Abstract
Background
Randomized trials have shown the benefits of injecting bone marrow-derived mesenchymal stem cells (BmMSCs) after standard hip decompression in patients with osteonecrosis of the femoral head. However, the combination of BmMSCs and platelet-rich plasma (PRP) injected into the femoral head after decompression has not been reported previously. This study reports the results in a preliminary series of patients with osteonecrosis of the femoral head treated with BmMSCs plus PRP.
Questions/purposes
(1) What is the survivorship free from reoperation, hip arthroplasty, and femoral head collapse in a preliminary series of patients with osteonecrosis of the femoral head treated with BmMSCs plus PRP? (2) Is there a change in the degree of femoral head involvement based on modified Kerboul angle? (3) What were the scores observed for pain and function at last followup? (4) Was there a difference in survivorship free from reoperation as a function of in vitro MSC count and viability?
Methods
Twenty-two consecutive patients (35 hips; 11 men and 11 women) with corticosteroid-induced osteonecrosis who met study inclusion criteria were enrolled; none declined participation, and none was lost to followup, although one patient (two hips) died within a year of the procedure for reasons unrelated to it, and five patients (seven hips) did not undergo MRI at the 1-year followup. All patients had precollapse osteonecrosis, rated either University of Pennsylvania Stage 1 (n = 4) or Stage 2 (n = 31 hips). Mean age and body mass index were 43 years and 31 kg/m2, respectively. Patients underwent pre- and postoperative radiographs and MRI to assess femoral head involvement using the modified Kerboul angle. Absolute cell count and colony-forming unit (CFU) assays were used to assess MSC abundance and viability of the bone marrow obtained at the time of surgery. Patients were followed at regular intervals to assess clinical response to treatment with a mean followup of 3 years (range, 2-4 years). The change in femoral head involvement was assessed with the modified Kerboul angle; the Harris hip score was used to assess clinical outcome; and conversion to THA, reoperation, and survivorship free from femoral head collapse were analyzed with the Kaplan-Meier method on a per-hip basis.
Results
Survivorship free from THA, any procedure, and femoral head collapse was 84% (95% confidence interval [CI], 75%-93%), 67% (95% CI, 55%-79%), and 93% (95% CI, 76%-98%), respectively, at 3 years postoperatively; two patients (four hips) underwent a second decompression and MSC injection for persistent pain without signs of radiographic collapse. All patients with collapse underwent THA. The mean modified Kerboul angle improved from 205° ± 47° to 172° ± 48° postoperatively (mean change -30° ± 6°, p = 0.01). A greater proportion of patients who underwent an additional procedure had a modified Kerboul grade of 3 or 4 preoperatively (80% [four of five] versus 13% [four of 30 Grade 1 or 2; odds ratio, 26; 95% CI, 2-296; p = 0.005). Preoperatively the mean Harris hip score was 57 ± 12, which improved to 85 ± 15 (mean change 28 ± 3, p < 0.001) at most recent followup. Patients undergoing a reoperation or THA had a lower mean concentration of nucleated cells/mL (5.5 x 106 ± 2.8 x 106 cells/mL versus 2.3 x 107 ± 2.2 x 107 cells/mL, p = 0.02) and lower mean CFUs (13 ± 6 versus 19 ± 7, p = 0.04) compared with those who did not.
Conclusions
Core hip decompression with injection of concentrated bone marrow plus PRP improved pain and function; > 90% of hips in this series were without collapse at a minimum of 2 years. In this preliminary study, successful results were seen when nucleated cell count was high and modified Kerboul grade was low. Further randomized studies are needed to determine this procedure’s efficacy versus core decompression or nonoperative treatment alone.
Level of Evidence:
Level II, therapeutic study.
