Abstract
Background: Portable activity sensing devices (PASDs) have received significant interest as tools for objectively measuring activity-related parameters and promoting health-related outcomes. Studies of PASDs suggest the potential value of integrating them with behavioral interventions to improve intermediate and downstream clinical outcomes.
Objectives: This systematic review describes and evaluates evidence from controlled studies of interventions using PASDs on their effectiveness in health-related outcomes. Study quality was also assessed.
Methods: A systematic literature search was performed of MEDLINE, Cochrane Central Register of Controlled Trials, PsycINFO, EMBASE, and CINAHL databases. We included English-language papers of controlled trials through 2015 reporting the effectiveness of PASDs in improving health-related outcomes in any population. We extracted and analyzed data on study characteristics including design, target population, interventions, and findings.
Results: Seventeen trials met the inclusion criteria from a total of 9553 unique records. Study objectives varied greatly, but most sought to increase physical activity. Studies with a “passive” intervention arm using a PASD with minimal behavioral support generally did not demonstrate effectiveness in improving health-related outcomes. Interventions integrating PASDs with multiple behavioral change techniques were more likely to be effective, particularly for intermediate outcomes such as physical activity and weight loss. Trials had small sample sizes but were generally free of bias, except for blinding and selection bias.
Conclusion: There is insufficient evidence to draw a conclusion about the general health-related benefits of PASD interventions. PASD interventions may improve intermediate outcomes when coupled with multiple behavioral change techniques. Devices alone or with minimal behavioral change support are insufficient to change health-related outcomes.
Keywords: physical activity tracker, activity sensor, portable sensor, wearable device, mobile health (mHealth) technology
INTRODUCTION
Portable activity sensing devices (PASDs), including wearable accelerometers and pedometers, have generated considerable interest from health care researchers.1,2 These devices objectively measure and estimate physical activity, balance control, exercise adherence, activity intensity, and energy expenditure more easily and accurately than self-reporting questionnaires or diaries.3–6 Mobile information technology (IT) advances – including wireless connectivity, real-time messaging, advanced visualization, and context awareness – also permit these devices to motivate and inform users.7–9 With decreasing technology costs, PASDs have also penetrated the consumer marketplace with activity trackers (eg, Fitbit devices) and smartphones/smartwatches with sensor-based health applications (eg, Apple Health, Google Fit).10–12
PASDs can play 2 important roles in health care delivery and health promotion. First, they provide increasingly powerful measurement, storage, and communication of health-related variables, such as the total amount, duration, frequency, timing, and intensity of physical activity, body postures, and body movements. These are interpreted into activity levels, step counts, fall risk estimates, and other constructs associated with cardiovascular disease,13 diabetes,14–16 cancer,17,18 hypertension,13 neurological disorders,19 gait disturbance,20 and balance impairment and falls.21,22 Regular assessment or remote monitoring of these measures could result in more timely, personalized, and appropriate therapeutic interventions or preventive strategies, implemented by clinicians or patients themselves.10,23 Individual-level PASD data can also be aggregated for population health surveillance and subgroup comparisons, as demonstrated by the use of pedometers in US and Japanese national health studies.23
The second role PASDs can play in health and health care is in “behavioral informatics” interventions.24 This involves integrating PASDs into behavioral interventions to improve physical activity or decrease health-related risks.24,25 For example, devices can help individuals self-monitor physical activity over time,26 motivate individuals to reduce sedentary behaviors,25 or provide coaching to improve body balance.27–29 These behavioral change techniques can be and often are combined and delivered through mobile, web, or desktop IT software linked to PASDs.30 Such software can facilitate longitudinal tracking, feedback, motivational communication, goal setting, exercise planning, time management, and social media support.23,31–35
Enough literature has accumulated to assess whether patients indeed experience better health-related outcomes when exposed to behavioral interventions using PASDs. Prior reviews demonstrated the validity,26,36,37 feasibility,37,38 reproducibility,37 potential efficiency,3,26,30,38 and acceptance39 of PASDs. However, the present study is, to our knowledge, the first to systematically review and synthesize evidence from controlled trials testing the health-related effectiveness of PASD-enabled behavioral interventions.
Objectives
The primary aim of this systematic review is to describe and evaluate controlled studies of interventions using PASDs. The review examines the effect of device-based interventions on intermediate outcomes such as physical activity levels and downstream clinical outcomes such as organ function. We hypothesize that intervention effectiveness varies depending on the nature of the behavioral change techniques used. A secondary objective was to evaluate the quality of reviewed trials.
METHODS
We conducted this systematic review of the English-language scholarly literature in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. One reviewer (HA) performed study selection, data extraction, and quality assessment and another (RJH) independently monitored these processes for completeness and accuracy.
Data sources and search queries
We searched 5 online databases from inception to December 30, 2015: MEDLINE (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO (EBSCO), EMBASE, and CINAHL (EBSCO). Queries covered 3 domains: (1) sensing devices, (2) portability or wearability, and (3) physical (body) activities (for details, see Supplementary Table S1). In addition, reference lists of relevant reviews and empirical studies were checked for eligible studies.
Study selection
Records were downloaded into an EndNote X7.7 (Clarivate Analytics, Philadelphia, PA, USA) library. After removing duplicate records, we screened titles and abstracts for inclusion and exclusion criteria (for details, see Supplementary Table S2). We used a “topic-collated” approach to accelerate screening: instead of sequentially reviewing publications in author or chronological order, we collated records by topics using keyword searches in the EndNote library prior to full-text review.
Studies were included in the systematic review if they were in English, randomized, studied humans of any age, and compared the health-related effectiveness of PASDs in the target population against a control group. Journal and peer-reviewed conference articles were included.
Data extraction
Data were extracted from eligible articles into a spreadsheet. Data included study design, objectives, target population, participant characteristics, interventions, device type, device data capture and processing, principal findings, and adverse events.
Quality assessment
Articles included in the final review were assessed for quality and risk of bias using updated criteria from the Cochrane Consumers and Communication Review Group.40 Studies were graded as low, unclear, or high for each Cochrane bias domain: selection, performance, detection, attrition, reporting, and other (Cochrane Handbook, Table 8.5.d).41
RESULTS
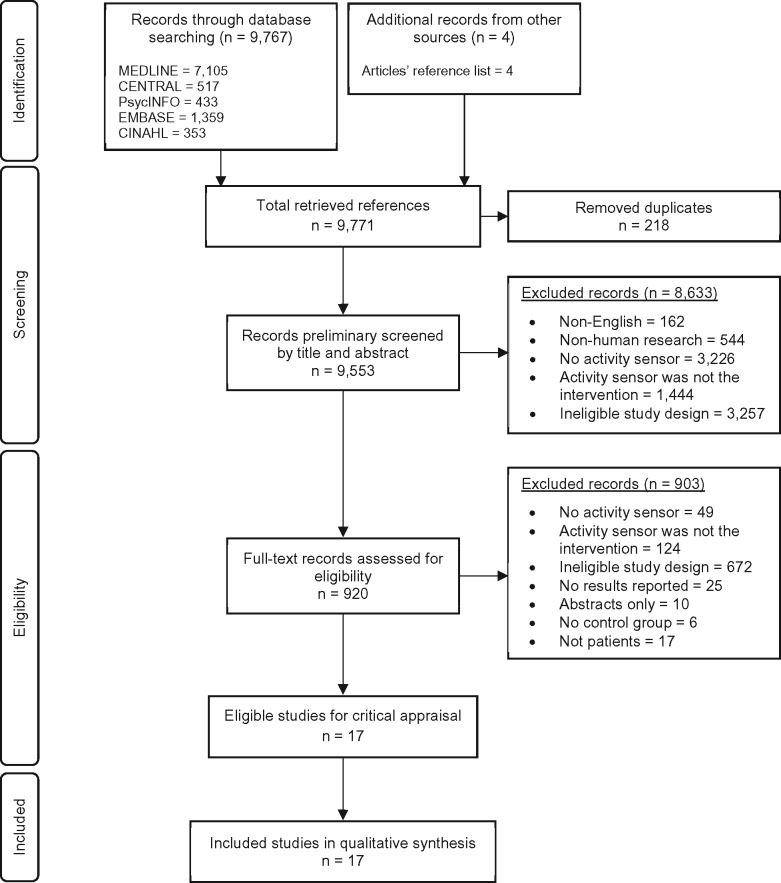
We identified 9771 search records and 4 additional papers through cited reference search (Figure 1 ). After removing duplicates, we reviewed titles and abstracts for 9553 records. Of these, 920 underwent full-text review. Most articles were excluded because the PASD was used not in the main intervention but to detect objective physical activity levels. A final total of 17 articles met inclusion and exclusion criteria, each using a PASD in the main intervention (Table 1; for more details, see Supplementary Table S3). All 17 met minimal quality standards for review inclusion.
