Abstract
Background
Once touted as the future of hip arthroplasty, metal-on-metal (MoM) bearing surfaces have fallen sharply from favor with the emergence of a strong body of evidence demonstrating unacceptably high premature implant failure rates. The previously unpredictable development of adverse local tissue reactions (ALTRs) has been a substantive contributor to this. Although the underlying pathophysiology of these so-called “pseudotumors” is now well understood, the fundamental predisposing patient risk factors have remained elusive.
Questions/purposes
The aim of this research, as a clinical-genotype correlation analysis, was to identify specific alleles (genes) associated with the development of ALTRs in patients with in situ MoM THAs.
Methods
A case-control study of patients who received a large-head, primary MoM THA between 2005 and 2008 was performed with a minimum followup of 5 years. Twenty-six patients who had undergone revision of a primary MoM THA secondary to symptomatic ALTRs were recruited. The mean timeframe from primary MoM THA to symptomatic revision was 5.5 years (range, 1-10 years). Twenty-eight control subjects were randomly selected asymptomatic patients with no evidence of ALTRs on protocol-specific screening. Baseline demographics and high-resolution genotype (human leukocyte antigen [HLA] Class II) were collected for all patients. Cohorts were similar with respect to age at the time of primary MoM THA (mean, 54.8 versus 54.9 years, p = 0.95) and serum cobalt (mean, 5.5 versus 8.5 μg/L, p = 0.09) and chromium concentrations (mean, 2.9 versus 4.2 μg/L, p = 0.27). The association between genotype and revision surgery secondary to ALTRs was determined with gender as a covariate.
Results
The prevalence of the risk genotype was 30% (16 of 54) among the entire cohort. Adjusting for sex, the odds of revision were 6.1 times greater among patients with the risk genotype present than among patients without (95% confidence interval [CI], 1.5-25.4; p = 0.01). Among females, the specificity of the risk genotype was 1.0 (95% CIexact, 0.5-1.0; pexact = 0.03), and for males, it was 0.8 (95% CIexact, 0.6-0.9; pexact < 0.01).
Conclusions
The findings of this study suggest that, among patients with a primary MoM THA, allelic variation within the HLA Class II loci may be a strong, independent risk factor associated with the need for subsequent revision surgery secondary to pseudotumor formation.
Clinical Relevance
Given the hypothesis-generating nature of this novel undertaking, confirmatory prospective clinical studies are required to further elucidate this correlation and to explore the clinical utility of targeted genetic screening in this specific population. This research may, however, represent a key missing piece in the puzzle that is metal ion-induced pseudotumor formation.
Introduction
Metal-on-metal (MoM) bearing surfaces in hip surgery have been used for decades with initial reports of use dating back to the 1960s [75]. Driven by purported favorable biomechanical characteristics and championed as the future of hip arthroplasty and the panacea for polyethylene wear-induced osteolysis (aseptic loosening) [3, 26, 38, 74], their use saw a dramatic resurgence in the early 2000s, accounting for up to 35% of the THA market share in the United States alone [10]. Despite early hope [4, 8, 13, 36, 62], MoM hips fell sharply out of favor with the emergence of a strong body of evidence demonstrating associated unacceptably high rates of premature implant failure [7, 64, 76] with a more than fivefold greater risk of revision at 8 years as compared with metal-on-polyethylene articulations [7, 76]. The periarticular lesions quickly associated with these failures—so-called “pseudotumors”—are nonneoplastic, aseptic, soft tissue masses, now broadly defined as “adverse local tissue reactions” (ALTRs) [80]. The specific histologic characteristics of these lesions are unique to MoM bearings [11, 15]. Although reported to occur in 25% to 61% of patients [24, 35, 83] with in situ MoM THAs [35], the true prevalence remains undefined [2].
Recent investigations have identified Th-1 cell-mediated immune processes as the primary pathway in the pathogenic evolution of ALTRs [28-30, 81]. Interface wear generates micron-sized debris [18, 42, 43], producing metal ions that subsequently bind to serum proteins [50, 63, 85, 86]. In a subset of patients, these metal-protein complexes are immunogenic, activating naïve T-lymphocytes [1, 6, 15, 20, 21, 28, 44, 54, 66, 79, 81, 82] and signaling macrophage recruitment [69] through antigen-dependent processes. Importantly, the metal ion sensitivity responsible for ALTRs in this setting is not the function of a preexisting metal allergy per se, but rather represents an acquired immune response [23, 29-31, 52, 81].
Attempts to identify patient-specific factors implicated in pseudotumor development have previously been unfruitful. It was initially postulated that a direct correlation between metal ion burden and ALTR development may exist [24, 34, 45, 48, 58, 65]. This theory gained traction when component wear and various technical factors were shown to increase synovial and serum metal ion concentrations [14, 16, 17, 25, 33, 45-47]. Recent data, however, have failed to establish a clear association between ALTR development and ion concentrations, component position, or the magnitude of component wear [19, 27, 35, 83]. Moreover, nearly half of patients with a MoM implant and a documented ALTR have ion concentrations less than comparative patients with similar prostheses, but no evidence of a pseudotumor [34, 59, 83].
Much research has been directed toward identifying at-risk patients according to postoperative parameters, at which point little can be done to avoid the deleterious effects of revision surgery. Conversely, the ability to identify “at-risk” patients through preoperative screening affords the potential opportunity to avoid the morbid sequelae resultant from revision arthroplasty. Given the well-accepted immunologic role in pseudotumor development, genetic screening may provide the answer as to why some patients function well and remain lesion-free with MoM bearings, whereas others, in seemingly identical circumstances, experience ATLR development and early implant construct failure. Exemplified by work done in the field of pharmacogenetics, the power of human leukocyte antigen (HLA) type screening lies in the ability to identify individuals genetically susceptible to this devastating outcome before exposure.
