Abstract
The AgCl, AgOAc, AuCl, and AuOAc complexes of the new bis(imino)acenaphthene(BIAN)-supported N-heterocyclic carbene ligand and the precursor imidazolium salt have been investigated with respect to their antimicrobial activities against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Psudomonas aeruginosa. The most active antimicrobial is the precursor imidazolium salt, which has a minimum inhibitory concentration (MIC) value of <40 μg/mL. The MIC values for the silver complexes IPr(BIAN)AgCl and IPr(BIAN)AgOAc against Gram-positive S. aureus are comparable to that for AgNO3, while those against Gram-negative E. coli and P. aeroginosa are significantly larger. Similar behavior was evident for the gold acetate complex IPr(BIAN)AuOAc. However, in the case of the gold chloride analogue, the MIC values are virtually identical for both the Gram-positive and the Gram-negative bacteria.
Keywords: N-heterocyclic carbene, gold, silver, antimicrobial
1. Introduction
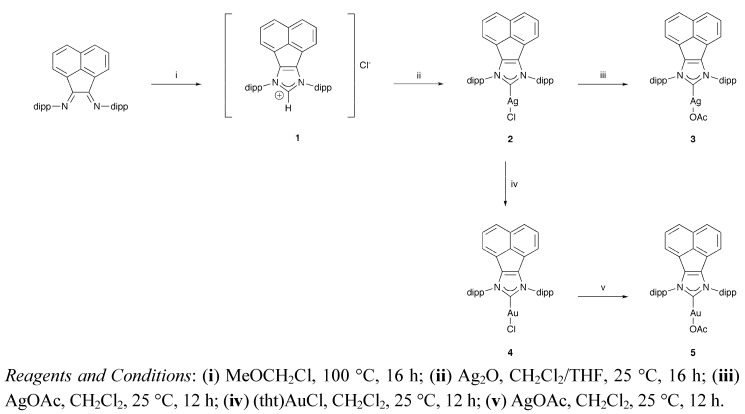
Recently, we reported the synthesis and structure of the first example of an N-heterocyclic carbene supported by a bis(imino)acenaphthene (BIAN) ligand [1], along with a number of Ag, Au and Ir coordination compounds. Given the usefulness of NHC complexes of these and other metals as anti-microbials [2], we became interested in exploring the properties of some of the corresponding BIAN compounds. The BIAN class of ligand, which features the fusion of a naphthalene moiety to a diimine, has a number of interesting and distinctive properties [3]. One such property is its extensive redox behavior. For example, BIAN ligands are capable of sequentially accommodating up to four electrons. These redox properties of the BIAN ligand class could, in principle, be useful for controlling the metal release from e.g. antimicrobials and anti-cancer drugs. An additional desirable property common to both NHC and BIAN carbene ligands is that the nitrogen substituents can be varied over a wide range. Lipophilicity can also play an important role in antimicrobial activity. For example, it is known that the activity of NHC imidazolium salts is enhanced significantly if long chain alkyl or bulky aryl groups are employed as N-substituents [4]. In the present paper, we explore the antimicrobial properties of the precursor IPr-BIAN imidazolium salt (1), along with those of the AgCl (2), AgOAc (3), AuCl (4) and AuOAc (5) complexes of the new BIAN carbene ligand.
2. Results and Discussion
2.1. Syntheses of 1-5 and the X-ray Crystal Structure of 5
Compounds 1, 2 and 4 were prepared as described previously [1]. Compounds 3 and 5 are new and were prepared by treatment of 2 and 4, with AgOAc and AuOAc, respectively, in CH2Cl2 solution (Scheme 1). Both compounds were characterized on the basis of MS, HRMS and multinuclear NMR data.
Scheme 1.
Synthetic pathways to 1-5.