Figure 1.
Flow chart of the systematic review.
Table 1.
Study characteristics of reviewed randomized clinical trials
| Reference | Outcome | Intervention duration | Group size | Intervention and comparison group characterization |
|---|---|---|---|---|
| Paschali et al. (2005)42 | Exercise adherence | 3 months | 13 | Intervention: accelerometer; recorded daily activity in a paper diary, received individualized exercise plan |
| 13 | Comparison: accelerometer with no access to activity data, received individualized exercise plan | |||
| Butler et al. (2009)43 | Physical activity adherence | 6 months | 44 | Intervention: pedometer; recorded daily steps, received motivating phone calls, set activity goals based on step counts, given brochure on physical activity |
| 46 | Comparison: given brochure about physical activity | |||
| Newton et al. (2009)44 | Physical activity level | 12 weeks | 38 | Intervention: unblinded pedometer; 10 000 steps/day goal, recorded daily step counts, received reminder text messages |
| 40 | Comparison: closed pedometer; received standard care | |||
| McMurdo et al. (2010)45 | Physical activity | 6 months | 60 | Pedometer plus behavioral intervention: pedometer; recorded daily pedometer counts in diaries, attended counseling sessions to increase physical activity, received individualized activity plan and barrier coping plans, received motivational phone calls |
| 53 | Behavioral intervention: no pedometer; recorded walking time outdoors, received motivational phone calls | |||
| 66 | Comparison: usual care; no pedometer and behavioral intervention | |||
| Maturi et al. (2011)46 | Physical activity and body measures | 12 weeks | 32 | Intervention: pedometer; 10 000 steps/week goal, recorded step counts on a calendar, received individualized motivational consultations, received pamphlet on weight loss |
| 32 | Comparison: usual care; no pedometer | |||
| Shuger et al. (2011)34 | Weight loss and waist circumference | 9 months | 26 | SenseWear armband alone (SWA): SenseWear; had access to real-time web-based personalized weight management, received regular feedback on energy expenditure through web, received weight loss manual, instructed to monitor diet, physical activity |
| 28 | Group-based behavioral weight loss education (GWL): attended weight loss counseling sessions, received weight loss manual, instructed to monitor diet, physical activity | |||
| 37 | Combined GWL and SWA group (GWL + SWA) | |||
| 32 | Comparison: weight loss manual; instructed to monitor nutrition and physical activity | |||
| Kovelis et al. (2012)47 | Daily physical activity | 1 month | 23 | Intervention: pedometer; 10 000 steps/day goal, recorded daily step counts |
| 17 | Comparison: booklet on advantages of having an active life, disadvantages of smoking | |||
| Adams et al. (2013)48 | Physical activity | 6 months | 10 | Intervention: open pedometer; set daily goals based on actual step count data, received motivational emails and short text messages, given motivational brochures and motivating feedback, given financial incentives for achieving daily goals |
| 10 | Comparison: pedometer; 10 000 steps/day goal, received motivational emails and short text messages, given brochures to motivate physical activity, given feedback and incentives for submitting pedometer data (but not for meeting daily goals) | |||
| Bickmore et al. (2013)49 | Step counts | 12 months | 132 | Intervention: pedometer; interacted daily with embodied conversational agent software, which provided 5-minconversation consisting of motivational feedback, troubleshooting discussion (if any), and individualized exercise counseling |
| 131 | Comparison: pedometer; recorded step counts daily | |||
| Wijsman et al. (2013)35 | Physical activity level and metabolism | 3 months | 114 | Intervention: accelerometer with access to DirectLife web-based physical activity program; received individualized motivational feedback, set daily goals based on current activity |
| 112 | Comparison: no activity monitor; given no instructions about daily physical activity | |||
| Choi et al. (2015)50 | Physical activity | 12 weeks | 15 | Intervention: open accelerometer with access to activity data; received motivational messages or short videos from an application, set individualized weekly goals |
| 15 | Comparison: open accelerometer with access to data; set goal to reach 8500 steps/day | |||
| Grewal et al. (2015)28 | Postural stability and daily physical activity | 4 weeks | 18 | Intervention: LegSys; did balance training exercises, visualized movement errors during exercises, received visual and auditory (positive sound) feedback |
| 16 | Comparison: no intervention; no training program | |||
| Mansfield et al. (2015)32 | Walking activity and gait performance | 3–26 days | 29 | Intervention: accelerometer with access to data; physiotherapist and participant collaboratively assigned walking activity goals based on analyzed activity data |
| 28 | Comparison: accelerometer with access to data; physiotherapist discussed daily walking goals based on participant’s self-reported walking activity | |||
| Martin et al. (2015)33 | Physical activity | Phase 1: 2 weeks | 32 | Unblinded, no text message: accelerometer with access to data through Fitbug application |
| 16 | Blinded, with no text messages: accelerometer with no access to activity information | |||
| Phase 2: 2 weeks | 16 | Unblinded, personalized messages: accelerometer; had access to data through Fitbug, received personalized texts from physician, set 10 000 steps/day goal, received motivational messages | ||
| 16 | Unblinded, no text messages: accelerometer with access to data through Fitbug | |||
| 16 | Blinded, no text message: accelerometer with no access to activity information | |||
| Oh et al. (2015)51 | Weight loss | 24 weeks | 118 | Intervention: pedometer with access to data; entered step counts into mobile application; received clinical decision support system algorithm-based feedback, received health education and nutrition consults over the phone based on activity levels and lifestyle |
| 210 | Comparison: pedometer; kept body weight journals, recorded daily step counts on a log sheet, visited hospitals to meet physicians and get consultation about nutrition and exercise | |||
| Schwenk et al. (2016)29 | Balance and gait performance | 4 weeks | 9 | Intervention: LegSys; did balance training exercises, visualized movement errors during exercises, received visual and auditory (positive sound) feedback |
| 10 | Comparison: no intervention; no formal exercise program | |||
| Ginis et al. (2016)52 | Body balance and quality of life | 6 weeks | 20 | Intervention: CuPiD system; used auditory feedback and freeze-of-gait training smartphone applications with 2 inertial measurement units, received positive verbal feedback on optimal gait performance, cued continuously during walking, received booklet of personalized instructions, recorded frequency and duration of training in diary |
| 18 | Comparison: no application or device; received training and recommendations, recorded frequency and duration of training sessions |
Study characteristics
All 17 reviewed studies were randomized clinical trials with a PASD incorporated into the main intervention (Table 1). A majority (76%) were published after 2010. Eight studies (47%) were conducted in the United States,28,29,33,34,42,48–50 1 was a collaboration between researchers in Belgium and Israel,52 and others were reported by investigators in Australia,43 New Zealand,44 Scotland,45 Iran,46 Brazil,47 the Netherlands,35 Canada,32 and South Korea.51 Sample sizes of the trials ranged from 19 to 328, with a median of 57 participants.
