Abstract
Background
Pelvic ring reconstruction after resection of pelvic malignancies or aggressive benign tumors remains challenging, especially when the tumor invades periacetabular bone, resulting in a Type II resection as classified by Enneking and Dunham (removal of part or all of the acetabulum). Although numerous treatment approaches are in use, none is clearly superior to the others. An alternative involving use of the ipsilateral proximal femur as an autograft has not been well characterized, so we present our preliminary experience with this approach.
Questions/purposes
(1) What were the oncologic outcomes after using an ipsilateral proximal femur autograft for reconstruction after Type II pelvic resection in a small series of patients who underwent this reconstructive approach? (2) What were the Musculoskeletal Tumor Society (MSTS) scores after this reconstruction? (3) What complications were observed?
Methods
Between October 2006 and May 2016, we treated 67 patients with Type II malignant or aggressive benign tumors of the ilium. Of those, we used an ipsilateral proximal femur and a prosthesis as a reconstruction method for 11 patients with pelvic tumors. In general, we performed this approach in young or middle-aged patients with primary malignant or aggressive benign tumors involving pelvic area II and in whom the tumor did not invade the hip. The method used for resection of pelvic tumors included osteotomy of the femoral shaft, harvesting the proximal femur as a graft. The length of the femoral graft was determined by the extent of the pelvic defect. The proper placement was selected after a comparison of the proximal femur and the pelvic defect. A curved reconstruction plate and cancellous bone screws were used for pelvic fixation. The operative duration and total blood loss were recorded. Of the 11 patients who underwent this approach, all but one had at least 2 years of followup unless death occurred earlier, and all but one have been seen within the last year for evaluation. Functional outcomes were assessed using the MSTS scoring system. Local recurrence, metastases, and deaths were recorded as were complications including infection, bone nonunion, mechanical failure and sciatic nerve palsy.
Results
The followup was a mean of 37 months (range, 13-96 months). One patient was lost to followup. Three patients died of disease owing to local recurrence or lung metastasis. The other seven patients lived without evidence of tumor. The main complications included mechanical failure in two patients, nonunion in one patient, infection in two patients, and sciatic nerve palsy in one patient. The median MSTS function score was 70% (21 of 30 points; range, 11-25 points).
Conclusions
Our preliminary results show that this technique of using the ipsilateral proximal femur may be an alternative method for reconstruction of pelvic bone defects after tumor resection. Even with this short followup, complications were common, but short-term function appears to be comparable to studies of other options. Longer term followup with more patients is necessary to confirm our results.
Level of Evidence:
Level IV, therapeutic study.
Introduction
Pelvic ring reconstruction after resection of pelvic bone tumors is challenging. When the tumor involves the acetabulum (Type II resection [9]), the hip is often reconstructed if the patient is to have a strong likelihood of being able to walk, although there are reports of reasonable function with no reconstruction and a flail hip [13, 21]; current methods for pelvic ring reconstruction include customized or modular hemipelvic prostheses, saddle prostheses, pelvic allografts, or allograft-prosthetic reconstructions [2, 7, 15, 20]. However, there is no agreement on which reconstructive approach might be superior, and all have shortcomings [2, 7, 22].
Biologic pelvic reconstruction has the potential advantage of long-term pelvic stability but is associated with a high likelihood of complications [14, 26]. In the 1980s, Puget and Uthéza [19] proposed an innovative approach: upshifting the ipsilateral proximal femur. They procured the proximal part of the ipsilateral femur to replace the resected pelvic bone and fixed it to the remaining bone by screws and plates. An acetabular cup was cemented into the transplanted bone, which itself was replaced by a femoral prosthesis (Fig. 1A-C). The goals of this technique are to ensure long-term fixation through integration of a cortical-cancellous autograft because of restored pelvic continuity and to implant a more conventional total hip prosthesis in the appropriate anatomic position in an attempt to optimize function [17]. In this study, we reviewed our preliminary experience in 11 patients with pelvic malignancies or aggressive benign tumors who underwent ipsilateral proximal femoral autograft reconstruction. We sought to describe the surgical procedure in detail and report on the oncologic and functional outcomes.
