Abstract
Background
Pasteurized autograft is regarded as a biologic reconstructive option for managing bone defects after tumor resection; however, reports on long-term outcomes from large patient series are scarce. Contrary to previous favorable reports, we have observed many patients with failures, in particular as the duration of followup increased. Because pasteurized autografts are used in many countries as a reconstruction option, we wished to formally evaluate patients who underwent this approach at one specialty center.
Questions/purposes
(1) What is the graft survival and what proportion of patients achieved union when pasteurized autografts were used for bone defects after tumor resection? (2) What are the complications and causes of graft removal? (3) What factors are related to the likelihood of union and graft survival? (4) What is the survival and cause of failure by type of pasteurized autograft reconstruction?
Methods
Over a 26-year period from 1988 to 2013, we performed 1358 tumor resections in our center. Of these, 353 were reconstructed with pasteurized autograft. Other reconstructions included endoprostheses (508 patients), instant arthrodesis using an intramedullary nail and bone cement (286 patients), allografts (97 patients), and resection only (114 patients). During the period in question, we generally used this approach when tumor showed an osteoblastic pattern and less than one-third cortical destruction in osteolytic tumor. We generally avoided this approach when the tumor showed an extensive osteolytic pattern. We excluded 75 (21% [75 of 353]) patients, 21 (6% [21 of 353]) for incomplete clinical data and 54 (15% [54 of 353]) with a followup < 2 years or those lost to followup leaving 278 autografts eligible. The mean followup was 113 months (range, 25–295 months). Of these 278 patients, 242 patients had primary bone sarcomas, 22 patients had soft tissue tumor invading bone, seven patients had metastatic carcinoma, and seven patients had aggressive benign bone tumors. From a chart review, we obtained the age, sex, location, tumor volume, histologic diagnosis, use of chemotherapy, graft length, fixation modality, type of pasteurized bone used, proportion of union, complications, and oncologic outcome of the patients. In total, 377 junctional sites were assessed for union with serial radiographs. We defined junctions showing union < 2 years as union and > 2 years as delayed union. We grouped our patients into type of pasteurized bone use: pasteurized autograft-prosthesis composites (PPCs) were performed in 149, intercalary grafts in 71, hemicortical grafts in 15, osteoarticular in 12, and fusion of a joint in 31 patients. The endpoint of interest included removal of the autograft with implant loosening, infection, fracture of the graft, or any reoperation resulting in removal. Survival of the graft was determined by Kaplan-Meier plot and intergroup differences were determined using log-rank test.
Results
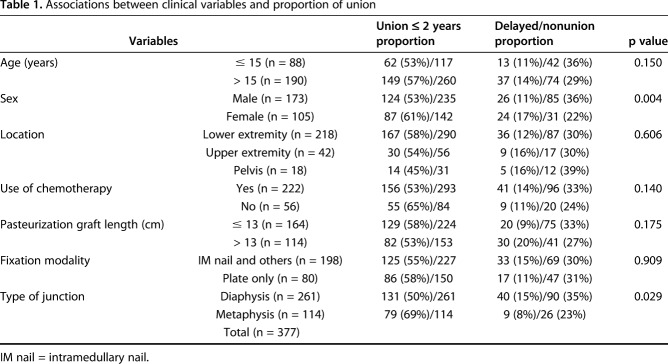
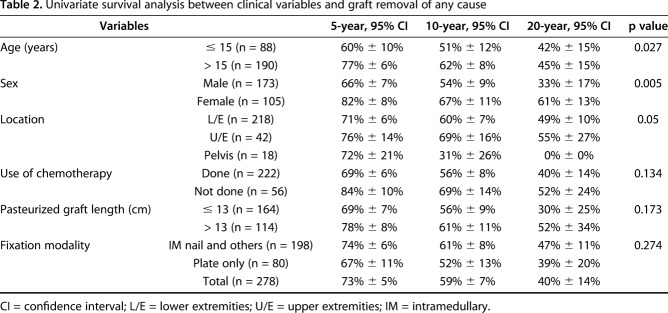
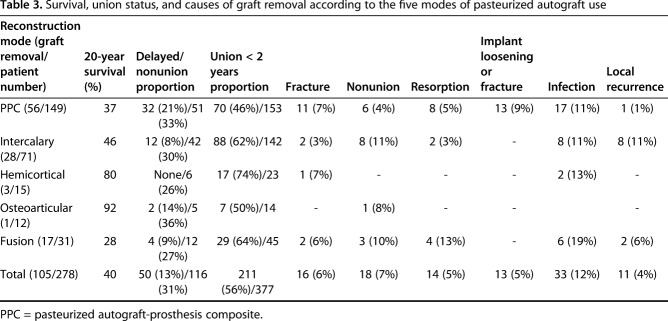
Five, 10-, and 20-year survival of 278 autografts was 73% ± 5.5%, 59% ± 6.7%, and 40% ± 13.6%, respectively. Of 278 autografts, 105 (38%) were removed with complications. Cause of removal included infection in 13% (33 patients), nonunion in 7% (18 patients), fracture of graft in 6% (16 patients), resorption of the graft in 5% (14 patients), and local recurrence in 4% (11 patients). Univariate survival analysis revealed that patient age ≤ 15 years (p = 0.027; hazard ratio [HR], 1.541), male sex (p = 0.004; HR, 1.810), and pelvic location (p = 0.05; HR, 2.518) were associated with graft removal. The 20-year survival rate of osteoarticular and hemicortical methods was 92% (95% confidence interval, -15.6% to +8.3%) and 80% ± 20%, respectively. For intercalary and fusion, it was 46% ± 15% and 28% ± 22%, respectively, although for PPC, it was 37% ± 22%. Log-rank survival analysis showed the osteoarticular and hemicortical groups had better graft survival compared with other types of reconstruction (p = 0.028; HR, 0.499). The most prevalent cause of graft removal in three major types of reconstruction was as follows: (1) PPC type was infection (30% [17 of 56]); (2) intercalary graft was infection, nonunion, and local recurrence in even proportions of 29% (86% [24 of 28]); and (3) fusion was infection (35% [six of 17]). Two hundred ten (56%) of 377 junctional sites showed union within 2 years (average, 14 months), 51 (13%) junctions showed delayed union after 2 years (average, 40 months), and the remaining 116 (31%) junctions showed nonunion. Diaphyseal junction (p = 0.029) and male sex (p = 0.004) showed a higher proportion of nonunion by univariate analysis.