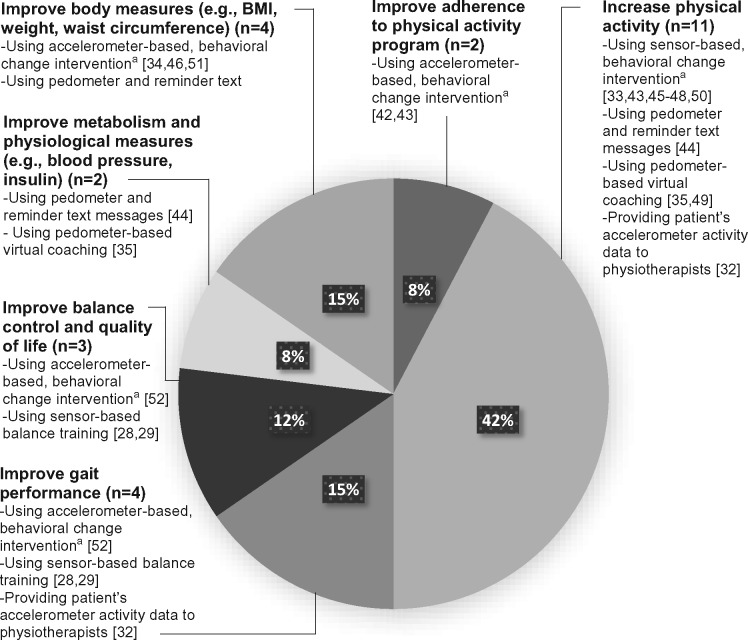
The reviewed studies mainly aimed to improve physical activity levels, metabolism, physiologic measures, body measures, balance control, and quality of life (Figure 2 ).
Figure 2.
Objectives and studied interventions of the 17 reviewed trials. aFor example, self-monitoring, motivational messages, goal setting, and exercise planning.
Three trials (18%) studied multiple intervention groups, comparing the effectiveness of PASDs integrating different behavioral change techniques.33,34,45 In 9 studies (53%), intervention participants wore PASDs and had access to device data, while controls did not use the device.28,29,34,35,43,45–47,52 In 3 trials (18%), both intervention and control participants used PASDs, but only the intervention group could access device data.33,42,44 Six additional studies (35%) provided PASDs and access to device data in both trial arms, but intervention participants received individualized exercise plans based on device-assessed activity data, while controls were provided static or no exercise planning.33,34,48–51
Target population characteristics
Included studies evaluated the effectiveness of accelerometer-based activity programs among 1665 total participants. Thirteen trials (76%) included men and women,28,29,32–35,42–44,47–49,51 3 (18%) studied women only,45,46,50 and 1 did not specify gender distribution.52
Five studies (29%) enrolled younger and older adults,28,32,43,47,51 4 trials (24%) enrolled older adults only,29,35,45,49 6 trials (35%) included younger adults only,33,34,42,46,48,50 1 enrolled adolescents,44 and 1 did not specify age group.52 Age distribution was poorly reported, with only 4 trials providing complete age information.28,29,32,42 Limited information was also reported on race and ethnicity of participants, with only 4 studies reporting distributions of white vs non-white participants,33,48–50 and 3 reporting Hispanic vs non-Hispanic distributions.48–50
Reviewed studies recruited individuals with different diseases or health-related conditions, including type 1 diabetes,44 type 2 diabetes,28,42 cardiovascular disease,33,43 sedentary lifestyle,34,35,45,48,49 postpartum care,46 pregnancy,50 smoking,47 stroke,32 metabolic syndrome,51 chemotherapy-induced peripheral neuropathy (CIPN),29 and Parkinson’s disease.52
Utilized portable activity sensing devices
All interventions involved 1 form of PASD to objectively track participants’ activity (Table 2). Eight studies (47%) used pedometers to record daily step counts; devices included Omron45,46,48,49 and Yamax Digiwalker.43,47 Six trials (35%) used accelerometers to measure motion along 3 axes; devices included BioTrainer,42 SenseWear,34 Fitbit Ultra,50 Fitbug Orb,33 Tracmor,35 and X6‐2mini.32 Three studies (18%) used inertial measurement unit (IMU) sensors to measure kinematic parameters for balance assessment; these included LegSys28,29 and EXLs3.52 Two trials did not disclose the PASD model used.44,51 Two studies used a separate PASD to measure physical activity outcomes (eg, step counts) independent of the device that was part of the intervention.35,45
Table 2.
Technological characteristics of reviewed studies
| Technology | No. of studies | References |
|---|---|---|
| Portable activity sensing devices | ||
| Pedometer | 8 | 43–49,51 |
| Accelerometer | 6 | 32–35,42,50 |
| Inertial measurement unit | 3 | 28,29,52 |
| Behavioral informatics software | ||
| No software | 6 | 32,43–48 |
| Desktop application | 3 | 28,29,42 |
| Web-based application | 4 | 33–35,50 |
| Smartphone application | 4 | 33,50–52 |
| Tablet application | 1 | 49 |
| Real-time physical activity feedback | 5 | 33–35,50,51 |
| Interactive virtual coaching | 1 | 49 |
| Audiovisual training program | 3 | 28,29,52 |
| Sensor data retrieval and storage | ||
| Manually entered into diary | 5 | 43–47 |
| Manually entered into software | 2 | 50,51 |
| Manually downloaded from sensor to software | 5 | 32,34,35,42,48,49 |
| Real-time transfer from sensor to software | 5 | 28,29,33,52 |
| Sensor data processing and display | ||
| To measure physical activity level, eg, step counts, energy expenditure, and activity duration | 15 | 32–35,42–52 |
| To provide graphical feedback on physical activity | 4 | 33,42,50,51 |
| To provide audio feedback on gait performance and individualized gait advice | 1 | 52 |
| To provide visual feedback on joint position | 2 | 28,29 |
| To set individualized activity goals | 6 | 32,43,45,48–50 |
| Used EHR-integrated CDSS algorithm for individualized feedback | 1 | 51 |
EHR = electronic health record; CDSS = clinical decision support system.
Studied interventions
We identified 6 possible categories of PASD interventions, depicted in Figure 3 , based on the nature of behavioral change techniques used and whether IT software was used separate from the device itself. The 3 categories of behavior change techniques were: (1) passive self-monitoring, where participants wore a device and its data were available for self-monitoring; (2) goal-based self-monitoring, where participants self-monitored device data with a specific goal in mind; and (3) integrative interventions, involving a PASD and at least 3 behavioral change techniques, including goal-based self-monitoring, motivational messages, coaching or training programs, group-based education, planning, incentives, and combinations thereof.