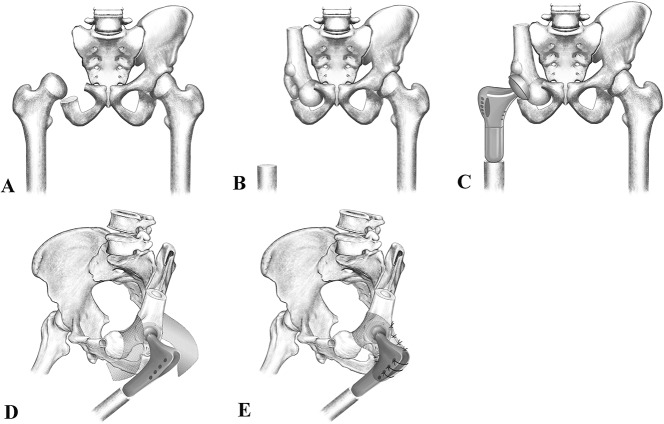
Fig. 1A-E.
We created a drawing to show pelvic reconstruction after tumor resection with an ipsilateral proximal femur. (A) The pelvic tumor is resected with a bone defect (zone I + II). (B) The ipsilateral proximal femur is harvested and transferred to fill the space. (C) The hip is rebuilt with a femoral prosthesis. (D-E) The hip capsule is reconstructed with hernia mesh to prevent dislocation. We crossed the mesh through the pelvic ring and sutured its ends to the proximal prosthesis.
Specifically, we asked the following questions: (1) What were the oncologic outcomes after using an ipsilateral proximal femur autograft for reconstruction after Type II pelvic resection in a small series of patients who underwent this reconstructive approach? (2) What were the Musculoskeletal Tumor Society (MSTS) scores after this reconstruction? (3) What complications were observed?
Patients and Methods
The retrospective study was approved by the Human Research Ethics Committee of our hospital. Between October 2006 and May 2016, 67 patients were treated for malignant or aggressive benign tumors involving the acetabulum (Type II resections as defined by Enneking and Dunham [9]). Of these, 11 patients underwent hemipelvic resection, reconstruction of the pelvic ring by upshifting the ipsilateral proximal femur, and reconstruction of the hip with a tumor-typed proximal femur prosthesis (Table 1; Fig. 1).
Table 1.
Patient information
The indications for selecting this reconstruction were young or middle-aged patients with primary malignant or aggressive benign pelvic tumors involving zone II that did not directly involve the hip, including isolated acetabular tumors, tumors that extended into the obturator ring (zone II + III), the wing of the ilium (zone I + II), and even all three zones. It was especially useful when part of the acetabulum remained (so-called partial Type II resection), because in these patients, it was easier to anchor the proximal femur to the pelvis. During the period in question, we considered a number of other approaches for reconstruction among our patients who underwent Type II resections. Fifty-six patients underwent Type II resections and were treated in other ways including amputation, no reconstruction (flail hip), and reconstruction by artificial hemipelvic prosthesis. In terms of our experience, the following four key dimensions need to be considered when upshifting the ipsilateral proximal femur. First, we consider age and tumor type. This technique is associated with a relatively long time to achieve bone healing. We therefore thought it was not applicable to older patients (> 60 years old) with poor ability of bone healing and those with a short life expectancy (metastatic tumor). Instead, a hemipelvic prosthesis or no reconstruction was considered for those patients. Second, for patients with a pelvic tumor extensively involving zone I in addition to zone II, it was a great challenge to use the traditional pelvic prosthesis, so the upshifting approach seemed more appropriate to us. Apart from a three-dimensional-printed prosthesis, upshifting the proximal femur for reconstruction seemed a good choice. Third, the desires and cooperation of the patient were considered. Compared with reconstruction after tumor resection, no reconstruction with a flail hip was a choice with a low incidence of complications. If patients were unwilling to take risks of postoperative complications and were able to accept the problems with hip function and leg length discrepancy, we would not use this technique. Fourth, we favored this reconstruction if part of the acetabulum remained. This technique was especially appropriate if part of the acetabulum remained (so-called partial Type II resection), because in these patients, it was easier to anchor the proximal femur to the pelvis, its location was easier to determine, and less bone graft was needed.