Introduction
Osteonecrosis (ON) of the femoral head occurs in 10,000 to 20,000 new patients in the United States each year, predominantly in patients younger than 40 years of age [28, 32, 33]. The disease is characterized by trabecular and subchondral bone death, leading to fracture and collapse of the overlying articular surface [9, 16, 34, 35]. Once collapse of the articular surface occurs, the disease course rarely regresses, often leading to severe pain, functional disability, and sometimes THA in young patients [4, 5, 17, 20, 21, 25, 34]. Currently, it is not clear what leads to osteocyte death; however, oral corticosteroid use is a known risk factor for the disease [7, 23].
Hip decompression alone has been used to treat ON in precollapse stages [3, 8, 9, 11, 12, 18, 22, 24, 30, 31, 42, 43] with a 10-year hip preservation rate of 96% patients with Ficat Stage I disease [8]. Although patients with Ficat Stage I disease are often effectively treated with core decompression alone, patients with more advanced precollapse disease (Ficat or University of Pennsylvania Stage II) treated with core decompression alone have a reported failure rate of up to 77% [24, 42]. To improve rates of hip preservation, the addition of different adjuvants to decompression including the injection of autologous mesenchymal stem cells (MSCs) obtained from iliac crest bone marrow concentrate (BMC) has been investigated [10, 12, 18, 22, 30, 43]; however, these approaches are not universally successful. Platelet-rich plasma (PRP) is an easily obtainable, autologous source of additional growth factors such as vascular endothelial growth factor, platelet-derived growth factor, transforming growth factor-β, and fibroblast growth factor, which has been shown to increase the rates of bone healing [13, 26, 38]. The combination of concentrated bone marrow-derived MSCs (BmMSCs) and PRP injected into the femoral head after decompression has not, to our knowledge, been reported previously.
We therefore asked: (1) What is the survivorship free from reoperation, hip arthroplasty, and femoral head collapse in a preliminary series of patients with ON of the femoral head treated with BmMSCs plus PRP? (2) Is there a change in the degree of femoral head involvement based on modified Kerboul angle? (3) What were the scores observed for pain and function at last followup? (4) Was there a difference in survivorship free from reoperation as a function of in vitro MSC count and viability?
Patients and Methods
After obtaining approval from our institutional review board, we invited all patients with precollapse corticosteroid-induced ON who met prespecified inclusion criteria to participate in this prospective study. Patients in this series underwent hip decompression augmented with BmMSC and PRP from June 1, 2012, until December 31, 2013. This consecutive cohort consisted of 22 patients (35 hips).
Patients were identified in the senior author’s (RJS) clinic as having precollapse ON of the femoral head, rated either University of Pennsylvania Stage I or II [40]. This was determined through the use of preoperative MRI and plain radiographs. To be enrolled in the study patients had to meet the following criteria: (1) patients consented to receive core decompression for a diagnosis of femoral head ON, University of Pennsylvania Stage I and II, based on preoperative MRI; (2) ages 18 to 70 years; (3) absence of a concurrent diagnosis of osteomyelitis; (4) normal bone marrow function, as defined by absolute neutrophil count > 1500/μL; and (5) radiographic and clinically confirmed ON of the femoral head.
Exclusion criteria consisted of (1) pregnant females; (2) active infection, HIV, hepatitis C, hepatitis B, or syphilis; (3) patients receiving active bisphosphonate therapy; (4) patients with poorly controlled diabetes (HgA1C > 8%), peripheral neuropathy, or vascular problems; (5) patients receiving hematopoietic growth factors or antiangiogenesis products; and (6) patients with collapse of the femoral head on preoperative imaging.
