturally in all-trans form [56]. The red color of lycopene is mainly due to many conjugated carbon double bonds, as it absorbs more visible spectrum compared to other carotenes [57]. Lycopene contains seven double bonds which can be isomerized to mono-cis- or poly-cis-isomers [58]. Based on the isomeric conformation of lycopene, 5-cis-lycopene was the most stable isomer, followed by all-trans- and 9-cis-lycopene [59]. Besides, 5-cis-lycopene has the lowest isomerization energy among other lycopene cis-isomers, and its very large rotational barrier restricts it to form all-trans structure [45]. More studies on isomerization energy are needed to explain the rationale on the conversion of all-trans-carotene to its cis-isomers by thermal processing, under low pH condition and exposure to light.
Lycopene cis-isomers are more soluble in oil or organic solvents than all-trans-lycopene [60]. There is dissimilarity between the isomerization of β-carotene and lycopene [61]. Lycopene isomerization occurs under the simulated gastric digestion, thermal processing and low pH [62], but the effect of these conditions on lycopene isomerization is unclear. Boileau et al. [56] reviewed that isomerization of lycopene was found to occur in human body due to the effect of gastric juice in the stomach. However, Blanquet-Diot et al. [59] reported that no cis-trans isomerization of lycopene has occurred using gastrointestinal tract model. Heating at 60 °C and 80 °C favored the isomerization of lycopene [63]. The formation of 9-cis-lycopene is more favorable at low pH condition while 13-cis-lycopene is the major degradation product formed from thermal processing [62].
The uptake of cis-lycopene by intestinal cells is known to exceed those of all-trans-lycopene, which was in agreement with the study by Tyssandier et al. [64] that cis-lycopene had greater bioaccessibility compared to its all-trans form. Lycopene cis-isomers also found to have greater bioactivity and bioavailability than their all-trans counterpart [65]. Besides, lycopene is less bioavailable than β-carotene and lutein [66]. Processing method could help to release the lycopene from the matrix in fruits and vegetables, and thus increases bioavailability [12].
2.3. Xanthophylls
Xanthophylls are the oxidized derivatives of carotenes. Xanthophylls, with a general chemical formula C40H56O2, contain hydroxyl groups and are more polar than carotenes [67]. In Nature, xanthophylls are found in the leaf of most plants and are synthesized within the plastids [68], which occur as yellow to red colored pigments. They are also considered accessory pigments, along with anthocyanins, carotenes, and sometimes phycobiliproteins [69].
Commonly found xanthophylls include lutein, zeaxanthin, and cryptoxanthin. In plant, violaxanthin, antheraxanthin and zeaxanthin participate in xanthophyll cycle, which involves the conversion of pigments from a non-energy-quenching form to energy-quenching forms [6]. Lutein is one kind of xanthophyll found abundently in fruits and vegetables [44,65]. It is a fat soluble compound and very stable in emulsion [66]. Although, lutein and zeaxanthin are isomers but they are not stereoisomers. In addition, lutein is one of the xanthophyll discovered in egg yolk [67]. As animals cannot produce xanthophylls, xanthophylls found in animals are known to be ingested from food [68].
The isomers of xanthophyll are not well studied. Since the development of the C30 HPLC analytical column, the determination of xanthophyll isomers is becoming a hot issue. Identification of xanthophyll isomers has been carried out using different polymeric columns [69]. Study also reported that cis-isomers of xanthophyll determined using a C30 stationary phase were relatively higher than accessed using C18 column [70]. Tóth and Szabolcs [71] had identified 9-cis- and 9’-cis-isomers of antheraxanthin, capsanthin, lutein and lutein epoxide in several higher plants. They found that 9-cis-isomers of antheraxanthin and lutein epoxide occurred without their 9’-cis counterparts in non-photosynthetic tissues. This could be explained by the non-stereoselective biosynthesis or stereomutation, while the 9-cis form is protected stereoselectively against photoisomerization.
Isomers of violaxanthin namely, 9-cis-, 13-cis- and di-cis-violaxanthin have been identified in orange juice [72,73]. Besides, lutein epoxide has been identified in dandelion petal, with high amounts of the 9-cis- and 9’-cis-isomers, with the all-trans form as the major carotenoid [74]. Moreover, 13-cis-zeaxanthin was found as the major isomerization products of all-trans form, which was induced by light and temperature (35–39 °C) [75]. In one study by Kishimoto et al. [76], sixteen xanthophylls were isolated from the petals of chrysanthemum. These xanthophylls were mainly the isomers of violaxanthin, luteoxanthin, lutein, and also lutein epoxides. They also concluded that chrysanthemum petals have a unique carotenoid characteristic compared to the flowers of other species. Furthermore, Yahia et al. [77] reported that the saponified crude extract of mango fruit has all-trans-violaxanthin and 9-cis-violaxanthin present in the esterified form. In ripening fruit, esterification of xanthophylls occurs [10], but the mechanism and biosynthetic pathways of esterification are still to be explored.
3. Carotenoid Pigments in Fruits and Vegetables
Carotenoids are widely distributed in the cellular tissues of plants [78]. The distribution of carotenoids in human tissues is originated from plant sources. Therefore, fruits and vegetables constitute the major source of carotenoids in human diet [79,80]. In plant, carotenoids are found as fat soluble and colored-pigments [81,82]. Carotenoids can be isolated from the grana of chloroplasts in the form of carotenoprotein complexes, which give various colors to the outer surfaces of the plants [83]. The visible colors of the plant are due to the conjugated double bonds of carotenoids that absorb light. The more number of double bonds results in the more absorbance of red color wavelength. The occurrences of carotenoids in plants are not as a single compound. Most of the carotenoids are bound with chlorophyll, and a combination of carotene-chlorophyll and xanthophyll-chlorophyll occurs often. The binding of carotenoids to chlorophylls can give rise to a variety of colors in plants, fruits and vegetables. However, as fruit matures, the chlorophyll content decreases, and results in colored-carotenoid pigments [84]. Besides, study had carried out to improve carotenoids color retention during ripening [85].
In nature, fruits have lesser xanthophyll contents compared to vegetables. Some fruits such as papaya (Carica papaya L.) and persimmon (Diospyros sp.) have high amount of xanthophylls (lutein and zeaxanthin), like that found in vegetables [44]. In fruits and vegetables, β-carotene is found to be bound to either chlorophylls or xanthophylls, forming chlorophyll-carotenoid complexes, which absorb light in the orange or red light spectrum and give rise to green, purple or blue coloration [86], These complexes could decrease the bioavailability of β-carotene and further weaken its bioefficacy for the conversion to vitamin A. However, this setback can be resolved by saponifying the plant extract to yield all-trans-β-carotene in a free-state form [77]. In vegetables, provitamin A carotenoids have lower bioavailability as compared to fruits [87], which may be due to their protein-complex structures in chloroplasts [54]. In this review, a comprehensive data for the typical carotenoids content in fruits and vegetables are given in Table 2 and Table 3, where the carotenoids contents in fruits and vegetables are summarized. The data from this compilation are useful for comparison of the ongoing study with other previous reports.
Table 2.
Carotenoid contents (mg/100 g fresh weight) of some common fruits.
