Abstract
Background
Instability is the most common complication after reverse total shoulder arthroplasty (rTSA). In the native glenohumeral joint, in addition to full dislocations, more subtle forms of instability exist. However, the incidence of more subtle forms of instability, the factors associated with instability, and the effect of instability on validated outcome scores after rTSA remain poorly understood.
Questions/purposes
(1) After rTSA, what is the risk of instability, including more subtle forms of subjective instability? (2) What are the factors associated with instability? (3) Are more subtle forms of instability associated with lower American Shoulder and Elbow Surgeons (ASES) functional outcome scores than those patients without instability?
Methods
A total of 168 rTSAs were performed during the study period. Six patients had died at the time of study initiation. Thirty patients were excluded, nine because rTSA was performed for an acute proximal humeral fracture, one because a lateralized humeral component was used, 17 because a retaining liner was used, and three because a lateralized glenosphere was used. One hundred thirty-two patients met inclusion and exclusion criteria. Thirty-five patients were lost to followup. Thus, 97 patients with a minimum of 2 years followup were included in the final cohort (74% of included patients). Followup was 47 ± 22 months (mean ± SD). The cohort included 23 men and 74 women with an age of 70 ± 9 years who underwent 78 primary and 19 revision rTSAs. Primary and revision patients were combined for subsequent analyses. A postoperative questionnaire was used to assess instability symptoms. Although it has not been validated, it is simple and we believe has high face validity. Briefly, it scored instability as (1) none; (2) feelings of instability; (3) probable dislocation/subluxation–self-reduced; and (4) dislocation with surgical reduction or dislocation with closed reduction (such as in the emergency department or the doctor’s office). ASES scores were collected specifically for this study. The preoperative and postoperative β angle was measured to determine glenoid inclination. Larger β angles denote more superior inclination, whereas smaller β angles denote more inferior inclination. Thus, a positive change in β angle from preoperatively to postoperatively denotes a change into more superior inclination, whereas a negative change in β angle from preoperatively to postoperatively denotes a change into more inferior inclination. Associations between instability symptoms and patient, implant, and surgical factors were evaluated in a multivariate model that considered age, sex, body mass index, and whether it was a primary or a revision procedure.
Results
A total of 13 of 97 (13%) patients reported some instability (Grades 2-4); four of 97 patients (4%) had full dislocations with reduction (Grade 4), four of 97 patients (4%) reported subluxations (Grade 3), and five of 97 patients (5%) reported feelings of instability or apprehension (Grade 2). After controlling for potential confounding variables like age, sex, body mass index, and revision versus primary procedure, the only factors associated with instability were greater superior baseplate inclination (larger β angle; odds ratio [OR], 1.15 [95% confidence interval {CI}, 1.042-1.258]; p = 0.005) and a greater change into superior inclination from preoperative to postoperative (greater positive change in ß angle; OR, 1.08 [1.009-1.165]; p = 0.027). Patients with any instability (Grades 2-4) reported lower final ASES scores than did patients without instability (Grade 1) (61 ± 16 versus 72 ± 19 mean difference 11 [95% CI, 0-22]; p = 0.032).
Conclusions
When more subtle instability after rTSA is included, instability may occur in up to 13% of patients. Instability is associated with greater superior baseplate inclination and less inferior correction of the β angle and thus surgeons should consider inferiorly inclining the baseplate to avoid postoperative instability. Although our study only demonstrates an association and not causation, the authors hypothesize that superior baseplate inclination increases inferior impingement, which leads to instability. Instability negatively influences final ASES score.
Level of Evidence
Level III, therapeutic study.
Introduction
As many as one in five patients who undergoes a reverse total shoulder arthroplasty (rTSA) will experience a surgical complication.[1, 5, 6, 9, 24, 27]. Shoulder instability is the most common postoperative complication [6, 7, 9, 11, 25]. Instability occurs after rTSA in 0% and 16% of patients with previously identified risk factors including male sex, obesity, revision surgery, humeral shortening, glenoid medialization, inadequate soft tissues, and heterotopic ossification [2, 4, 10, 26].