Conclusions
Compared with the favorable short-term and small cohort reports, survival of pasteurized autograft in this long-term large cohort was disappointing. We believe that pasteurized autograft should be used with caution in children and adolescents, in the pelvic region, and in PPC form. When bone stock destruction is minimal, it is worth considering this approach for small intercalary or distal long bone reconstruction. We believe this procedure is best indicated after hemicortical resection of long bone.
Level of Evidence
Level III, therapeutic study.
Introduction
Currently, endoprostheses are commonly used for the reconstruction of large skeletal defects after tumor resection; however, in some tumor locations and for certain tumors such as parosteal osteosarcoma, biologic or composite biologic reconstruction has been used as an alternative [2-4, 7, 18, 22]. The main purpose of biologic reconstruction is to decrease tumor prosthesis-related complications and potentially to improve longevity of the implant. For biologic materials, bone allografts are used and, when unavailable, recycled autograft is an alternative. Compared with allograft, recycled autograft has advantages such as accessibility, perfect size matching, reduced disease transmission, and cost. Methods for recycling of autograft after removal of the involved bony segment include autoclaving, irradiating, pasteurization, and freezing with liquid nitrogen [5, 9, 13, 25]. Of these, pasteurization of bone at 65° C for 30 minutes intends to kill tumor cell while preserving bone morphogenic protein. An experimental study on various sterilization methods for recycled bone autograft suggests pasteurization has the best callus formation ability because of its osteocyte preservation and bone marrow cellularity [28]. The frozen method is popular in Japan and is reported to show an improved union rate and mechanical strength compared with other types of recycled autograft; however, its superiority is still controversial [24, 27]. To choose a proper biologic reconstructive option, knowledge about the method’s limitations and long-term fate of the graft is important.
Since 1988, we have applied pasteurized autograft after resections of bone and soft tissue tumors invading bone. Initially, it was used primarily for intercalary resections. Later, we expanded its use to pasteurized autograft-prosthesis composite (PPC), arthrodesis (distal radius or distal tibia), hemicortical resection, and osteoarticular (acetabulum or glenoid) resection. Our previous reports on smaller groups of patients and other reports demonstrated encouraging survival of graft [7, 10-12]. However, tumor-bearing bone is partially destroyed and any type of tumor sterilization method will change its structural integrity. Accordingly, we presumed mechanical strength of recycled bone would be inferior to that of allograft. In our previous study, the average union time of pasteurized autograft was approximately 15 months, which is longer than that reported for allograft (9 months) [12, 21]. However, we noticed that with longer followup, there was a number of patients with graft removal, especially in patients who are still growing and in composite reconstruction. Therefore, we wanted to assess the method in a large population over a longer followup.
In a review of 278 pasteurized autografts, we asked: (1) What is the graft survival and what proportion of patients achieved union when pasteurized autografts were used for bone defects after tumor resection? (2) What are the complications and causes of graft removal? (3) What factors are related to the likelihood of union and graft survival? (4) What is the survival and cause of failure by type of pasteurized autograft reconstruction?