| Taxonomy | Common name | α-Carotene | β-Carotene | Lycopene | References | ||
|---|---|---|---|---|---|---|---|
| Family | Genus | Species | |||||
| Anacardiaceae | Mangifera | indica L. | Mango | − | 0.553 | 0.353 | [91] |
| − | 1.71(0.95) | − | [90] | ||||
| 0.017[0.001] | 0.445[0.016] | − | [44] | ||||
| var. Black-gold | ND | 0.615 | ND | [65] | |||
| var. Gedong | 0.061(0.086) | 3.267(2.075) | − | [92] | |||
| var. Manalagi | ND | 0.19(0.123) | − | [92] | |||
| var. Indramayn | 0.067(0.005) | 1.606(0.166) | − | [92] | |||
| var. Harum manis | 0.055(0.001) | 1.08(0.264) | − | [92] | |||
| var. Golek | 0.055(0.003) | 1.237(0.626) | − | [92] | |||
| Spondias | dulcis L. | Hog plum | − | 0.201 | 0.364 | [91] | |
| Actinidiaceae | Actinidia | deliciosa C.F.Liang.& A.R.Ferguson. | Kiwifruit | ||||
| var. Hayward | ND | 0.074[0.021] | ND | [93] | |||
| var. Zespri gold | ND | 0.092[0.008] | ND | [93] | |||
| Bromeliaceae | Ananas | comosus (L.) Merr. | Pineapple | ND | 0.056[0.005] | ND | [93] |
| ND | 0.17 | ND | [94] | ||||
| Caricaceae | Carica | papaya L. | Papaya | ND | 0.23-1.981 | 1.477-5.75 | [65,94,95] |
| − | 1.05(0.44) | − | [90] | ||||
| ND | 0.276[0.245] | − | [44] | ||||
| var. Fruit tower | ND | 0.409[0.027] | 2.481[0.692] | [93] | |||
| var. Sun rise | ND | 1.981[0.059] | 1.477[0.302] | [93] | |||
| var. Yellow sweet | ND | 1.048[0.026] | 1.987[0.851] | [93] | |||
| var. Hawaiian | ND | 0.5 | 1.7 | [94] | |||
| Cucurbitaceae | Citrullus | lanatus (Thunb.) Matsum. & Nakai | Watermelon | 0-0.76 | 0.14-6.806 | 0.071-11.389 | [65,91,94,95] |
| ND | 0.59[0.033] | 6.184[0.152] | [93] | ||||
| Ebenaceae | Diospyros | sp. | Persimmon | − | 0.253 | − | [44] |
| ND | 0.129[0.003] | 0.415[0.013] | [93] | ||||
| Ericaceae | Vaccinium | spp. | Blueberries | ND | 0.035 | ND | [44] |
| ND | 0.027[0.005] | ND | [93] | ||||
| Malvaceae | Durio | zibethinus L. | Durian | 0.006 | 0.023 | − | [44] |
| Moraceae | Artocarpus | heterophyllus Lam. | Jackfruit | ND | 0.026-0.36 | 0.037 | [65,91,94,96] |
| − | 0.16(0.06) | − | [90] | ||||
| Musaceae | Musa | spp. | Banana | 0.005[0.005] | 0.021[0.014] | − | [44] |
| 0.058[0.007] | 0.058[0.006] | ND | [93] | ||||
| paradisiaca L.var. Ambon | Banana | − | 0.097 | 0.114 | [91] | ||
| sapientum Linn. | |||||||
| var. Emas | − | 0.04 | − | [65] | |||
| var. Tanduk | − | 0.092 | − | [65] | |||
| Myrtaceae | Psidium | guajava L. | Guava | − | 0.001 | 0.114 | [91] |
| − | 0.001 (0.0001) | − | [90] | ||||
| var. Pink | Pink guava | − | − | 5.4 | [95] | ||
| ND | 0.359[0.015] 5.027[0.08] | 2.307[0.058] 4.383[0.371] | [93] | ||||
| Oxalidaceae | Averrhoa | carambola L. | Starfruit | ND | 0.028-0.042 | 0-0.042 | [65,91,96] |
| ND | ND | ND | [93] | ||||
| Passifloraceae | Passiflora | edulis Sims | Passion fruit | 0.035 | 0.53 | − | [44] |
| ND | 0.156[0.02] | 0.057[0.003] | [93] | ||||
| Rosaceae | Eriobotrya | japonica(Thunb.) Lindl. | Loquat | − | 0.207 | − | [97] |
| Fragaria | ananassa Duchesne | Strawberry | 0.005 | − | − | [44] | |
| Malus | domestica Borkh. | Apple | 0.001-0.03 | 0.031-0.072 | 0.209 | [96,98,99] | |
| var. Fuji | ND | 0.036[0.003] | ND | [93] | |||
| Rosaceae | Prunus | armeniaca L. | Apricot | ND | 2.554 | 0.005 | [44] |
| salicina Lindl. | Nectarine | ||||||
| var. Red Jim | − | 0.073(0.016) | − | [100] | |||
| var. August red | − | 0.128(0.005) | − | [100] | |||
| var. Spring bright | − | 0.085(0.006) | − | [100] | |||
| var. May glo | − | 0.058(0.005) | − | [100] | |||
| var. September red | − | 0.131(0.023) | − | [100] | |||
| persica (L.) Batsch | Peach | 0.001[0.001] | 0.097[0.013] | − | [44] | ||
| var. Summer sweet | − | 0.04(0.01) | − | [100] | |||
| var. Snow king | − | 0.008(0.002) | − | [100] | |||
| var. Snow giant | − | 0.006(0.001) | − | [100] | |||
| var. Champagne | − | 0.007(0.001) | − | [100] | |||
| var. September snow | − | 0.004(0.001) | − | [100] | |||
| var. Hakuto | ND | 0.048[0.032] | ND | [93] | |||
| var. Kanto 5 go | ND | 0.036[0.006] | ND | [93] | |||
| var. Mochizuki | ND | ND | ND | [93] | |||
| var. Nishiki | ND | 0.16[0.005] | ND | [93] | |||
| var. Ogonto | ND | 0.121[0.008] | ND | [93] | |||
| domestica L. | Plum | − | 0.098 | − | [44] | ||
| var. Red | ND | 0.127 | ND | [65] | |||
| var. Wickson | − | 0.04(0.004) | − | [100] | |||
| var. Black Beaut | − | 0.188(0.017) | − | [100] | |||
| var. Red Beaut | − | 0.064(0.012) | − | [100] | |||
| var. Santa Rosa | − | 0.049(0.012) | − | [100] | |||
| var. Angeleno | − | 0.057(0.009) | − | [100] | |||
| var. Ponteroza | ND | 0.218[0.019] | ND | [93] | |||
| var. Soldam | ND | 0.439[0.029] | ND | [93] | |||
| Rosaceae | Prunus | spp. | Cherry | ND | ND | ND | [94] |
| − | 0.14(0.06) | − | [90] | ||||
| − | 0.028 | − | [44] | ||||
| var. Domestic | 0.018(0.004) | 0.071(0.004) | ND | [93] | |||
| var. USA | ND | 0.037(0.004) | ND | [93] | |||
| Pyrus | sp. | Pear | 0.006 | 0.027 | ND | [44] | |
| Rubus | sp. | Raspberry | 0.012 | 0.008 | − | [44] | |
| Rutaceae | Citrus | aurantium L. | Orange | − | 0.17(0.08) | − | [90] |
| maxima Merr. | Pummelo | 0.014 | 0.32 | − | [44] | ||
| microcarpa Bunge | Musk lime | − | 0.012 | − | [65] | ||
| nobilis L. | Orange | − | 0.025 | − | [65] | ||
| paradisiaca Macfad. | Grapefruit | ||||||
| var. Star ruby | ND | 0.452[0.019] | 1.869[0.654] | [93] | |||
| var. Pink | 0.005[0.005] | 0.603[0.152] | − | [44] | |||
| − | − | 3.36 | [95] | ||||
| var. White | 0.008 | 0.014 | − | [44] | |||
| sinensis (L.) Osbeck | Orange | 0.016 | 0.051 | − | [44] | ||
| var. Navel | 0.019[0.002] | 0.139[0.014] | ND | [93] | |||
| var. Valencia | 0.015[0.001] | 0.051[0.004] | ND | [93] | |||
| reticulata Blanco | Mandarin orange | − | 0.081 | − | [65] | ||
| ND | 0.03 | ND | [94] | ||||
| Sapindaceae | Nephelium | lappaceum L. | Rambutan | − | ND | 0.148 | [91] |
| Vitaceae | Vitis | vinifera Linnaeus | Grape | − | 0.039 | − | [44] |
| var. Deraware | ND | 0.058[0.004] | ND | [93] | |||
| Taxonomy | Common name | β-cryptoxanthin | Lutein | Zeaxanthin | References | ||
| Family | Genus | Species | |||||
| Anacardiaceae | Mangifera | indica L. | Mango | 0.137 | − | − | [91] |
| 0.011[0.009] | − | − | [44] | ||||
| var. Black-gold | ND | ND | − | [65] | |||
| Spondias | dulcis L. | Hog plum | 0.309 | − | − | [91] | |
| Actinidiaceae | Actinidia | deliciosa L. | Kiwifruit | ||||
| var. Hayward | ND | 0.153(0.005) | ND | [93] | |||
| var. Zespri gold | ND | 0.156(0.005) | 0.113(0.006) | [93] | |||
| Bromeliaceae | Ananas | comosus (L.) Merr. | Pineapple | 0.089 | ND | ND | [93] |
| Caricaceae | Carica | papaya L. | Papaya | 0.18-3.182 | 0.016-0.063 | 0.165-0.564 | [65,91,95] |
| 0.076[0.225] | 0.075c | − | [44] | ||||
| var. Fruit tower | 0.725[0.012] | 0.016[0.001] | 0.165[0.001] | [93] | |||
| var. Sun rise | 3.182[0.117] | 0.063[0.001] | 0.564[0.01] | [93] | |||
| var. Yellow sweet | 1.629[0.064] | 0.029[0.001] | 0.303[0.007] | [93] | |||
| var. Hawaiian | − | − | − | [94] | |||
| Cucurbitaceae | Citrullus | lanatus (Thunb.) Matsum. & Nakai | Watermelon | 0.09-0.48 | 0, 0.017c | ND | [65,91,95] |
| ND | ND | ND | [93] | ||||
| Ebenaceae | Diospyros | sp. | Persimmon | 1.45 | 0.834c | 0.49 | [44] |
| 0.52[0.02] | ND | 0.238[0.01] | [93] | ||||
| Ericaceae | Vaccinium | spp. | Blueberries | − | − | − | [44] |
| 0.011[0.006] | 0.042[0.011] | ND | [93] | ||||
| Malvaceae | Durio | zibethinus L. | Durian | ND | − | − | [44] |