Current clinical understanding of instability after rTSA is limited. There continues to be substantial debate as to the incidence of instability, which seems to range from 0% to 16% [2, 4, 10, 26]. Prior reports have focused on complete dislocation events often with closed or open reduction, often with component revision, and have not reported on lower levels of instability. Few prior comparative studies exist with regard to risk factors and thus it remains unclear whether these factors truly associate with instability [6, 7, 9, 11, 25]. The association between instability and patient-reported outcomes is also unclear because of the lack of prior comparative studies. Certainly, no prior studies have evaluated patient-perceived instability, either apprehension or subluxation, after rTSA.
Therefore, we asked: (1) After rTSA, what is the risk of instability, including more subtle forms of subjective instability? (2) What are the factors associated with instability? (3) Are more subtle forms of instability associated with lower American Shoulder and Elbow Surgeons (ASES) functional outcome scores than those patients without instability?
Patients and Methods
This is a retrospective study of all patients who underwent primary or revision rTSA by a single surgeon (RZT) from February 2007 when the surgeon began practice to September 2013 to allow a minimum followup of 2 years at the University of Utah. Only patients from a single surgeon were included and thus a consistent set of indications was used for this patient population. Minimum followup was 2 years. Institutional review board approval from the University of Utah was obtained before initiation of the study (IRB #71740).
Any patient undergoing rTSA as a primary or revision procedure was included with the exception of patients in whom proximal humerus fracture was an indication for rTSA (because the lesser tuberosity was repaired in all of these patients), use of a constrained humeral component liner, use of a lateralized onlay humeral component, or use of a lateralized glenosphere system. A total of 168 rTSAs were performed during the study period. Six patients had died at the time of study initiation. Thirty patients were excluded, nine because rTSA was performed for an acute proximal humeral fracture, one because a lateralized humeral component was used, 17 because a retaining liner was used, and three because a lateralized glenosphere was used. One hundred thirty-two patients met inclusion and exclusion criteria. Thirty-five patients were lost to followup. Thus, 97 patients with a minimum of 2 years followup were included in the final cohort (74% of included patients).
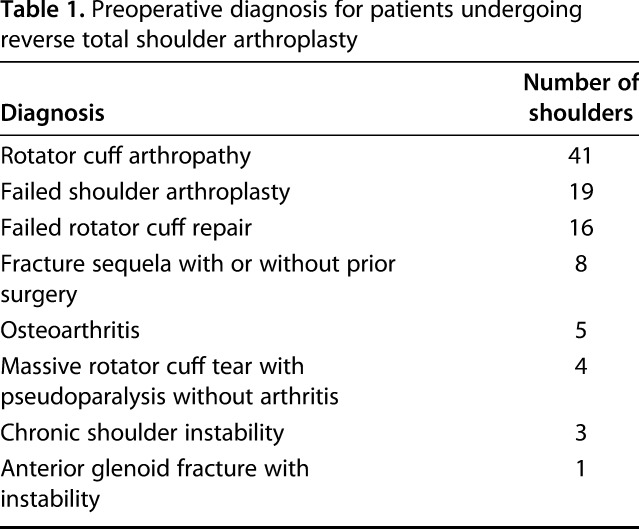
Patients were evaluated at a mean of 47 ± 22 months postoperatively. The mean age at the time of surgery was 70 ± 9 years. There were 23 men and 74 women. The mean patient body mass index (BMI) was 28 ± 8 kg/m2. There were 78 primary and 19 revision rTSAs. The most common preoperative diagnosis for rTSA was rotator cuff arthropathy (Table 1). Nine patients had the humeral component placed in 0° of retroversion and 88 patients had the humeral component placed in 10° of retroversion. In all 97 patients, a 36-mm glenosphere was implanted. All glenospheres were either flush or inferior to the inferior glenoid margin. Preoperative glenoid morphology was classified using the Favard system: E0 (64), E1 (13), E2 (15), E3 (three) [21]. The preoperative β angle of the native glenoid was measured as previously described [15]. Larger β angles denote more superior inclination, whereas smaller β angles denote more inferior inclination. Thus, a positive change in β angle from preoperatively to postoperatively denotes a change into more superior inclination, whereas a negative change in β angle from preoperatively to postoperatively denotes a change into more inferior inclination. Two glenoids could not be classified because one was for a failed anatomic TSA and the other was for a failed rTSA. Nine patients had glenoid grafting at the time of rTSA as a result of severe glenoid erosion. Six patients (6%) had major complications, four of whom underwent revision surgery. One patient underwent irrigation and débridement for an early postoperative wound hematoma. One patient underwent revision for a loose baseplate with concomitant glenoid bone grafting at the time of revision rTSA. One patient underwent open reduction and polyethylene liner exchange for a complete dislocation. This patient initially underwent revision of a failed hemiarthroplasty to an rTSA with a proximal humeral bone graft and became unstable within the first 5 months from surgery. She underwent revision with increased polyethylene liner thickness and a constrained liner. A second patient underwent two surgical procedures to stabilize an unstable rTSA. At the first surgery, the glenosphere was enlarged from a 36-mm to a 42-mm glenosphere and a thicker constrained polyethylene component was utilized. A second revision surgery was performed, which included both further increased length on the humeral side with a constrained polyethylene liner and a custom 10-mm lateralized 42-mm glenosphere. Two other patients who sustained complete dislocations were reduced in the emergency department or in the clinic in a closed manner and no further surgical treatment was performed. All completely unstable rTSAs are currently stable without further instability episodes.
