Abstract
Background
Pelvic resections are challenging, and reconstruction of the resected acetabulum to restore mobility and stability is even more difficult. Extracorporeal radiation therapy (ECRT or extracorporeal irradiation) of autograft bone and reimplantation allows for a perfect size match and has been used with some success in the extremities. Although the risk of wound complications in pelvic surgery has discouraged surgeons from using ECRT of autografts in that anatomic site, we believe it may be a reasonable option.
Questions/purposes
In a small series, we asked: (1) What was the median surgical time and blood loss for these procedures, and what early complications were observed? (2) Is there evidence of osteonecrosis or cartilage loss at a minimum of 2 years after ECRT of acetabular autografts, and what functional scores were achieved? (3) What were the oncologic outcomes after ECRT?
Methods
Between March 2007 and September 2016, one surgeon performed 12 ECRT acetabular autografts and reimplantations after resections of pelvic or acetabular tumors. Of those, 10 with minimum 2-year followup are reported on here with respect to oncologic, functional, and radiographic assessment; all 12 are reported on for purposes of surgical parameters and early complications. During that period, we generally performed this approach when we judged it possible to achieve a tumor-free margin, adequate bone stock, and sufficient remaining hip musculature to allow use of the bone as an autograft with restoration of hip mobility. We generally did not use this approach when we anticipated a difficult resection with uncertain margins or where remaining bone was judged of poor strength for use as a graft or if both iliopsoas and abductors were sacrificed. Since 2010, this series represents seven of the 21 pelvic resections with reconstruction that we performed (five patients in this series had the procedure performed before 2010). Followup was at a median of 65 months (range, 33-114 months) for nine patients whose functional outcomes were evaluated. The median patient age was 30 years (range, 10-64 years). Clinical parameters were recorded from chart review; radiographic analysis for assessment of cartilage was performed by looking for any obvious loss of joint space when compared with the opposite side. Functional scoring was done using the Musculoskeletal Tumor Society score, which was obtained from chart review. Oncologic assessment was determined for local recurrence as well as metastases.
Results
Median surgical time was 8.6 hours and median blood loss was 2250 mL. There were no perioperative wound-related complications. Two patients underwent a second surgical procedure during the postoperative period, one for a femoral artery thrombus and another for a complete sciatic nerve deficit. No patients developed avascular necrosis of the femoral head. None of the patients who underwent osteoarticular grafting showed radiographic evidence of joint space narrowing. The median Musculoskeletal Tumor Society score was 28 (range, 17-30). No fractures in the radiated segment of reimplanted bone were seen in this small series.
Conclusions
Results from this small series suggest that ECRT is a potential option in selected patients who have good bone stock and adequate soft tissue coverage. Although technically challenging, ECRT is a low-cost alternative to prostheses in providing a mobile and stable hip. Although we did not observe cartilage wear on plain radiographs, followup here was short term; it may appear as we continue to follow these patients. Future studies from retrieval specimens may shed light on the actual status of cartilage on the acetabulum.
Level of Evidence
Level IV, therapeutic study.
Introduction
Pelvic resections present a challenge to any orthopaedic surgeon because of the extensive surgical approach involved as well as blood loss, long surgical time, and complication risks. Patients without reconstruction have been reported to reach satisfactory functional levels after prolonged and intensive rehabilitation [8, 14]. Efforts at improving function by reconstruction aimed at providing a mobile and stable hip have raised concerns about a high likelihood of complications, residual functional limitations, and reoperations (both early and later on) [1]. Although results with newer approaches to endoprosthetic reconstruction—such as navigation, three-dimensional (3-D) printed jigs, and implants using new approaches to osseointegration—have been exciting, concerns remain about potential shortcomings of these approaches [4, 11, 13].
Extracorporeal irradiation (ECRT) and reimplantation is a potential biologic alternative to reconstruction. The obvious advantage of a perfectly size-matched autograft has been exploited in reconstruction of the extremities [2, 5, 10, 15, 17, 18]. However, the risks of wound complications in pelvic reconstructions involving allograft in particular have discouraged surgeons from using allografts/ECRT autografts as a reconstruction method [6]. Various small series have reported outcomes with ECRT in the pelvis [3, 16, 18]. Considering very limited access to a pelvic allograft or prosthesis, ECRT was an attractive option to us and we had already used it for the extremities.