There were 11 males and 11 females with a mean age and body mass index of 43 years (range, 22-66 years) and 31 kg/m2 (range, 22-41 kg/m2), respectively, at the time of decompression. All patients had painful precollapse ON, rated either University of Pennsylvania Stage 1 (n = 4) or Stage 2 (n = 31 hips) [40]. In addition to all patients having a history of oral corticosteroid use, 14 patients were still taking oral corticosteroids at the time of decompression with a mean daily dose of 13 ± 9 mg. Seven patients were current tobacco users. An accompanying labral tear was observed in 20 of 35 hips and may have contributed to pain in some patients (Table 1). The labral tear was treated with arthroscopic débridement (n = 3) or repair (n = 2). In the remaining patients (n = 15), it was treated nonoperatively. Underlying diagnoses that required a history of corticosteroid use included asthma (n = 4), adrenal insufficiency (n = 3), polyarthralgia (n = 3), ulcerative colitis (n = 2), organ transplant (n = 2), chronic pain (n = 2), rheumatoid arthritis (n = 1), psoriasis (n = 1), Sjögren's syndrome (n = 1), leukemia (n = 1), encephalitis (n = 1), and dermatomyositis (n = 1).
Table 1.
Preoperative characteristics of patients undergoing core decompression augmented with bone marrow concentrate and platelet-rich plasma
All patients encountered in the senior author’s clinic who met the eligibility criteria were enrolled in the study. No patient who was approached to be involved in the study refused. One patient (two hips) died before his 1-year followup examination for reasons related to his underlying comorbidities; therefore, no postoperative Harris hip score was calculated in this patient. At his last postoperative visit (6 months postoperatively), there were no signs of disease progression. Five patients (seven hips) did not undergo MRI at the 1-year followup. Reasons for patients not having the MRI performed included claustrophobia (n = 2), patient refusal (n = 1), death before 1 year (n = 1), and conversion to THA before 1-year followup (n = 1). The remaining 17 patients (28 hips) underwent postoperative MRI.
Surgical Procedure
The procedure was performed on a radiolucent table in the supine position under general anesthesia in all patients. After induction, 60 or 120 cc (bilateral hips) of anticoagulated blood was obtained and the blood was placed into a 60-cc vial and centrifuged for 15 minutes using the BioCUE™ System (Biomet Biologics, Warsaw, IN, USA). From this 6 or 12 cc of PRP was obtained. During the centrifugation process, bone marrow was aspirated from the anterior aspect of both iliac crests utilizing a 2- to 3-mm incision. A trocar (Biomet Biologics) was inserted into each iliac crest with gentle taps of a mallet between the two tables of ilium, allowing the aspiration of 60 to 120 cc of bone marrow. The bone marrow was then concentrated 10 x using the BioCUE System (Biomet Biologics), yielding 6 to 12 cc of BMC per crest. The goal of the procedure was to inject 6 cc of PRP and 12 cc of concentrated bone marrow into each femoral head.
During the bone marrow centrifugation, hip decompression was performed using biplanar (AP and frog-leg lateral) fluoroscopy. Through a 1-cm incision, the starting point for the 6-mm trocar was identified above the level of the lesser trochanter distal to the vastus ridge on the lateral aspect of the femur. The lateral cortex of the femur was then breached and the decompression was performed by hand through advancing the trocar with gentle taps of a mallet from lateral to medial, utilizing fluoroscopy to ensure the position of the trocar. Once the tip of the trocar entered the necrotic lesion, which was typically accompanied by a change in pitch of the mallet strikes, the position was confirmed with biplanar fluoroscopy. Care was taken to avoid advancing the trocar to within 5 mm of the articular surface because this may be a risk factor for postoperative subchondral collapse. When the femoral head lesion was not visible radiographically, the surgeon used the MR images to approximate the level where the trocar should sit in the femoral head.
When the trocar was in proper position, the inner rod of the trocar was removed, leaving a 6-mm trocar sleeve in the area of ON. The trocar was retracted a few millimeters to open up the area of decompression and free the trocar from bone at its tips and the 12 cc of BMC and 6 cc of PRP were mixed in a 30-cc syringe and injected into the trocar with the hip in flexion to avoid backflow of the fluid. To prevent retrograde backflow of the BMC and PRP, the trocar was removed and reinserted at a different angle to push cancellous bone into the tract or demineralized bone matrix was injected into the trocar to plug the tract. The incision was then closed in a standard fashion.