Table 1.
Preoperative diagnosis for patients undergoing reverse total shoulder arthroplasty
In all patients, a deltopectoral approach was used. Both the Trabecular Metal (TM) Reverse Total Shoulder Arthroplasty (Zimmer, Warsaw, IN, USA) and Aequalis Reversed Shoulder Arthroplasty (Tornier, Bloomington, MN, USA) systems were used. The humeral head was cut using the respective cutting guide and the humerus was prepared as per the manufacturer’s recommendations. The proximal humerus was cut between 0° and 10° of retroversion in all patients as based on the surgeon’s experience; this maximizes postoperative rotational ROM. The glenoid was then prepared and the baseplate was placed such that the inferior margin of the baseplate was flush with the inferior margin of the glenoid. Attempts were made to place the baseplate in a neutral alignment or inferior tilt up to 10°. A glenosphere was then impacted onto the baseplate. The humeral component was cemented in all patients and then a polyethylene spacer was chosen. Parameters used to choose the correct spacer thickness included moderate difficulty encountered in reducing the prosthesis, the inability to create a gap between the humeral and glenoid components with distal pressure on the humeral component with the arm in adduction and neutral rotation, good tension within the conjoint tendon, the absence of extreme tension within the axillary nerve, and moderate difficulty in dislocating the prosthesis with lateral pressure on the humeral component. The subscapularis was not repaired in any patient, even if repairable.
For each patient, the following data were collected from the preoperative documentation: sex, BMI, and preoperative diagnosis (rotator cuff arthropathy, osteoarthritis, failed shoulder arthroplasty, massive rotator cuff tear with pseudoparalysis, failed rotator cuff repair, chronic shoulder instability, fracture sequelae with or without prior surgery, anterior glenoid fracture with instability). The following data were collected from the intraoperative documentation: procedure, implant type, humeral component version, glenosphere diameter, intraoperative complications, and if glenoid bone grafting was performed. The following data were collected at final followup specifically for this study: postoperative complications, any revision surgery, ASES score, length of followup, and a questionnaire regarding subjective shoulder instability. Although this has not been validated, it is simple and we believe has high face validity. In addition, no prior patient-reported outcome instruments are available specifically with regard to instability after rTSA. The questionnaire asked the following question: “Have you experienced a dislocation since your surgery?” Answers included: (1) none; (2) feelings of instability; (3) probable dislocation/subluxation–self-reduced; and (4) dislocation with surgical reduction or dislocation with closed reduction (such as in the emergency department or the doctor’s office). For statistical analyses, Grades 2 through 4 were considered “unstable.” To reduce loss of followup, we attempted to contact patients by all available means including physical mail, electronic mail, and by phone on at least three separate occasions at all available numbers. Preoperative scores were only available on 32 of 97 (33%) patients and thus these data were not included.