| Moraceae | Artocarpus | heterophyllus Lam. | Jackfruit | 0.017-0.036 | 0.095 | − | [65,91,96] |
| Musaceae | Musa | spp. | Bananas | ND | NDc | − | [44] |
| ND | 0.113(0.008) | ND | [93] | ||||
| paradisiaca L.var. Ambon | 0.003 | − | − | [91] | |||
| Myrtaceae | Psidium | guajava L. | Guava | 0.012, 0.464 | 0.044 | ND | [91,95] |
| var. Pink | Pink guava | 0.012[0.003], 0.464[0.015] | 0.044[0.002] | ND | [93] | ||
| Oxalidaceae | Averrhoa | carambola L. | Starfruit | 0.036-1.066 | 0.066 | ND | [65,91,96] |
| ND | ND | ND | [93] | ||||
| Passifloraceae | Passiflora | edulis Sims. | Passion fruit | 0.046 | − | − | [44] |
| 0.027[0.001] | 0.042[0.002] | [93] | |||||
| Rosaceae | Eriobotrya | japonica(Thunb.) Lindl. | Loquat | 0.518 | − | − | [97] |
| Fragaria | ananassa Duchesne | Strawberry | − | − | − | [44] | |
| Malus | domestica Borkh. | Apple | 0.001-0.106 | 0.017 | 0.0019 | [91,96,99] | |
| var. Fuji | ND | ND | ND | [93] | |||
| Prunus | armeniaca L. | Apricot | ND | − | − | [44] | |
| salicina Lindl. | Nectarine | ||||||
| var. Red Jim | 0.014(0.005) | − | − | [100] | |||
| var. August red | 0.014(0.003) | − | − | [100] | |||
| var. Spring bright | 0.021(0.002) | − | − | [100] | |||
| var. May glo | 0.008(0) | − | − | [100] | |||
| var. September red | 0.015(0.006) | − | − | [100] | |||
| persica (L.) Batsch | Peach | 0.024 | 0.057c | − | [44] | ||
| var. Summer sweet | 0.012(0) | − | − | [100] | |||
| var. Snow king | ND | − | − | [100] | |||
| var. Snow gaint | ND | − | − | [100] | |||
| var. Champagne | ND | − | − | [100] | |||
| var. September snow | ND | − | − | [100] | |||
| Rosaceae | Prunus | persica (L.) Batsch | Peach | ||||
| var. Hakuto | ND | ND | ND | [93] | |||
| var. Kanto 5 go | 0.283[0.003] | ND | 0.51[0.015] | [93] | |||
| var. Mochizuki | 0.081[0.011] | ND | 0.028[0.002] | [93] | |||
| var. Nishiki | 0.074[0.003] | 0.051[0.005] | 0.116[0.005] | [93] | |||
| var. Ogonto | 0.025[0.008] | 0.029[0.002] | 0.104[0.002] | [93] | |||
| domestica L. | Plum | 0.016 | − | − | [44] | ||
| var. Red | 0.04 | 0.149 | − | [65] | |||
| var. Wickson | 0.05(0.01) | − | − | [100] | |||
| var. Black Beaut | 0.13(0.01) | − | − | [100] | |||
| var. Red Beaut | 0.03(0.01) | − | − | [100] | |||
| var. Santa Rosa | 0.07(0.03) | − | − | [100] | |||
| var. Angeleno | 0.03(0) | − | − | [100] | |||
| var. Ponteroza | 0.05[0.008] | 0.133[0.024] | 0.049[0.006] | [93] | |||
| var. Soldam | 0.077[0.009] | 0.207[0.011] | 0.026[0.002] | [93] | |||
| spp. | Cherry | − | − | − | [44] | ||
| var. Domestic | 0.021[0.001] | 0.112[0.008] | 0.042[0.005] | [93] | |||
| var. USA | 0.014[0.002] | 0.091[0.004] | 0.027[0.001] | [93] | |||
| Rutaceae | Citrus | maxima Merr. | Pummelo | 0.103 | − | − | [44] |
| paradise Macfad. | Grapefruit | ||||||
| var. Star ruby | ND | ND | ND | [93] | |||
| var. Pink | 0.012[0.009] | − | − | [44] | |||
| var. White | − | − | − | [44] | |||
| nobilis L. | Orange | − | 0.275 | ND | [91] | ||
| sinensis (L.) Osbeck | Orange | ||||||
| var. Navel | 0.462[0.031] | 0.059[0.006] | 0.164[0.013] | [93] | |||
| var. Valencia | 0.278[0.001] | 0.071[0.002] | 0.019[0.001] | [93] | |||
| 0.122 | 0.187c | − | [44] | ||||
| Sapindaceae | Nephelium | lappaceum L.var. Deraware | Rambutan | ND | − | − | [91] |
| ND | 0.103[0.014] | 0.028[0.004] | [93] | ||||
a ND, Not detected; − data not available; var., variety; b mean(standard deviation), mean[standard error]; c content of lutein + zeaxanthin
Table 3.
Carotenoid contents (mg/100 g fresh weight) of common leafy and non-leafy vegetables.
| Taxonomy | Common name | α-Carotene | β-Carotene | Lycopene | References | |||
|---|---|---|---|---|---|---|---|---|
| Family | Genus | Species | ||||||
| Leafy Vegetables | ||||||||
| Alliaceae | Allium | fistulosum L. | Spring onion leaves | − | 1.28 | − | [65] | |
| sativum L. | Garlic leaves | − | 5.0 | − | [42] | |||
| cepa L. | Onion leaves | − | 4.9(0.15) | − | [90] | |||
| Apiaceae | Apium | graveolens L. | Celery | ND | 0.77 | ND | [100] | |
| ND | 0.15 | − | [43] | |||||
| Coriandrum | sativum L. | Coriander leaves | ND | 3.17 | ND | [65] | ||
| Coriander | − | 4.8(0.16) | − | [90] | ||||
| Foeniculum | vulgare Mill. | Fennel common | − | 4.4 | − | [101] | ||
| Amaranthaceae | Amaranthus | spp. | Amaranth | − | 1.96-8.6 | − | [42,101,102] | |
| spinosus L. | Mulla thotakura | − | 10.9(1.25) | − | [90] | |||
| sp. | Yerramolakakaura | − | 11.9(1.48) | − | [90] | |||
| Spinacia | oleracea L. | Spinach | ND | 3.177, 36.53(6.4) | ND | [65,103] | ||
| var. Red | ND | 5.088 | ND | [65] | ||||
| − | 1.1(0.36) | − | [90] | |||||
| ND | 5.597[0.561] | − | [44] | |||||
| Asteraceae | Lactuca | sativa L. | Lettuce | ND | 0.097 | ND | [65] | |
| − | 1.4(0.28) | − | [90] | |||||
| var. Cos or Romaine | ND | 1.272 | − | [44] | ||||
| var. Iceberg | 0.002 | 0.192[0.069] | − | [44] | ||||
| Brassicaceae | Brassica | juncea (L.) Czern. | Chinese mustard leaves | ND | 2.93 | ND | [65] | |
| oleracea L. | ||||||||
| var. Acephala | Kale | ND | 9.23 | ND | [44] | |||
| var.Alboglabra | Chinese kale | ND | 4.09 | ND | [65] | |||
| var. Capitata | Cabbage | ND | 0.01-3.02 | ND | [44,101] | |||
| var. Chinensis | − | 2.703 | − | [65] | ||||
| var. Pekinensis | − | 0.01(0.01) | − | [104] | ||||
| papaya L. | Papaya leaves | 0.424(0.355) | 5.229(2.195) | [92] | ||||
| aquatica Forssk. | Swamp cabbage | ND | 1.895 | ND | [61] | |||
| Water spinach | 0.014(0.026) | 2.73 (1.013) | − | [92] | ||||
| Cucurbitaceae | Momordica | Charantia Descourt. | Bitter melon leaves | − | 3.4 | − | [101] | |
| Euphorbiaceae | Manihot | esculenta Crantz | Cassava leaves | 0.038(0.054) | 9.912(2.503) | − | [92] | |
| Fabaceae | Sesbania | grandiflora (L.) Poiret | Sesbania | ND | 13.61, 13.28(3.2) | ND | [65,103] | |
| Trigonella | foenum-graecum L. | Fenugreek | − | 9.2(1.48), 12.13(4.1) | − | [91,103] | ||
| Lamiaceae | Mentha | arvensis L. | Pudina | − | 4.3(2.0) | − | [90] | |
| Meliaceae | Azadirachta | indica L. | Neem tree leaves | − | 0.92 | − | [101] | |
| Moringaceae | Moringa | oleifera Lam. | Drumstick leaves | ND | 5.2, 7.54 | ND | [65,102] | |
| − | 19.7(5.55), 22.89(6.8) | − | [91,103] | |||||
| Phyllanthaceae | Sauropus | androgynus L. | Sweet shoot leaves | ND | 13.35 | ND | [65] | |
| 1.335(0.878) | 10.01(2.189) | − | [92] | |||||
| Solanaceae | Solanum | nigrum L. | Black nightshade | ND | 7.05 | ND | [65] | |
| Rutaceae | Murraya | koenigii (L.) Sprengel | Curry leaves | − | 7.1(2.36) | − | [90] | |
| Non-leafy Vegetables | ||||||||
| Alliaceae | Allium | schoenoprasum L. | Chive | ND | 0.83, 3.51 | ND | [42,65] | |
| Apiaceae | Daucus | carota L. | Carrot | 3.41-6.2 | 6.5-21 | ND | [44,65,93] | |
| Araceae | Colocasia | esculenta (L.) Schott | Taro | − | − | − | − | |
| Asparagaceae | Asparagus | officinalis L. | Asparagus | 0.012 | 0.493 | − | [44] | |
| Brassicaceae | Brassica | oleracea L. | ||||||
| var. Calabrese | Broccoli | − | 0.898 | − | [97] | |||
| 0.001[0.001] | 0.779[0.19] | − | [44] | |||||
| Brassicaceae | Brassica | var. Italica Plenck. | − | 0.81(0.2) | − | [103] | ||
| var. Gemmiferae | Brussels sprout | ND | 0.14 | ND | [42] | |||
| 0.006 | 0.45[0.057] | − | [44] | |||||
| − | 0.14(0.02) | − | [104] | |||||
| var. Botrytis | Cauliflower | − | 0.08 | − | [42] | |||
| − | 0.08(0.03) | − | [104] | |||||
| − | 6.5(1.46) | − | [90] | |||||
| Convolvulaceae | Ipomea | batatas (L.) Lam | Sweet potato | 0.002 | 0.058, 9.18 | ND | [44,92] | |