Patients and Methods
From 1988 to 2013, we performed 1358 tumor resections including bone for musculoskeletal tumors. After resection, the type of reconstructions included tumor prosthesis (508 patients), resection arthrodesis using intramedullary nail and bone cement (286 patients), pasteurized autograft (353 patients), allograft (97 patients), and resection only (114 patients). From our database we extracted 353 patients who underwent reconstructions using pasteurized autograft. We excluded 75 patients, 21 for incomplete clinical data, 27 with followup < 2 years without an event, and 27 patients were lost to followup. Therefore, 278 patients were enrolled in this study. The mean followup was 113 months (range, 25–295 months). This retrospective study was approved by our institutional review board. The indication for pasteurized autograft was as follows: (1) predominance of an osteoblastic pattern on plain radiograph; (2) less than one-third cortical bone destruction on axial MRI or CT for osteolytic tumor; and (3) soft tissue tumors encroaching on the neighboring cortical bone. Contraindications included (1) tumors showing extensive an osteolytic pattern; and (2) patients with a limited life expectancy. From our database, we extracted the patients’ age, sex, location of the tumor, initial tumor volume, histologic diagnosis, length of the graft, fixation modality of the pasteurized bone, adjuvant chemotherapy, local recurrence, distant metastasis, and final survival status of the patients. There were 173 male and 105 female patients with an average age of 24 years (range, 5-72 years). Pathologic diagnoses included osteosarcoma (n = 201), chondrosarcoma (n = 23), Ewing’s sarcoma (n = 11), malignant fibrous histiocytoma of bone (n = 7), metastatic carcinoma (n = 7), benign aggressive bone tumor (n = 7), and soft tissue sarcoma invading bone (n = 22). Tumor volume was calculated using three parameters (length, width, and depth) on MRI using the ellipsoid formula: [V = (4π/3)abc]. Average tumor volume was 187 cm3 (range, 4-499 cm3). There were 137 tumors in the femur, 81 in the tibia, 34 in the humerus, 18 in the pelvis, five in the forearm, and three in the scapula (Fig. 1). Neoadjuvant and adjuvant chemotherapy was performed in 222 (80%) patients. There were 22 (8%) local recurrences and 80 (29%) distant metastases. The surgical margin status of 22 patients with local recurrence was wide in 13, marginal in seven, and intralesional in two.
Fig. 1.
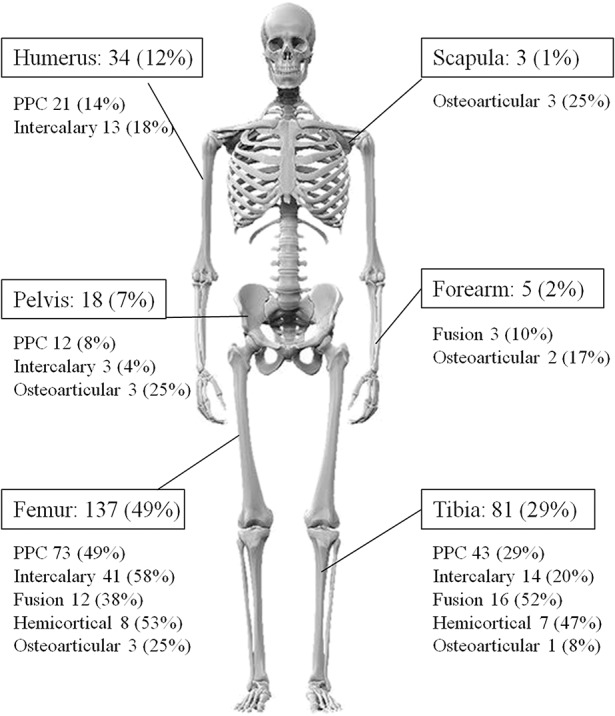
Figure of a skeleton shows locations of 278 grafts and types of reconstruction.
Patients’ final statuses were continuously disease-free in 185 (66%), no evidence of disease in 35 (13%), died of disease in 50 (18%), alive with disease in seven (3%), and one patient died of another disease.
After resection of the tumor, the pasteurized bone was prepared as previously described [12]. Briefly, (1) the bone was cleared of soft tissue and extraosseous tumor; (2) the medullary cavity was reamed and intraosseous tumor was removed; (3) the bone was then kept in preheated saline at 65° C for 30 minutes, retrieved, and prepared on a different table; and (4) the pasteurized bone was returned and fixed to host bone using internal fixation. An intramedullary nail was used for fixation in 174, a plate in 80, both in nine, and screws only in 15 patients. According to the type of reconstruction, there were 149 patients with PPC, 71 intercalary grafts, 15 hemicortical reconstructions, 12 osteoarticular, and 31 fusions.