Preoperative and immediate postoperative radiographs included AP, Grashey AP, scapular-Y lateral, and axillary lateral views. These were independently evaluated by an attending surgeon fellowship-trained in shoulder and elbow surgery (PNC) who did not perform the rTSAs and who was blinded to the patient’s reported level of instability. Glenoid deficiency was classified on preoperative radiographs and CT scans using the Favard system [21]. Glenoid inclination (β angle) was measured on the postoperative true AP view as a measure of final baseplate inclination (Fig. 1) [15]. To demonstrate reliability, 20 postoperative radiographs were evaluated by two attending surgeons fellowship-trained in shoulder and elbow surgery (PNC, RZT) and the intraclass correlation was 0.943 [95% confidence interval, 0.862–0.977].
Fig. 1.
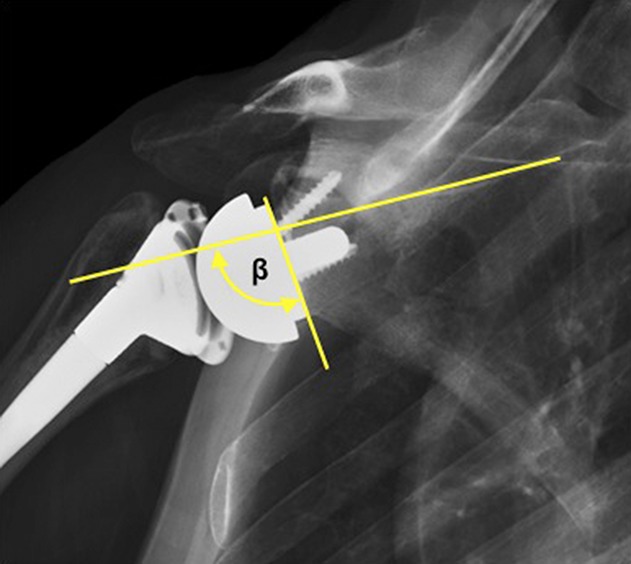
This AP radiograph demonstrates the methodology for measurement of the β angle, which is the angle subtended between the scapular spine and the baseplate.
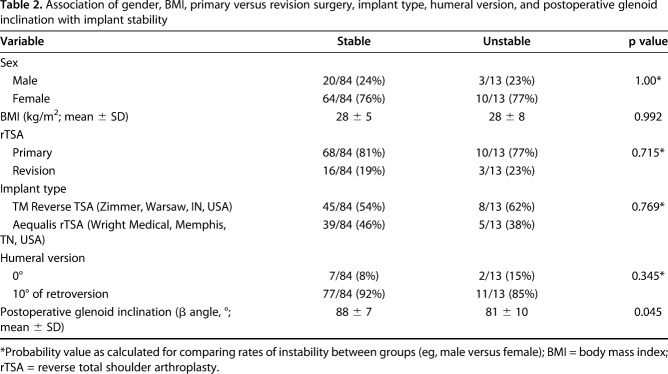
Various patient, implant, and surgical factors were evaluated regarding their association with instability including implant system (Zimmer TM Reverse Total Shoulder Arthroplasty versus Tornier Aequalis Reversed Shoulder Arthroplasty), primary versus revision surgery, humeral version (0° versus 10° of retroversion), BMI, postoperative β angle (Fig. 2), change in β angle from preoperative to postoperative, and sex (Table 2). The mean postoperative glenoid inclination (β angle) for the entire cohort was 87° ± 8°.
Fig. 2 A-B.
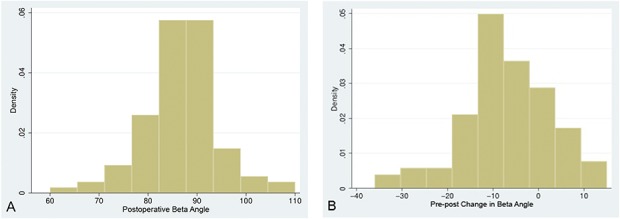
These histograms demonstrate the distribution of the (A) postoperative β angle and the (B) preoperative–postoperative change in β angle.
Table 2.