The aim of this research, as a clinical-genotype correlation analysis, was to identify specific genes (alleles) associated with the development of ALTRs in patients with in situ MoM THAs.
Patients and Methods
A case-control study was performed using prospectively collected data at a single, tertiary referral center. A target cohort of 372 patients was identified from a comprehensive, local database that consisted of all patients who had undergone primary THA with the use of a large head, stemmed, MoM bearing couple between January 2005 and December 2008 performed by one of four senior arthroplasty surgeons on staff. This represented 11.9% (372 of 3119) of the total number of THAs performed during this timeframe. Enrollment of all participants took place during the period January 2014 to July 2015 after approval by the institutional human subject research ethics board.
Given the novel concept of the current study, and its hypothesis-screening nature, no formal sample size calculation was performed. A target sample size of 40 patients (that is, 20 patients and 20 control subjects) was identified a priori as sufficient to satisfy the objectives of the study.
Eligibility for inclusion in the study included patients 18 years of age or older with a unilateral, large-head, primary MoM THA with a minimum of 5 years followup. Exclusion criteria included documented periprosthetic joint infection (15), known autoimmune disease (six), joint arthroplasty implants in addition to the THA of interest—including in situ bilateral THA or TKA(s) (68), and a history of implanted metal in situ for a duration > 6 weeks (medical device/osteosynthesis construct for fracture or other foreign body), not including the THA prosthesis of interest (27). After exclusions, 256 patients were identified as potential candidates for inclusion. Of these, incomplete or unavailable medical records or final pathology reports limited the final cohort size to 198 patients.
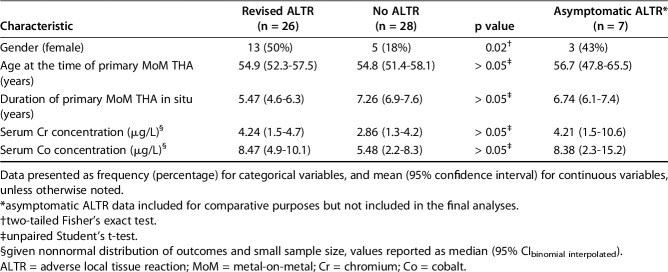
A total of 26 patients and 28 control subjects were enrolled, representing 26 of 198 (13%) and 28 of 198 (14%) of the available cohort, respectively. Baseline demographics were comparable between the study groups (Table 1), with the exception of the relative gender distribution. A higher proportion of females (50% [13 of 26]) was observed in the case group versus the control group (18% [five of 28]; p = 0.02). To ensure representativeness of the final patient and cohort samples, the basic demographics of age and gender were compared with the total initial sample identified (all MoM THAs during the study period = 372). The mean age of this whole group was 54.3 years, comprising 262 males (262 of 372 [70%]) with an average age of 54 years and 110 females (110 of 372 [30%]) with an average age of 54.8 years. There were no differences identified in comparisons between the patients in the total initial sample (n = 372) and case groups (n = 26) (all p values > 0.05), other than the gender ratios whereby a higher proportion of females was seen in the case group (29% [110 of 372] versus 50% [13 of 26]; p = 0.03).
Table 1.
Subject demographics and clinical characteristics
Cases were defined as patients who had undergone revision of a primary MoM THA prosthesis for the management of a symptomatic ALTR. The decision to revise was based on clinical findings in concert with evidence of an ALTR on ultrasonography and/or metal artefact reduction (MAR) magnetic resonance imaging (MRI) scan. The patients comprised the study group “revised ALTR”.
Control subjects were defined as subjects with a single, primary MoM THA in situ and no evidence of an ALTR on study-specific ultrasonography or other imaging available at the time of enrollment. To avoid selection bias, potential control subjects were randomly selected from the study database using simple random number generation tables. Again, to minimize the potential confounding effect of targeted selection bias, no attempt was made to match demographic characteristics. All control subjects were asymptomatic with respect to the hip of interest. Once consented enrollment had been achieved, ultrasound was performed to evaluate for presence of an ALTR. Subjects classified as control subjects were referred to as “no ALTR.” Seven patients (11.5% [seven of 61]) were initially recruited as potential control subjects, but were subsequently found to have ALTRs on screening imaging (“asymptomatic ATLR”). To avoid data contamination, these individuals were excluded from the final analyses of an association between HLA genotype and revision, but their demographic information was included for comparative analysis.
Data pertaining to baseline characteristics and clinical course were collected from the database for all patients enrolled. Genotype data from all study participants were also compared with healthy, matched population norms derived from the Canadian HLA gene frequency database (Canadian Blood Services). This comparison functioned as a population control reference. Determination of case-control status was made independent of HLA genotype.