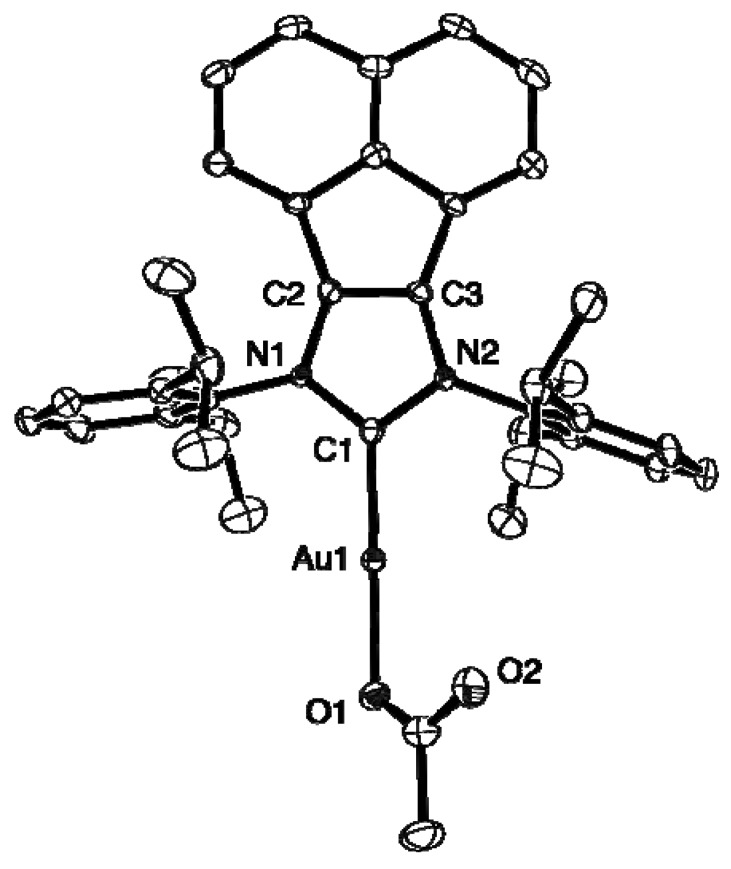
The gold complex 5 was also characterized by single-crystal X-ray diffraction. A summary of X-ray data collection details appears in Table 1 and a selection of pertinent metrical parameters is presented in Table 2. The C(2)-C(3) bond distance for the NHC moiety of 5 (1.352(6) Å) is virtually identical to that of the AuCl analogue 4 (1.358(4) Å) and indicative of some double bond character, as are the average C-N bond distances of the NCCN fragment (Figure 1).
Table 1.
Selected crystal data, data collection and refinement parameters for compound 5.
| Formula | C40H45AuCl2N2O2 |
| Formula weight | 853.65 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 12.764(2) |
| b/Å | 19.274(3) |
| c/Å | 15.025(3) |
| α/° | 90.0 |
| β/° | 93.849(5) |
| γ/° | 90.0 |
| Z | 4 |
| Dc/g cm-3 | 1.537 |
| F(000) | 1712 |
| Crystal size/nm | 0.27x0.11x0.10 |
| Θ range/° | 3.03-26.00 |
| Collected reflections | 7234 |
| Independent reflections | 7234 |
| R1 [I>2σ(I)] | 0.0318 |
| wR2 (all data) | 0.0796 |
Table 2.
Selected bond distances (Å) and angles (°) for compound 5.
| Bond distances/Å | Bond angles/° | ||
|---|---|---|---|
| C(1)-N(1) | 1.357(5) | N(1)-C(1)-N(2) | 105.6(3) |
| C(1)-N(2) | 1.365(5) | N(1)-C(2)-C(3) | 107.8(4) |
| C(2)-C(3) | 1.352(6) | C(2)-C(3)-N(2) | 106.9(3) |
| C(2)-N(1) | 1.360(5) | C(1)-N(1)-C(2) | 110.1(3) |
| C(3)-N(2) | 1.377(5) | C(1)-N(2)-C(3) | 109.6(3) |
| C(1)-Au(1) | 1.949(4) | N(1)-C(1)-Au(1) | 129.5(3) |
| Au(1)-O(1) | 2.023(3) | N(2)-C(1)-Au(1) | 124.9(3) |
| C(1)-Au(1)-O(1) | 177.62(14) | ||
Figure 1.