Association of gender, BMI, primary versus revision surgery, implant type, humeral version, and postoperative glenoid inclination with implant stability
Statistical Analysis
Postoperative ASES scores, BMI, and radiographic data were assessed for normality using the Kolmogorov-Smirnov test. Mann-Whitney U tests were used to compare postoperative ASES score and glenoid inclination (β angle) between the stable (ie, those who answered “A” to our questionnaire) and unstable (ie, pooled B, C, and D groups) patients. Fisher’s exact tests and independent t-tests were used to compare stable and unstable groups with regard to sex, BMI, primary versus revision, implant type, and humeral version. Simple logistic regression was used to estimate the probability for patient-reported instability as a function of postoperative β angle, adjusting for differences resulting from indication (coded as “primary rTSA” or “removal rTSA”), age, sex, and BMI. A similarly adjusted model was used to estimate the probability for instability as a function of change in pre- to postoperative β angle. Marginal plots for postoperative β angle (Fig. 3) and change in β angle (Fig. 4) were derived from the logistic models by setting the covariates to their means. We initially inspected a scatterplot of the continuous β angle predictor variables with the instability outcome measures. The association was linear throughout the bulk of the data without any clear discontinuity suggestive of an empiric threshold. However, we chose to group the β angle predictor variables into quintiles to facilitate clinical use and ease of interpretation. We thus created a categorical variable for β angle (or change in β angle) by sorting their values into quintiles. Probability values ≤ 0.050 were considered statistically significant.
Fig. 3.
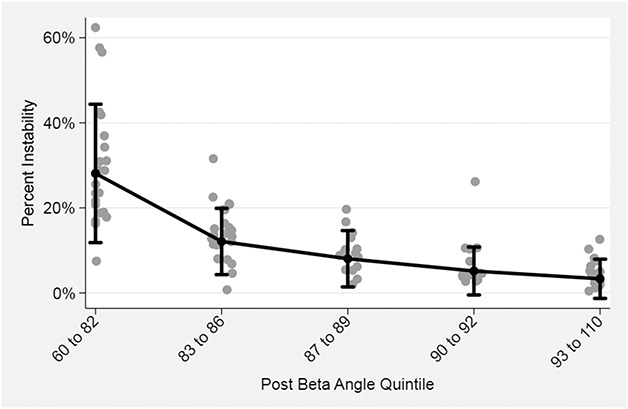
This marginal plot was derived from the logistic model by setting the covariates to their means and plotting these in comparison to a categorical variable created for β angle by sorting their values into quintiles. Adjusted for age, sex, BMI, and diagnosis.
Fig. 4.
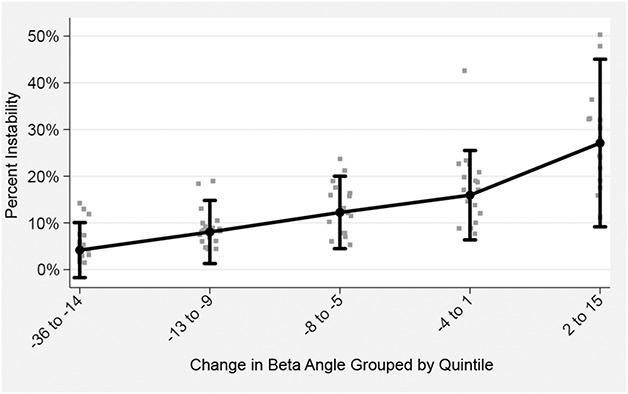
This marginal plot was derived from the logistic model by setting the covariates to their means and plotting these in comparison to a categorical variable created for change in β angle by sorting their values into quintiles. Adjusted for age, sex, BMI, and diagnosis.
Results
A total of 13 of 97 (13%) patients reported instability; four of 97 patients (4%) had full dislocations with reduction, four of 97 patients (4%) reported subluxations, and five of 97 patients (5%) reported feelings of instability or apprehension.