Figure 3.
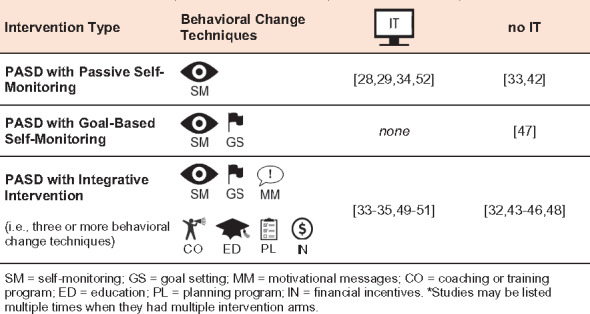
Categorization of reviewed interventions using portable activity sensing devices (PASDs), based on their use of behavioral change techniques and information technology (IT) software.
Technology characteristics
Table 2 summarizes the hardware, software, data retrieval and storage, data processing, and display characteristics of the technology used in reviewed studies.
The IT software involved in behavioral informatics interventions varied in terms of platform (eg, desktop vs smartphone) and type (eg, real-time feedback vs virtual counseling agent). One study42 used a desktop application to provide graphical feedback of weekly physical activity to participants based on sensor data. In 3 trials, sensor data were stored in a web-based data-management application that provided real-time feedback to participants.33–35,50 Smartphone applications were also used to collect sensor data and give feedback to participants.33,50,51 Two studies provided feedback through both smartphone and web applications.33,50 In another trial, sensor data were downloaded to a tablet-based interactive counseling application, where an animated character delivered personalized exercise coaching.49 In one study, patients with Parkinson’s disease received gait-related audiovisual feedback from smartphone-based training applications.52 Another interactive training program provided real-time visual feedback on joint positions and angles through processing of inertial sensor-based kinematic data, helping patients understand motor errors and better control balance.28,29 Other studies did not specify the use of software.32,43–48
In all studies, retrieval of data from the device was important to assess study outcomes or deliver the interventions. There were 4 ways to retrieve or transfer data from devices (Table 2). In 5 trials (29%), participants manually entered step counts from a pedometer into daily, weekly, or monthly paper diaries.43–47 In other studies, sensor data were manually downloaded from the pedometer or accelerometer into desktop software or a web application by participants or investigators,32,34,35,42,48,49 or participants entered step counts into a smartphone application.50,51 In fewer cases, sensor data were automatically transferred in real time to a smartphone application33,52 or a balance training program28,29 for further processing.
Approaches to processing sensor data (Table 2) included applying algorithms to estimate step counts,32,33,35,42–52 energy expenditure,34 or physical activity duration.32–35,50,52 Mansfield et al.32 also processed accelerometer data using a customized, trained machine-learning algorithm to detect step counts, walking bouts, and activity duration. Other studies converted data into graphical feedback on physical activity level33,42,50,51 or audio feedback with individualized gait advice.52 Kinematic data in 1 study were used for visual feedback on joint positions and angles.28,29 Moreover, collected data from sensors were often used to set individualized activity goals.32,43,45,48–50 In one study, sensor data were integrated with the health care center’s server to generate individualized feedback based on a clinical decision support system algorithm.51
Effects of interventions on health-related outcomes
Tables 3 and 4 summarize the effects of reviewed interventions on physical activity (Table 3) and other intermediate and downstream health-related outcomes (Table 4). To test the hypothesis that PASD intervention effectiveness depends on the nature of the intervention, these tables organize interventions by the type of behavioral change techniques employed.
Table 3.
Reported effectiveness of portable activity sensing devices (PASDs) on physical activity, organized by category of employed behavioral change techniques
| Outcomes and Behavioral Interventions | PASD with Passive Self-Monitoring |
PASD with Goal-Based Self-Monitoring |
PASD with Integrative Intervention |
|||
|---|---|---|---|---|---|---|
| Improved | No change | Improved | No change | Improved | No change | |
| Physical activity level | ||||||
| Self-monitoring | 42 | |||||
| Self-monitoring, goal setting | 47 | |||||
| Self-monitoring, motivational messages, goal setting | ||||||
| a. Under 6 months | 46 | 32 , 44 | ||||
| b. 6 months or more | 45 , 48 | |||||
| Self-monitoring via computer software | 33 | |||||
| Computerized goal setting, motivational message, self-monitoring | ||||||
| a. Under 6 months | 33 , 35 , 49 | 50 | ||||
| b. 6 months or more | 49 | |||||
| Computerized balance training program, self-monitoring | 28 | |||||
| Physical activity duration | ||||||
| Self-monitoring | 42 | |||||
| Self-monitoring, motivational messages, goal setting | ||||||
| a. Under 6 months | 43 | 32 , 44 | ||||
| b. 6 months or more | 43 | 45 | ||||
| Self-monitoring via computer software | 33 | |||||
| Computerized goal setting, motivational message, self-monitoring | 33 , 35 | |||||
| Number of physical activity sessions | ||||||
| Self-monitoring, motivational messages, goal setting | ||||||
| a. Under 6 months | 43 | |||||
| b. 6 months or more | 43 | |||||
| Metabolic equivalents | ||||||
| Self-monitoring, motivational messages, goal setting | 43 | |||||
Effectiveness refers to statistically significant differences between intervention and comparison groups.
Table 4.
Reported effectiveness of portable activity sensing devices (PASDs) on outcomes besides physical activity, organized by category of employed behavioral change techniques
| Outcomes and Behavioral Interventions | PASD with Passive Self-Monitoring |
PASD with Goal-Based Self-Monitoring |
PASD with Integrative Intervention |
|||
|---|---|---|---|---|---|---|
| Improved | No change | Improved | No change | Improved | No change | |
| Body mass index | ||||||
| Self-monitoring, motivational messages, goal setting | 46 | 43,44 | ||||
| Self-monitoring via computer software | 34 | |||||
| Computerized goal setting, motivational message, self-monitoring | 51 | 34 | ||||
| Hip circumference | ||||||
| Self-monitoring, motivational messages, goal setting | 46 | |||||
| Computerized goal setting, motivational message, self-monitoring | 35 | |||||
| Waist circumference | ||||||
| Self-monitoring, motivational messages, goal setting | 46 | 43 | ||||
| Self-monitoring via computer software | 34 | |||||
| Computerized goal setting, motivational message, self-monitoring | ||||||
| a. Under 6 months | 35 | |||||
| b. 6 months or more | 34 | |||||
| Body fat percentage | ||||||
| Computerized goal setting, motivational message, self-monitoring | 35 | 34 | ||||
| Systolic and diastolic blood pressure | ||||||
| Self-monitoring, motivational messages, goal setting | 44 | |||||
| Computerized goal setting, motivational message, self-monitoring | 35,51 | |||||
| HbA1c | ||||||
| Self-monitoring, motivational messages, goal setting | 44 | |||||
| Computerized goal setting, motivational message, self-monitoring | 35 | |||||
| Serum lipid profile | ||||||
| Computerized goal setting, motivational message, self-monitoring | 35,51 | |||||
| Framingham 10-year risk | ||||||
| Computerized goal setting, motivational message, self-monitoring | 35 | |||||
| Fasting insulin level | ||||||
| Computerized goal setting, motivational message, self-monitoring | 35 | |||||
| Fasting glucose serum level | ||||||
| Computerized goal setting, motivational message, self-monitoring | 35 | |||||
| Weight loss | ||||||
| Self-monitoring, motivational messages, goal setting | 46 | |||||
| Self-monitoring via computer software | 34 | |||||
| Computerized goal setting, motivational message, self-monitoring | ||||||
| a. Under 6 months | 35 | 34 | ||||
| b. 6 months or more | 34,51 | |||||
| Insulin total daily dose reduction | ||||||
| Self-monitoring, motivational messages, goal setting | 44 | |||||
| Balance control, postural stability, and gait performance | ||||||
| Computerized balance training program, self-monitoring | 28,29,52 | |||||
| Quality of life | ||||||
| Self-monitoring, motivational messages, goal setting | 44,45 | |||||
| Computerized balance training program, self-monitoring | 28 | 29,52 | ||||
| Smoking behavior | ||||||
| Self-monitoring, goal setting | 47 | |||||
| Computerized goal setting, motivational message, self-monitoring | 51 | |||||
| Lung function | ||||||
| Self-monitoring, goal setting | 47 | |||||
| Lower extremities function | ||||||
| Self-monitoring, motivational messages, goal setting | 45 | |||||
Effectiveness refers to statistically significant differences between intervention and comparison groups.