ORTEP diagram of 5 with 30% probability thermal ellipsoids. Hydrogen atoms are omitted for clarity.
2.2. Antimicrobial Testing of Compounds 1-5
Compounds 1-5 were tested for their antimicrobial activities against the Gram-positive bacterial strains Staphylococcus aureus and Bacillus subtilis and the Gram-negative bacterial strains Escherichia coli and Pseudomonas aeruginosa by determination of their corresponding minimum inhibitory concentration (MIC) values [5] in Mueller-Hinton (MH) broth. Compounds 1-5 were dissolved initially in a 4% dimethyl sulfoxide (DMSO)-water solution, followed by serial dilutions with the MH broth while adding additional DMSO in order to maintain a 2% DMSO concentration in each dilution.
The results of the MIC tests for 1-5 are summarized in Table 3. Clearly, the most active antimicrobial for both the Gram-positive and Gram-negative bacteria is the BIAN imidazolium salt 1 with a MIC value of < 40 μg/mL for each pathogen. Interestingly, and despite the efficacy of imidazolium salts, relatively little has been published in this area. Early work by Pernak et al. [4] established that the MIC values for N-alkylthiomethyl-substituted imidazolium salts are dependent upon the length of the hydrocarbon chain attached to the sulfur atom. The optimum chain lengths were found to be C10H21 and C12H25 and the use of longer or shorter chains resulted in diminished antibacterial activity. The MIC ranges were found to be 1.13−11.9 and 0.69−46 μg/mL for the C10H21 and C12H25 derivatives, respectively. Somewhat similar results have been reported more recently by Lee et al. [6]. In a subsequent publication, Pernak et al. [7] reported that the MIC values for the 3-alkoxymethyl analogues were not particularly sensitive to the type of counter-anion. Working with saturated 1,3-diazolidinium salts, Çetkinkaya et al. [8] discovered that, while there was a dependence of activity on the length of the n-alkyl chains, the attachment of mesityl or mesitylmethyl substituents to the N atoms resulted in the most significant increase of activity towards both Gram-positive and Gram-negative bacteria. Interestingly, however, in contrast to the work of Pernak et al. [4] the activity of these derivatives was found to depend on the identity of the counter anion. For example, the value for the mesitylmethyl chloride salt was 3.12 μg/mL.
Table 3.
Minimum inhibitory concentrations (MIC) for compounds 1-5 in μg/mL.
| Compound | S. aureus | B. subtilis | E. coli | P. aeruginosa |
|---|---|---|---|---|
| Imidazolium Salt (1) | < 40 | < 40 | < 40 | < 40 |
| IPr(BIAN)AgCl (2) | 310 | 310 | > 20,000 | 5,000 |
| IPr(BIAN)AgOAc (3) | 630 | 1,300 | 10,000 | 10,000 |
| IPr(BIAN)AuCl (4) | 630 | 630 | 630 | 630 |
| IPr(BIAN)AuOAc (5) | 630 | 630 | > 20,000 | 10,000 |
The first examples of Ag (I)-substituted NHC complexes featuring antimicrobial activity against some common pathogens were reported by Youngs et al. [2] and involve a one-dimensional polymeric arrangement of pyridine-supported pincer ligands featuring pendant (NHC)Ag+ moieties. These polymers were tested against E. coli, S. aureus and P. aeruginosa and in each case the activity against the pathogen exceeded that of AgNO3 (for AgNO3, a MIC value of 433 μg/mL was cited). Comparison with the present results (Table 3) reveals that while the MIC values for IPr(BIAN)AgCl and IPr(BIAN)AgOAc [9] against Gram-positive S. aureus are comparable to that for AgNO3 , those for Gram-negative E. coli and P. aeroginosa are larger.