Serum Metal Ion Analysis
Serum ion concentrations of cobalt (Co) and chromium (Cr) were collected in all study patients at the time of enrollment. For participants in the revised ALTR group, only serum Cr and Co ion concentrations sampled within 3 months of revision surgery were considered. One vial of blood was collected from each patient using a plastic 7-mL nonadditive, red label, blue top BD Vacutainer® tube (Trace Element, Serum, Reference 368380; Becton Dickinson, Franklin Lakes, NJ, USA). Blood was allowed to clot for 20 minutes before being centrifuged with the stopper on for 15 minutes. The serum was then transferred using a polypropylene transfer pipette into a 7-mL Sarstedt polypropylene tube (Sarstedt, Nümbrecht, Germany). It was then stored at -20° C before analysis. All specimens were shipped to the Trace Elements Laboratory at Western University, Canada, under strictly controlled medical specimen transport conditions. The laboratory used the Thermo Fisher Element 2 high-resolution sector field inductively coupled plasma mass spectrometer (Thermo Scientific, Waltham, MA, USA) for measurement of metal ions. This device is considered the gold standard for trace metal ion analysis [56, 57]. All samples were collected at the same laboratory, by the same phlebotomist, trained in this specific procedure.
Hip Ultrasound Protocol
Using a previously described and validated imaging protocol [24], shown to have a sensitivity of 100% and a specificity of 96%, outpatient ultrasonographic assessment was performed screening for the presence of an ALTR among asymptomatic patients (potential control subjects) with a primary MoM THA in situ. A single sonographer with experience in over 500 protocol-specific studies performed all of the ultrasound examinations. Each study was performed using a Siemens Antares Ultrasound System (Siemens Medical Solutions USA, Mountain View, CA, USA) and the results recorded utilizing a standardized template. The Siemens VFX9-4 linear transducer and/or the Siemens CH6-2 curvilinear transducer were used for all studies based on the body habitus of the patient being evaluated. All studies provided images satisfactory for diagnosis. Acquisition time was approximately 20 minutes. Twenty-two (22 of 26 [85%]) of the patients and 17 (17 of 28 [61%]) of the control group underwent study protocoled ultrasounds. Four (four of 26 [15%]) patients also underwent a MAR MRI scan, which was ordered independent of the study protocol by referring physicians. These results were available at the time of ultrasound, but the reporting radiologists were blinded to these findings for study image review. In each of the four patients, the MRI and ultrasound diagnosis of pseudotumor was concordant. There were no MRIs performed for control subjects. Two patients (one in the case group and one in the control group) also had CT scans performed but both occurred after ultrasound investigation and did not change the final diagnostic result.
All images were interpreted by one of four senior fellowship-trained musculoskeletal radiologists experienced with ultrasonography of hip prostheses and ALTR pathology. The radiologists were blinded to both the clinical status of the patient and the HLA genotype results. The presence, size, and position of any fluid, cystic mass, or solid mass related to the hip were recorded along with the involvement of any local neurovascular structures. Independently, each radiologist was asked to provide a definitive statement on whether the scan was normal or abnormal and to confirm the presence or absence of an ALTR. In the event of an abnormal study, each radiologist categorized the findings according to one or more of the following: (1) simple fluid collection; (2) cystic mass or a complex fluid collection; or (3) solid mass. Simple fluid collections were defined as anechoic with increased through-transmission and a well-defined posterior wall, whereas a complex fluid collection/cystic mass contained debris (internal echoes), septations, or both. The diagnosis of an ALTR was based on the presence of a complex fluid collection, cystic mass, or solid mass. When a definitive diagnosis could not be made, a second radiologist, blinded from the report of the first, interpreted the study followed by open discussion and consensus agreement on the findings.
HLA Genotyping
A second 7-mL peripheral blood specimen was collected from each participant in EDTA BD Vacutainer tubes (Becton Dickinson Reference #367863) and stored in a medical refrigerator at 4° C. Samples were then transferred to an on-site College of American Pathologists/American Society for Histocompatibility and Immunogenetics-certified immunology laboratory at Vancouver General Hospital (Vancouver, British Columbia, Canada). Genomic DNA for each participant was extracted using an automated robotic method (Quiasymphony, Quiagen, Canada) and polymerase chain reaction amplification performed using an Applied Biosystems thermocycler (Foster City, CA, USA). HLA typing was performed using Luminex sequence-specific oligonucleotide typing kits (Luminex, Austin, TX, USA) to determine HLA Class II DRB1, DQA1, and DQB1 allelic specificities at intermediate and high resolution as required using a polymerase chain reaction amplification technique with group-specific primers. These loci were selected because of their known relationship with inflammatory disorders [51], as defined by the CDW 2.0.0 catalog [78].
Study endpoints are reported as frequency and percentages (95% confidence interval [CI]) for categorical variables and mean (95% CI) values for continuous variables, unless otherwise noted. A test for normality of study endpoints was performed using a combined skewness and kurtosis test, when possible. Homogeneity of demographic and clinical characteristics across the study groups was performed using one-way analysis of variance for continuous, parametric endpoints, Kruskal-Wallis test for nonparametric continuous endpoints, and Fisher’s exact test for categorical endpoints. Pairwise comparisons between “revised ALTR” and “no ALTR” groups were completed according to traditional parametric and nonparametric hypothesis tests.
The frequency of a particular allele was arranged in a two-by-two contingency according to case/control status and was evaluated using a Pearson chi-square test. No correction for multiple testing was performed given the colinearity of the allelic variants at the loci examined. Evaluation of differences in observed and expected allelic carriage rates among patients and control subjects was performed using a one-tailed, binomial probability test. Matched-population norms were used to derive expected allele frequencies.
An odds ratio (OR) was used to quantify the clinical association between HLA genotype and the need for revision secondary to symptomatic ALTR. Statistical significance of the OR was set at 0.10 a priori and determined using a Fisher’s exact test given the small sample size. A 90% CI for the OR was calculated according to Woolf’s method.
The level of statistical significance was set at p < 0.05, unless otherwise noted. Statistical analysis was performed with STATA (Version 13.1; StataCorp, College Station, TX, USA).