Physical activity
Merely providing objective feedback of step counts and activity level to participants for self-monitoring purposes did not effectively increase physical activity level and duration (Table 3).33,42 Kovelis et al.47 reported similar results when adding goal setting along with objective step counts for physically inactive smokers, concluding that the interventions were not sufficient to motivate physical activity.
Use of 3 or more behavioral change techniques in the integrative interventions was associated with more promising, though still mixed, evidence for improved physical activity levels. Six of 10 trials reported such improvements with a combination of PASD, motivational messages, exercise plans, and objective activity feedback33,35,45,46,48,49; 4 reported no effect.32,44,49,50 In a study by Bickmore et al.49 on the effect of a virtual coaching computer program with individualized exercise goals and motivational messages, the intervention was successful at 2 months for increasing daily step counts, but the effect did not last after 1 year. Martin et al.33 also found no significant effect on activity level and duration when participants received only device feedback; however, there was a significant increase in step counts per day and duration of exercise after the group received motivational text messages, planned exercise goals, and activity-level feedback compared to an attention control group using a device with no access to device data. In contrast, Newton et al.44 reported that weekly text messages to participants reminding them to wear the pedometer and stay active did not improve either activity level or amount of exercise. McMurdo et al.45 found an increase in physical activity at 6 months among sedentary elderly women by providing individualized activity plans and motivational messages but no accelerometer.
Three studies reported that interventions with 3 or more behavioral change techniques increased the duration of physical activity33,35,43; 3 others reported no effect.32,44,45 For example, Butler et al.43 showed a long-term effect of integrative interventions among adult patients participating in cardiac rehabilitation programs, whereas McMurdo et al.45 reported no effect in sedentary elderly women after 6 months. In the 1 study assessing reported exercise tolerance in metabolic equivalents, the intervention was not effective.43
Body measures
Three trials concluded that an intervention using PASDs does not affect body mass index,34,43,44 while 2 studies reported body mass index improvements in postpartum women46 and obese adults (Table 4).51
Studies reported mixed outcomes of PASDs incorporating motivational messages, goal setting, and objective activity feedback on hip circumference. Maturi et al.46 found the intervention effective among postpartum women, and Wijsman et al.35 reported positive results in sedentary older adults. Two studies showed no effect on waist circumference.34,43
Physiological measures
Few studies evaluated the impact of PASD interventions on physiological measures, and results were mixed (Table 4). Shuger et al.34 reported no reduction in body fat percentage after providing objective activity feedback alongside motivational messages and exercise planning to sedentary obese adults, but Wijsman et al.35 reported a positive change among sedentary older adults. Wijsman et al.35 also found improvement in HbA1c, while Newton et al.44 observed no change in HbA1c among type 1 diabetes patients. One study also found that a computerized intervention with motivational messages, when combined with self-monitoring and individualized goal setting, improved fasting serum insulin levels but had no effect on fasting serum glucose levels.35 Other trials reported no effect on blood pressure,35,44,51 serum lipid profile,35,51 or Framingham 10-year risk of heart attack.35
Weight loss
Four reports showed that use of PASDs could help patients lose weight (Table 4).34,35,46,51 The integrative interventions of PASD with self-monitoring, motivational messages, and goal setting were successful even without providing software.46 Wijsman et al.35 and Oh et al.51 studied the impact of computerized self-monitoring programs with individualized exercise planning and motivational feedback on weight in sedentary elders and obese adults with metabolic syndrome. Participants in the intervention groups lost, on average, 1.5 kg of weight after 3 months35 and 2.2 kg after 6 months,51 significantly more than control group participants. Maturi et al.46 conducted similar interventions, but without IT software, with postpartum women and found a significant mean weight decrease of 2.1 kg after 3 months. Shuger et al.34 studied 2 types of interventions in the short and long term with physically inactive obese adults. They reported that providing activity-level feedback alone did not result in weight loss; however, adding computerized real-time self-monitoring along with group-based education resulted in an average 9-month weight decrease of 6.59 kg.
Balance control, postural stability, and gait performance
All 3 studies investigating the effect of IMUs on balance control reported positive change (Table 4).28,29,52 Grewal et al.28 and Schwenk et al.29 examined the same computerized system on patients with diabetic peripheral neuropathy and CIPN. By teaching patients better motor and posture control, the intervention resulted in significant reduction of hip, ankle, and center-of-mass sway in both groups. In another study by Ginis et al.,52 2 smartphone applications provided real-time feedback on gait abnormalities and freezing-of-gait occurrence alerts to patients with Parkinson’s disease. They concluded that the intervention improved balance and gait performance compared to conventional gait training for people with Parkinson’s disease.
Quality of life
No improvement in quality of life was observed in 2 trials investigating the effect of pedometers and accelerometers within a behavioral intervention.44,45 Grewal et al.28 found that their computerized balance training program and IMU measuring device might improve quality of life in diabetic peripheral neuropathy patients, but not in CIPN patients.29 Ginis et al.52 reported no effect of a gait-alerting smartphone-based application on quality of life among patients with Parkinson’s disease (Table 4).
Other outcomes
Only 1 study evaluated the effect of interventions on total daily insulin dose and found no significant impact.44 No improvement in smoking behavior was reported by 2 studies.47,51 Another study evaluating the effect of self-monitoring plus goal-setting on lung function concluded that there was no improvement among smokers.47 McMurdo et al.45 found no improvement in lower extremity function among sedentary older women (Table 4).
Risk of bias in included studies
We evaluated study quality by assessing the risk of bias in the 17 included studies (Figures 4 and 5 ). Randomization was adequate in 14 studies (82%),28,29,32–35,42,43,45–48,50,51 but the randomization method was unclear in 3 others. Allocation was concealed in 10 studies (59%),28,29,32,33,45–48,50,52 but other studies did not provide enough information to assess allocation concealment. Most studies were not successful in blinding participants and research personnel to the allocated interventions.28,29,32,34,35,42–51 Only 1 study33 clearly stated that participants and research personnel were blinded; blinding was unclear in another.52 Blinding the assessors of outcomes was achieved in 7 studies (41%).29,32–35,42,49 Seven trials (41%)44–48,50–52 explicitly stated that they did not blind research personnel, and 2 (12%) did not clearly report this.28,43 The completeness of outcome data was adequate in 16 trials (94%), whereas 1 study could not sufficiently collect outcomes due to participant nonadherence.47 With respect to selective reporting, we identified 5 trials (29%) with available study protocols reporting all preselected outcomes32–34,45,48; however, not enough information was available to judge selective reporting bias in other studies. In assessing other potential sources of bias, we identified 15 trials (88%) at risk of bias due to small sample size and lack of generalizability.28,29,32–34,42–44,47–50,52 Some of the trials were also at high risk of selection bias due to self-selected participants42,43 or imbalanced education level between intervention and control groups.51 One study had adequate statistical power and was deemed to be free of other bias.45
Figure 4.