| − | 1.87(0.14) | − | [90] | |||||
| ND | 9.18[1.272] | − | [44] | |||||
| Cucurbitaceae | Coccinea | grandis (L.) J. Voigt | Ivy gourd | − | 3.2-4.1 | − | [42] | |
| Cucumis | sativus L. | Cucumber | 0.008 | 0.031-0.14 | ND | [44] | ||
| ND | ND | ND | [94] | |||||
| Cucurbita | maxima Duch. | Pumpkins | 0.03-7.5 | 0.06-14.85 | ND | [65,94] | ||
| (12 varieties) | 0-7.5 | 1.4-7.4 | − | [105] | ||||
| minima L. | − | 1.16(0.057) | − | [90] | ||||
| moschata Duch. | − | 9.29(7.5) | − | [106] | ||||
| (4 varieties) | 0.98-5.9 | 3.1-7.0 | − | [105] | ||||
| pepo L. (5 varieties) | 0.03-0.17 | 0.06-2.3 | − | [105] | ||||
| Momordica | charantia Descourt. | Bitter gourd | ND | ND | ND | [94] | ||
| Euphorbiaceae | Manihot | esculenta Crantz | Cassava | ND | 0.008 | − | [44] | |
| var. Monroe | ND | 0.52 | ND | [94] | ||||
| var. Beqa | ND | 0.43 | ND | [94] | ||||
| var. Common | ND | <0.02 | ND | [94] | ||||
| utilissima Pohl. | Tapioca shoot | ND | 5.72 | ND | [65] | |||
| Phaseolus | vulgaris L. | French bean | ND | 0.24 | ND | [65] | ||
| var. Red | Common Bean | 0.28 | 0.8 | ND | [94] | |||
| var. Yellow | ND | ND | − | [94] | ||||
| var. French | 0.72 | 0.78 | ND | [94] | ||||
| Vigna | unguiculata (L.) Walp. | |||||||
| subsp. unguiculata | Cow pea | − | − | − | [107] | |||
| subsp. sesquipedalis | Long bean | ND | 0.41-0.57 | ND | [65] | |||
| Malvaceae | Abelmoschus | esculentus (L.) Moench | Okra | 0.028 | 0.43 | − | [44] | |
| Marantaceae | Maranta | arundinacea L. | Arrowroot | ND | 0.01 | − | [44] | |
| Poaceae | Zea | mays L. | Maize | − | 0.014 | − | [44] | |
| (13 varieties) | 0.003-0.086 (0-0.009) | 0.037-0.879 (0-0.028) | − | [92] | ||||
| Solanaceae | Capsicum | annuum L. | ||||||
| var. Cayenne | Chilies | 3.41 | 0.47-6.77 | ND | [65] | |||
| var. Grossa | Capsicum | − | 1.13(0.8) | − | [90] | |||
| ND | 0.27 | ND | [65] | |||||
| sp. | Pepper | 0.022-0.059 | 0.2-2.38 | ND | [44] | |||
| − | 0.11 (0.04) | − | [91] | |||||
| Solanum | betaceum Cav. | Tree tomato | ND | 0.6 | ND | [65] | ||
| lycopersicum L. | Tomatoes | 2.5 | 0.365-1.3 | 0.009-2.0 | [65,94,95] | |||
| − | 0.62(0.19) | − | [90] | |||||
| melongena L. | Red eggplant | ND | ND | ND | [94] | |||
| tuberosum L. | Potato | − | 0.006 | − | [44] | |||
| Taxonomy | Common name | β-Cryptoxanthin | Lutein | Zeaxanthin | References | |||
| Family | Genus | Species | ||||||
| Leafy Vegetables | ||||||||
| Alliaceae | Allium | fistulosum Linnaeus | Spring onion leaves | ND | 0.323 | − | [65] | |
| Amaranthaceae | Spinacia | oleracea L. | Spinach | − | 77.58(6.6) | 1.51(0.4) | [103] | |
| Apiaceae | Apium | graveolens L. | Celery | ND | 0.23c | 0.003 | [44] | |
| Spinacia | oleracea L. | Spinach | ND | 4.175 | − | [65] | ||
| var. Red | ND | 2.047 | − | [65] | ||||
| ND | 11.938c | − | [44] | |||||
| Asteraceae | Lactuca | sativa L. | Lettuce | ND | 0.073 | − | [65] | |
| var. Cos or Romaine | ND | 2.635c | − | [44] | ||||
| var. Iceberg | ND | 0.352c | − | [44] | ||||
| Brassicaceae | Brassica | juncea (L.) Czern. | Chinese mustard | ND | 1.02 | − | [65] | |
| oleracea L. | ||||||||
| var. Acephala | Kale | ND | 39.55c | − | [44] | |||
| var. Alboglabra | Chinese kale | ND | 1.54 | − | [65] | |||
| var. Capitata | Cabbage | ND | 0.02, 0.31c | − | [44,101] | |||
| rapa L. | Chinese cabbage | |||||||
| var. Chinensis | − | 2.703 | − | [65] | ||||
| var. Pekinensis | − | 0.02(0.01) | − | [104] | ||||
| Convolvulaceae | lpomoea | aquatica Forssk. | Swamp cabbage | ND | 0.335 | − | [65] | |
| Fabaceae | Sesbania | grandiflora (L.) Poiret | Sesbania | ND | 20.21, 16.9(3.7) | 0.57(0.7) | [65,103] | |
| Moringaceae | Moringa | oleifera Lam. | Drumstick leaves | ND | 7.13, 50.4(0.8) | 4.13(0.7) | [102,103] | |
| Phyllanthaceae | Sauropus | androgynus L. | Sweet shoot leaves | ND | 29.91 | − | [65] | |
| Rutaceae | Murraya | koenigii (L.) Sprengel | Curry leaves | ND | 5.25 | − | [65] | |
| Solanaceae | Solanum | nigrum L. | Black nightshade | ND | 2.89 | − | [65] | |
| Non-leafy Vegetables | ||||||||
| Alliaceae | Allium | schoenoprasum L. | Chive | ND | 1.08 | − | [42,65] | |
| Apiaceae | Daucus | carota L. | Carrot | ND | ND | − | [44] | |
| Araceae | Colocasia | esculenta (L.) Schott | Taro | − | 0.16 | 0.006 | [107] | |
| Brassicaceae | Brassica | oleracea L. | ||||||
| var. Calabrese | Broccoli | ND | 1.28, 2.45c | [44,97] | ||||
| var. Italica Plenck | − | 0.68(0.22) | − | [104] | ||||
| var. Gemmiferae | Brussels sprout | ND | 0.43 | − | [42] | |||
| ND | 1.59C | − | [44] | |||||
| − | 0.43(0.06) | − | [104] | |||||
| var. Botrytis | Cauliflower | − | 0.13 | − | [42] | |||
| − | 0.05(0.02) | − | [104] | |||||
| Convolvulaceae | Ipomea | batatas (L.) Lam | Sweet potato | ND | ND | − | [44] | |
| Cucurbitaceae | Coccinea | grandis (L.) J. Voigt | Ivy gourd | − | 0.99 | ND | [107] | |
| Cucumis | sativus L. | Cucumber | − | 0.544 | 0.009 | [107] | ||
| Cucurbita | maxima Duch. | Pumpkins | ND | 0.94-17.0 | 0.278 | [65,107] | ||
| (12 varieties) | − | 0.8c-17.0c | − | [105] | ||||
| minima L. | − | 1.16(0.057) | − | [90] | ||||
| moschata Duch. | − | 9.29(7.5) | − | [106] | ||||
| (4 varieties) | − | 0.08c-1.1c | − | [105] | ||||
| pepo L. (5 varieties) | − | 0c-1.8c | − | [105] | ||||
| Euphorbiaceae | Manihot | esculenta Crantz | Cassava | ND | − | − | [44] | |
| var. Monroe | − | − | − | [94] | ||||
| var. Beqa | − | − | − | [94] | ||||
| var. Common | − | − | − | [94] | ||||
| utilissima Pohl. | Tapioca shoot | ND | 1.68c | − | [65] | |||
| Fabaceae | Phaseolus | vulgaris L. | French bean | ND | 0.171-0.46 | 0.02 | [65,107] | |
| Vigna | unguiculata (L.) Walp. | |||||||
| subsp. unguiculata | Cow pea | − | 0.24 | 0.009 | [107] | |||
| subsp. sesquipedalis | Long bean | ND | 0.3-0.42 | − | [65] | |||
| Malvaceae | Abelmoschus | esculentus (L.) Moench | Okra | − | 0.347 | 0.008 | [44] | |
| Marantaceae | Maranta | arundinacea L. | Arrowroot | ND | − | − | [44] | |
| Poaceae | Zea | mays L. (13 varieties) | Maize | 0.037-0.988 (0.001-0.015) | 0-2.047 (0-0.075) | 0.173-2.07 (0.004-0.073) | [92] | |
| Solanaceae | Capsicum | annum L. | ||||||
| var. Cayenne | Chilies | 1.75 | 0.39-1.902 | 0.063 | [65,107] | |||
| var. Grossa | Capsicum | − | 0.425 | 0.005 | [107] | |||
| spp. | Pepper | 2.21 | 0.22 | − | [44,65] | |||
| Solanum | betaceum Cav. | Tree tomato | 1.24 | ND | − | [65] | ||
| lycopersicum L. | Tomatoes | ND | 0.13-0.289 | 0.014 | [65,107] | |||
| melongena L. | Red eggplant | − | 0.065-1.8 | 0.005-0.016 | [107] | |||
| tuberosum L. | Potato | − | − | − | − | |||
| Phaseolus | vulgari L. | French bean | ND | 0.171-0.46 | 0.02 | [65,107] | ||
a ND, Not detected; − data not available; var., variety; b mean(standard deviation), mean[standard error]; c content of lutein + zeaxanthin
3.1. Orange and yellow pigment carotenoids
Naturally occurring β-carotene, with 11 double bonds, is orange in color [55]. Takyi [83] reported β-carotene occurs as an orange pigment, while α-carotene is a yellow pigment, which can be found in fruits and vegetables. Yellow colored fruits that contain low or trace amounts of β-carotene are mainly from the genera Ananas, Averrhoa, Citrus, Durio, Malus, Musa, Nephelium, Pyrus, Rubus and Vitis or vegetables from the genera Apium, Cucumis, Manihot, Vigna and Maranta (Table 2 and Table 3). Besides, yellow maize (Zea mays L.) is a good source of β-carotene [88].