In a small series, we therefore asked: (1) What was the median surgical time and blood loss for these procedures, and what early complications were observed? (2) Is there evidence of osteonecrosis or cartilage loss at a minimum of 2 years after ECRT of acetabular autografts, and what functional scores were achieved? (3) What were the oncologic outcomes after ECRT?
Patients and Methods
Patients and Indications
The technical difficulties, complications, oncologic, and functional outcomes were analyzed for 12 acetabulum involving pelvic resections in patients aged 10 to 64 years who underwent ECRT between 2007 and 2016 (Table 1). Resections were classified as per Enneking and Dunham classification (Table 1). Indications for the procedure included ability to achieve adequate margins, adequate strength in the tumor-bearing bone, and presence of motors after reconstruction to control the hip. We chose tumors with smaller intrapelvic soft tissue masses (Table 1) to target completing the resection within 4 hours to minimize the wound complications related to length of surgery. However, in five of our patients, the resection took longer than anticipated; even so, we went ahead with ECRT as planned, because during surgery, those patients were deemed fit to tolerate the extended procedure. Contraindications included massive bony destruction rendering the bone unsuitable for autografting, need to resect the hip abductors and the iliopsoas for margin, patients with a large intrapelvic soft tissue mass, which would complicate a resection, or a patient unwilling or unfit to undergo this surgery. Our institute waived approval for the human protocol for this retrospective study. Followup at the last visit ranged from 12 to 114 months. Ten patients with minimum 33-month followup (median, 65 months) or until death were analyzed for oncologic and functional outcomes, whereas all 12 patients were analyzed for associated complications.
Table 1.
Patient data
Surgical Technique
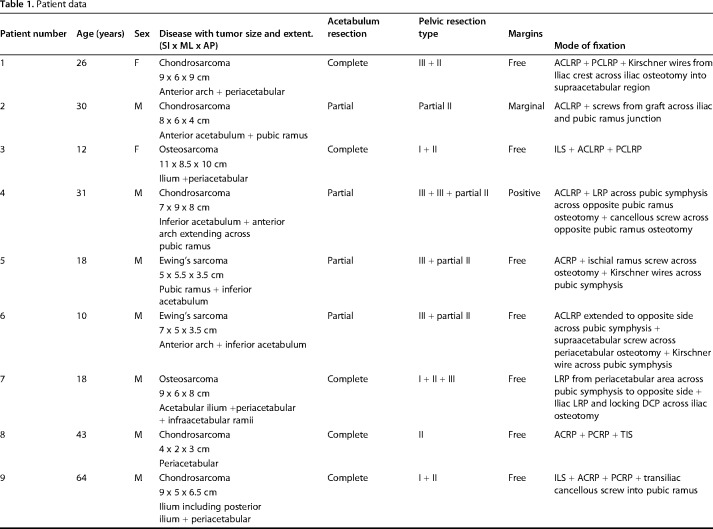
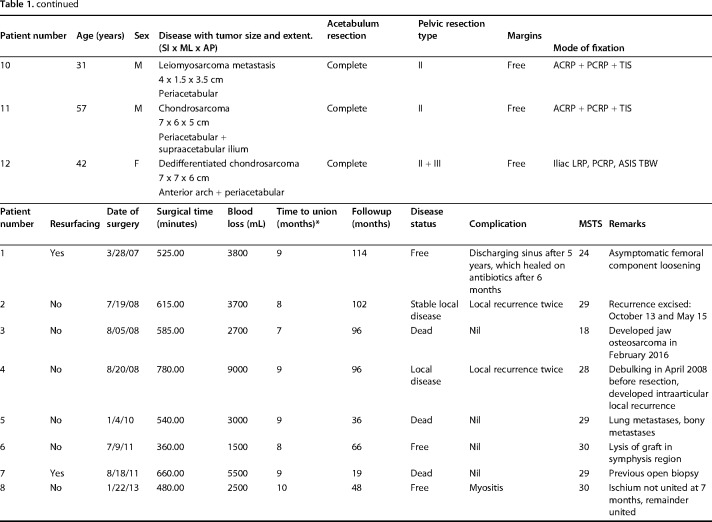
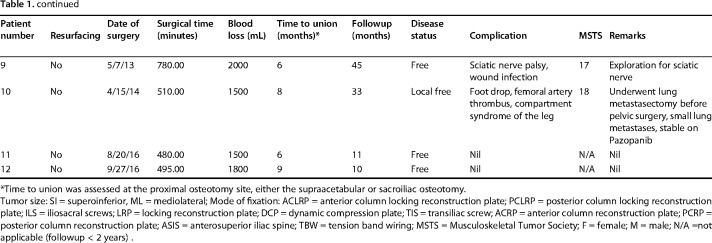
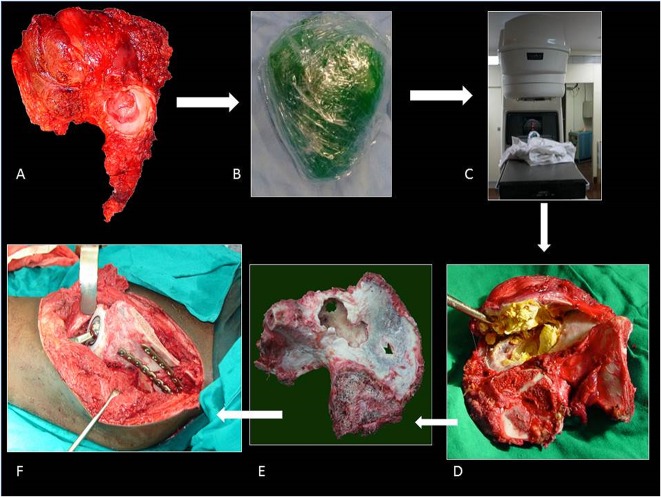
A standard utilitarian incision (T-shaped two-incision) was used in our initial five patients. All others were done through a single ilioinguinal approach. The resection margins were planned using CT and MRI so as to ensure a layer of normal tissue over the soft tissue component and 2-cm bony margin. On the medial side, an iliacus cover was left on the soft tissue mass wherever it was present. Similarly, abductors directly covering the tumor were resected preserving the parts away from the tumor with the aim to preserve as much of these main motors of the hip as possible without compromising the resection margin. The bony cuts