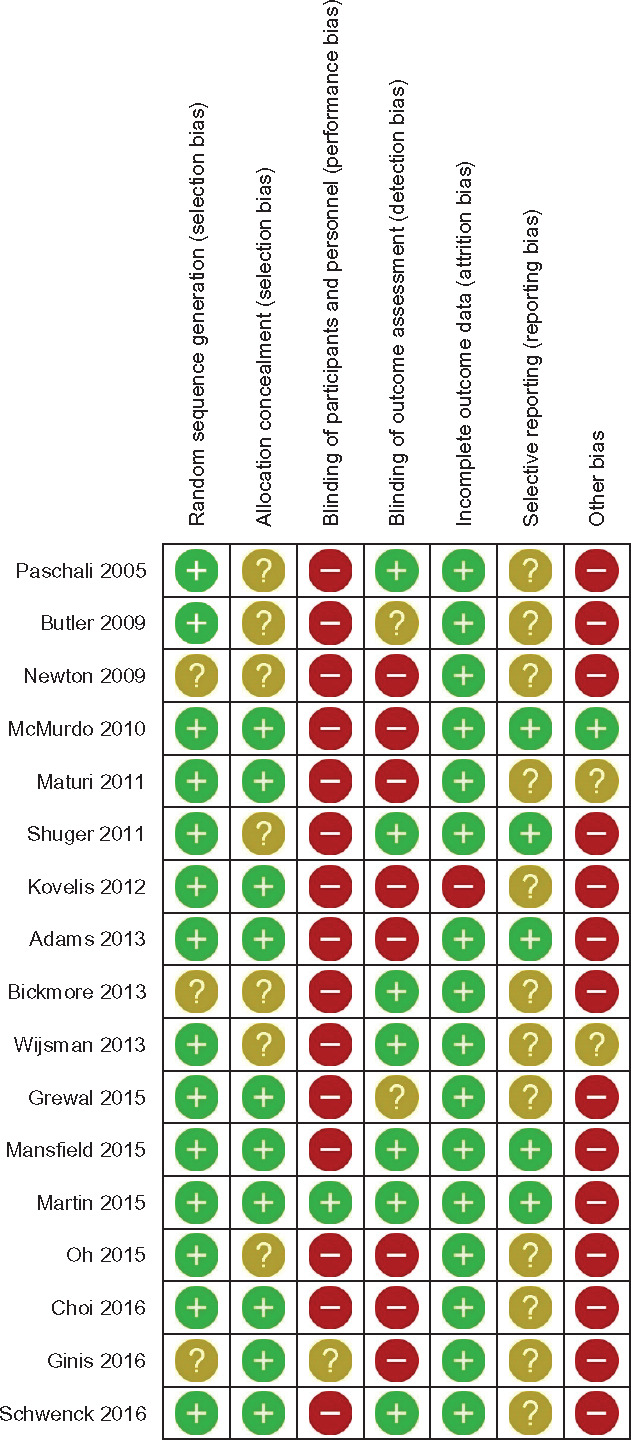
Summary of authors’ consensus judgment about of risk of bias for each included study, by various sources of potential bias.
Figure 5.
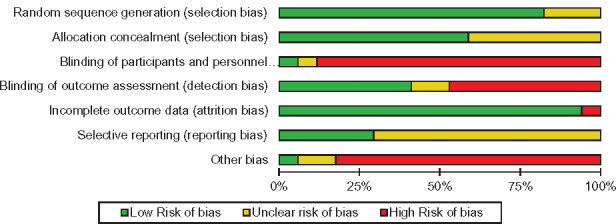
Risk of bias graph based on authors’ judgment about risk of bias items, presented as percentages across all included studies.
DISCUSSION
Findings on the health-related impact of PASDs are highly diverse, but the majority of research supports a benefit from combining sensors with an integrative set of behavioral change techniques. In contrast to more passive interventions, where the burden is on participants to meaningfully use device data, integrative interventions support the active use of data through training or coaching, motivational feedback, and other behavioral change techniques alongside self-monitoring and goal setting.
The importance of behavioral change techniques
One implication of these findings is that passive interventions – ones where people receive a PASD with minimal further assistance, reinforcement, or additional behavioral change techniques – may not be effective. In other words, the device alone may be insufficient to effect a change in health. This echoes a general concern about the use of health IT as a stand-alone intervention and implies that there is a need to better explicate the role of health IT in behavioral theory.53,54
In contrast, when paired with an appropriate array of behavioral change techniques, PASDs can produce benefits, particularly for intermediate outcomes such as physical activity or weight loss. Even so, it is unclear whether the PASD itself contributes to these outcomes beyond the behavioral change techniques. A recent study in which participants receiving an integrative weight-loss intervention were randomized to receive a PASD or continue the intervention with no PASD found no long-term differences between the 2 groups.55 However, further research is needed to test the hypothesis that PASDs provide independent value or interact with behavioral change techniques to amplify their effectiveness. Leveraging interactive and adaptive mobile applications, in particular, might be an appropriate way to investigate the effect of health behavior interventions.56
The role of behavioral informatics
In the reviewed studies, IT software often provided a convenient and efficient way of delivering interventions with techniques such as feedback and motivational messaging. Such behavioral informatics interventions were often, though not always, effective at increasing physical activity levels, activity duration, and weight loss. Given the small number of trials and the heterogeneity of software used, it is premature to make any conclusions about the value of IT software in addition to PASD hardware. Other studies report favorable but mixed findings in the literature concerning the effects of behavioral IT interventions, including mobile technology and text-messaging, on health outcomes.57 While several studies have reported improved HbA1c in patients with type 2 diabetes,58 adherence to blood glucose measurement and glycemic control in type 1 diabetic adults,59,60 smoking cessation behavior,61,62 nutrition education and healthy diet,62,63 and psychological outcomes,62 other studies did not report benefits of such interventions for physical activity,62,64 weight loss,62 and dietary behavior.65 This could be the result of the health IT design approaches employed by those studies, with successful health IT being more likely to follow user-centered design principles and therefore yielding higher usability, user satisfaction, acceptability, and performance.66 For example, systems produced through user-centered design have been effective at improving quality of life in HIV patients67 and self-management behaviors among lung transplant recipients.68 Further research should investigate specific software characteristics as well as software design approaches, eg, user-centered design,66,69,70 that promote effectiveness of PASD interventions.
Health-related outcomes
Notably, few PASD intervention studies assessed downstream clinical outcomes such as organ function and quality of life, and none found an effect of PASD interventions. This generally conforms with the strength of evidence for intermediate as opposed to endpoint effectiveness in the overall literature on consumer-facing health IT.71,72 More research is needed to assess downstream outcomes and specific PASD intervention approaches most likely to improve clinical outcomes, such as hospitalizations, disease onset, and health-related quality of life.