All patients were discharged home the day of surgery and allowed to weightbear as tolerated immediately with the use of crutches for approximately 2 weeks or until they no longer had pain. Patients were also started on 20 mg atorvastatin daily if they were able to tolerate the medication.
MSC Content
An additional 30 cc of bone marrow was aspirated from the patient and 3 cc of BMC was obtained and used to quantify the MSC content. Nucleated cell quantity within the BMC was determined using a CountessTM (Invitrogen, Grant Island, NY, USA) automated cell counter with cells stained with 0.4% trypan blue (Invitrogen).
The nucleated cells in the BMC were suspended and plated with MSCs identified as cells with the ability to proliferate in culture with an adherent, spindle-shaped morphology in expansion media supplemented with 10% fetal bovine serum (FBS). Cell cultures were passaged when the cells reached 80% confluence using 0.05% Trypsin (Invitrogen). After each passage, 30,000 cells were replated on new 100-mm plates with expansion media and 10% FBS. The process was repeated for a total of two cell passages before the start of growth experiments.
To assess MSC yield, fibroblast colony-forming unit (CFU) assays were used as previously described [36]. Human MSC precursors were quantified by washing cells and staining with 0.5% Crystal Violet (Sigma Aldrich, St Louis, MO, USA) in methanol for 5 minutes at room temperature after 2 weeks of expansion. Visible colonies were counted and the experiments were performed in triplicate and reported as mean.
Lesion Analysis on MRI
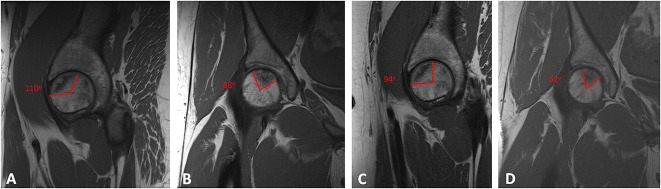
Patients underwent preoperative and 12-month postoperative MRI to assess change in the size of the necrotic lesion using the modified Kerboul angle [14]. The modified Kerboul grade was used to assess the overall involvement of the femoral head on preoperative imaging by measuring the necrotic angle on midcoronal and midsagittal images. Lesions were graded as 1 (< 200°), 2 (200°-249°), 3 (250°-299°), and 4 (≥ 300°) as previously described [14] (Fig. 1). Patients were prospectively followed over the course of the study with regular postoperative visits to assess clinical response to treatment. At the 12-month time point, patients underwent repeat MRI to evaluate changes in the ON lesion.
Fig. 1A-D.
Selected MR images show a female patient with a history of oral corticosteroid use. On preoperative sagittal (A) and coronal (B) T1-weighted images, the mean modified Kerboul angle was 198°. At the time of aspiration, the mean cell count was 6.25 x 107 cells/mL and the mean CFUs was 29. One year postoperatively, a repeat T1-weighted MRI showed the mean modified Kerboul angle on sagittal (C) and coronal (D) images had improved to 146°. Likewise, her Harris hip score improved from 69 preoperatively to 100 postoperatively.
Clinical Evaluation
Functional outcome after the procedure was calculated using the Harris hip score [15], on which 0 is the worst possible score and 100 is the best. Mean followup after the decompression was 3 years (range, 2-4 years). Progression in ON stage to collapse and subsequent procedures including repeat decompression and THA were collected in all patients.
Statistical Analysis
Continuous variables were assessed using the Student’s t-test and categorical variables were compared using the Fisher’s exact test. Correlation between MRI changes was made with Spearman's rank correlation coefficient. Survivorship free from conversion to THA, any procedure, and femoral head collapse was made using the Kaplan-Meier method at the 2- and 3-year postoperative time points. Because this was a preliminary study and to capture all hips for failure in patients with bilateral disease, each hip was analyzed independently. Statistical significance was set at a p value of < 0.05.