were planned using readily identifiable landmarks like the superior or inferior rim of the acetabulum or lower part of the sacroiliac joint at the sciatic notch. We did not use navigation or patient-specific jigs. Acetabular resection was partial in four and complete in eight patients (Table 1). This was based on tumor extent; part of the acetabulum was saved if possible without compromising the margin. One patient received radiotherapy postoperatively because of contaminated margins. The technique for ECRT that was followed was similar to what we followed for the long bones (Fig. 1). The resected segment (Fig. 1A) is soaked in vancomycin solution (2 g vancomycin in 1 L of normal saline) and then double plastic-wrapped over an impervious drape (Fig. 1B) to maintain sterility while being transported to the radiation department for a single fraction of 50 to 60 Gy using a linear accelerator (Fig. 1C), which was delivered over 20 to 25 minutes. The radiated bone was stripped of attached soft tissues and all visible tumor from bone (Fig. 1D). Debulked tumor after ECRT was sent for histopathologic examination. Margins were wide in 10, marginal in one, and contaminated in one based on gross assessment by the surgeon during tumor debulking postradiation treatment. Histopathologic evaluation of the margins was not done because the tumor was debulked and reimplanted after radiation. The void after tumor clearance (Fig. 1E) was reconstructed with bone cement to avoid any weak zones in the radiated graft and finally the graft was soaked in a fresh solution of vancomycin before we fixed it in the gap with buttressing plates and screws (Fig. 1F). Resurfacing of the acetabulum was done in our first patient and in another where tumor infiltrated the cartilage. Both of these patients had complete acetabular resection. A constrained liner was used in the two resurfaced patients. In subsequent patients we preserved the articular cartilage with the intention of reducing the operating time as well as delaying a prosthetic replacement until arthritis had set in. Based on previous reports [5, 9, 18], we were hopeful that it would be many months before needing a replacement. We also reasoned that not having a large metallic prosthesis may make management of a wound-related problem easier should it arise. The hip capsule was repaired with No. 5 (7.0 metric) polyester braided Ethibond (Ethicon Inc, manufactured in Aurangabad, India, by Johnson & Johnson Pvt Ltd) sutures. Hip and knee mobilization was started immediately postoperatively. Patients were kept nonweightbearing until radiographic evidence of bony union and then gradually moved to full weightbearing ambulation. Patients on chemotherapy were restarted on chemotherapy between 10 and 14 days after surgery.