Quality of reviewed randomized controlled trials
Most examined trials did not follow CONSORT guidelines,73 the globally accepted standard for efficiently and accurately reporting randomized controlled trial results. Although most reviewed trials reported randomization and measured outcomes, reporting quality for other categories was not acceptable. Participant demographics were poorly reported; only 24% of trials reported age distribution,28,29,32,42 1 study did not specify gender distribution,52 and race/ethnicity information was minimal in 24% of trials.33,48–50 Poor reporting of key methodological elements may not directly indicate the quality of the trials but limits the ability to assess the validity and generalizability of findings.73–75 We therefore urge researchers to adopt CONSORT guidelines in reporting randomized controlled trials of PASD effectiveness.76–78
Limitations
It is possible that eligible trials were inadvertently excluded from the review. The literature lacks accepted terminology for PASDs and PASD research. PASDs are also referred to as wearable devices, activity monitors, activity sensors, portable devices, wireless activity sensors, and wearable motion detectors. To mitigate the risk of excluding eligible trials, our search queries used these and other search terms, including the names of common PASDs (eg, Actigraph, Fitbit). We recommend using the term “PASD,” which refers to the device’s primary function (activity sensing) and includes portable devices that are not wearable (eg, phones or devices carried in a pocket or purse). Further, only 1 reviewer screened the publications, increasing the risk of selection bias. However, a second reviewer monitored the process for accuracy to mitigate this risk. Classifying interventions by the nature of behavioral change techniques and IT software integration (Figure 3) is novel to this review and may not accurately represent the taxonomy of PASD interventions. However, there was no other existing taxonomy, and ours was consistent with the intervention groups used in individual studies, which differed by the number of behavioral change techniques and use of software.28,29,32–35,42–52 Lastly, the outcomes of reviewed studies were too heterogeneous to perform quantitative meta-analysis of the effectiveness of PASD interventions.
Future work
Future research should assess the impact of specific PASD characteristics – including hardware, software, and data transfer and processing elements – on outcomes. Study designs should permit disambiguation of the effect of the PASD itself vs other elements of the intervention, including behavioral change techniques. Studies can also test the hypothesis that integration of PASD with behavioral change techniques is more effective than use of the PASD or behavioral change techniques alone. An important future direction is the study of longitudinal effects, especially given evidence of a lack of lasting effects of PASD interventions.49 This will also permit assessment of downstream outcomes such as disease onset or resolution.
CONCLUSION
There is insufficient volume or quality of evidence to conclude that PASD interventions are generally beneficial for health. However, PASD interventions might be effective in improving intermediate outcomes when coupled with multiple behavioral change techniques. Devices alone or with minimal behavioral change support appear to be insufficient to change health-related outcomes.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Competing Interests
Authors have no competing interests to declare.
Contributors
HA and RJH contributed to conceptualization, design, analysis, and writing the manuscript. HA performed study selection, data extraction, and quality assessment, monitored by RJH for completeness and accuracy.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
References
- 1. Matthews CE, Hagstromer M, Pober DM et al. . Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Case MA, Burwick HA, Volpp KG et al. . Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;3136:625–26. [DOI] [PubMed] [Google Scholar]
- 3. Mathie MJ, Coster AC, Lovell NH et al. . Accelerometry: providing an integrated, practical method for long-term, ambulatory monitoring of human movement. Physiol Meas. 2004;252:R1–20. [DOI] [PubMed] [Google Scholar]
- 4. Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev Chronic Dis. 2012;9:E113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ainsworth B, Cahalin L, Buman M et al. . The current state of physical activity assessment tools. Prog Cardiovasc Dis. 2015;574:387–95. [DOI] [PubMed] [Google Scholar]
- 6. Prince SA, Adamo KB, Hamel ME et al. . A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;51:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar S, Nilsen WJ, Abernethy A et al. . Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med. 2013;452:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varshney U. Pervasive healthcare and wireless health monitoring. Mobile Networks Appl. 2007;12(2–3):113–27. [Google Scholar]
- 9. Loo Gee B, Griffiths KM, Gulliver A. Effectiveness of mobile technologies delivering Ecological Momentary Interventions for stress and anxiety: a systematic review. J Am Med Inform Assoc. 2016;231:221–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piwek L, Ellis DA, Andrews S et al. . The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016;132:e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandhi M, Wang T. Digital Health Consumer Adoption. 2015. https://rockhealth.com/reports/digital-health-consumer-adoption-2015. Accessed October 9, 2016.
- 12. Krebs P, Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR Mhealth Uhealth. 2015;34:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Heart Association Nutrition C, Lichtenstein AH, Appel LJ et al. . Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;1141:82–96. [DOI] [PubMed] [Google Scholar]
- 14. Hu FB, Leitzmann MF, Stampfer MJ et al. . Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;16112:1542–48. [DOI] [PubMed] [Google Scholar]
- 15. Wing RR, Goldstein MG, Acton KJ et al. . Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care. 2001;241:117–23. [DOI] [PubMed] [Google Scholar]
- 16. Despres J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;4447121:881–87. [DOI] [PubMed] [Google Scholar]
- 17. Marchand LL, Wilkens LR, Kolonel LN et al. . Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;5721:4787–94. [PubMed] [Google Scholar]
- 18. Fader AN, Arriba LN, Frasure HE et al. . Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;1141:121–27. [DOI] [PubMed] [Google Scholar]
- 19. Rovio S, Kåreholt I, Helkala E-L et al. . Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;411:705–11. [DOI] [PubMed] [Google Scholar]
- 20. Baezner H, Blahak C, Poggesi A et al. . Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;7012:935–42. [DOI] [PubMed] [Google Scholar]
- 21. Ensrud KE, Nevitt MC, Yunis C et al. . Correlates of impaired function in older women. J Am Geriatr Soc. 1994;425:481–89. [DOI] [PubMed] [Google Scholar]
- 22. Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc. 2000;488:883–93. [DOI] [PubMed] [Google Scholar]
- 23. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;3311:1769–83. [DOI] [PubMed] [Google Scholar]
- 24. Pavel M, Jimison HB, Korhonen I et al. . Behavioral informatics and computational modeling in support of proactive health management and care. IEEE Trans Biomed Eng. 2015;6212:2763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bravata DM, Smith-Spangler C, Sundaram V et al. . Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;29819:2296–304. [DOI] [PubMed] [Google Scholar]
- 26. Pitta F, Troosters T, Probst VS et al. . Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;275:1040–55. [DOI] [PubMed] [Google Scholar]
- 27. Sveistrup H. Motor rehabilitation using virtual reality. J Neuroeng Rehabil. 2004;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grewal GS, Schwenk M, Lee-Eng J et al. . Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: a randomized controlled trial. Gerontology. 2015;616:567–74. [DOI] [PubMed] [Google Scholar]
- 29. Schwenk M, Grewal GS, Holloway D et al. . Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology. 2016;625: 553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mercer K, Li M, Giangregorio L et al. . Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR Mhealth Uhealth. 2016;42:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyons EJ, Lewis ZH, Mayrsohn BG et al. . Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014;168:e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mansfield A, Wong JS, Bryce J et al. . Use of accelerometer-based feedback of walking activity for appraising progress with walking-related goals in inpatient stroke rehabilitation: a randomized controlled trial. Neurorehabil Neural Repair. 2015;299:847–57. [DOI] [PubMed] [Google Scholar]