The primary tumor types included seven patients with chondrosarcoma, two patients with primitive neuroectodermal tumor/Ewing’s sarcoma, and two patients with giant cell tumor. Five patients with tumor involved pelvic area I + II, four involved pelvic area II + III, one involved pelvic I + II + III, and one involved pelvic II (Table 1).
Surgical Technique
The method used for the resection of pelvic tumors was the same as that used in prior reports [9, 16]. The patients were lying in a sloppy lateral position. A curved incision was made from the pubic symphysis through the groin and along the iliac crest to the sacroiliac joint. A second incision was made from the iliac spine to the greater trochanter that extended along the direction of the femur to the midthigh. Flaps were made to dissect the tumor and expose the pelvis for osteotomies. Osteotomies were performed in the ilium and pubis and/or ischium depending on the extent of the tumor. The proximal femur was exposed through the lateral incision. The vastus lateralis muscle was dissected from the femoral shaft and the iliopsoas tendon was released from the lesser trochanter. The osteotomy level of the femoral shaft was determined by the extent of the pelvic defect to obtain enough graft to fill the defect. The proximal femur, including the femoral head and intertrochanteric region and sufficient shaft, was used as a graft. The proper direction and angle were selected after comparison of the proximal femur and the pelvic defects. If possible, the remaining acetabulum was used as a reference to locate the acetabulum region in the intertrochanteric area. A curved reconstruction plate and cancellous bone screws were used for pelvic fixation. Attempts were made to avoid gaps at the junctions to provide good bone-on-bone contact. Large defects were filled with cancellous bone obtained from the intertrochanteric area of the femur. The new acetabulum was reamed as close to the original location of the acetabulum as possible. If possible, the remaining acetabulum was used as a reference to control the direction and depth of the acetabulum. An X-CHANGE reinforcement ring (Howmedica Osteonics Corp, Mahwah, NJ, USA) was used in one patient (Fig. 2). The acetabular cup was cemented on the newly reamed femoral "acetabulum." A proximal femoral prosthesis was implanted in the proximal femoral canal after the canal was reamed. The hip was repositioned. The capsule was reconstructed with hernia mesh to prevent dislocation of the femoral prosthesis (Fig. 2). In detail, the hernia mesh was folded into a rectangle. Then we crossed the mesh through the pelvic ring and sutured its two ends to the proximal femoral prosthesis, which served as a new artificial hip capsule (Fig. 1D-E). The iliopsoas residual was sutured to the hole of the prosthesis. The gluteus medius was sutured to the vastus lateralis and reinforced by suturing it to a hole on the greater trochanter of the prosthesis. Three suction drainage tubes were routinely placed before the incision was closed.
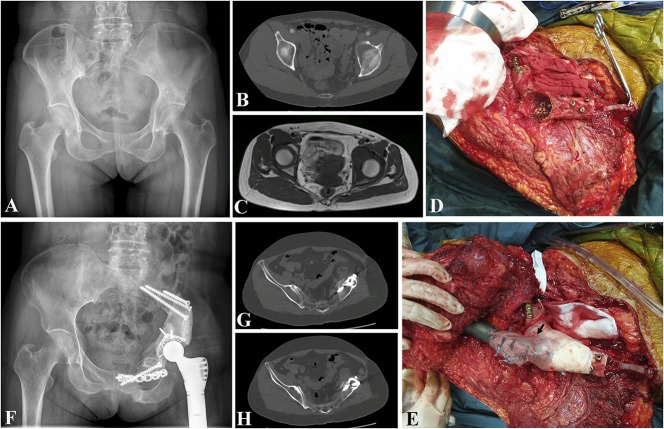
Fig. 2A-H.
Patient 9 was a 52-year-old woman who had a chondrosarcoma of the pelvis (zone I + II). She underwent pelvic reconstruction by upshifting the ipsilateral proximal femur after tumor resection. (A-C) Radiograph, CT, and MR images show a chondrosarcoma in the pelvis. Intraoperative photographs show (D) the proximal femur was fixed to the pelvic ring and the acetabulum was built; (E) the hip was repositioned and the capsule was reconstructed with hernia mesh (black arrow). (F) Fifteen months after the operation, the prosthesis was stable. (G-H) CT scan demonstrates that the autograft has united to the pelvis.