Fig. 1 A-F.
The ECRT technique. (A) The specimen is resected as per oncologic principles after which (B) the specimen is wrapped in vancomycin-saline mops and plastic wrap to maintain sterility while being transported to the radiation department. (C) Radiotherapy: single-fraction 60 Gy with 6-mV photons at 3 Gy/min is delivered to the specimen. (D) The specimen is received on a separate sterile trolley, stripped of all soft tissue, and (E) cleaned thoroughly with vancomycin mixed with saline. (F) The specimen is fixed with 3.5-mm reconstruction plates. Resurfacing with a cemented constrained liner is done for tumor invasion into acetabulum.
Assessment of Endpoints
Images, hospital files, and charts were reviewed and all data were entered into an Excel spreadsheet (Microsoft Inc, Redmond, WA, USA). Patients were reviewed every 3 months with radiographs of the pelvis. AP views of the pelvis with both hips and Judet’s views were done to look for bridging across the junctions, for any signs of local recurrence like bony destruction or soft tissue mass or calcification/ossification, or signs of secondary osteoarthritis. Union at the proximal osteotomy junction (supraacetabular, sacroiliac) was easier to appreciate and of greater clinical importance because it dictated the weightbearing ambulation for the patient. Nonunions at the ischium or pubic symphysis do not seem to affect function as much so no effort was made to assess these specifically. The joint space was visually compared with the normal hip. Chest radiographs (posteroanterior view) were obtained every 6 months alternating with the CT scan of the chest to screen for metastatic nodules. CT scans of the pelvis were done along with the chest for 3 years to screen for any local recurrence. Radiographs were performed every 6 months after 3 years had elapsed from surgery. Functional scoring was done using the Musculoskeletal Tumor Society (MSTS) score 2 years after surgery and then every 3 months at followup.
Results
The median surgical time was 8.6 hours (range, 6-13 hours) and median blood loss was 2250 mL (range, 1500-9000 mL). There were no wound-related acute complications. One patient had a delayed discharging sinus after 5 years, which subsided after removing the screw found dislodged in subcutaneous tissue. The cultures were sterile and the patient continues to be functional without any clinical or radiologic signs of infection. There were two major acute postoperative complications. One patient had sciatic nerve palsy, which began to improve at 6 months and currently the patient is independently mobile with a walking stick. Another patient had a femoral artery thrombus, which was noted the next postoperative day. Embolectomy and leg fasciotomy were done. The patient developed extensive leg muscle necrosis and fasciotomy wound infection needing débridement and skin grafting. He is able to independently ambulate without support.
None of the eight of 10 patients with followup > 24 months who had the procedure without a hip arthroplasty developed osteonecrosis based on radiograms and CT scans, and none showed any evidence of joint space reduction based on radiograms when compared visually with the opposite side. The median MSTS score was 28 (range, 17-30). All proximal osteotomy junctions (supraacetabular, periacetabular, or sacroiliac) united within 10 months. No fractures occurred in the radiated segments of reimplanted bone in this small series.