Several vegetables are known to contain β-carotene. For example, β-carotene is present in carrot, sweet potato and tomato which are from the genera of Daucus, Ipomea and Solanum, respectively. Carrot is the major contributor of β-carotene in the diet, along with green leafy vegetables. Rajyalakshmi et al. [89] reported that the β-carotene contents in 70 edible wild green leafy vegetables ranged from 0.4–4.05 mg per 100 gram edible portion. A few underutilized green leafy vegetables from India were also found to have 0.68–12.6 mg/100 g β-carotene [90]. Therefore, other than carotene-rich yellow-orange colored vegetables (e.g., carrot, pumpkin and sweet potato), green leafy vegetables are good sources of β-carotene. The β-carotene contents of some green leafy vegetable grown in the wild such as black nightshade (Solanum nigrum) and Mulla thotakura (Amaranthus spinosus) are comparable to carrot or sweet potato (Table 3).
Orange colored fruits such as apricot (Prunus armeniaca L.), grapefruit (Citrus paradise Macfad.), mango (Mangifera indica L.), papaya (Carica papaya L.), persimmon (Diospyros sp.), pink guava (Psidium guajava L. var. Pink) and watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai] are rich in β-carotene. Khoo et al. [108] reported that orange colored underutilized fruits contained high amount of β-carotene. Although papaya is orange in color, certain cultivars have shown to contain low β-carotene [93,94]. Furthermore, Levy et al. [98] reported some of the orange colored fruits had low amount of β-carotene.
Naturally, most of xanthophylls are yellow-orange colored pigments, especially lutein and zeaxanthin which can be found in most of the fruits and vegetables [82]. As lutein can absorb blue light, it appears as yellow color; while zeaxanthin appears yellow-orange color. Cryptoxanthins are other types of yellow-orange colored carotenoids. Takyi [82] reported α-cryptoxanthin appears as yellow colored pigment, while β-cryptoxanthin is orange in color. As shown in Table 3, lutein is found to be in higher amounts in green leafy and yellow colored non-leafy vegetables as compared to fruits.
Green leafy vegetables that contain high amount of xanthophylls are mainly from the genera of Brassica, Coriandrum, Lactuca, Moringa, Murraya, Sauropus, Sesbania, Solanum and Spinacia, while the non-leafy vegetables are from the genera of Allium, Brassica, Capsicum, Cucurbita and Zea (Table 3). These green vegetables contain mainly lutein and zeaxanthin [44,65]. Kale (Brassica oleracea L. var. Acephala), lettuce (Lactuca sativa L. var. Cos or Romaine), Sesbania (Sesbania grandiflora L. Poiret), spinach (Spinicia oleracea L. var. Red) and sweet shoot leaves (Sauropus androgynus L.) are the example of the leafy vegetables that have high lutein content (Table 3); while other lutein-rich non-leafy vegetables are red eggplant (Solanum melongena L.), chili (Capsicum annum L. var. Cayenne), ivy gourd [Coccinea grandis (L.) J. Voigt], and pumpkin (Cucurbita maxima Duch.) [107].
Muzhingi et al. [88] reported that 36 genotypes of yellow maizes (Zea mays L.) contained lutein and zeaxanthin, whereas saponification significantly decreased the xanthophyll contents. In some cases, zeaxanthin-rich fruits [e.g., papaya (Carica papaya L.) and persimmon (Diospyros sp.)] and zeaxanthin-rich non-leafy vegetables [e.g. pumpkin (Cucurbita maxima Duch.) and maize (Zea mays L.)] were found to have high amount of β-carotene (Table 2 and Table 3). Cryptoxanthin is another yellow colored carotenoid, which is closely related to carotene [9,82]. Cryptoxanthin has approximately half of provitamin A activity as compared to β-carotene [109]. Cryptoxanthins have been identified in various types of fruits and vegetables [9,82,110]. Besides, the level of β-cryptoxanthin is high in fruits such as papaya (Carica papaya L.), persimmon (Diospyros sp.) and starfruit (Averrhoa carambola L.) (Table 2) and non-leafy vegetables such as chili (Capsicum annum L. var. Cayenne), maize (Zea mays L.), pepper (Capsicum sp.) and tree tomato (Solanum betaceum Cav.) (Table 3).
3.2. Red pigment carotenoids
Lycopene is one of the naturally occurring red colored carotenoids [58]. The all-trans-isomer of lycopene is the most predominant geometrical isomer in fruits and vegetables [111]. Lycopene has two more double bonds than β-carotene, hence it appears red. Beside lycopene, δ-carotene pigment is red-orange in color, while astaxanthin is a red colored pigment [82].
This review shows that red lycopene pigment is abundant in fruits such as papaya (Carica papaya L.), pink grapefruit (Citrus paradise Macfad. var. Pink), pink guava (Psidium guajava L. var. Pink) and watermelon [Citrullus lanatus(Thunb.) Matsum. & Nakai] [93]. For non-leafy vegetables, USDA database [112] showed that raw red cabbage (Brassica oleracea var. Capitata) and boiled asparagus (Asparagus officinalis L.) contained 20 and 30 μg lycopene per 100 g edible portions. Red colored pigments in fruits and vegetables are believed to be originated from lycopene, which might also account for xanthophylls [113,114,115] and anthocyanins [116,117].
In fruits, dry persimmon (Diospyros sp.) contains the highest amount of lycopene (53.21 mg/100 g dry weight), which is two times higher than dry tomato [118]. A review by Bramley [95] has shown that pink guava and watermelon had comparable amounts of lycopene, which are even higher than fresh tomato (Table 2 and Table 3). Lycopene content in tomato products such as tomato ketchup is 5.5-time higher than in fresh ripe tomato [44]. Lycopene content in fresh tomato is influenced by the cultivars, agricultural practices, maturity and environmental factors [61]. Besides, mutant tomatoes have an almost two-fold increase in lycopene content [119].
4. Conclusions
Carotenoids are colorful pigments found in fruits and vegetables. The geometric isomers of carotenoids are present in all-trans and cis forms, together with carotenoid epoxides. Although all-trans-isomer is the major form of carotenoid, the cis isomers are available in small quantities. Heating and thermal processing could increase the amount of carotenoid cis-isomers. The degradation of carotenoids in fruits and vegetables is a major issue due to carotenoid loss. More attention should be given to the control of carotenoid geometry isomer degradation, and to improve the quality of dietary carotenoids. The color changes during geometric isomerization of carotenoids, thermal processing and even fruit ripening have been widely studied. However, there is still lack of information on the chemical and kinetic pathways of color changes during carotenoid degradation and isomerization. In the future, more studies are needed to focus on the isomerization of carotenoids in relation to the colorful pigments in the biodiversity of fruits and vegetables.
Footnotes
Sample Availability: Samples are available from the authors.