Postoperatively, three drains were maintained until the volume of drainage was < 50 mL per day. Intravenous antibiotic treatment was maintained for 2 weeks after surgery. Patients were immobilized in bed for 8 weeks and partial weightbearing using crutches was started 8 weeks after surgery. Full weightbearing was not allowed until bone union of the pelvic ring was seen on plain radiographs and CT scans at followup. All patients were followed up at 1 month, 3 months, and every 3 months for the first 2 years, every 6 months between 2 and 5 years, and yearly thereafter. The average followup was 37 months (range, 13-96 months). One patient was lost to followup 46 months after surgery.
Local recurrence was screened by history and examination, radiographs, and bone scans. The presence of metastasis was determined by routine chest CT scan and bone scan at 3 and 6 monthly intervals separately. The functional outcomes, which included pain, function, emotional acceptance, support, walking ability, and gait, were assessed with the 1993 MSTS system [8] by chart review at last followup (data were collected when the patients were still surviving for the ones who died). Chart review was performed by a physician (YL) who was not involved in the care of the patients.
Results
At last followup, two patients (Patients 3 and 5) experienced local recurrences and two developed lung metastasis (Patients 1 and 5). Three patients (Patients 1, 3, and 5) had died of the disease 39 months, 28 months, and 13 months after surgery separately. One patient (Patient 4) was lost to followup 46 months after surgery. The other seven patients lived without evidence of tumor (Table 2).
Table 2.
Followup results
The median MSTS 93 score was 70% (21 of 30 points; range, 11-25 points). All but one (Patient 11) had at least 2 years of followup unless death occurred earlier, and all but one (Patient 4 lost to followup) have been seen within the last year for evaluation. We provided gait videos of seven patients (Patients 1, 2, 6-10) to show postoperative function. In these videos, patients could walk without assistance (see Supplemental Digital Content 1, Supplemental Digital Content 2, Supplemental Digital Content 3, Supplemental Digital Content 4, Supplemental Digital Content 5, Supplemental Digital Content 6, and Supplemental Digital Content 7).
The main complications included mechanical failure (two patients), nonunion (one patient), infection (two patients), and sciatic nerve palsy (one patient). One patient experienced a local superficial infection 1 month after surgery and was successfully treated by dressing changes and antibiotics with good functional recovery (Patient 1). One patient sustained a deep infection 44 months after surgery and was treated with débridement and original implants were retained (Patient 6). One patient experienced postoperative sciatic nerve palsy and bone nonunion 15 months after surgery. The internal fixation was broken. No treatment was administered with no recovery of the nerve function (Patient 3). One patient experienced loosening of the acetabular prosthesis 26 months after surgery, and a revision operation was performed. The patient was alive without evidence of tumor at last followup before she was lost (Patient 4).
Discussion
Reconstruction after resection of a periacetabular tumor is one of the most technically demanding procedures in orthopaedic oncology. Various methods of pelvic reconstruction after tumor resection have emerged, including customized pelvic prostheses, modular pelvic prostheses, allografts with or without prostheses, reimplantation of autologous pelvic bone after devitalization of the pelvic bone tumor, and autologous fibular grafting [2, 7, 15, 25, 26]. Hemipelvic prostheses have been the preferred option for the reconstruction of large defects by some centers [7, 15, 23]. In the 1980s, Puget and Uthéza [19] proposed to treat bone defects after pelvic tumor resection with reconstruction of the pelvic ring by upshifting the ipsilateral proximal femur. This technique aimed to restore the continuity of the pelvic ring with a femoral autograft and to implant a more conventional femoral prosthesis in the appropriate anatomic position in an attempt to optimize function. In our small series, the resected ipsilateral proximal femur was upshifted for pelvic reconstruction, an approach we believe is advantageous for several reasons. First, compared with pelvic prostheses, it restores the continuity of the pelvic ring owing to the natural curvature of the proximal femur that fits adequately into the defect, giving a good chance for a stable, biologic pelvic ring in the long term. Limb length discrepancy is avoided in comparison with a flail hip or hip transposition. Second, this technique is adapted for extensive defects in zone I combined zone II, in which it is a great challenge for pelvic prostheses as a result of lack of a bone block. Third, femoral autograft instead of allograft could result in a lower infection rate. A massive allograft is very attractive because it provides anatomic reconstruction. Nevertheless, a limited source of bone bank, fracture, infection, transmission of infectious diseases, and the absence of incorporation in the long term are issues that deter some surgeons from using allografts. However, the major disadvantage of upshifting the proximal femur lies in the disturbance of gait owing to loss of the bony attachment of the gluteus in the greater trochanter of the femur. However, that is not apparent when tumor invades zone I combined zone II, in which the origin of the gluteus has to be resected. Our preliminary findings suggest that upshifting the ipsilateral proximal femur may be a reasonable option for combined Type II and II defects and combined Type I and II defects. However, we did observe many complications, and function in the short term seems comparable to other approaches.