- 33. Martin SS, Feldman DI, Blumenthal RS et al. . mActive: a randomized clinical trial of an automated mHealth intervention for physical activity promotion. J Am Heart Assoc. 2015;411:e002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shuger SL, Barry VW, Sui X et al. . Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys. Act 2011;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wijsman CA, Westendorp RG, Verhagen EA et al. . Effects of a web-based intervention on physical activity and metabolism in older adults: randomized controlled trial. J Med Internet Res. 2013;1511:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tudor-Locke C, Williams JE, Reis JP et al. . Utility of pedometers for assessing physical activity: convergent validity. Sports Med. 2002;3212: 795–808. [DOI] [PubMed] [Google Scholar]
- 37. de Vries SI, Bakker I, Hopman-Rock M et al. . Clinimetric review of motion sensors in children and adolescents. J Clin Epidemiol. 2006;597: 670–80. [DOI] [PubMed] [Google Scholar]
- 38. de Bruin ED, Hartmann A, Uebelhart D et al. . Wearable systems for monitoring mobility-related activities in older people: a systematic review. Clin Rehabil. 2008;22(10–11):878–95. [DOI] [PubMed] [Google Scholar]
- 39. Schaefer SE, Van Loan M, German JB. A feasibility study of wearable activity monitors for pre-adolescent school-age children. Prev Chronic Dis. 2014;11:E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins JP, Altman DG, Gotzsche PC et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration; 2011. www.cochrane-handbook.org. Accessed February 25, 2016. [Google Scholar]
- 42. Paschali AA, Goodrick GK, Kalantzi-Azizi A et al. . Accelerometer feedback to promote physical activity in adults with type 2 diabetes: a pilot study. Percept Mot Skills. 2005;1001:61–68. [DOI] [PubMed] [Google Scholar]
- 43. Butler L, Furber S, Phongsavan P et al. . Effects of a pedometer-based intervention on physical activity levels after cardiac rehabilitation: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2009;292:105–14. [DOI] [PubMed] [Google Scholar]
- 44. Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care. 2009;325:813–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McMurdo MET, Sugden J, Argo I et al. . Do pedometers increase physical activity in sedentary older women? A randomized controlled trial. J Am Geriatr Soc. 2010;5811:2099–106. [DOI] [PubMed] [Google Scholar]
- 46. Maturi MS, Afshary P, Abedi P. Effect of physical activity intervention based on a pedometer on physical activity level and anthropometric measures after childbirth: a randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kovelis D, Zabatiero J, Furlanetto KC et al. . Short-term effects of using pedometers to increase daily physical activity in smokers: a randomized trial. Respir Care. 2012;577:1089–97. [DOI] [PubMed] [Google Scholar]
- 48. Adams MA, Sallis JF, Norman GJ et al. . An adaptive physical activity intervention for overweight adults: a randomized controlled trial. PLoS One. 2013;812:e82901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bickmore TW, Silliman RA, Nelson K et al. . A randomized controlled trial of an automated exercise coach for older adults. J Am Geriatr Soc. 2013;6110:1676–83. [DOI] [PubMed] [Google Scholar]
- 50. Choi J, Lee JH, Vittinghoff E et al. . mHealth physical activity intervention: a randomized pilot study in physically inactive pregnant women. Matern Child Health J. 2016;205:1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oh B, Cho B, Han MK et al. . The effectiveness of mobile phone-based care for weight control in metabolic syndrome patients: randomized controlled trial. JMIR Mhealth Uhealth. 2015;33:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ginis P, Nieuwboer A, Dorfman M et al. . Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat Disord. 2016;22:28–34. [DOI] [PubMed] [Google Scholar]
- 53. Sawesi S, Rashrash M, Phalakornkule K et al. . The impact of information technology on patient engagement and health behavior change: a systematic review of the literature. JMIR Med Inform. 2016;41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kukafka R, Johnson SB, Linfante A et al. . Grounding a new information technology implementation framework in behavioral science: a systematic analysis of the literature on IT use. J Biomed Inform. 2003;363:218–27. [DOI] [PubMed] [Google Scholar]
- 55. Jakicic JM, Davis KK, Rogers RJ et al. . Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. JAMA. 2016;31611:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Riley WT, Rivera DE, Atienza AA et al. . Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011;11:53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Free C, Phillips G, Galli L et al. . The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;101:e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Quinn CC, Clough SS, Minor JM et al. . WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;103:160–68. [DOI] [PubMed] [Google Scholar]
- 59. Kirwan M, Vandelanotte C, Fenning A et al. . Diabetes Self-Management Smartphone Application for Adults With Type 1 Diabetes: Randomized Controlled Trial. J Med Internet Res. 2013;1511:e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cafazzo AJ, Casselman M, Hamming N et al. . Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res. 2012;143:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med. 2010;51(3–4):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Portnoy DB, Scott-Sheldon LAJ, Johnson BT et al. . Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988–2007. Prev Med. 2008;471:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brug J, Campbell M, van Assema P. The application and impact of computer-generated personalized nutrition education: a review of the literature. Patient Educ Couns. 1999;362:145–56. [DOI] [PubMed] [Google Scholar]
- 64. Neville LM, O’Hara B, Milat A. Computer-tailored physical activity behavior change interventions targeting adults: a systematic review. Int J Behav Nutr Phys Act. 2009;61:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Norman GJ, Zabinski MF, Adams MA et al. . A review of eHealth interventions for physical activity and dietary behavior change. Am J Prev Med. 2007;334:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Johnson CM, Johnson TR, Zhang J. A user-centered framework for redesigning health care interfaces. J Biomed Inform. 2005;381:75–87. [DOI] [PubMed] [Google Scholar]
- 67. Gustafson DH, Hawkins R, Boberg E et al. . Impact of a patient-centered, computer-based health information/support system. Am J Prev Med. 1999;161:1–9. [DOI] [PubMed] [Google Scholar]
- 68. De Vito Dabbs A, Song MK, Myers BA et al. . A randomized controlled trial of a mobile health intervention to promote self-management after lung transplantation. Am J Transplant. 2016;167: 2172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Preece J, Rogers Y, Sharp H. Interaction Design: Beyond Human-Computer Interaction. New York: Wiley; 2015. [Google Scholar]
- 70. De Vito Dabbs A, Myers BA, Mc Curry KR et al. . User-centered design and interactive health technologies for patients. Comput Inform Nurs. 2009;273:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Finkelstein J, Knight A, Marinopoulos S et al. . Enabling patient-centered care through health information technology. Evid Rep Technol Assess (Full Rep). 2012;206:1–1531. [PMC free article] [PubMed] [Google Scholar]
- 72. Gibbons M, Samal RFWL et al. . Impact of Consumer Health Informatics Applications. Evidence Report/Technology Assessment No. 188. Rockville, MD: AHRQ Publication No. 09(10)-E019; 2009. [PMC free article] [PubMed] [Google Scholar]
- 73. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;81:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soares HP, Daniels S, Kumar A et al. . Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ. 2004;3287430:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huwiler-Müntener K, Jüni P, Junker C et al. . Quality of reporting of randomized trials as a measure of methodologic quality. JAMA. 2002;28721:2801–04. [DOI] [PubMed] [Google Scholar]
- 76. Plint AC, Moher D, Morrison A et al. . Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;1855:263–67. [DOI] [PubMed] [Google Scholar]
- 77. Moher D, Jones A, Lepage L. Use of the consort statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;28515:1992–95. [DOI] [PubMed] [Google Scholar]
- 78. Kane RL, Wang J, Garrard J. Reporting in randomized clinical trials improved after adoption of the CONSORT statement. J Clin Epidemiol. 2007;603:241–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.