In patients with postoperative chemotherapy, plain AP and lateral radiograph examinations were performed monthly until the completion of chemotherapy. The radiographic union at the junctional site was judged by one radiologist (JYY) and two of the authors (D-GJ, SYL). The site of the osteotomy was considered radiographically healed when the callus was seen to bridge the osteotomy line in both the AP and lateral planes. Three hundred seventy-seven junctional sites were analyzed for union (eight patients with pasteurization of the whole affected long bone were excluded). In the present study, average union time was 18 months and that of autoclaved bone was 24 months [13]. Because an autoclaved one will show longer union time, we defined junctions showing union 2 years after the index operation as delayed union. Patients showing symptomatic nonunion within 2 years and nonunion until last followup were regarded as nonunion. Patients with symptomatic nonunion underwent an osteosynthesis procedure. Asymptomatic patients with nonunion or loosening after 2 years from their operation did not undergo an additional procedure. Graft failure was defined as removal of the original autograft for any cause. Time to failure was defined as the elapsed time between the first surgery and the date of autograft or prosthetic removal. After completion of chemotherapy or in patients undergoing surgery only, radiologic examination was performed every 3 months for the first 2 years and biannually thereafter. Survival curves for graft survival were determined using the Kaplan-Meier method and intergroup differences in survival were determined using the log-rank test. Analyses were performed using SPSS Version 13.0 (SPSS Inc, Chicago, IL, USA), and p values < 0.05 were considered significant.
Results
Overall, with graft removal as the primary endpoint, the 5-, 10-, and 20-year survival rates of the 278 pasteurized bones calculated using the Kaplan-Meier method were 73% ± 5.5%, 59% ± 6.7%, and 40% ± 13.6%, respectively (Fig. 2).
Fig. 2.
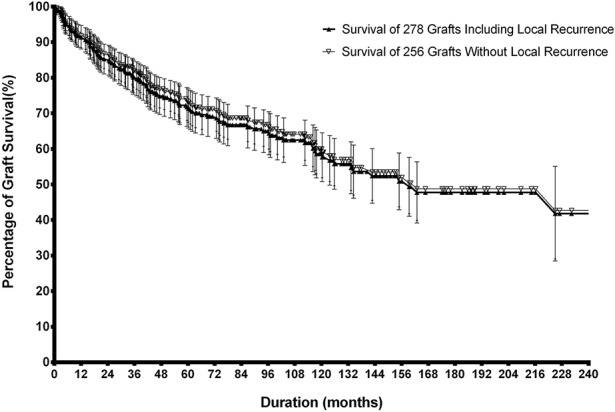
Survival of the 278 pasteurized autografts with removal of the graft for any cause and that of 256 grafts excluding 22 patients with local recurrence is plotted. Error bars denote upper and lower 95% CI.
Of 278 pasteurized autografts, 105 (38%) were removed because of major complications. Major complications included infection in 33 (13%), nonunion in 18 (7%), fracture of the graft in 16 (6%), failure of fixation in 13 (5%), graft resorption in 14 (5%), and local recurrence in 11 (4%). Minor complications include superficial wound infection in 14 (5%), dislocation in six (2%), and one each of deep vein thrombosis and flap necrosis.
Two hundred ten (56%) of 377 junctional sites showed union within 2 years (average, 14 months), 51 (13%) junctions showed delayed union after 2 years (average, 40 months), and the remaining 116 (31%) junctions showed nonunion. Of 118 sites showing nonunion, an additional osteosynthesis procedure was performed in 21 patients and 10 (48%) of 21 junctions achieved secondary union at an average of 23 months (range, 4-61 months).
We observed a higher proportion of nonunion with the diaphyseal compared with metaphyseal junction (65% versus 77%, p = 0.029) and male compared with female (64% versus 78%, p = 0.004) (Table 1). The proportion of nonunion among five types of reconstruction was PPC in 44% (51 of 116 nonunion), intercalary in 36% (42 of 116), fusion in 10% (12 of 116), hemicortical in 5% (six of 116), and osteoarticular in 4% (five of 116) in decreasing order.
Table 1.
Associations between clinical variables and proportion of union
Univariate survival analysis revealed patient age ≤ 15 years (p = 0.027; hazard ratio [HR], 1.541), male sex (p = 0.005; HR, 1.810), and pelvic location (p = 0.05; HR, 2.518) were related to graft removal because of complications (Table 2).
Table 2.
Univariate survival analysis between clinical variables and graft removal of any cause
The 20-year survival rate of osteoarticular and hemicortical methods was 92% (95% confidence interval [CI], -15.6% to +8.3%) and 80% ± 20%, respectively. For intercalary and fusion, it was 46% ± 15% and 28% ± 22%, respectively, although for PPC, it was 37% ± 22%. Log-rank survival analysis of five types of graft reconstruction showed osteoarticular and hemicortical groups have better graft survival compared with the other options (p = 0.028) (Fig. 3). The most prevalent cause of graft removal in three major types of reconstruction is as follows: (1) PPC type was infection (30% [17 of 56]); (2) intercalary graft was infection, nonunion, and local recurrence in even proportions of 29% (86% [24 of 28]); and (3) fusion was infection (35% [six of 17]) (Table 3).