Results
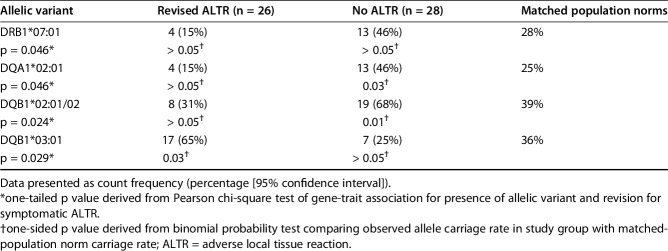
Genetic analysis at the major histocompatibility complex Class II loci showed clear differences in allelic carriage rates between patient groups (Table 2). The frequency of DQB1*03:01 was increased in participants with ALTR, being present in 65% (17 of 26) compared with only 25% (seven of 28) of those without disease (p = 0.02), or 36% of the normal population (p = 0.03) [78]. In contrast, HLA-DRB1*07:01, DQA1*02:01, and DQB1*02:01/02 were negatively associated with the incidence of revision for symptomatic ALTR in the study cohort. HLA-DRB1*07:01 was expressed by only 15% (four of 26) of patients with ALTR compared with 46% (13 of 28) without (p = 0.05) and 28% of the normal population (p = 0.20) [78]. HLA-DQA1*02:01 was also expressed by 15% (four of 26) of affected patients compared with 46% (13 of 28) free from ALTR (p = 0.05) and 25% of normal control subjects (p = 0.28). HLA-DQB1*02:01/02 was expressed by 31% (eight of 26) of patients with ALTR compared with 68% (19 of 28) of disease-free participants (p = 0.02) and 39% of the normal population (p = 0.31) [78]. These alleles occur in high linkage disequilibrium with their respective frequencies of association above that which would be expected if the loci were independent and associated randomly [49]. There was no effect with the numbers available of homozygosity or specific allelic combinations at any of these loci within the population tested.
Table 2.
Allelic variant carriage rates
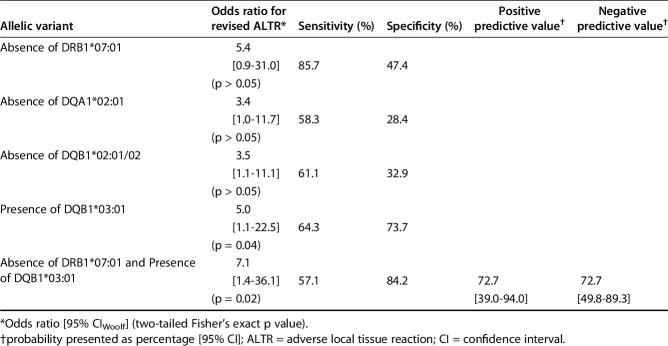
Among participants with the DQB1*03:01 allele present, the OR for undergoing revision secondary to ALTR was 5.0 (95% CIWoolf, 1.1-22.5). Among participants without the DRB1*07:01, DQA1*02:01, and DQB1*02:01/02 alleles, the OR (95% CIWoolf) for undergoing revision was 5.4 (0.9-31.0), 3.4 (1.0-11.7), and 3.5 (1.1-11.1), respectively. The greatest observed increase in clinical risk profile was noted when the “at-risk” DQB1*03:01 allele was present and “protective” DRB1*07:01 allele was simultaneously absent, resulting in an OR for revision of 7.1 (95% CIWoolf, 1.4-36.1). Diagnostic performance of the alleles of interest according to the study data was collated and summarized (Table 3).
Table 3.
Clinical association and diagnostic performance of various alleles
Serum Cr and Co concentrations, age at primary MoM THA, and gender were not independently associated, with the numbers available, with subsequent revision surgery secondary to symptomatic ATLR development.
Discussion
The previously unpredictable development of pseudotumors in patients with primary MoM THAs has long remained a perplexing clinical conundrum. Recent histopathologic studies have defined key Th-1 cell-mediated immune pathways in the evolution of these lesions [30] driven by acquired, antigen-dependent processes. These findings suggest a potential underlying genetic predisposition at a patient-specific level. The purpose of the current study, therefore, was to explore gene frequencies—based on alleles known to have Th-1 modulating linkage—associated with ALTR formation in patients with in situ MoM hip replacements. We found that the alleles DRB1*07:01, DQA1*02:01, DQB1*02:01/02, and DQB1*03:01 were associated with revision hip surgery secondary to symptomatic ALTR among patients with a primary MoM THA when compared with patients without evidence of an ALTR. Although the presented data suggest that the presence of DQB1*03:01 confers an increased risk of pseudotumor development, the DRB1*07:01 allele conversely appears to afford some genetically ascribed protection against the development of a symptomatic ALTR and consequent need for revision surgery. Independent of serum metal ion concentrations and of patient age at the time of the primary MoM THA, those patients lacking the DRB1*07:01 allele, but harboring the DQB1*03:01 allele, were found to have an increased odds of undergoing revision surgery.