References and Notes
- 1. WHO . World Health Organization; Geneva, Switzerland: 2003. [(accessed on 3 May 2010)]. Fruit, Vegetables and NCD Disease Prevention. Available online: http://www.who.int/hpr/NPH/fruit_and_vegetables/fruit_vegetables_fs.pdf, [Google Scholar]
- 2.Cooper D.A. Carotenoids in health and disease: Recent scientific evaluations, research recommendations and the consumer. J. Nutr. 2004;134:221–224. doi: 10.1093/jn/134.1.221S. [DOI] [PubMed] [Google Scholar]
- 3.Young C.Y.F., Yuan H.Q., He M.L., Zhang J.Y. Carotenoids and prostate cancer risk. Mini-Rev. Med. Chem. 2008;8:529–537. doi: 10.2174/138955708784223495. [DOI] [PubMed] [Google Scholar]
- 4.Shahidi F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009;20:376–387. doi: 10.1016/j.tifs.2008.08.004. [DOI] [Google Scholar]
- 5.Bartley G.E., Scolnik P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell. 1995;7:1027–1038. doi: 10.1105/tpc.7.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janik E., Grudziński W., Gruszecki W.I., Krupa Z. The xanthophyll cycle pigments in Secale cereale leaves under combined Cd and high light stress conditions. J. Photochem. Photobiol. B. 2008;90:47–52. doi: 10.1016/j.jphotobiol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster J.E., Lister C.E., Reay P.F., Triggs C.M. Influence of pigment composition on skin color in a wide range of fruit and vegetables. J. Am. Soc. Hortic. Sci. 1997;122:594–598. [Google Scholar]
- 8.Rodriguez-Amaya D.B., Kimura M. HarvestPlus Technical Monograph, Series 2. International Food Policy Research Institute and International Center for Tropical Agriculture; Washington, DC, USA: 2004. HarvestPlus Handbook for Carotenoid Analysis. [Google Scholar]
- 9.Rodriguez-Amaya D.B. A Guide to Carotenoid Analysis in Foods. International Life Sciences Institute, ILSI Press; Washington, DC, USA: 2001. [Google Scholar]
- 10.Hornero-Méndez D., Mínguez-Mosquera M.I. Xanthophyll esterification accompanying carotenoid overaccumulation in chromoplast of Capsicum annuum ripening fruits is a constitutive process and useful for ripeness index. J. Agric. Food Chem. 2000;48:1617–1622. doi: 10.1021/jf9912046. [DOI] [PubMed] [Google Scholar]
- 11.Minguez-Mosquera M.I., Hornero-Mendez D. Changes in carotenoid esterification during the fruit ripening of Capsicum annuum Cv. Bola. Bola. J. Agric. Food Chem. 1994;42:640–644. doi: 10.1021/jf00039a007. [DOI] [Google Scholar]
- 12.Schieber A., Carle R. Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci. Technol. 2005;16:416–422. doi: 10.1016/j.tifs.2005.03.018. [DOI] [Google Scholar]
- 13.Mertz C., Brat P., Caris-Veyrat C., Gunata Z. Characterization and thermal lability of carotenoids and vitamin C of tamarillo fruit (Solanum betaceum Cav.) Food Chem. 2010;119:653–659. [Google Scholar]
- 14.Zepka L.Q., Mercadante A.Z. Degradation compounds of carotenoids formed during heating of a simulated cashew apple juice. Food Chem. 2009;117:28–34. doi: 10.1016/j.foodchem.2009.03.071. [DOI] [Google Scholar]
- 15.Shi J., Yi C., Ye X., Xue S., Jiang Y., Ma Y., Liu D. Effects of supercritical CO2 fluid parameters on chemical composition and yield of carotenoids extracted from pumpkin. LWT – Food Sci. Technol. 2010;43:39–44. [Google Scholar]
- 16.Niedzwiedzki D.M., Sandberg D.J., Cong H., Sandberg M.N., Gibson G.N., Birge R.R., Frank H.A. Ultrafast time resolved absorption spectroscopy of geometric isomers of carotenoids. Chem. Phys. 2009;357:4–16. doi: 10.1016/j.chemphys.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu D., Chen Z.-R., Li H.-R. Effect of heating on solid β-carotene. Food Chem. 2009;112:344–349. doi: 10.1016/j.foodchem.2008.05.071. [DOI] [Google Scholar]
- 18.Liu R.S.H., Asato A.E. The primary process of vision and the structure of bathorhodopsin: a mechanism for photoisomerization of polyenes. Proc. Natl. Acad. Sci. USA. 1985;82:259–263. doi: 10.1073/pnas.82.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton G. Overview of carotenoid biosynthesis. In: Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids: Biosynthesis and Metabolism. Birkhäuser Verlag; Basel, Switzerland: 1998. pp. 13–147. [Google Scholar]
- 20.Vásquez-Caicedo A.L., Sruamsiri P., Carle R., Neidhart S. Accumulation of all-trans-β-carotene and its 9-cis and 13-cis stereoisomers during postharvest ripening of nine Thai mango cultivars. J. Agric. Food Chem. 2005;53:4827–4835. doi: 10.1021/jf048168h. [DOI] [PubMed] [Google Scholar]
- 21.Aman R., Schieber A., Carle R. Effects of heating and illumination on trans-cis isomerization and degradation of β-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 2005;53:9512–9518. doi: 10.1021/jf050926w. [DOI] [PubMed] [Google Scholar]
- 22.Rickman J.C., Bruhn C.M., Barrett D.M. Nutritional comparison of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. J. Sci. Food Agric. 2007;87:1185–1196. [Google Scholar]
- 23.Parker R. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10:542–551. [PubMed] [Google Scholar]
- 24.De Rigal D., Gauillard F., Richard-Forget F. Changes in the carotenoid content of apricot (Prunus armeniaca, var Bergeron) during enzymatic browning: β-carotene inhibition of chlorogenic acid degradation. J. Sci. Food Agric. 2000;80:763–768. doi: 10.1002/(SICI)1097-0010(20000501)80:6<763::AID-JSFA623>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Bohm V., Puspitasari-Nienaber N.L., Ferruzzi M.G., Schwartz S.J. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002;50:221–226. doi: 10.1021/jf010888q. [DOI] [PubMed] [Google Scholar]
- 26.Chen B.H., Tang Y.C. Processing and stability of carotenoid powder from carrot pulp waste. J. Agric. Food Chem. 1998;46:2312–2318. doi: 10.1021/jf9800817. [DOI] [Google Scholar]
- 27.Goula A.M., Adamopoulos K.G., Chatzitakis P.C., Nikas V.A. Prediction of lycopene degradation during a drying process of tomato pulp. J. Food Eng. 2006;74:37–46. doi: 10.1016/j.jfoodeng.2005.02.023. [DOI] [Google Scholar]
- 28.Mordi R.C., Walton J.C., Burton G.W., Hughes L., Keith I.U., David L.A., Douglas M.J. Oxidative degradation of β-carotene and β-apo-8'-carotenal. Tetrahedron. 1993;49:911–928. [Google Scholar]
- 29.Wacheä Y., Bosser-Deratuld A.L., Lhuguenot J.-C., Belin J.-M. Effect of cis/trans isomerism of β-carotene on the ratios of volatile compounds produced during oxidative degradation. J. Agric. Food Chem. 2003;51:1984–1987. doi: 10.1021/jf021000g. [DOI] [PubMed] [Google Scholar]
- 30.Lin C.H., Chen B.H. Determination of carotenoids in tomato juice by liquid Chromatography. J. Chromatogr. A. 2003;1012:103–109. doi: 10.1016/S0021-9673(03)01138-5. [DOI] [PubMed] [Google Scholar]
- 31.Britton G. UV/Visible Spectroscopy. In: Brotton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids Spectroscopy. Birkhäuser Verlag; Basel, Switzerland: 1995. [Google Scholar]
- 32.Krinsky N.I. Carotenoid protection against oxidation. Pure Appl. Chem. 1979;51:649–660. doi: 10.1351/pac197951030649. [DOI] [Google Scholar]
- 33.Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 34.Marx M., Schieber A., Carle R. Quantitative determination of carotene stereoisomers in carrot juices and vitamin supplemented (ATBC) drinks. Food Chem. 2000;70:403–408. doi: 10.1016/S0308-8146(00)00096-0. [DOI] [Google Scholar]
- 35.Khoo H.-E., Prasad K.N., Ismail A., Mohd-Esa N. Carotenoids from Mangifera pajang and their antioxidant capacity. Molecules. 2010;15:6699–6712. [Google Scholar]
- 36.De Faria A.F., Hasegawa P.N., Chagas E.A., Pio R., Purgatto E., Mercadante A.Z. Cultivar influence on carotenoid composition of loquats from Brazil. J. Food Compos. Anal. 2009;22:196–203. doi: 10.1016/j.jfca.2008.10.014. [DOI] [Google Scholar]
- 37.Chen J.P., Tai C.Y., Chen B.H. Improved liquid chromatographic method for determination of carotenoids in Taiwanese mango (Mangifera indica L.) J. Chromatogr. A. 2004;1054:261–268. [PubMed] [Google Scholar]
- 38.Chen J.P., Tai C.Y., Chen B.H. Effects of different drying treatments on the stability of carotenoids in Taiwanese mango (Mangifera indica L.) Food Chem. 2007;100:1005–1010. [Google Scholar]
- 39.Lessin W.J., Schwartz S.J. Quantification of cis-trans isomers of provitamin A carotenoids in fresh and processed fruits and vegetables. J. Agric. Food Chem. 1997;45:3728–3732. doi: 10.1021/jf960803z. [DOI] [Google Scholar]