Results
Survivorship free from THA at the 2- and 3-year time points was 97% (95% confidence interval [CI], 83%-99%) and 84% (95% CI, 56%-95%). The overall survival of articular cartilage collapse at the 2- and 3-year time points was 97% (95% CI, 83%-99%) and 93% (95% CI, 76%-98%). In addition to these four failures to THA, two patients (four hips) underwent a second decompression with BMC and PRP injection for persistent pain. These two patients have not undergone THA or progression in disease stage. The survivorship free from any reoperation at the 2- and 3-year time points was 97% (95% CI, 83%-99%) and 67% (95% CI, 42%-85%). There were no complications related to the procedure including infections, aspiration or injection-related complications, or femoral fractures.
The mean modified Kerboul angle improved from 205° ± 47° to 172° ± 48° postoperatively (mean change -30° ± 6°, p = 0.01). Preoperatively based on the modified Kerboul grade, Grade 2 (47% [16 of 35]) was most common with a greater proportion of patients with Kerboul Grade 3 or 4 compared with Kerboul Grade 1 or 2 (80% [four of five] versus 13% [four of 30]; odds ratio, 26; 95% CI, 2-296; p = 0.005) undergoing an additional surgical procedure (Table 2). Three progressed to THA; one underwent redecompression and the other has not progressed.
Table 2.
Postoperative patient characteristics of patients undergoing core decompression augmented with bone marrow concentrate and platelet-rich plasma
At the patients’ final followup, the mean preoperative Harris hip score was 57 ± 12, which improved to 85 ± 15 at latest followup (mean change 28 ± 3, p < 0.001). Twenty-seven (77%) hips had a good to excellent functional outcome (≥ 80) as rated by the Harris hip score.
The mean CFU was 19 ± 6. Patients who underwent an additional surgical procedure (THA or repeat core decompression) had a lower mean concentration of nucleated cells per milliliter of BMC (5.5 x 106 ± 2.8 x 106 cells/mL versus 2.3 x 107 ± 2.2 x 107 cells/mL, p = 0.02) and lower mean CFUs (13 ± 6 versus 19 ± 7, p = 0.04) compared with those who did not.
Discussion
Precollapse ON of the femoral head can be adequately treated with core decompression alone [24, 42]; however, in patients with advanced precollapse disease, the rate of failure of core decompression alone is high. To improve survivorship of the hips in these patients, adjuvants including MSCs have been used; however, failure still occurs. PRP is an easily obtainable source of growth factors that has the potential to improve bone healing; however, its use in combination with BmMSC to treat ON of the femoral head has not been investigated. In this preliminary series, we found that 93% of patients treated with a combination of BmMSC and PRP were free of femoral head collapse and 84% were free of conversion to THA at 3 years of followup.
The results of this study should be taken in light of certain limitations. Because this study only includes patients with corticosteroid-induced ON, results may not be translatable to patients with ON secondary to an alternative risk factor. Furthermore, there is no comparison to patients undergoing decompression alone. A randomized trial would be ideal, because the use of hip decompression augmented with BMC remains controversial. We are unable to comment if BMC versus PRP versus core decompression alone is superior to the other. Followup here was at short term; with more time, there may be an increased rate of conversion to THA and articular surface collapse. Likewise, the cost associated with the collection of BmMSCs and PRP was not analyzed. Future studies will need to evaluate whether the added costs of using BmMSCs is justified in light of the results over time; ideally, such studies should compare BmMSCs with the available alternatives and with core decompression alone. In addition, 13 patients had bilateral disease; however, we analyzed each hip independently. It is possible that if only one hip per patient was analyzed in terms of survival free of THA, reoperation, or femoral head collapse, the outcome of the study would be different.