Analogously to the present work, the AgCl complex of 1-benzyl-3-tert-butylimidazol-2-ylidene reported by Ghosh et al. [10] was found to be active against Gram-positive B. subtilis, (MIC = 25 ± 3.2 μM). However, there was no evidence of antimicrobial activity against Gram-negative E. coli. Interestingly however, the precursor imidazolium salt was found to be inactive. The corresponding AuCl complex was found to be more active against B. subtilis (MIC = 15 ± 2.3 μM) than the AgCl analogue. The reason cited for the superior antimicrobial activity of the gold complex is based on the fact that DFT calculations revealed that the NHC-Au bond strength is superior to that of the Ag analogue. Relatively little has been published on the antimicrobial behavior of other NHC gold (I) complexes [11]. In 2004, Çetinkaya et al. [12] synthesized and explored the antimicrobial behavior of gold salts of the general type [Au(NHC)2][Cl] in which the NHCs were imidazolidine-2-ylidenes bearing alkyl or aryl-N-substituents. A marked dependence was noted on the nature of the nitrogen substituents in the sense that changing the para substituent of the N-benzyl groups resulted in substantial differences in activity with respect to both Gram-positive and Gram-negative bacteria. Thus, while the para-trimethoxyphenyl and para-tert-butylphenyl derivatives had MIC values of 12.5 μg/mL toward S. aureus, that of the para-pentamethylphenyl analogue was 200 μg/mL, which is comparable to those found in the present work for 4 and 5 (630 μg/mL). In the most recent work, three substituted benzimidazole gold(I) chloride complexes were synthesized and tested against a selection of Gram-positive and Gram-negative bacteria and fungi. A marked dependence of antimicrobial activity on the nature of the N-aryl substituents was evident. Thus, while the bis(trimethoxybenzyl) and the trimethoxybenzyl/para-tert-butyl derivatives exhibited MIC values of 12.5 μg/mL, that of the bis(pentamethyl)benzyl analogue was 200 μg/mL [13] which is comparable with the 630 μg/mL MIC values found for 4 and 5 in the present work. Based on the foregoing, it is clear that the stereoelectronics of the NHC ligands can also play a very important role in terms of the antimicrobial activity.
3. Experimental
3.1. General
All glassware was oven-dried before use. All reagents were obtained commercially and used without further purification. Toluene and hexanes were dried over Na and freshly distilled prior to use. The dichloromethane was dried over CaH2 and freshly distilled prior to use. Compounds 1, 2 and 4 were synthesized according to literature procedures [1].
3.2. Physical Measurements
Low-resolution CI mass spectra were obtained on a Thermo Scientific TSQ Quantum GC mass spectrometer and high-resolution CI mass spectra were recorded on a magnetic sector Waters Autospec Ultima instrument. 1H- and 13C{1H}-NMR spectra were recorded at 295 K in CDCl3 on a Varian Unity 300 (1H, 300 MHz; 13C, 75 MHz) or a Varian INOVA 500 spectrometer (1H, 500 MHz; 13C, 125 MHz) immediately following sample preparation. Deuterated chloroform was obtained from Cambridge Isotopes and stored over 4 Å molecular sieves prior to use. 1H and 13C{1H} chemical shift values are reported in parts per million (ppm) relative to SiMe4 (δ 0.00), using the solvent resonance as the internal standard.
3.3. X-Ray Crystallography
For compound 5, a crystal of suitable quality was removed from a vial, covered with mineral oil and mounted on a nylon thread loop. The X-ray diffraction data were collected on a Rigaku AFC-12 Saturn 724+ CCD diffractometer equipped with a graphite-monochromated Mo Kα radiation source (λ = 0.71073 Å) and a Rigaku XStream low temperature device cooled to 100 K. Corrections were applied for Lorentz and polarization effects. The structure was solved by direct methods and refined by full-matrix least-squares cycles on F2 using the Siemens SHELXTL PLUS 5.0 (PC) software package [14]. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in fixed, calculated positions using a riding model. The CCDC reference number for compound 5 is 793378.