- 40.Li F., Murillo C., Wurtzel E.T. Maize Y9 encodes a product essential for 15-cis-ζ-carotene isomerization. Plant Physiol. 2007;144:1181–1189. doi: 10.1104/pp.107.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulshof P.J.M., Kosmeijer-Schuil T., West C.E., Hollman P.C.H. Quick screening of maize kernels for provitamin A content. J. Food Compos. Anal. 2007;20:655–661. doi: 10.1016/j.jfca.2006.04.014. [DOI] [Google Scholar]
- 42.Rodriguez-Amaya D.B. The Retention of Provitamin A Carotenoids in Prepared, Processed, and Stored Foods. John Snow Inc; Rio de Janeiro, Brazil: 1997. Carotenoids and Food Preparation. [Google Scholar]
- 43.During A., Smith M.K., Piper J.B., Smith J.C. Carotene 15,15’-dioxygenase activity in human tissues and cells: Evidence of an iron dependency. J. Nutr. Biochem. 2001;12:640–647. doi: 10.1016/S0955-2863(01)00184-X. [DOI] [PubMed] [Google Scholar]
- 44.Holden J.M., Eldridge A.L., Beecher G.R., Buzzard I.M., Bhagwat A.S., Davis C.S., Douglass L.W., Gebhardt E.S., Haytowitz D., Schakel S. Carotenoid content of U.S. foods: An update of the database. J. Food Compos. Anal. 1999;12:169–196. [Google Scholar]
- 45.Guo W.-H., Tu C.-Y., Hu C.-H. Cis-trans isomerizations of β-carotene and lycopene: A theoretical study. J. Phys. Chem. 2008;112:12158–12167. doi: 10.1021/jp8019705. [DOI] [PubMed] [Google Scholar]
- 46.ESA . Carotenoid Isomers. ESA Application Note, 5600A. ESA Inc; Chelmsford, MA, USA: 2009. [(accessed on 7 October 2009)]. Available online: http://www.esainc.com. [Google Scholar]
- 47.Kuki M., Koyama Y., Nagae H. Triplet-sensitized and thermal isomerization of all-trans, 7-cis, 9-cis, 13-cis and 15-cis isomers of β-carotene: Configurational dependence of the quantum yield of isomerization via the T1 state. J. Phys. Chem. 1991;95:7171–7180. [Google Scholar]
- 48.Vásquez-Caicedo A.L., Schilling S., Carle R., Neidhart S. Effects of thermal processing and fruit matrix on beta-carotene stability and enzyme inactivation during transformation of mangoes into purée and nectar. Food Chem. 2007;102:1172–1186. doi: 10.1016/j.foodchem.2006.07.005. [DOI] [Google Scholar]
- 49.Lozano-Alejo N., Carrillo G.V., Pixley K., Palacios-Rojas N. Physical properties and carotenoid content of maize kernels and its nixtamalized snacks. Innov. Food Sci. Emerg. Technol. 2007;8:385–389. doi: 10.1016/j.ifset.2007.03.015. [DOI] [Google Scholar]
- 50.Tang Y.C, Chen B.H. Pigment change of freeze-dried carotenoid powder during storage. Food Chem. 2000;69:11–17. [Google Scholar]
- 51.Chen B.H., Huang J.H. Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chem. 1998;62:299–307. doi: 10.1016/S0308-8146(97)00201-X. [DOI] [Google Scholar]
- 52.Marx M., Stuparic M., Schieber A., Carle R. Effects of thermal processing on trans-cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chem. 2003;83:609–617. doi: 10.1016/S0308-8146(03)00255-3. [DOI] [Google Scholar]
- 53.Breitenbach J., Sandmann G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta. 2005;220:785–793. doi: 10.1007/s00425-004-1395-2. [DOI] [PubMed] [Google Scholar]
- 54.Castenmiller J.J.M., West C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998;18:19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- 55.Rao A.V., Rao L.G. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Boileau T.W.M., Boileau A.C., Erdman J.W., Jr. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 57.Schulz H., Baranska M., Baranski R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers. 2005;77:212–221. doi: 10.1002/bip.20215. [DOI] [PubMed] [Google Scholar]
- 58.Kong K.-W., Khoo H.-E, Prasad K.N., Ismail A., Tan C.-P., Rajab N.F. Revealing the power of the natural red pigment lycopene. Molecules. 2010;15:959–987. doi: 10.3390/molecules15020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanquet-Diot S., Soufi M., Rambeau M., Rock E., Alric M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J. Nutr. 2009;139:876–883. doi: 10.3945/jn.108.103655. [DOI] [PubMed] [Google Scholar]
- 60.Failla M.L., Chitchumroonchokchai C., Ishida B.K. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J. Nutr. 2008;138:482–486. doi: 10.1093/jn/138.3.482. [DOI] [PubMed] [Google Scholar]
- 61.Shi J., Maguer M.L., Bryan M. Lycopene from Tomatoes. In: Shi J., Maguer M.L., Bryan M.L., editors. Functional Food: Biochemical & Processing Aspects. Vol. 2. CRC Press LCC; Danvers, MA, USA: 2002. pp. 135–167. [Google Scholar]
- 62.Moraru C., Lee T.-C. Lycopene isomerization at gastric pH; Nutraceuticals & Functional Foods Session of IFT Annual Meeting. Las Vegas, NV, USA; Jul 12–16, 2004. [Google Scholar]
- 63.Lee M.T., Chen B.H. Stability of lycopene during heating and illumination in a model system. Food Chem. 2002;78:425–432. doi: 10.1016/S0308-8146(02)00146-2. [DOI] [Google Scholar]
- 64.Tyssandier V., Reboul E., Dumas J.-F., Bouteloup-Demange C., Armand M., Marcand J., Sallas M., Borel P. Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am. J. Physiol-Gastr. L. 2003;284:G913–G923. doi: 10.1152/ajpgi.00410.2002. [DOI] [PubMed] [Google Scholar]
- 65.Tee E.-S., Lim C.-L. Carotenoid composition and content of Malaysian vegetables and fruits by the AOAC and HPLC methods. Food Chem. 1991;41:309–339. doi: 10.1016/0308-8146(91)90057-U. [DOI] [Google Scholar]
- 66.Losso J.N., Khachatryan A., Ogawa M., Godber J.S., Shih F. Random centroid optimization of phosphatidylglycerol stabilized lutein-enriched oil-in-water emulsions at acidic pH. Food Chem. 2005;92:737–744. doi: 10.1016/j.foodchem.2004.12.029. [DOI] [Google Scholar]
- 67.Matsuno T., Hirono T., Ikuno Y., Maoka T., Shimizu M., Komori T. Isolation of three new carotenoids and proposed metabolic pathways of carotenoids in hen's egg yolk. Comp. Biochem. Physiol. B. 1986;84:477–481. doi: 10.1016/0305-0491(86)90110-0. [DOI] [PubMed] [Google Scholar]
- 68.Handelman G.J. The evolving role of carotenoids in human biochemistry. Nutrition. 2001;17:818–822. doi: 10.1016/S0899-9007(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 69.Emenhiser C., Sande L.C., Schwartza S.J. Capability of a polymeric C30 stationary phase to resolve cis-trans carotenoid isomers in reversed-phase liquid chromatography. J. Chromatogr. A. 1995;707:205–216. doi: 10.1016/0021-9673(95)00336-L. [DOI] [Google Scholar]
- 70.Sander L.C., Sharpless K.E., Pursch M. C30 stationary phases for the analysis of food by liquid chromatography. J. Chromatogr. A. 2000;880:189–202. doi: 10.1016/S0021-9673(00)00121-7. [DOI] [PubMed] [Google Scholar]
- 71.Tóth G., Szabolcs J. Occurrence of some mono-cis-isomers of asymmetric C40-carotenoids. Phytochem. 1981;20:2411–2415. [Google Scholar]
- 72.Meléndez-Martínez A.J., Vicario I.M., Heredia F.J. Geometrical isomers of violaxanthin in orange juice. Food Chem. 2007;104:169–175. doi: 10.1016/j.foodchem.2006.11.017. [DOI] [Google Scholar]
- 73.Gross J. Pigments in Fruits: Food Science and Technology. Academic Press; Orlando, FL, USA: 1987. [Google Scholar]
- 74.Meléndez-Martínez A.J., Britton G., Vicario I.M., Heredi F.J. HPLC analysis of geometrical isomers of lutein epoxide isolated from dandelion (Taraxacum officinale F. Weber ex Wiggers) Phytochemistry. 2006;67:771–777. doi: 10.1016/j.phytochem.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Milanowska J., Gruszecki W.I. Heat-induced and light-induced isomerization of the xanthophyll pigment zeaxanthin. J. Photochem. Photobiol. B. 2005;80:178–186. doi: 10.1016/j.jphotobiol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Kishimoto S., Maoka T., Nakayama M., Ohmiya A. Carotenoid composition in petals of chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura) Phytochemistry. 2004;65:2781–2787. doi: 10.1016/j.phytochem.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 77.Yahia E.M., Ornelas-Paz J.J., Gardea A. Extraction, separation and partial identification of ‘Ataulfo’ mango fruit carotenoids. Acta Hortic. 2006;712:333–338. [Google Scholar]
- 78.Furr H.C., Clark R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997;8:364–377. doi: 10.1016/S0955-2863(97)00060-0. [DOI] [Google Scholar]
- 79.Scott K.J., Thurnham D.I., Hart D.J., Bingham S.A., Day K. The correlation between the intake of lutein, lycopene and β-carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50-65 years in the UK. Brit. J. Nutr. 1996;75:409–418. doi: 10.1079/bjn19960143. [DOI] [PubMed] [Google Scholar]
- 80.Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Simpson K.L. Chemical Changes in Natural Food Pigments. In: Richardson T., Finley J.W., editors. Chemical Changes in Food during Processing. Van Nostrand Reinhold Company, Inc.; New York, NY, USA: 1985. pp. 409–437. [Google Scholar]