Previous studies have shown a decreased concentration of BmMSCs in the proximal femur of patients with ON; as such, the addition of BmMSC, which contains osteogenic and angiogenic progenitor cells, was thought to be able to heal the avascular, necrotic regions of the femoral head [6, 19]. Augmenting hip decompression with BmMSC was first described in 2002 by Hernigou and Beaujean [18], after which multiple prospective randomized and retrospective studies comparing core decompression with decompression augmented with BmMSC have reported success with more patients in the BmMSC arm able to avoid THA [10, 12, 18, 37, 39]. That being said, the outcome of treatment is mixed with series also failing to demonstrate better results in the cell therapy arm [29]. Previous studies have not evaluated the combination of PRP and BmMSC. The preliminary results of this prospective cohort study are comparable to previous reports in terms of hip preservation [10, 12, 18, 37, 39] and highlight the risk factors for progression in this group of patients with corticosteroid-induced ON such as a high initial Kerboul angle and low BmMSC count and function.
The modified Kerboul angle has been shown to be associated with collapse of the femoral head in patients with ON [14]. That study separated the patients into “low risk” and “high risk” for collapse based on the preoperative grade and randomized to nonoperative treatment or core decompression alone [14]. There was no difference in outcome between the operative and nonoperative groups and among the high-risk (modified Kerboul Grade 3 and 4) patients, 100% of patients collapsed, whereas only 29% of the low-risk patients (modified Kerboul Grade 1 and 2) progressed to collapse [14]. The results of our study are similar with 80% of patients classified as “high risk” undergoing an additional surgical procedure (THA or repeat decompression); however, no low-risk patients progressed to collapse. This study supports randomized trials showing an improvement in femoral head involvement after hip decompression augmented with cell therapy [10, 39]. Further followup and repeat MRI at later intervals may show further improvements in femoral head involvement and are needed to determine whether the initial healing continues or halts.
After core decompression augmented with BMC, multiple reports have shown improvements in patient function [10, 12, 18, 39]. In a prospective comparative study, Gangji et al. [10] noted improvements in the visual analog pain score and a decrease in joint symptoms based on the Lequesne index between patients undergoing core decompression augmented with BMC compared with those without BMC. Although there was a reduction in the pain patients were having, the authors failed to notice a major difference in the WOMAC between patients augmented with BMC and those without [10]. Sen et al. [39] noted improvements in the Harris hip score after core decompression augmented with BMC. In the present series, 80% of patients who did not go on to have THA had a good or excellent outcome as measured by the Harris hip score. Likewise, there was no difference in the mean Harris hip score between patients with “low-risk” or “high-risk” lesions based on the modified Kerboul angle; therefore, the degree of pain is not a good indicator as to who would be a good candidate for the procedure.
The ability of the BMC injected into the area of necrosis to differentiate into bone is not fully understood. Gangji et al. was able to show that after the injection of BMC into the necrotic lesion, a majority of the cells remain in the femoral head 24 hours after injection [10]; however, no studies have definitively shown these cells differentiate into bone and may instead exert a paracrine effect in the environment orchestrating the healing response. In their original paper, Hernigou and Beaujean hypothesized that osteogenesis and angiogenesis were related to the number of MSCs injected [18]. For patients with corticosteroid-induced ON, there was a major difference in the number CFUs for patients who progressed to THA compared with those who did not [18]. The results of this study are similar with an increase in mean CFU, but also the total number of nucleated cells isolated from the bone marrow in patients who underwent an additional procedure and those who did not. Although patients who went on to have a reoperation received fewer cells, recent studies have shown that although MSCs are isolated from BMC in patients with ON, they are unable to differentiate into bone with the same proclivity as MSCs isolated from patients without ON [22]. As a result of the progression of disease even with injection of MSCs, alternative cell sources such as adipose-derived MSCs (AdMSCs), which have been shown to be phenotypically superior to BmMSC to differentiate into bone in the setting of ON [41], may hold promise to increase rates of healing, especially in those in whom the BMC may not be the best source of MSC. Further studies demonstrating the superiority of AdMSCs over BmMSCs in the setting of hip decompression have yet to be performed.