3.4. Preparations
IPr(BIAN)[AgOAc] (3). Dichloromethane (30 mL) was added to a 50 mL round bottom flask that had been covered with aluminum foil, then charged with 2 (0.115 g, 0.175 mmol) and AgOAc (0.030 g, 0.179 mmol). The reaction mixture was stirred for 12 h at ambient temperature, after which the solvent was removed under reduced pressure. The crude product was digested in toluene, filtered and the solvent stripped under reduced pressure to afford analytically pure 3 as a bright yellow solid (0.0775 g, 65.1%). MS (CI+, CH4): m/z 679 [M+H]+; HRMS (CI+, CH4): calcd for C39H44N2O 2Ag m/z 679.2454; found, 679.2449; 1H-NMR: δ 1.11 (d, 12H, CH3), 1.33 (d, 12H, CH3), 1.88 (s, 3H, OAc-CH3), 2.83 (sept, 4H, -CH), 7.02 (d, 2H, Naph-H), 7.40 (d, 4H, Ar-H), 7.43 (t, 2H, Naph-H), 7.59 (t, 2H, Ar-H), 7.81 (d, 2H, Naph-H); 13C-NMR: δ 22.66, 23.83, 24.69, 28.88, 121.05, 124.57, 125.27, 127.79, 128.37, 129.86, 130.70, 130.84, 133.37, 139.19, 139.25, 145.63, 178.07; mp (decomp): 268-270 °C.
IPr(BIAN)[AuOAc] (5). Dichloromethane (30 mL) was added to a 50 mL aluminum foil-wrapped round bottom flask containing 4 (0.120 g, 0.161 mmol) and AgOAc (0.0280 g, 0.168 mmol). The reaction mixture was stirred for 12 h at ambient temperature, after which the solvent was removed under reduced pressure. The crude product was digested in toluene, filtered and the solvent stripped under reduced pressure to afford analytically pure 5 as a bright yellow solid (0.0735 g, 59.3%). A suitable crystal for X-ray diffraction analysis was grown by slow evaporation of a saturated DCM/hexanes solution of 5. MS (CI+, CH4): m/z 769 [M+H]+; HRMS (CI+, CH4): calcd for C39H44N2O2Au m/z 769.3068; found, 769.3068; 1H-NMR: δ 1.10 (d, 12H, CH3), 1.41 (d, 12H, CH3), 1.81 (s, 3H, OAc-CH3), 2.82 (sept, 4H, -CH), 7.01 (d, 2H, Naph-H), 7.40 (d, 4H, Ar-H), 7.44 (t, 2H, Naph-H), 7.60 (t, 2H, Ar-H), 7.81 (d of d, 2H, Naph-H); 13C-NMR: δ 23.91, 23.96, 24.38, 29.05, 121.21, 124.52, 125.36, 127.80, 128.55, 129.78, 130.29, 130.87, 132.83, 138.05, 145.67, 176.35; mp (decomp): 340-343 °C.
3.5. Antimicrobial Activities of 1-5
Antimicrobial activities of compounds 1-5 were determined using the Microtiter-Based Minimum Inhibitory Concentration (MIC) Test. S. aureus ATCC 6538, B. subtilis ATCC 19659, E. coli ATCC 11229 and P. aeruginosa ATCC 15442 were grown to approximetly 105 CFU/mL in Mueller-Hinton broth. The stock solutions of compounds 1-5 were prepared with DI water supplemented with dimethyl sulfoxide (DMSO). The concentrations of the tested compounds ranged from 2% to 0.0039% using the Mueller-Hinton broth as the diluent, supplemented with DMSO to a final concentration of 2%. The plates were incubated at 36.0 ± 1 °C for 18-24 h. The MIC was taken to be the last well in the dilution series that did not exhibit growth, determined on the basis of turbidity.
4. Conclusions
The first examples of silver and gold antimicrobial compounds supported by the BIAN ligand class are reported. The antimicrobial activities of these complexes and the precursor imidazolium salt have been investigated. In terms of Minimum Inhibitory Concentration (MIC) values, the most active compound against both Gram-positive and Gram-negative bacteria is the precursor imidazolium salt. Comparison of the MIC values for the AgCl and AgOAc ligated complexes reveals that their activities against Gram-positive S. aureus are similar to that of AgNO3. On the other hand, these complexes are significantly less active toward Gram-negative E. coli and P. aeroginosa.