Fig. 3.
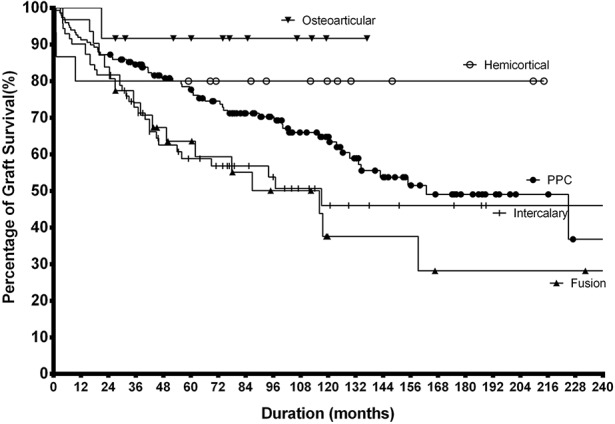
Log-rank survival analysis of five types of graft shows osteoarticular and hemicortical forms have better graft survival over other types (p = 0.028).
Table 3.
Survival, union status, and causes of graft removal according to the five modes of pasteurized autograft use
Discussion
In limb-sparing surgery for malignant musculoskeletal tumors, biologic reconstruction is a viable option, and in some locations or tumor types, this modality may offer superior longevity of reconstruction [2-4, 17]. Recycled autograft was introduced for situations in which an allograft is not readily available and its principal advantage is anatomic conformation [16, 19]. Although there is a variety of ways to prepare an autograft after tumor resection, we believe the preponderance of the evidence supports pasteurization [12, 23, 26]. Accordingly, we expanded its indication from intercalary reconstruction to other types such as PPC for bone stock restoration. However, as followup duration increased, we experienced failures with this procedure. Therefore, we questioned the efficacy of this method and wished to evaluate it more formally. In our study, we were disappointed with the long-term graft survival of patients with composite reconstruction (PPC) and patients who were still growing. We believe, based on our study, that pasteurized autograft should be used with caution for patients with skeletal growth remaining, tumors in the pelvic region, and in PPC form. It may be useful for intercalary or distal long bone (distal tibia or distal radius) reconstructions. We observed the fewest complications and nonunions in patients treated with hemicortical resection of long bone.
Our retrospective study has limitations such as heterogeneity in diagnosis, diversity in location, use of chemotherapy, amount of bone and soft tissue resection, differences in postoperative management, and mode of internal fixation. Additionally, over the long study period, improvement in technical skill may have improved our results, and readers should consider this when interpreting our findings. Some patients were lost to followup; in general, those missing may have more likely experienced complications or reoperations, and so our results may be construed as a best case scenario for this reconstructive approach. We did not have a comparison group to document whether this technique compares favorably or is worse than other reconstruction methods. We were unable to perform a multivariate analysis to look at specific factors that might relate to failure. We did not use a competitive risk analysis, which would have improved the accuracy of our survival observations. However, in our data, 18% of our patients died of disease and their average followup was 48 months. We believe 4 years is an acceptable period to estimate the survival of the graft and it had a minor effect on the accuracy of survival. Finally, because this study spans > 20 years and indications may have evolved, there is an issue of selection bias. Retrospectively, in the early 10 years, high-grade osteolytic tumor involving one cortex on plain radiographs was regarded as a candidate for autograft. At present, we believe initial bone quality is of utmost importance. Most of the patients showing long-term graft survival had low-grade surface lesions (eg, parosteal osteosarcoma) or small tumors with minimal bone destruction.