Although not investigated in this series, the cost associated with the use of BmMSC compared with decompression alone is greater. In addition, nonoperative treatment with bisphosphonates has been shown to be efficacious in the treatment of ON of the femoral head with improvements in pain and function [1, 2, 27]. This has been supported by a prospective randomized study that showed bisphosphonate therapy to be effective in preventing progression of osteonecrotic lesions in the femoral head [27]. As a result of the cost associated with all treatments, and the risks associated with surgery, future studies analyzing costs of care as well as risks versus benefits both in surgical and nonsurgical approaches are needed.
Overall, in this preliminary study, core decompression augmented with BmMSC and PRP preserved the femoral head articular surface in 93% of patients and 84% of patients were free of THA in this cohort at short-term followup. Future studies are needed to compare the outcome of BmMSC and PRP compared with core decompression and nonoperative treatment alone to determine superiority of treatment. Patients with a high modified Kerboul angle and low concentration of nucleated cell count in the BmMSC had risk of collapse or continued pain requiring THA or repeat decompression.
Acknowledgments
We thank Ruben Crespo-Diaz MD, PhD, and Paul Stalboerger MS, PMP, for their assistance in the laboratory work.
Footnotes
One of the authors (RJS), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of less than USD 10,000, and he and his institution have received research support funding from Zimmer/Biomet Orthopedics (Warsaw, IN, USA). The institution of two authors (MTH, CCW) has received, during the study period, funding from Zimmer/Biomet Orthopedics. One of the authors (RJS) or his immediate family has or may receive payments or benefits, during the study period, from Zimmer/Biomet Orthopedics and Link Orthopedics (Rockaway, NJ, USA) in the amount of USD 10,000 to USD 100,000.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxford). 2005;44:352–359. [DOI] [PubMed] [Google Scholar]
- 2.Agarwala S, Sule A, Pai BU, Joshi VR. Alendronate in the treatment of avascular necrosis of the hip. Rheumatology (Oxford). 2002;41:346–347. [DOI] [PubMed] [Google Scholar]
- 3.Aldridge JM, 3rd, Urbaniak JR. Avascular necrosis of the femoral head: etiology, pathophysiology, classification, and current treatment guidelines. Am J Orthop (Belle Mead NJ). 2004;33:327–332. [PubMed] [Google Scholar]
- 4.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–209. [DOI] [PubMed] [Google Scholar]
- 5.Cheng EY, Thongtrangan I, Laorr A, Saleh KJ. Spontaneous resolution of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86:2594–2599. [DOI] [PubMed] [Google Scholar]
- 6.Connolly J, Guse R, Lippiello L, Dehne R. Development of an osteogenic bone-marrow preparation. J Bone Joint Surg Am. 1989;71:684–691. [PubMed] [Google Scholar]
- 7.Cruess RL. Steroid-induced osteonecrosis: a review. Can J Surg. 1981;24:567–571. [PubMed] [Google Scholar]
- 8.Fairbank AC, Bhatia D, Jinnah RH, Hungerford DS. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42–49. [PubMed] [Google Scholar]
- 9.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed] [Google Scholar]
- 10.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49:1005–1009. [DOI] [PubMed] [Google Scholar]
- 11.Gangji V, Hauzeur JP. Treating osteonecrosis with autologous bone marrow cells. Skeletal Radiol. 2010;39:209–211. [DOI] [PubMed] [Google Scholar]
- 12.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86:1153–1160. [DOI] [PubMed] [Google Scholar]
- 13.Griffin XL, Smith CM, Costa ML. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40:158–162. [DOI] [PubMed] [Google Scholar]
- 14.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88(Suppl 3):35–40. [DOI] [PubMed] [Google Scholar]
- 15.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 16.Herndon JH, Aufranc OE. Avascular necrosis of the femoral head in the adult. A review of its incidence in a variety of conditions. Clin Orthop Relat Res. 1972;86:43–62. [DOI] [PubMed] [Google Scholar]