The findings reported here must be considered with the context of the limitations of the study. First, given the hypothesis-generating nature of this study, the relatively small sample size limits the confidence in which these findings can be more broadly generalized. Moreover, the limited statistical power may have compromised our ability to identify associations between certain allotypic variants and the incidence of the need for surgical revision, which, given a larger number of participants, may be found to be associated with revision surgery secondary to ALTRs. Nonetheless, despite this, we were able to demonstrate discrepancies in allele carriage rates between the “no ALTR” and “revised ALTR” patients. Of the eligible cohort size (372 patients who underwent MoM THAs during the study collection period), 174 (47%) were excluded, the majority resulting from the presence of an in situ second (or multiple) joint arthroplasty or other implanted metallic device or component. Although this approximately halved the potential eligible group for sampling, we maintain this was a necessary and important step to minimize the potentially confounding effect of metal debris/incited tissue reaction from sources other than the single joint of interest. The relative risk increase posed to patients of ALTR development in the setting of multiple in situ implants remains unclear and represents another potential avenue for investigation. Our decision to exclude seven patients with an “asymptomatic ALTR” from the primary analysis also warrants further discussion. When performing gene-trait association studies, it is of utmost importance to precisely define the phenotype of interest and accurately delineate patients according to the defined phenotype to ensure internal and external validity. For this study, we chose to focus on the most clinically relevant phenotype, patients who developed symptomatic ALTRs who underwent revision. Although we can be reasonably confident that patients with “no ALTR” represent a discrete phenotype from those within the “revised ALTR” group, we cannot be certain as to which phenotype best describes the “asymptomatic ALTR” patients. This consideration provides an avenue for future research.
Further complicating the classification of these patients is the use of ultrasound for diagnosis. With a previously demonstrated and published 100% sensitivity for detecting the presence of an ALTR in asymptomatic patients [24], we are confident in the negative predictive value of the technique used. However, given a reported diagnostic false-positive rate of 4% (0%–12%), it may be reasonably postulated that of the asymptomatic patients subsequently identified as having an ALTR lesion, and therefore excluded from further analysis (that is, patients with “asymptomatic ALTRs”), a small likelihood of mischaracterization exists. The potential effect on resultant data analysis is unclear but is likely to be small.
It is important to note that disparity did exist with regard to the relative gender distribution between the two study groups. The overall cohort showed a male predominance (67% [36 of 54]), although 50% (13 of 26) of the included patients were female. Why such a gender imbalance existed remains unclear although it may simply have reflected the small overall sample size and random sampling error. This represents another potential avenue for future investigation utilizing a larger cohort size. Any potential confounding bias resulting from the disproportionate female representation was ameliorated by the a priori control of gender as an independent covariate in the final data analyses.
Although the important contribution of acetabular cup malpositioning has been well documented as a precursor to wear debris generation, the volume of particulate debris and the resultant synovial and serum metal ion levels [59] have independently been demonstrated not to directly influence subsequent pseudotumor development. Like in all THA construct types, the importance of correct component positioning (both acetabular and femoral) in influencing implant survivorship is clear; however, in the setting of MoM bearings, this has not been correlated with ALTR formation.
Lastly, the population allelic carriage rates used to determine the expected frequencies for the study participants were derived from a predominantly North American white population. Although representative of the ethnic distribution of our study participants, there may be some discrepancy between the true carriage rates for the underlying study population and the population norms used for the study. This again provides an avenue for future related research centered among different racial and ethnic cohorts.
Support for this type of biophysiologic predictive model can be found in recent immunogenetics work [32]. Among patients with Type 1 diabetes mellitus [84, 86], Graves’ disease [87], scleroderma, and multiple sclerosis [5], allelic frequencies for DRB1*07, DQA1*02, and DQB1*02 are lower among individuals displaying the condition of interest, as compared with those who do not (ie, matched control subjects). Such findings suggest that the individual presence of these specific alleles may offer protection against Th-1-mediated autoimmune processes [55] similar to the development of ALTRs in the setting of metal ion exposure.
With regard to the potential clinical utility of our findings, there is no better example than the rapidly evolving field of pharmacogenetics. Over the past decade, gene-trait association studies have shed light on immunologically mediated drug reactions long thought to be randomly evolved and otherwise unpredictable. To date more than 50 specific HLA alleles have been implicated in the development of drug and environmental antigen-related hypersensitivities [9, 70] biologically similar to the acquired immune response to metal ions in the setting of ALTR formation [9, 28-30, 70, 81]. As a result of such breakthroughs, the FDA [12, 22] and the US Department of Health [37, 60, 67] recently provided recommendations for HLA genotype screening before administration of medications known to be associated with life-threatening adverse reactions. Such preintervention screening has been shown to be both an efficacious [61, 72] and cost-effective [41, 53, 73] means for the primary prevention of medication-induced Type IV hypersensitivities. The extrapolation of such applications to the prospective identification of patients potentially at risk for the development of periprosthetic pseudotumors through exposure to MoM joint arthroplasties, after the clear characterization of high-risk genotypes, holds much clinical promise and obvious potential benefit.
Similar to its current use in the field of pharmacogenetics, prospective HLA typing may soon be capable of identifying patients genetically predisposed to the development of metal ion-induced pseudotumors, before surgery, allowing the selection of implant and bearing combinations known to place the individual patient at the lowest possible risk. As a preoperative screening tool, from an alternative line of thinking, this result may even provide surgeons with the confidence to exploit the superior biomechanical and wear properties of MoM bearing couples [71] without the fear of the catastrophic sequelae associated with ALTR development, particularly in the demonstrated presence of a “protective” allele combination. Moreover, as we continue to gain an appreciation for metal ion-induced ALTRs within the context of various modular femoral stem designs [39, 40, 68, 77], the findings of this study may also demonstrate value in reducing the incidence of failures attributed to such implants.