Our findings need to be interpreted in light of the following limitations. First, the number of patients is small, and so the degree to which the findings might generalize to different patients or different tumors is unknown. Second, there was no group of pelvic prostheses for comparison, but there are published outcomes using the pelvic prosthesis, which we discuss subsequently, that can serve as a basis for comparison. Related to that, in a retrospective study, there always is the issue of selection bias. Other treatments including amputation, no reconstruction, and hemipelvic prostheses were used during this time for patients with Type II resections. In general, we used upshifting of the proximal femur in young or middle-aged patients with primary malignant or aggressive benign tumors involving pelvic zone II; it is especially useful when part of the acetabulum remains. Third, the followup is short; it is possible, and perhaps likely, that more complications will accrue and more revision procedures may be performed, reflecting the complex nature of these reconstructions. Finally, there is no perfect way to measure function in such a heterogeneous group of patients with complex reconstructions; we have used the MSTS score, which is not as detailed perhaps as it could be. However, we supplement that with videos of seven of our patients (see Supplemental Digital Content 1, Supplemental Digital Content 2, Supplemental Digital Content 3, Supplemental Digital Content 4, Supplemental Digital Content 5, Supplemental Digital Content 6, and Supplemental Digital Content 7).
We found we could achieve adequate local control using this approach. We observed two (18%) local recurrences and two (18%) lung metastases. Three patients (27%) died 39 months, 28 months, and 13 months after surgery separately. One patient was lost to followup 46 months after surgery. The other seven patients (63%) remained disease-free. The result of local tumor control in our series is comparable to results in other reports. Ji et al. [15] retrospectively reviewed 100 patients who were treated by reconstruction with modular hemipelvic endoprostheses. They reported that 20 patients (20%) had local recurrence, 28 patients (28%) developed distant metastasis, 36 patients (36%) died, and 58 patients (58%) were disease-free. Guo et al. [10] examined 45 patients with pelvic chondrosarcoma involving the periacetabulum who received reconstruction of modular hemipelvic endoprostheses, saddle endoprostheses, devitalized tumor bone, or iliofemoral arthrodeses. The proportions of both local recurrence and distant metastasis were 22.2%. Thirty patients (66.7%) were alive without evidence of disease.
The short-term functional scores from the followup in this study appear to be comparable to those of pelvic prostheses reported by others [4, 12, 15]. Further observation is required to determine the long-term effects. The median MSTS score was 70% (21 of 30 points; range, 11-25 points) in our series, in keeping with the results of other reconstruction methods, and no reconstruction with a flail hip or hip transposition has a stable long-term effect with few complications. Schwartz et al. [21] reported on resection arthroplasty of the hemipelvis and found a mean MSTS score of 73.3% (22 of 30 points; range, 53.3%-80%) for eight patients after a mean followup of 9.8 years. Hoffmann et al. [13] compared the function of endoprosthetic replacement and hip transposition and found better functional results in a hip transposition group with a mean score of 60.7% (range, 16.6%-83.3%). However, limb length discrepancy was a major concern, ranging from 2 cm to 12 cm [13, 18]. The MSTS score in patients with modular endoprostheses ranged from 57.2% to 63.3% [10, 11, 15]. On the other hand, the function with some other new endoprostheses including LUMiC(R) (implantcast, Buxtehude, Germany) [4], ice cream cone [1], and pedestal cup [12] ranged from 63.3% to 71%. Biologic reconstruction is advocated because of its good biocompatibility and the ability to restore the continuity of the pelvic ring. Wafa et al. [26] used extracorporeally irradiated autografts for pelvic reconstruction in 16 patients and reported the mean MSTS score was 77% (range, 50%-90%). Tang et al. [24] retrospectively reviewed 13 patients with bulk femoral head autografts and found a high mean MSTS score of 83% (range, 63%-97%). In another study [17] involving 10 patients with proximal femur for reconstruction, similar in some ways to the approach we used, the mean MSTS score was also generally high, 83% (range, 67%-97%). In our study, most patients could walk without support. However, the interference with gait is inevitable as a result of loss of the bony attachment of the gluteus to the greater trochanter of the femur.