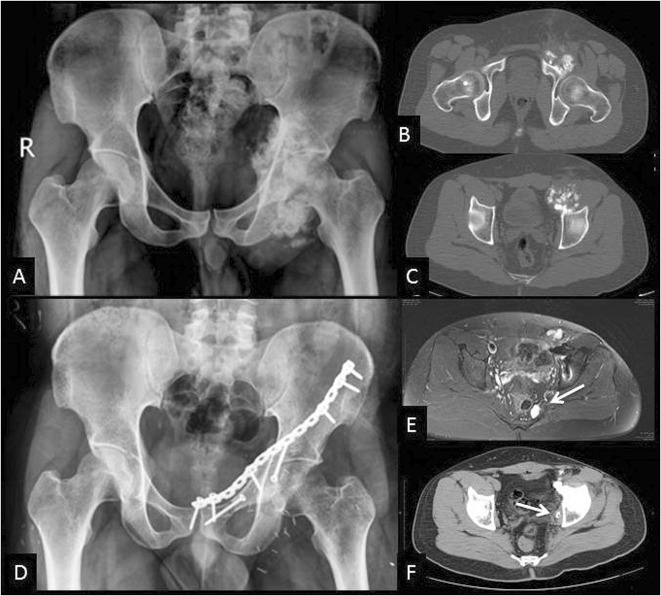
Two patients with high-grade sarcomas have died of metastatic disease 19 and 36 months after surgery. Another patient developed jaw osteosarcoma 7 years after the original surgery for osteosarcoma and died of metastatic disease within 1 year. The patient operated on for metastatic leiomyosarcoma of the pelvis was also operated on for solitary lung metastases. He developed small lung nodules again and this is currently controlled on pazopanib (selective multitargeted receptor tyrosine kinase inhibitor) therapy. None of these four patients had any local disease. Two patients developed local recurrence of disease. Both of these patients had low-grade secondary chondrosarcomas arising in exostoses. Both patients had prior debulking surgeries. One had a contaminated margin in the region of the bladder and prostate. He was given radiation and twice developed intracapsular recurrence in the hip, which has been excised, and he is currently disease-free. The other patient (Fig. 2A-D) developed small recurrences near the inner pelvic wall (Fig. 2F) and sacrum (Fig. 2E) and again was operated on twice to remove the recurrence and is currently disease-free. Thus, six of 10 patients with followup > 24 months are alive and disease-free and one is alive with metastatic disease.
Fig. 2 A-F.
Secondary chondrosarcoma arising on an exostoses from the anterior column. This patient underwent attempted debulking before presenting to us. (A) Radiograph and (B-C) CT scans showing the tumor arising purely from the left anterior column. He was treated with ECRT and reimplantation of the anterior column. A plate was used to buttress the graft as seen on the (D) followup radiograph after 2 years showing the healed graft. (E) Local recurrence seen in the parasacral area (white arrow) was excised completely. (F) Local recurrence in the pelvic wall (white arrow) too was completely excised. The recurrences were probably from the extensive contamination caused by the prior debulking.
Discussion
Reconstruction after total or partial acetabular resection for tumor is challenging. A high risk of wound complications has been a major deterrent for any major pelvic reconstruction [7, 9, 19] and while some patients achieve adequate function after rehabilitation without reconstruction [8, 14], many do not. Many reconstructive approaches have been described [1] including endoprostheses, 3-D printed jigs, and implants using new approaches to osseointegration; concerns remain about the shortcomings of all of these approaches [4, 11, 13]. Pelvic allografts are hard to get and have been reported to have infection rates of 39% [6] and also are at risk for delayed union, nonunion, and fracture. ECRT is a potential biologic alternative to reconstruction; its advantages appear to be size matching (as shown in the extremities) [2, 5, 10, 15, 17, 18] and low cost. However, it has not been well studied in the pelvis [3, 16, 18]. In our small study at short-term followup, we found ECRT to be a viable option, with a median MSTS score of 28, complications comparable to other large pelvic reconstructions, and no progressive arthritis as yet.