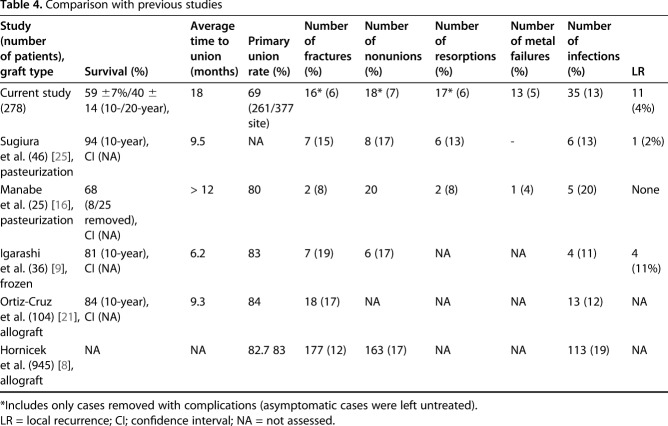
Our present study on reconstruction using the pasteurization method showed a 10-year survival rate of approximately 59%, which was lower than that in other recent long-term studies of 94%, 81% in autograft series, and 84% in allograft. Furthermore, although absence of 20-year data in other series does not allow for comparison, at 20 years, this dropped to 40% (Table 4). Lower 10-year survival compared with other recycled autograft series may be interpreted by a difference in the use of graft or augmentation bone graft. In Sugiura et al.’s [25] series, only four (9% versus 54% in our study) patients underwent the PPC form of reconstruction and 39% (18 of 46) had an additional osteosynthesis procedure using vascularized fibula. However, in Igarashi et al.’s [9] series, they reported a 10-year survival rate of 80% (seven of 36 graft removed) despite graft fracture in 19% (seven of 36), infection in 11% (four of 36), and nonunion in 17% (six of 36). Statistically, an early event for graft removal (< 2 years) may contribute to the high overall survival. Chronologically, the cause of revision of pasteurized autograft was infection followed by nonunion or implant loosening/fracture, graft fracture, and resorption of graft. Although we cannot directly compare our results with others, the union rate (56% [211 of 377 junctions]) in our study is seemingly lower than that of Sugiura et al. (83% [38 of 46 patients]) or Igarashi et al. (72% [26 of 36 patients]) [9, 25]. The high union rate in other studies may have resulted from a small sample size or a concomitant osteosynthesis procedure [6, 14, 20, 25]. Infection is one of the major causes of revision in biologic reconstruction. The infection rate of 13% in our series was lower than that of a large allograft series by Hornicek et al. [8] (18% [113 of 945 allografts]). Although direct comparison is not possible, possible reasons are (1) our cohort may have smaller tumors (mean tumor volume < 200 cc), that is, the pasteurized group may have less extensive soft tissue resection; (2) the allograft group had a high proportion of osteoarticular-type reconstruction; and (3) heat treatment itself may have an additional sterilizing effect against microorganisms. Graft fracture is another factor in graft survival. The mechanical strength of the graft, implant loosening or fracture, nonunion, and graft resorption are predisposing factors for graft fracture. The graft fracture rate of 6% (16 of 278) in our study is lower than that of Igarashi et al. (19% [seven of 36]) or Sugiura et al. (15% [seven of 46]) using frozen or pasteurized autograft [9, 25]. In a series of allografts, Hornicek et al. [8] and Ortiz-Cruz et al. [21] reported graft fracture rates of 12% (177 of 945) and 17% (18 of 104), respectively. Although we cannot directly compare our findings with those of others, a reason why we experienced a lower proportion of graft fracture is not clear. However, tumor-bearing bone is partially destroyed and, in addition, it loses elasticity after heat treatment. For these reasons, we believe that pasteurized bone is mechanically weaker than allograft. Bone resorption is a precursor of fracture and the high resorption rate of 14% (14 of 40 resorptions removed) in our study suggests that a substantial portion of the pasteurized bone grafts was in a state of impending fracture. Regarding the mode of fixation, we presumed that the use of intramedullary nailing would decrease mechanical complications, but with the numbers we had, we found no difference between nailing and plate fixation. Pasteurized autografts in the pelvic region showed a high risk of removal and our current principle for pelvic tumor is resection hip arthroplasty (pseudojoint formation by preserving the femoral head) [15].
Table 4.
Comparison with previous studies
In univariate analysis, young age (≤ 15 years) and male sex were factors associated with graft failure. Because there are many confounders for graft failure such as tumor type, graft bone quality, method of fixation, and type of autograft used, it is not clear whether those factors were related to graft failure. The observed finding is young patients (≤ 15 years) show a higher revision rate with nonunion (9% [eight of 88] versus 5% [10 of 190]) or resorption (9% [eight of 88] versus 3% [six of 190]) than older patients (> 15 years). However, the type of graft use in both groups was different; the fusion proportion was 23% (20 of 88) in the younger age group, whereas it was 6% (11 of 190) in the older age group and all the 15 hemicortical resections were performed in the older age group. Therefore, age itself may not be related to nonunion. On the contrary, resorption showed a different figure. The younger patient group showed a three times higher revision rate resulting from resorption. Regardless of revision, in younger patients, the overall resorption rate was twice as high as in older patients (23% [20 of 88] versus 11% [20 of 190]). Radiologic findings of resorption ranged from moderate cortical atropy to vanishing bone disease-like resorption. We presume those findings are a spectrum of consequence according to initial bone quality. Patients with initial good bone stock may show mild to moderate cortical atropy, which stabilizes approximately 3 years after operation, whereas patients with poor initial bone stock may experience more marked osteolysis. Because cortical thickness of patients during the years of growth is thinner than that of adults, patients still growing may show more frequent or a higher degree of resorption. Although we do not have an innovative way to decrease the infection or local recurrence rate, if we can increase the proportion of primary union, we may increase the graft survival rate.