Acknowledgements
The authors are grateful to the Robert A. Welch Foundation (Grant F-0003) for financial support of this work.
References and Notes
- 1.Vasudevan K.V., Butorac R.R., Abernethy C.D., Cowley A.H. Synthesis and coordination compounds of a bis(imino)acenaphthene (BIAN)-supported N-heterocyclic carbene. Dalton Trans. 2010;39:7401. doi: 10.1039/c0dt00278j. [DOI] [PubMed] [Google Scholar]
- 2.Hindi K.M., Panzer M.J., Tessier C.A., Cannon C.L., Youngs W.J. The medicinal applications of imidazolium carbene-metal complexes. Chem. Rev. 2009;109:3859. doi: 10.1021/cr800500u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill N.J., Vargas-Baca I., Cowley A.H. Recent developments in the coordination chemistry of bis(imino)acenaphthene (BIAN) ligands with s- and p-block elements. Dalton Trans. 2009:240. doi: 10.1039/b815079f. [DOI] [PubMed] [Google Scholar]
- 4.Pernak J., Skrzpezak A. 3-alkylthiomethyl-1-ethylimidazolium chlorides. Correlation between critical micelle concentrations and minimum inhibitory concentrations. Eur. J. Med. Chem. 1996;31:901. doi: 10.1016/S0223-5234(97)89853-4. [DOI] [Google Scholar]
- 5.Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicrobial Chemother. 2001;48(Suppl. 1):S5. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 6.Demberelnyamba D., Kim K.-S., Choi S., Park S.-Y., Lee H., Kim C.-J., Yoo I.-D. Synthesis and antimicrobial properties of imidazolium and pyrrolidinonium salts. Bioorg. Med. Chem. 2004;12:853. doi: 10.1016/j.bmc.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Pernak J., Sobaszkiewicz K., Mirska I. Anti-microbial activities of ionic liquids. Green Chem. 2003;5:52. doi: 10.1039/b207543c. [DOI] [Google Scholar]
- 8.Çetinkaya E., Denizci A., Özdemir I., Öztürk H. T., Karaboz I., Çetinkaya B. Remarkable substituent effects on antimicrobial activities of 1,3-diorganylimidazolidinium salts. J. Chemother. 2002;14:241. doi: 10.1179/joc.2002.14.3.241. [DOI] [PubMed] [Google Scholar]
- 9.Patil S., Deally A., Gleeson A., Müller-Bunz H., Paradisi F., Tacke M. Novel benzyl-substituted N-heterocyclic carbene-silver acetate complexes: synthesis, cytotoxicity and antibacterial studies. Metallomics. 2011;3:74. doi: 10.1039/c0mt00034e. [DOI] [PubMed] [Google Scholar]
- 10.Ray S., Mohan R., Singh J.K., Samantaray M.K., Shaikh M.M., Panda D., Ghosh P. Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J. Am. Chem. Soc. 2007;129:15042. doi: 10.1021/ja075889z. [DOI] [PubMed] [Google Scholar]
- 11.Barnard P. J., Berners-Price S. J. Targeting the mitochondrial cell death pathway with gold compounds. Coord. Chem. Rev. 2007;251:1889. an interesting review of NHC-Au complexes that target mitochondrial function. [Google Scholar]
- 12.Özdemir I., Denizci A., Öztürk H. T., Çetinkaya B. Synthetic and antimicrobial studies on new gold(I) complexes of imidazolidin-2-ylidenes. Appl. Organomet. Chem. 2004;18:318. [Google Scholar]
- 13.zdemir I., Temelli N., Günal S., Demir S. Gold(I) complexes of N-heterocyclic carbene ligands containing benzimidazole: synthesis and antimicrobial activity. Molecules. 2010;15:2203. doi: 10.3390/molecules15042203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheldrick G.M. SHELXTL-PC, version 5.03. Siemens Analytical X-ray Instruments, Inc.; Madison, WI: 1994. See also Sheldrick, G. M. Acta Crystallogr Sect A. 2008, A 64, 112. [Google Scholar]