The most important limitation of this study was the low number of patients with relatively short followup. It is possible that other complications could arise than were observed in a small study group and, in particular, that more complications could arise over time. In particular, it seems likely that some of the patients will develop progressive arthritis in the autograft; although it is possible that they may develop further local recurrences, long-term data from patients undergoing extremity ECRT have not raised any concerns [2, 10]. Fatigue failure is another concern over long-term followup and for all these reasons, these patients should receive continued followup. We selected patients who were willing to undergo rigorous rehabilitation and remain nonweightbearing while the osteotomy junctions unites; we indicated the procedure for tumors with adequate bone stock for structural strength. These tight criteria may account for the generally favorable outcomes we observed. We were limited in our assessment of the hip after ECRT to plain radiographs and comparative analysis to the opposite hip. Information from sophisticated imaging may be of great value to assess cartilage status and osteonecrosis. Although we feel this procedure offers the benefits of hip function preservation (as compared with hip transposition) while reducing risks associated with alternative reconstruction methods (endoprostheses, allograft), future studies with comparative data are needed.
ECRT of acetabular autografts after resection of pelvic tumors is a relatively large procedure, associated in this small series with prolonged surgical times, substantial amounts of blood loss, and some major complications. We had one patient who developed sciatic nerve palsy and one patient with femoral artery thrombosis. Similar complications have been reported by Davidson et al. [5] and Delloye et al. [6]. We believe these may be the result of inadvertent injury during bone manipulation in these difficult patients. Unlike an allograft, nonunion in the iliac region or the sacroiliac region does not seem to be a problem with ECRT of bone, also reported by Wafa et al. in their series [18]. Nonunions and bony resorption at the pubic symphysis and ischium can occur but do not seem to cause any functional compromise. Asymptomatic heterotopic bone formation was seen in some of our patients. Infection rates have been reported to be as high as 30% by Wafa et al. in 18 cases [18] and 0% by Chan et al. in nine cases [3]. We have had one delayed infection after 5 years of surgery (Table 2).
Table 2.
Comparative data with other published series
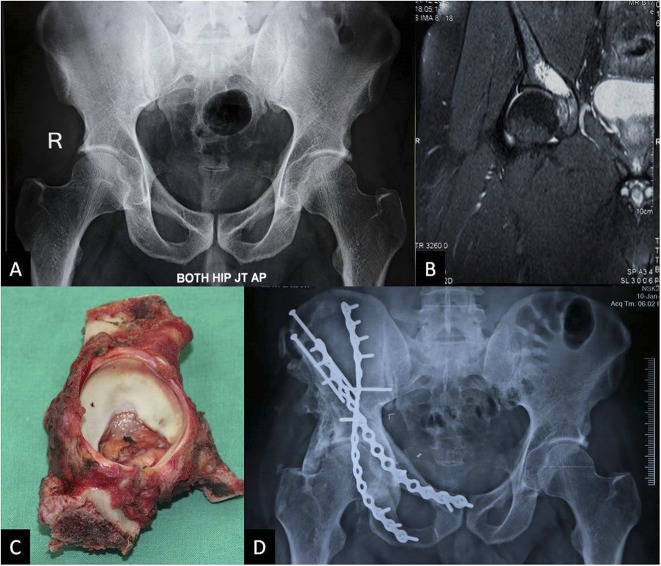
In this small series, we did not observe degeneration of the cartilage of the autografts in the eight patients with followups > 24 months whose hips were left unresurfaced. Although the followup was relatively short term (minimum, 33 months; median, 5.25 years), we are cautiously optimistic. Opinion is again divided about acetabular resurfacing. Uyttendaele et al.’s group resurfaced the acetabulum for tumor invasion or when they thought that the risk of femoral avascular necrosis was high [16, 17]. They report mechanical complications in three of their five patients who underwent resurfacing. Wafa et al. resurfaced hips in all skeletally mature patients and did not report any complications related to it [18]. In our series, we resurfaced our first patient’s hip with the belief that cartilage would be destroyed by radiation. Subsequently, we resurfaced only one patient’s hip for tumor invasion; for the rest, we believed it would delay an arthroplasty for a few years and also reduce the prosthesis-related complications. We have not made any objective measurements of cartilage thickness, but none of our patients has shown any joint narrowing on radiographs when visually compared with the opposite side (Fig. 3A-D). Our longest followup has been 114 months with complete acetabular resection and 102 months with partial acetabular resection. We do not have an explanation as to why the joint has not collapsed in any patients. Six of 10 evaluable patients have MSTS scores of > 90%; of the other four, poor functional scores are seen in the patient with sciatic nerve palsy and one with compartment syndrome and myonecrosis after femoral artery thrombosis. One other patient has had complete resection of the abductors. Although this is a small series, we have also not encountered any acute wound-related complications in our 12 cases, which were a major concern in choosing this form of reconstruction in the past. We have not selected very large tumors nor those with large intrapelvic masses for ECRT (Table 1 lists the tumor sizes) and this may be a factor in lesser complications and better functional scores. In the modern era of 3-D printing, efforts have been made to create and implant a 3-D-printed pelvic prosthesis with an internal structure similar to normal bone to aid osteointegration and soft tissue reattachment [4, 11, 13]. We believe ECRT of bone offers the advantages of a cost-effective osteointegrable scaffold, which is perfectly size-matched to the defect.