The findings of the current study present evidence suggesting a strong association between HLA allotype and the incidence of revision surgery for the management of symptomatic ALTRs (pseudotumors) among patients with a single, primary, MoM THA prosthesis. To our knowledge, this work represents the first published demonstration of such a clinical association and has potentially wide-reaching clinical application in future prospective patient management. Although these results will require wider ratification and further expansion, this work certainly provides foundation data for an exciting new frontier within the field of adult hip arthroplasty, which may show future benefit in appropriately prospectively identifying “at-risk” patient genotypes. Extrapolating this work further, diagnosis of the “at-risk” DRB1*07:01, DQA1*02:01, DQB1*02:01/02, or DQB1*03:01 alleles in patients with known in situ MoM THAs may be influential in guiding clinical decision-making when considering the role of early intervention approaches.
Acknowledgments
We thank and recognize the contributions of Ms Raman Johal and Ms Azarm Akhavien for their hard work and effort in the consolidation and preparation of the samples and data utilized in this work. We also thank the staff of the Department of Radiology, Vancouver General Hospital (Vancouver, British Columbia, Canada) for their generous contribution of time, resources, and expertise in the scanning and image interpretation phases of this study.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Vancouver Coastal Health Authority, Vancouver General Hospital, University of British Columbia, Vancouver, British Columbia, Canada.
References
- 1.Abbas A, Lichtman A, Pober J. Cellular and Molecular Immunology. 4th ed. Philadelphia, PA, USA: Saunders; 2000:34–36. [Google Scholar]
- 2.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumours in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013;471:3814–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992;276:7–18. [PubMed] [Google Scholar]
- 4.Amstutz HC, Su EP, Le Duff MJ. Surface arthroplasty in young patients with hip arthritis secondary to childhood disorders. Orthop Clin North Am. 2005;36:223–230. [DOI] [PubMed] [Google Scholar]
- 5.Arnett FC, Gourh P, Shete S, Ahn CW, Honey RE, Agarwal SK, Tan FK, McNearney T, Fischbach M, Fritzler MJ, Mayes MD, Reveille JD. Major histocompatibility complex (MHC) class II alleles, haplotypes and epitopes which confer susceptibility or protection in systemic sclerosis: analyses in 1300 Caucasian, African-American and Hispanic cases and 1000 controls. Ann Rheum Dis. 2010;69:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aroukatos P, Repanti M, Repantis T, Bravou V, Korovessis P. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Australian Orthopaedic Association National joint replacement registry (AOANJRR): annual report. Available at: https://aoanjrr.sahmri.com/. Accessed February 15, 2017.
- 8.Berton C, Girard J, Krantz N, Migaud H. The durom large diameter head acetabular component: early results with a large-diameter metal-on-metal bearing. J Bone Joint Surg Br. 2010;92:202–208. [DOI] [PubMed] [Google Scholar]
- 9.Bharadwaj M, Illing P, Theodossis A, Purcell AW, Rossjohn J, McCluskey J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401–403. [DOI] [PubMed] [Google Scholar]
- 10.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. [DOI] [PubMed] [Google Scholar]
- 11.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumour-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. [DOI] [PubMed] [Google Scholar]
- 13.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. [DOI] [PubMed] [Google Scholar]
- 14.Davda K, Lali FV, Sampson B, Skinner JA, Hart AJ. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg Br. 2011;93:738–745. [DOI] [PubMed] [Google Scholar]
- 15.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. [DOI] [PubMed] [Google Scholar]
- 16.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. [DOI] [PubMed] [Google Scholar]
- 17.Desy NM, Bergeron SG, Petit A, Huk OL, Antoniou J. Surgical variables influence metal ion levels after hip resurfacing. Clin Orthop Relat Res. 2011;469:1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res. 1998;42:103–111. [DOI] [PubMed] [Google Scholar]
- 19.Ebramzadeh E, Campbell P, Tan TL, Nelson SD, Sangiorgio SN. Can wear explain the histological variation around metal-on-metal total hips? Clin Orthop Relat Res. 2015;473:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans EM, Freeman MA, Miller AJ, Vernon-Roberts B. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Joint Surg Br. 1974;56:626–642. [DOI] [PubMed] [Google Scholar]
- 21.Fang CS, Harvie P, Gibbons CL, Whitwell D, Athanasou NA, Ostlere S. The imaging spectrum of peri-articular inflammatory masses following metal-on-metal hip resurfacing. Skeletal Radiol. 2008;37:715–722. [DOI] [PubMed] [Google Scholar]
- 22.Ferrell PB, Jr, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9:1543–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frigerio E, Pigatto PD, Guzzi G, Altomare G. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273–279. [DOI] [PubMed] [Google Scholar]
- 24.Garbuz DS, Hargreaves BA, Duncan CP, Masri BA, Wilson DR, Forster BB. The John Charnley Award: Diagnostic accuracy of MRI versus ultrasound for detecting pseudotumours in asymptomatic metal-on-metal THA. Clin Orthop Relat Res. 2014;472:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glyn-Jones S, Roques A, Taylor A, Kwon YM, McLardy-Smith P, Gill HS, Walter W, Tuke M, Murray D. The in vivo linear and volumetric wear of hip resurfacing implants revised for pseudotumour. J Bone Joint Surg Am. 2011;93:2180–2188. [DOI] [PubMed] [Google Scholar]