To improve the proportion of primary union, rigid fixation and addition of an osteosynthesis procedure are important. For intercalary reconstruction, dual plate fixation covering two osteotomy sites seems to be mandatory. In PPC-type reconstruction, rotational instability and small bone-to-bone contact area may cause nonunion and subsequent loosening. To increase rotational stability and bone-to-bone contact area, we tried a V osteotomy at the time of tumor resection; however, its role against nonunion was not determined. Therefore, the addition of plate fixation at the junctional site may be considered when remaining host bone segment is shorter than the PPC construct, thereby decreasing the long lever arm and related loosening or nonunion. Considering most of the patients with recycled autograft have malignancies and chemotherapy is imperative for survival, routine application of autogenous iliac bone graft or free vascularized fibula transfer at the time of index operation may impose additional morbidity. We believe it is probably best to revise nonunion of the osteosynthesis sites until chemotherapy has been completed.
Since the introduction of the recycled autograft, we have used five types of autograft according to tumor location within the bone or type of reconstruction. For small diaphyseal tumors (resection length < 10 cm) showing minimal cortical destruction, reconstruction using a pasteurization method may be a reasonable option to consider. The use of autograft in PPC form is a technically complex procedure. In the pelvic region, our experience suggests that the high failure rate precludes its use and we no longer use pasteurized grafts for this purpose. In the extremities, the survival of the composite reconstruction at 10 and 20 years is 66% and 41%, respectively, in our series, raising the question of whether it offers any advantage compared with tumor endoprostheses. However, in the proximal femur, when greater trochanter can be spared, application of PPC may contribute to preserving abductor function. Additionally, in economically compromised patients, PPC may be a cheaper substitute. In the distal tibia, where a durable prosthesis has not yet been designed, use of recycled autograft may be a reasonable option to consider [1, 17]. When we applied a plate for graft fixation, we experienced difficulty in soft tissue closure and long-term external support was used until bony union. Accordingly, we switched to retrograde intramedullary nail fixation from the calcaneal side and this appeared to improve the fixation problem. We reserve the osteoarticular form of pasteurization for glenoid reconstruction and it serves as an excellent spacer while maintaining shoulder function. In low- to intermediate-grade tumors involving less than half of the bone circumference, we found that hemicortical excision and reposition after heat treatment are reasonably successful. A large contact area with host bone appears to enable early union at approximately 6 months and the highest rate of union along with satisfactory long-term graft survival.
In conclusion, compared with the favorable short-term and small cohort reports [7, 10-12], our long-term graft survival results from a large cohort of pasteurized autograft were disappointing, and complications were common. Although we cannot directly compare our results with other reconstructions, based on our experience, we believe that pasteurized autograft should be used with caution in children and adolescents, in the pelvic region, and in PPC form. When bone stock destruction is minimal, it is worth trying for small intercalary or distal long bone reconstruction. We believe this procedure is best indicated after hemicortical resection of long bone.
Acknowledgments
We thank Ji Young Yoo MD, for radiographic evaluation of this study.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution has approved the human protocol for their investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abudu A, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement of the distal tibia and ankle joint for aggressive bone tumours. Int Orthop. 1999;23:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL. Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res. 2012;470:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bus MP, Bramer JA, Schaap GR, Schreuder HW, Jutte PC, van der Geest IC, van de Sande MA, Dijkstra PD. Hemicortical resection and inlay allograft reconstruction for primary bone tumors: a retrospective evaluation in the Netherlands and review of the literature. J Bone Joint Surg Am. 2015;97:738–750. [DOI] [PubMed] [Google Scholar]
- 4.Capanna R, Campanacci DA, Belot N, Beltrami G, Manfrini M, Innocenti M, Ceruso M. A new reconstructive technique for intercalary defects of long bones: the association of massive allograft with vascularized fibular autograft. Long-term results and comparison with alternative techniques. Orthop Clin North Am. 2007;38:51–60, vi. [DOI] [PubMed] [Google Scholar]
- 5.Chen TH, Chen WM, Huang CK. Reconstruction after intercalary resection of malignant bone tumours: comparison between segmental allograft and extracorporeally-irradiated autograft. J Bone Joint Surg Br. 2005;87:704–709. [DOI] [PubMed] [Google Scholar]