Fig. 3 A-D.
A 43-year-old man with periacetabular chondrosarcoma treated with ECRT. The patient presented with hip pain. (A) Preoperative radiograph appears normal and the lesion is not visible, whereas (B) MRI shows the periacetabular lesion, which was diagnosed to be a Grade 2 chondrosarcoma by CT-guided biopsy. (C) Resected specimen was treated with ECRT and reimplantation with transiliac screws and two-column fixation as seen on the (D) 4-year postoperative radiograph showing the healed junctions. There is heterotopic bone, which did not cause any restriction of movement. The joint space is good. This man walks without a limp and is back to working full-time.
Although there is the potential for tumor cells to persist after ECRT, we were gratified that the only local recurrences were observed in patients who had prior complicated resections before the procedure studied in this report; these recurrences were seen in the soft tissue. These findings are similar to those reported in long-term followup studies after ECRT [2, 10]. Chondrosarcomas are known to be particularly resistant to radiation but have not recurred after ECRT [10]. It is unclear as to what dose of radiation is required for complete sterilization of the graft from the tumor. Doses > 50 to 60 Gy may not offer any benefit on tumor control but can damage the proteins in the bone matrix and adversely affect the revascularization and osteoconductive properties of the irradiated autograft [12]. A dose of 50 Gy delivered in a single fraction was equivalent to 250 Gy delivered by conventional fractionation and should be sufficient to produce 100% tumor kill [5]. We do not have unequivocal laboratory studies defining how much dose of radiation would be best for complete tumor kill and we do not know if this dose would be different for different tumors.
In summary, we found ECRT to be a useful option to reconstruct acetabulum involving defects after pelvic tumor resection in highly selected cases. The surgical procedure is complex and prolonged with potential for serious complications that can permanently compromise function. Although acute postoperative infection and progressive arthritis have not been observed in this small series with short-term followup, it may be seen at longer followup. Long-term data are therefore required to make recommendations about resurfacing the radiated acetabulum or not. As has already been reported in the extremities [2, 10], we hope not to face a situation of tumor recurrence with longer followup. The functional results we observed were encouraging in those patients who did not experience complications. Future studies to determine the optimal dose of radiation for complete tumor kill may alleviate some of the concerns today about using ECRT in the pelvis.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at P. D. Hinduja Hospital and Medical Research Center, Mumbai, India.