- 26.Goodman SB, Gibon E, Yao Z. The basic science of periprosthetic osteolysis. Instr Course Lect. 2013;62:201–206. [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin W, Fehring T, Kudrna J, Schmidt R, Christie M, Odum S, Dennos A. Are metal ion levels a useful trigger for surgical intervention? J Arthroplasty. 2012;27(Suppl):32–36. [DOI] [PubMed] [Google Scholar]
- 28.Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–391. [DOI] [PubMed] [Google Scholar]
- 29.Hallab NJ, Caicedo M, Epstein R, McAllister K, Jacobs JJ. In vitro reactivity to implant metals demonstrates a person-dependent association with both T-cell and B-cell activation. J Biomed Mater Res A. 2010;92:667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallab NJ, Caicedo M, Finnegan A, Jacobs JJ. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg Res. 2008;13;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallab NJ, Caicedo M, McAllister K, Skipor A, Amstutz H, Jacobs JJ. Asymptomatic prospective and retrospective cohorts with metal-on-metal hip arthroplasty indicate acquired lymphocyte reactivity varies with metal ion levels on a group basis. J Orthop Res. 2013;31:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handunnetthi L, Ramagopalan SV, Ebers GC, Knight JC. Regulation of major histocompatibility complex class II gene expression, genetic variation and disease. Genes Immun. 2010;11:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart AJ, Buddhdev P, Winship P, Faria N, Powell JJ, Skinner JA. Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after metal-on-metal hip resurfacing. Hip Int. 2008;18:212–219. [DOI] [PubMed] [Google Scholar]
- 34.Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B, Skinner JA. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br. 2011;93:1308–1313. [DOI] [PubMed] [Google Scholar]
- 35.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumours in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–325. [DOI] [PubMed] [Google Scholar]
- 36.Heilpern GN, Shah NN, Fordyce MJ. Birmingham hip resurfacing arthroplasty: a series of 110 consecutive hips with a minimum five-year clinical and radiological follow-up. J Bone Joint Surg Br. 2008;90:1137–1142. [DOI] [PubMed] [Google Scholar]
- 37.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, Fling ME, Stocum M, Bowman C, Thurmond LM, Roses AD. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. [DOI] [PubMed] [Google Scholar]
- 38.Howard JL, Kremers HM, Loechler YA, Schleck CD, Harmsen WS, Berry DJ, Cabanela ME, Hanssen AD, Pagnano MW, Trousdale RT, Lewallen DG. Comparative survival of uncemented acetabular components following primary total hip arthroplasty. J Bone Joint Surg Am. 2011;93:1597–1604. [DOI] [PubMed] [Google Scholar]
- 39.Howie DW, Cain CM, Cornish BL. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J Bone Joint Surg Br. 1991;73:29–32. [DOI] [PubMed] [Google Scholar]
- 40.Hsu AR, Gross CE, Levine BR. Pseudotumour from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ). 2012;41:422–426. [PubMed] [Google Scholar]
- 41.Hughes DA, Vilar FJ, Ward CC, Alfirevic A, Park BK, Pirmohamed M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics. 2004;14:335–342. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs JJ, Shanbhag A, Glant TT, Black J, Galante JO. Wear debris in total joint replacements. J Am Acad Orthop Surg. 1994;2:212–220. [DOI] [PubMed] [Google Scholar]
- 43.Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review of current knowledge and future strategies. J Bone Joint Surg Br. 2007;89:567–573. [DOI] [PubMed] [Google Scholar]
- 44.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–1191. [DOI] [PubMed] [Google Scholar]
- 45.Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg Br. 2010;92:356–361. [DOI] [PubMed] [Google Scholar]
- 46.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. [DOI] [PubMed] [Google Scholar]
- 47.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham hip resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. [DOI] [PubMed] [Google Scholar]
- 48.Lavigne M, Ganapathi M, Mottard S, Girard J, Vendittoli PA. Range of motion of large head total hip arthroplasty is greater than 28 mm total hip arthroplasty or hip resurfacing. Clin Biomech (Bristol, Avon). 2011;26:267–273. [DOI] [PubMed] [Google Scholar]
- 49.Lee WC. Testing for candidate gene linkage disequilibrium using a dense array of single nucleotide polymorphisms in case-parents studies. Epidemiology. 2002;13:545–551. [DOI] [PubMed] [Google Scholar]
- 50.Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans. A review of the potential sources and systemic health effects. Toxicology. 2017;387:43–56. [DOI] [PubMed] [Google Scholar]
- 51.Ling SF, Viatte S, Lunt M, Van Sijl AM, Silva-Fernandez L, Symmons DP, Young A, Macgregor AJ, Barton A. HLA-DRB1 amino acid positions 11/13, 71, and 74 are associated with inflammation level, disease activity, and the Health Assessment Questionnaire Score in patients with inflammatory polyarthritis. Arthritis Rheumatol. 2016;68:2618–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lisby S, Hansen LH, Menn T, Baadsgaard O. Nickel-induced proliferation of both memory and naive T cells in patch test-negative individuals. Clin Exp Immunol. 1999;117:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Locharernkul C, Shotelersuk V, Hirankarn N. Pharmacogenetic screening of carbamazepine-induced severe cutaneous allergic reactions. J Clin Neurosci. 2011;18:1289–1294. [DOI] [PubMed] [Google Scholar]
- 54.Lohmann CH, Meyer H, Nuechtern JV, Singh G, Junk-Jantsch S, Schmotzer H, Morlock MM, Pflüger G. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am. 2013;95:1561–1568. [DOI] [PubMed] [Google Scholar]
- 55.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. [DOI] [PubMed] [Google Scholar]
- 56.London Laboratory Services Group. Trace elements laboratory. Available at: www.lhsc.on.ca/lab/metals/icpms1.htm. Accessed November 15, 2014.