- 17.Hernigou P, Bachir D, Galacteros F. The natural history of symptomatic osteonecrosis in adults with sickle-cell disease. J Bone Joint Surg Am. 2003;85:500–504. [DOI] [PubMed] [Google Scholar]
- 18.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81:349–355. [DOI] [PubMed] [Google Scholar]
- 20.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–2572. [DOI] [PubMed] [Google Scholar]
- 21.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86:2589–2593. [DOI] [PubMed] [Google Scholar]
- 22.Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31:893–898. [DOI] [PubMed] [Google Scholar]
- 23.Hungerford DS, Zizic TM. Alcoholism associated ischemic necrosis of the femoral head. Early diagnosis and treatment. Clin Orthop Relat Res. 1978;130:144–153. [PubMed] [Google Scholar]
- 24.Iorio R, Healy WL, Abramowitz AJ, Pfeifer BA. Clinical outcome and survivorship analysis of core decompression for early osteonecrosis of the femoral head. J Arthroplasty. 1998;13:34–41. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Matsuno T, Omizu N, Aoki Y, Minami A. Mid-term prognosis of non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 2003;85:796–801. [PubMed] [Google Scholar]
- 26.Kanthan SR, Kavitha G, Addi S, Choon DS, Kamarul T. Platelet-rich plasma (PRP) enhances bone healing in non-united critical-sized defects: a preliminary study involving rabbit models. Injury. 2011;42:782–789. [DOI] [PubMed] [Google Scholar]
- 27.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. [DOI] [PubMed] [Google Scholar]
- 28.Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250–261. [DOI] [PubMed] [Google Scholar]
- 29.Lim YW, Kim YS, Lee JW, Kwon SY. Stem cell implantation for osteonecrosis of the femoral head. Exp Mol Med. 2013;45:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin JR, Houdek MT, Sierra RJ. Use of concentrated bone marrow aspirate and platelet rich plasma during minimally invasive decompression of the femoral head in the treatment of osteonecrosis. Croat Med J. 2013;54:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–178. [DOI] [PubMed] [Google Scholar]
- 32.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. [DOI] [PubMed] [Google Scholar]
- 33.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;335(Suppl):S314–335. [PubMed] [Google Scholar]
- 34.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92:2165–2170. [DOI] [PubMed] [Google Scholar]
- 35.Mwale F, Wang H, Johnson AJ, Mont MA, Antoniou J. Abnormal vascular endothelial growth factor expression in mesenchymal stem cells from both osteonecrotic and osteoarthritic hips. Bull NYU Hosp Jt Dis. 2011;69(Suppl 1):S56–61. [PubMed] [Google Scholar]
- 36.Penfornis P, Pochampally R. Isolation and expansion of mesenchymal stem cells/multipotential stromal cells from human bone marrow. Methods Mol Biol. 2011;698:11–21. [DOI] [PubMed] [Google Scholar]
- 37.Rastogi S, Sankineani SR, Nag HL, Mohanty S, Shivanand G, Marimuthu K, Kumar R, Rijal L. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: a preliminary study. Musculoskelet Surg. 2013;97:223–228. [DOI] [PubMed] [Google Scholar]
- 38.Sarkarat F, Kalantar Motamedi MH, Jahanbani J, Sepehri D, Kahali R, Nematollahi Z. Platelet-rich plasma in treatment of zoledronic acid-induced bisphosphonate-related osteonecrosis of the jaws. Trauma Mon. 2014;19:e17196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27:679–686. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 41.Wyles CC, Houdek MT, Crespo-Diaz RJ, Norambuena GA, Stalboerger PG, Terzic A, Behfar A, Sierra RJ. Adipose-derived mesenchymal stem cells are phenotypically superior for regeneration in the setting of osteonecrosis of the femoral head. Clin Orthop Relat Res. 2015;473:3080–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon TR, Song EK, Rowe SM, Park CH. Failure after core decompression in osteonecrosis of the femoral head. Int Orthop. 2001;24:316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–330. [DOI] [PubMed] [Google Scholar]