- 6.Ehara S, Nishida J, Shiraishi H, Tamakawa Y. Pasteurized intercalary autogenous bone graft: radiographic and scintigraphic features. Skeletal Radiol. 2000;29:335–339. [DOI] [PubMed] [Google Scholar]
- 7.Eid AS, Jeon DG, Song WS, Lee SY, Cho WH. Pasteurized autograft-prosthesis composite for proximal femoral reconstruction: an alternative to allograft composite. Arch Orthop Trauma Surg. 2011;131:729–737. [DOI] [PubMed] [Google Scholar]
- 8.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi K, Yamamoto N, Shirai T, Hayashi K, Nishida H, Kimura H, Takeuchi A, Tsuchiya H. The long-term outcome following the use of frozen autograft treated with liquid nitrogen in the management of bone and soft-tissue sarcomas. Bone Joint J. 2014;96:555–561. [DOI] [PubMed] [Google Scholar]
- 10.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Pasteurized autograft-prosthesis composite for distal femoral osteosarcoma. J Orthop Sci. 2007;12:542–549. [DOI] [PubMed] [Google Scholar]
- 11.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Pasteurized autograft-prosthesis composite for reconstruction of proximal tibia in 13 sarcoma patients. J Surg Oncol. 2007;96:590–597. [DOI] [PubMed] [Google Scholar]
- 12.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Pasteurized autograft for intercalary reconstruction: an alternative to allograft. Clin Orthop Relat Res. 2007;456:203–210. [DOI] [PubMed] [Google Scholar]
- 13.Khattak MJ, Umer M, Haroon ur R, Umar M. Autoclaved tumor bone for reconstruction: an alternative in developing countries. Clin Orthop Relat Res. 2006;447:138–144. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Kim KJ, Han I, Oh JH, Lee SH. The use of pasteurized autologous grafts for periacetabular reconstruction. Clin Orthop Relat Res. 2007;464:217–223. [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Jeon DG, Cho WH, Song WS, Kong CB. Comparison of pasteurized autograft-prosthesis composite reconstruction and resection hip arthroplasty for periacetabular tumors. Clin Orthop Surg. 2017;9:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manabe J, Ahmed AR, Kawaguchi N, Matsumoto S, Kuroda H. Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:258–266. [DOI] [PubMed] [Google Scholar]
- 17.Moore DR, Halpern JL, Schwartz HS. Allograft ankle arthrodesis: a limb salvage technique for distal tibial tumors. Clin Orthop Relat Res. 2005;440:213–221. [DOI] [PubMed] [Google Scholar]
- 18.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J Bone Joint Surg Am. 2005;87:2449–2455. [DOI] [PubMed] [Google Scholar]
- 19.Nishida Y, Tsukushi S, Wasa J, Urakawa H, Toriyama K, Kamei Y, Ishiguro N. Vascularized fibular flaps enhance histological repair in pasteurized autogenous bone graft. Ann Plast Surg. 2011;67:416–420. [DOI] [PubMed] [Google Scholar]
- 20.Ogura K, Miyamoto S, Sakuraba M, Fujiwara T, Chuman H, Kawai A. Intercalary reconstruction after wide resection of malignant bone tumors of the lower extremity using a composite graft with a devitalized autograft and a vascularized fibula. Sarcoma. 2015;2015:861575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz-Cruz E, Gebhardt MC, Jennings LC, Springfield DS, Mankin HJ. The results of transplantation of intercalary allografts after resection of tumors. A long-term follow-up study. J Bone Joint Surg Am. 1997;79:97–106. [DOI] [PubMed] [Google Scholar]
- 22.Sewell MD, Hanna SA, McGrath A, Aston WJ, Blunn GW, Pollock RC, Skinner JA, Cannon SR, Briggs TW. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg Br. 2011;93:1111–1117. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Masumi S, Yano H, Fukunaga T, Ikebe S, Shin S. Revascularization and new bone formation in heat-treated bone grafts. Arch Orthop Trauma Surg. 1999;119:57–61. [DOI] [PubMed] [Google Scholar]
- 24.Shimozaki S, Yamamoto N, Shirai T, Nishida H, Hayashi K, Tanzawa Y, Kimura H, Takeuchi A, Igarashi K, Inatani H, Kato T, Tsuchiya H. Pedicle versus free frozen autograft for reconstruction in malignant bone and soft tissue tumors of the lower extremities. J Orthop Sci. 2014;19:156–163. [DOI] [PubMed] [Google Scholar]
- 25.Sugiura H, Nishida Y, Nakashima H, Yamada Y, Tsukushi S, Yamada K. Evaluation of long-term outcomes of pasteurized autografts in limb salvage surgeries for bone and soft tissue sarcomas. Arch Orthop Trauma Surg. 2012;132:1685–1695. [DOI] [PubMed] [Google Scholar]
- 26.Sugiura H, Yamamura S, Sato K, Katagiri H, Nishida Y, Nakashima H, Yamada Y. Remodelling and healing process of moderately heat-treated bone grafts after wide resection of bone and soft-tissue tumors. Arch Orthop Trauma Surg. 2003;123:514–520. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchiya H, Nishida H, Srisawat P, Shirai T, Hayashi K, Takeuchi A, Yamamoto N, Tomita K. Pedicle frozen autograft reconstruction in malignant bone tumors. J Orthop Sci. 2010;15:340–349. [DOI] [PubMed] [Google Scholar]
- 28.Yasin NF, Ajit Singh V, Saad M, Omar E. Which is the best method of sterilization for recycled bone autograft in limb salvage surgery: a radiological, biomechanical and histopathological study in rabbit. BMC Cancer. 2015;15:289. [DOI] [PMC free article] [PubMed] [Google Scholar]