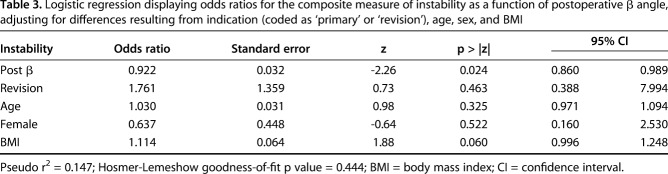
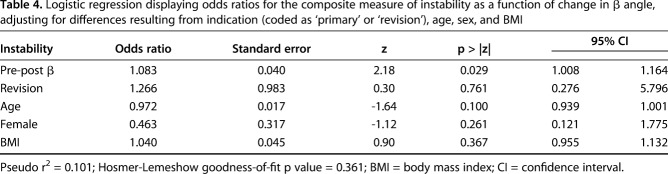
After controlling for potential confounding variables like age, sex, BMI, and revision versus primary procedure, the only factors associated with any instability (Grades 2-4) were greater superior baseplate inclination (ie, larger β angle) (odds ratio [OR], 1.15 [95% confidence interval {CI},1.043-1.258; p = 0.005; Table 3; Fig. 3) and a greater change into superior inclination from preoperative to postoperative (ie, larger change in β angle) (OR, 1.08 [1.009-1.165]; p = 0.027; Table 4; Fig. 4). Hosmer-Lemeshow goodness-of-fit test revealed no evidence of any poorly fitted model. The mean postoperative glenoid inclination (β angle) was lower for patients reporting instability (81° ± 10°) compared with those without any report of instability (88° ± 7°; p = 0.045).
Table 3.
Logistic regression displaying odds ratios for the composite measure of instability as a function of postoperative β angle, adjusting for differences resulting from indication (coded as ‘primary’ or ‘revision’), age, sex, and BMI
Table 4.
Logistic regression displaying odds ratios for the composite measure of instability as a function of change in β angle, adjusting for differences resulting from indication (coded as ‘primary’ or ‘revision’), age, sex, and BMI
Patients with any instability (Grades 2-4) reported lower final ASES scores than did patients without instability (72 ± 19 versus 61 ± 16, mean difference 11 [95% CI, 0-22; p = 0.032).
Discussion
Shoulder instability is the most common postoperative complication after rTSA [6, 7, 9, 11, 25]. However, the incidence of more subtle forms of instability, the factors associated with instability, and the effect of more subtle instability on validated outcomes scores after rTSA remain poorly understood. Prior authors have only reported complete dislocations with closed or open reduction [6, 7, 9, 11, 25]. If more subtle forms of instability exist after rTSA and if they negatively influence ASES scores, ASES scores for patients negatively influenced by more subtle forms of instability will never be improved without recognition of the contribution of these more subtle forms of instability to these scores. The effect of instability on ASES scores is also unclear because of the lack of prior comparative studies. Certainly, no prior studies of which we are aware have evaluated patient-perceived instability, either apprehension or subluxation, after rTSA. Therefore, we aimed to characterize the incidence of more subtle forms of instability, the associates with instability after rTSA, and the influence of more subtle forms of instability on postoperative ASES scores.
This study had several limitations. First, our study is limited by assessment bias. We used a nonvalidated questionnaire. Although the has not been validated, it is simple and we believe has high face validity. In addition, no prior patient-reported outcome instruments are available specifically with regard to instability after rTSA with which to compare our questionnaire. This questionnaire includes more subtle forms of instability such as apprehension and subluxations, both of which create patient symptoms and negatively influence ASES scores and thus were categorized as instability within our analysis. We did not consistently obtain preoperative clinical ASES scores in all patients because only 32% of patients had these data available. Thus, the association between preoperative ASES scores on instability after rTSA remains unknown. We used the β angle to measure inclination and demonstrated the interrater reliability for this measurement here. Second, some patients (26%) were lost to followup. Loss of followup may have resulted in an underestimation of the incidence of instability; the effect of loss to followup on research questions 2 and 3 is unknown and thus these estimates may be more imprecise than suggested by our CIs. Third, selection bias is possible because our indications for rTSA may differ from those of other surgeons. Briefly, the senior author generally feels that rTSA is indicated for elderly patients who have failed an extended trial of nonoperative treatment and who have one of the diagnoses listed (Table 1). However, these factors are not the focus of this study. Fourth, our study may be underpowered for some comparisons such as those with regard to gender and BMI. Fifth, our questionnaire gathers subjective patient feelings of instability and is thus limited by the patient’s understanding of this sensation and concept.