References
- 1.Beauchamp CP, Eckardt JJ, Schwartz AJ. Internal hemipelvectomy for musculoskeletal tumors—indications and options for reconstruction. Oncol Hematol Rev. 2011;7:123. [Google Scholar]
- 2.Bohm P, Fritz J, Thiede S, Budach W. Reimplantation of extracorporeal irradiated bone segments in musculoskeletal tumor surgery: clinical experience in eight patients and review of the literature. Langenbecks Arch Surg. 2003;387:355–365. [DOI] [PubMed] [Google Scholar]
- 3.Chan LW, Imanishi J, Ngan SY, Chander S, Chu J, Thorson R, Pang G, Choong P. Extracorporeal Irradiation and reimplantation with total hip arthroplasty for periacetabular pelvic resections: a review of 9 cases. Sarcoma. 2016;2016:2549616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Xu L, Wang Y, Hao Y, Wang L. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Programs Biomed. 2016;125:66–78. [DOI] [PubMed] [Google Scholar]
- 5.Davidson AW, Hong A, McCarthy SW, Stalley PD. En-bloc resection, extracorporeal irradiation, and re-implantation in limb salvage for bony malignancies. J Bone Joint Surg Br. 2005;87:851–857. [DOI] [PubMed] [Google Scholar]
- 6.Delloye C, Banse X, Brichard B, Docquier PL, Cornu O. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J Bone Joint Surg Am. 2007;89:579–587. [DOI] [PubMed] [Google Scholar]
- 7.Farfalli GL, Albergo JI, Ritacco LE, Ayerza MA, Muscolo DL, Aponte-Tinao LA. Oncologic and clinical outcomes in pelvic primary bone sarcomas treated with limb salvage surgery. Musculoskelet Surg. 2015;99:237–242. [DOI] [PubMed] [Google Scholar]
- 8.Gebert C, Gosheger G, Winkelmann W. Hip transposition as a universal surgical procedure for periacetabular tumors of the pelvis. J Surg Oncol. 2009;99:169–172. [DOI] [PubMed] [Google Scholar]
- 9.Han I, Lee YM, Cho HS, Oh JH, Lee SH, Kim HS. Outcome after surgical treatment of pelvic sarcomas. Clin Orthop Surg. 2010;2:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong AM, Millington S, Ahern V, McCowage G, Boyle R, Tattersall M, Haydu L, Stalley PD. Limb preservation surgery with extracorporeal irradiation in the management of malignant bone tumor: the oncological outcomes of 101 patients. Ann Oncol. 2013;24:2676–2680. [DOI] [PubMed] [Google Scholar]
- 11.Jentzsch T, Vlachopoulos L, Furnstahl P, Muller DA, Fuchs B. Tumor resection at the pelvis using three-dimensional planning and patient-specific instruments: a case series. World J Surg Oncol. 2016;14:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krieg AH, Mani M, Speth BM, Stalley PD. Extracorporeal irradiation for pelvic reconstruction in Ewing's sarcoma. J Bone Joint Surg Br. 2009;91:395–400. [DOI] [PubMed] [Google Scholar]
- 13.Liang H, Ji T, Zhang Y, Wang Y, Guo W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Joint J. 2017;99:267–275. [DOI] [PubMed] [Google Scholar]
- 14.Puri A, Pruthi M, Gulia A. Outcomes after limb sparing resection in primary malignant pelvic tumors. Eur J Surg Oncol. 2014;40:27–33. [DOI] [PubMed] [Google Scholar]
- 15.Sabo D, Bernd L, Buchner M, Treiber M, Wannenmacher M, Ewerbeck V, Parsch D. [Intraoperative extracorporeal irradiation and replantation in local treatment of primary malignant bone tumors] [in German]. Der Orthopade. 2003;32:1003–1012. [DOI] [PubMed] [Google Scholar]
- 16.Sys G, Uyttendaele D, Poffyn B, Verdonk R, Verstraete L. Extracorporeally irradiated autografts in pelvic reconstruction after malignant tumour resection. Int Orthop. 2002;26:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyttendaele D, De Schryver A, Claessens H, Roels H, Berkvens P, Mondelaers W. Limb conservation in primary bone tumours by resection, extracorporeal irradiation and re-implantation. J Bone Joint Surg Br. 1988;70:348–353. [DOI] [PubMed] [Google Scholar]
- 18.Wafa H, Grimer RJ, Jeys L, Abudu AT, Carter SR, Tillman RM. The use of extracorporeally irradiated autografts in pelvic reconstruction following tumour resection. Bone Joint J. 2014;96:1404–1410. [DOI] [PubMed] [Google Scholar]
- 19.Wirbel RJ, Schulte M, Mutschler WE. Surgical treatment of pelvic sarcomas: oncologic and functional outcome. Clin Orthop Relat Res. 2001:190–205. [DOI] [PubMed] [Google Scholar]