- 82.Minguez-Mosquera M.I., Gandul-Rojas B., Garrido-Fernandez J., Gallardo-Guerrero L. Pigments present in virgin olive oil. J. Am. Oil Chem. Soc. 1990;67:192–196. doi: 10.1007/BF02539624. [DOI] [Google Scholar]
- 83.Takyi E.E.K. Bioavailability of Carotenoids from Vegetables versus Supplements. In: Watson R.R., editor. Vegetables, Fruits, and Herbs in Health Promotion. CRC Press LCC; Danvers, MA, USA: 2001. pp. 19–31. [Google Scholar]
- 84.Marín A., Ferreres F., Tomas-Barberan F.A., Gil M.I. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.) J. Agric. Food Chem. 2004;52:3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- 85.Markus F., Daood H.G., Kapitany J., Biacs P.A. Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J. Agric. Food Chem. 1999;47:100–107. doi: 10.1021/jf980485z. [DOI] [PubMed] [Google Scholar]
- 86.Wieruszewski J.B. Simon Fraser University; Ottawa, Canada: 2002. Astaxanthin bioavailabity, retention efficiency and kinetics in Atlantic salmon (Salmo salar) as influenced by pigment concentration and method of administration (kinetics only) Master Thesis. [Google Scholar]
- 87.De Pee S., West C.E., Permaesih D., Martuti S., Muhilal , Hautvast J.G.A.J. Increasing intake of orange fruits is more effective than increasing intake of dark-green leafy vegetables in increasing serum concentrations of retinol and β-carotene in schoolchildren in Indonesia. Am. J. Clin. Nutr. 1998;68:1058–1067. doi: 10.1093/ajcn/68.5.1058. [DOI] [PubMed] [Google Scholar]
- 88.Muzhingi T., Yeum K.J., Russell R.M., Johnson E.J., Qin J., Tang G. Determination of carotenoids in yellow maize, the effects of saponification and food preparations. Intl. J. Vit. Nutr. Res. 2008;78:112–120. doi: 10.1024/0300-9831.78.3.112. [DOI] [PubMed] [Google Scholar]
- 89.Rajyalakshmi P., Venkatalaxmi K., Venkatalakshmamma K., Jyothsna Y., Devi K.B., Suneetha V. Total carotenoid and beta-carotene contents of forest green leafy vegetables consumed by tribals of south India. Plant Food Hum. Nutr. 2001;56:225–238. doi: 10.1023/A:1011125232097. [DOI] [PubMed] [Google Scholar]
- 90.Bhaskarachary K., Rao D.S.S., Deosthale Y.G., Reddy V. Carotene content of some common and less familiar foods of plant origin. Food Chem. 1995;54:189–193. doi: 10.1016/0308-8146(95)00029-I. [DOI] [Google Scholar]
- 91.Setiawan B., Sulaeman A., Giraud D.W., Driskell J.A. Carotenoid content of selected Indonesian fruits. J. Food Compos. Anal. 2001;14:169–176. doi: 10.1006/jfca.2000.0969. [DOI] [Google Scholar]
- 92.Hulshof P.J.M., Chao X., Van De Bovenkamp P., Muhilal, West C.E. Application of a validated method for the determination of provitamin A carotenoids in Indonesia foods of different maturity and origin. J. Agric. Food Chem. 1997;45:1174–1179. doi: 10.1021/jf9603137. [DOI] [Google Scholar]
- 93.Yano M., Kato M., Ikoma Y., Kawasaki A., Fukazawa Y., Sugiura M., Matsumoto H., Oohara Y., Nagao A., Ogawa K. Quantitation of carotenoids in raw and processed fruits in Japan. Food Sci. Technol. Res. 2005;11:13–18. [Google Scholar]
- 94.Lako J., Trenerry V.C., Wahlqvist M., Wattanapenpaiboon N., Sotheeswaran S., Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- 95.Bramley P.M. Is lycopene beneficial to human health? Phytochem. 2000;54:233–236. doi: 10.1016/s0031-9422(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 96.Charoensiri R., Kongkachuichai R., Suknicom S., Sungpuag P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009;113:202–207. doi: 10.1016/j.foodchem.2008.07.074. [DOI] [Google Scholar]
- 97.Granado-Lorencio F., Olmedilla-Alonso B., Herrero-Barbudo C., Blanco-Navarro I., Pérez-Sacristán B., Blázquez-García S. In vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007;102:641–648. doi: 10.1021/jf070301t. [DOI] [PubMed] [Google Scholar]
- 98.Levy A., Harel S., Palevitch D., Akiri B., Menagem E., Kanner J. Carotenoid pigments and β-carotene in paprika fruits (Capsicum spp.) with different genotypes. J. Agric. Food Chem. 1995;43:362–366. [Google Scholar]
- 99.Dias M.G., Filomena M., Camões G.F.C., Oliveira L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009;113:808–815. doi: 10.1016/j.foodchem.2008.08.002. [DOI] [Google Scholar]
- 100.Gil M.I., Tomás-Barberán F.A., Hess-Pierce B., Kader A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- 101.Speek A.J., Speek-Saichua S., Schreurs W.H.P. Total carotenoids and β-carotene contents of Thai vegetables and effects of processing. Food Chem. 1988;27:245–251. doi: 10.1016/0308-8146(88)90010-6. [DOI] [Google Scholar]
- 102.Begum A., Pereira S.M. The β-carotene content of Indian edible green leaves. Trop. Geogr. Med. 1977;29:47–50. [PubMed] [Google Scholar]
- 103.Lakshminarayana R., Raju M., Krishnakantha T.P., Baskaran V. Determination of major carotenoids in a few Indian leafy vegetables by high-performance liquid chromatography. J. Agric. Food Chem. 2005;53:2838–2842. doi: 10.1021/jf0481711. [DOI] [PubMed] [Google Scholar]
- 104.Singh J., Upadhyay A.K., Prasad K., Bahadur A., Rai M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007;20:106–112. doi: 10.1016/j.jfca.2006.08.002. [DOI] [Google Scholar]
- 105.Murkovic M.U., Mülleder U., Neunteufl H. Carotenoid content in different varieties of pumpkins. J. Food Comp. Anal. 2002;15:633–638. doi: 10.1006/jfca.2002.1052. [DOI] [Google Scholar]
- 106.Pandey S., Singh J., Upadhyay A.K., Ram D., Rai M. Ascorbate and carotenoid content in an Indian collection of pumpkin (Cucurbita moschata Duch. Ex Poir.) Cucurbit Gen. Coop. Rep. 2003;26:51–53. [Google Scholar]
- 107.Aruna G., Mamatha B.S., Baskaran V. Lutein content of selected Indian vegetables and vegetable oils determined by HPLC. J. Food Compos. Anal. 2009;22:632–636. doi: 10.1016/j.jfca.2009.03.006. [DOI] [Google Scholar]
- 108.Khoo H.E., Ismail A., Mohd-Esa N., Idris S. Carotenoid content of underutilized fruits. Plant Food Hum. Nutr. 2008;63:170–175. doi: 10.1007/s11130-008-0090-z. [DOI] [PubMed] [Google Scholar]
- 109.Yuan J.-M., Gao Y.-T., Ong C.-N., Ross R.K., Yu M.C. Prediagnostic level of serum retinol in relation to reduced risk of hepatocellular carcinoma. J. Natl. Cancer I. 2006;98:482–490. doi: 10.1093/jnci/djj104. [DOI] [PubMed] [Google Scholar]
- 110.Homnava A., Payne J., Koehler P., Eitenmiller R. Provitamin A (alpha-carotene, beta-carotene and beta-cryptoxanthin) and ascorbic acid content of Japanese and American persimmons. J. Food Quality. 1990;13:85–95. doi: 10.1111/j.1745-4557.1990.tb00009.x. [DOI] [Google Scholar]
- 111.Xianquan S., Shi J., Kakuda Y., Yueming J. Stability of lycopene during food processing and storage. J. Med. Food. 2005;8:413–422. doi: 10.1089/jmf.2005.8.413. [DOI] [PubMed] [Google Scholar]
- 112.USDA database . Agricultural Research Service, United States Department of Agriculture; Washington, DC, USA: 2011. [(accessed on 20 January 2011)]. USDA National Nutrient Database for Standard Reference, Release 23. Available online: http://www.ars.usda.gov. [Google Scholar]
- 113.Camara B., Hugueney P., Bouvier F., Kuntz M., Monéger R., Kwang W.J., Jonathan J. Biochemistry and molecular biology of chromoplast development. Int. Rev. Cytol. 1995;163:175–247. doi: 10.1016/S0074-7696(08)62211-1. [DOI] [PubMed] [Google Scholar]
- 114.Bouvier F., d'Harlingue A., Hugueney P., Marin E., Marion-Poll A., Camara B. Xanthophyll biosynthesis. J. Biol. Chem. 1996;271:28861–28867. doi: 10.1074/jbc.271.46.28861. [DOI] [PubMed] [Google Scholar]
- 115.Prasad K. N., Lyee C., Khoo H.E., Bao Y., Azlan A., Amin. I. Carotenoids and antioxidant capacities from Canarium odontophyllum Miq. fruits. Food Chem. 2011;124:1549–1555. doi: 10.1016/j.foodchem.2010.08.010. [DOI] [Google Scholar]
- 116.Tanaka Y. Flower colour and cytochromes P450. Phytochem. Rev. 2006;5:283–291. doi: 10.1007/s11101-006-9003-7. [DOI] [Google Scholar]
- 117.Bakker J., Timberlake C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997;45:35–43. doi: 10.1021/jf960252c. [DOI] [Google Scholar]
- 118.Ben-Amotz A., Fishler R. Analysis of carotenoids with emphasis on 9-cis β-carotene in vegetables and fruits commonly consumed in Israel. Food Chem. 1998;62:515–520. doi: 10.1016/S0308-8146(97)00196-9. [DOI] [Google Scholar]
- 119.Levin I., De Vos C.H.R., Tadmor Y., Bovy A., Lieberman M., Oren-Shamir M., Segev O., Kolotilin I., Keller M., Ovadia R. High pigment tomato mutants—more than just lycopene (a review) Isr. J. Plant Sci. 2006;54:179–190. doi: 10.1560/IJPS_54_3_179. [DOI] [Google Scholar]