We found that the overall risk of full dislocations with reduction is low (4%). This risk is similar to the dislocation risk reported by most authors [3, 18, 26]. These numbers are thus useful for preoperative discussions with patients regarding the incidence of postoperative complications after rTSA. However, select authors have reported this complication to occur in as many as 16% of patients [10]. Because this series was written in 2011 and likely reflects earlier experience with the implant, it may be that as surgeon experience with rTSA has grown, instability rates have decreased. However, we also found an additional 9% of patients undergoing rTSA experienced subjective instability for a global instability incidence of 13%. To the best of our knowledge, no prior other studies have reported on more subtle levels of instability after rTSA. Understanding and recognizing more subtle forms of instability will help surgeons to better understand the factors associated with instability after rTSA and how to mitigate these factors. Future studies in larger patient groups should focus on the association among implant geometry, implant position, subscapularis repair, and patient factors on more subtle forms of instability.
In our study, larger superior inclination of the baseplate and greater change into superior inclination were the only factors associated with instability. Although prior studies have shown that the primary factors identified with complete dislocations included male sex, increased BMI, revision surgery, and humeral shortening, we were not able to confirm any of these factors [3, 4, 13, 18, 26]. Several of the prior studies suggesting these factors are important are noncomparative studies, which may explain the difference in findings [3, 4, 13, 18, 26]. The status of the subscapularis has been debated with some clinical studies supporting the importance of repair to prevent instability, whereas others show no relative benefit; in our study, no subscapularis repairs were performed [6, 17, 19, 22]. Only one prior study has examined the effect of inclination on instability after rTSA. In this study, two patients with atraumatic instability were found to have greater superior tilt compared with 31 control subjects [20]. However, this study is limited by the extremely small sample size and the lack of inclusion of other variables thought to affect stability such as sex and BMI [20]. Baseplate inferior inclination may also be desired because it is connected to decreased micromotion and backside forces on the baseplate [12], increased adduction ROM in vitro [16], and increased internal and external rotation [14]. However, another study showed that inferior tilt did not have an effect on clinical scapular notching or patient-reported outcome scores, although the amount of inferior tilt was not radiographically controlled [8]. Within our cohort, instability was associated with greater superior inclination of the baseplate. However, because of overlap between groups--inclination in those without instability ranged from 81 to 95 and inclination in those with instability ranged from 71 to 91–we cannot recommend a specific cutoff value. The difference between groups was 7° using the β angle [15]. The β angle, as measured on radiographs, is a reliable measure of inclination compared with three-dimensional CT with the error being < 6° in 85% of the measurements on radiographs [23]. Consequently, the difference observed in our study is unlikely to be a result of measurement error. After controlling for age, sex, BMI, and revision versus primary procedure, we found that the final postoperative β angle and changes in β angle from preoperative to postoperative were independent associates of instability taking into consideration other factors known to be associated with instability. There appears to be a very clear dose-response effect of superior baseplate inclination and failure to correct superior inclination and the development of instability (Figs. 3, 4). Although our study only demonstrates an association and not causation, the authors hypothesize that superior baseplate inclination increases inferior impingement, which leads to instability.
Patients who reported any instability symptoms had worse final ASES scores compared with those patients who did not. Prior studies have reported ASES scores in patients with unstable rTSAs and have demonstrated that despite instability, patients will typically have improvement in function as compared with preoperatively [26]. However, most studies evaluating instability after rTSA have concentrated on identifying risk factors instead of evaluating ASES scores of unstable rTSAs as compared with stable rTSAs [6, 18, 26]. Consequently, avoiding even lower levels of subjective instability is important to improve ASES scores after rTSA.
Complete dislocation after rTSA was uncommon. Approximately one in eight patients reports some level of more subtle levels of instability. Instability was associated with greater superior baseplate inclination and less inferior correction of the β angle from preoperative to postoperative and negatively influenced final ASES scores. Thus, surgeons should consider inferiorly inclining the baseplate to avoid postoperative instability.