- 57.Loughead JM, Starks I, Chesney D, Matthews JN, McCaskie AW, Holland JP. Removal of acetabular bone in resurfacing arthroplasty of the hip: a comparison with hybrid total hip arthroplasty. J Bone Joint Surg Br. 2006;88:31–34. [DOI] [PubMed] [Google Scholar]
- 58.Mabilleau G, Kwon YM, Pandit H, Murray DW, Sabokbar A. Metal-on-metal hip resurfacing arthroplasty: a review of periprosthetic biological reactions. Acta Orthop. 2008;79:734–747. [DOI] [PubMed] [Google Scholar]
- 59.Malek IA, Rogers J, King AC, Clutton J, Winson D, John A. The interchangeability of plasma and whole blood metal ion measurement in the monitoring of metal on metal hips. Arthritis. 2015;2015:216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, Christiansen FT. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. [DOI] [PubMed] [Google Scholar]
- 61.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A; PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. [DOI] [PubMed] [Google Scholar]
- 62.McMinn DJ, Daniel J, Ziaee H, Pradhan C. Results of the Birmingham hip resurfacing dysplasia component in severe acetabular insufficiency: a six- to 9.6-year follow-up. J Bone Joint Surg Br. 2008;90:715–723. [DOI] [PubMed] [Google Scholar]
- 63.Merritt K, Rodrigo JJ. Immune response to synthetic materials. sensitization of patients receiving orthopaedic implants. Clin Orthop Relat Res. 1996;326:71–79. [PubMed] [Google Scholar]
- 64.National Joint Registry (NJR) for England and Wales. Available at: www.njrcentre.org.uk. Accessed November 15, 2014.
- 65.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. [DOI] [PubMed] [Google Scholar]
- 66.Pandit H, Vlychou M, Whitwell D, Crook D, Luqmani R, Ostlere S, Murray DW, Athanasou NA. Necrotic granulomatous pseudotumours in bilateral resurfacing hip arthroplasties: evidence for a type IV immune response. Virchows Arch. 2008;453:529–534. [DOI] [PubMed] [Google Scholar]
- 67.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. US Department of Health and Human Services. Available at: aidsinfo.nih.gov. Accessed November 15, 2014.
- 68.Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paukkeri EL, Korhonen R, Hämäläinen M, Pesu M, Eskelinen A, Moilanen T, Moilanen E. The inflammatory phenotype in failed metal-on-metal hip arthroplasty correlates with blood metal concentrations. PLoS One. 2016;11:e0155121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–1306. [DOI] [PubMed] [Google Scholar]
- 71.Rieker CB. Tribology of total hip arthroplasty prostheses–what an orthopaedic surgeon should know. EFORT Open Rev. 2016;1:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, Stancil B, Mosteller M, Brothers C, Wannamaker P, Hughes A, Sutherland-Phillips D, Mallal S, Shaefer M; Study of Hypersensitivity to Abacavir and Pharmacogenetic Evaluation Study Team. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111–1118. [DOI] [PubMed] [Google Scholar]
- 73.Schackman BR, Scott CA, Walensky RP, Losina E, Freedberg KA, Sax PE. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy for HIV. AIDS. 2008;22:2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 75.Singh G, Meyer H, Ruetschi M, Chamaon K, Feuerstein B, Lohmann CH. Large-diameter metal-on-metal total hip arthroplasties: a page in orthopedic history? J Biomed Mater Res A. 2013;101:3320–3326. [DOI] [PubMed] [Google Scholar]
- 76.Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379:1199–1204. [DOI] [PubMed] [Google Scholar]
- 77.Tallroth K, Eskola A, Santavirta S, Konttinen YT, Lindholm TS. Aggressive granulomatous lesions after hip arthroplasty. J Bone Joint Surg Br. 1989;71:571–575. [DOI] [PubMed] [Google Scholar]
- 78.The Common and Well-Documented (CWD) Alleles 2.0.0 catalog. Available at: http://igdawg.org/cwd.html. Accessed February 15, 2017.
- 79.Thierse HJ, Moulon C, Allespach Y, Zimmermann B, Doetze A, Kuppig S, Wild D, Herberg F, Weltzien HU. Metal-protein complex-mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel-specific human T cell activation. J Immunol. 2004;172:1926–1934. [DOI] [PubMed] [Google Scholar]
- 80.US Food and Drug Administration. Concerns about metal-on-metal hip implants. Available at: www.fda.gov. Accessed November 15, 2014.
- 81.Vermes C, Kuzsner J, Bardos T, Than P. Prospective analysis of human leukocyte functional tests reveals metal sensitivity in patients with hip implant. J Orthop Surg Res. 2013;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. [DOI] [PubMed] [Google Scholar]
- 83.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumour in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93:2164–2171. [DOI] [PubMed] [Google Scholar]
- 84.Yanagawa T, Mangklabruks A, Chang YB, Okamoto Y, Fisfalen ME, Curran PG, DeGroot LJ. Human histocompatibility leukocyte antigen-DQA1*0501 allele associated with genetic susceptibility to Graves' disease in a Caucasian population. J Clin Endocrinol Metab. 1993;76:1569–1574. [DOI] [PubMed] [Google Scholar]
- 85.Yang J, Black J. Competitive binding of chromium, cobalt and nickel to serum proteins. Biomaterials. 1994;15:262–268. [DOI] [PubMed] [Google Scholar]
- 86.Yang J, Merritt K. Production of monoclonal antibodies to study corrosion products of CO-CR biomaterials. J Biomed Mater Res. 1996;31:71–80. [DOI] [PubMed] [Google Scholar]
- 87.Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves' disease. Am J Med Genet. 2000;95:432–437. [DOI] [PubMed] [Google Scholar]