Footnotes
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (HBH). One of the authors (RZT) has been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: Wright Medical (Memphis, TN, USA), Conextions (Salt Lake City, UT, USA), IMASCAP (Plouzané, France), INTRAFUSE (Logan, UT, USA) Journal of Bone and Joint Surgery-American, Journal of Trauma, KATOR (Logan, UT, USA), Mitek (Raynham, MA, USA), Shoulder Innovations (Holland, MI, USA), and Zimmer (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Affonso J, Nicholson GP, Frankle MA, Walch G, Gerber C, Garzon-Muvdi J, McFarland EG. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157–168. [PubMed] [Google Scholar]
- 2.Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res. 2016;102:S33–43. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:737–744. [DOI] [PubMed] [Google Scholar]
- 5.Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19:439–449. [PubMed] [Google Scholar]
- 6.Clark JC, Ritchie J, Song FS, Kissenberth MJ, Tolan SJ, Hart ND, Hawkins RJ. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21:36–41. [DOI] [PubMed] [Google Scholar]
- 7.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–1251. [DOI] [PubMed] [Google Scholar]
- 8.Edwards TB, Trappey GJ, Riley C, O'Connor DP, Elkousy HA, Gartsman GM. Inferior tilt of the glenoid component does not decrease scapular notching in reverse shoulder arthroplasty: results of a prospective randomized study. J Shoulder Elbow Surg. 2012;21:641–646. [DOI] [PubMed] [Google Scholar]
- 9.Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O'Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. [DOI] [PubMed] [Google Scholar]
- 10.Gallo RA, Gamradt SC, Mattern CJ, Cordasco FA, Craig EV, Dines DM, Warren RF, Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement; J Shoulder Elbow Surg. 2011;20:584–590. [DOI] [PubMed] [Google Scholar]
- 11.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–1747. [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:732–739. [DOI] [PubMed] [Google Scholar]
- 13.Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:588–595. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Knutson Z, Choi D, Lobatto D, Lipman J, Craig EV, Warren RF, Gulotta LV. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:807–813. [DOI] [PubMed] [Google Scholar]
- 15.Maurer A, Fucentese SF, Pfirrmann CWA, Wirth SH, Djahangiri A, Jost B, Gerber C. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J Shoulder Elbow Surg. 2012;21:1096–1103. [DOI] [PubMed] [Google Scholar]
- 16.Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14:524–528. [DOI] [PubMed] [Google Scholar]
- 17.Oh JH, Shin S-J, McGarry MH, Scott JH, Heckmann N, Lee TQ. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1091–1098. [DOI] [PubMed] [Google Scholar]
- 18.Padegimas EM, Zmistowski BM, Restrepo C, Abboud JA, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Instability after reverse total shoulder arthroplasty: which patients dislocate? Am J. Orthop. 2016;45:E444–E450. [PubMed] [Google Scholar]
- 19.Pastor M-F, Kraemer M, Wellmann M, Hurschler C, Smith T. Anterior stability of the reverse shoulder arthroplasty depending on implant configuration and rotator cuff condition. Arch Orthop Trauma Surg. 2016;136:1513–1519. [DOI] [PubMed] [Google Scholar]
- 20.Randelli P, Randelli F, Arrigoni P, Ragone V, D’Ambrosi R, Masuzzo P, Cabitza P, Banfi G. Optimal glenoid component inclination in reverse shoulder arthroplasty. How to improve implant stability. Musculoskelet Surg. 2014;98(Suppl 1):15–18. [DOI] [PubMed] [Google Scholar]
- 21.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. [DOI] [PubMed] [Google Scholar]
- 22.Trappey GJ, O'Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Haver A, Heylen S, Vuylsteke K, DeClercq G, Verborgt O. Reliability analysis of glenoid component inclination measurements on postoperative radiographs and computed tomography-based 3D models in total and reversed shoulder arthroplasty patients. J Shoulder Elbow Surg. 2016;25:632–640. [DOI] [PubMed] [Google Scholar]
- 24.Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon's experience? J Shoulder Elbow Surg. 2012;21:1470–1477. [DOI] [PubMed] [Google Scholar]
- 25.Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. [DOI] [PubMed] [Google Scholar]
- 26.Welton KL, Kraeutler MJ, McCarty EC, Vidal AF, Bravman JT. Current pain prescribing habits for common shoulder operations: a survey of the American Shoulder and Elbow Surgeons membership. J Shoulder Elbow Surg. 2017. December 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Wierks C, Skolasky RL, Ji JH, McFarland EG. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res. 2009;467:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]