The main complications in our series were superficial or deep infection (18%), mechanical failure (18%), nonunion (9%), and sciatic nerve palsy (9%), but there was no dislocation. The complications associated with other reconstruction methods vary with the type of implant. Massive allografts are a valid reconstructive option and are associated with complications such as infection, dislocation, sciatic nerve palsy, and nonunion. Delloye et al. [6] reviewed 24 patients with pelvic allograft and reported five infections (21%), six neurologic deficits (25%), two dislocations (8%), and three nonunions (13%). In another study [5] of 33 patients with massive allograft, five infections (15%), six hip dislocations (18%), and eight sciatic nerve palsies (24%) were reported. Prosthetic reconstructions, including custom-made, modular prosthesis, saddle and pedestal cup, are generally the preferred approach with satisfactory functional recovery in the short term. However, they are expensive and are also associated with a high rate of complications, including infection, mechanical failure, and dislocation. Bus et al. [4] studied 47 patients with a LUMiC(R) prosthesis and found that infection (28%) and dislocation (22%) were relatively common. Hipfl et al. [12] reported that 40% patients with pedestal cups developed complications, including infection (17%), dislocation (15%), and aseptic loosening (6%). Although autograft reconstruction might have the natural advantage of good biocompatibility, complications are still encountered. In one study [3] involving 13 patients with the proximal femur used for pelvic reconstruction, four patients underwent revision surgery as a result of mechanical failure and infection. Four dislocations (31%) occurred. Laffosse et al. [17] also used the ipsilateral femur for reconstruction in 10 patients. The major complications were dislocation (30%) and infection (20%). The complications in our study are fairly similar to those of other reports except for dislocation. No hip dislocation occurred in our small group, although it was frequent in massive allograft (8%-18%) [5, 6], pelvic prosthesis (15%-22%) [4, 12], and femoral autograft (30%-31%) [3, 17]. We attribute this to the following factors. First, we reamed the new acetabulum as close to the original location of the acetabulum as possible to ensure the right abduction and anteversion angle. Second, the reconstruction of the hip capsule was performed by using hernia mesh in six patients, which helped maintain hip stability at the early stage and facilitated scar tissue growing in at the late stage. Third, patients were immobilized in bed for a long period (8 weeks) postoperatively.
The reconstruction of pelvic bone defects after pelvic tumor resection is difficult and no one reconstruction option has been shown to be predictably better than others. We report early experience using the ipsilateral proximal femur as a method for pelvic reconstruction. Longer followup and more patients treated using this method are necessary to know if this approach is superior to other types of reconstruction, but our early experience suggests it may be suitable for bone defects of both pelvic area II + III and pelvic area I + II. A high proportion of patients who undergo this complex procedure will experience major complications. The short-term function appears to be similar to that of pelvic prostheses, massive allografts, and autografts. Although a larger study with more patients and longer followup will be necessary to confirm the potential benefits of this technique, we believe that this approach might be particularly useful in young or middle-aged patients with primary malignant or aggressive benign tumors involving pelvic Type II and may provide satisfactory reconstruction of periacetabular pelvic bone defects.
Acknowledgments
We thank Yunxia Liu MD (Department of Oncology, Third People’s Hospital of Hangzhou) for help with chart review, Leiming Xu MD (Department of Radiology, Second Affiliated Hospital of Zhejiang University School of Medicine) for help with CT-guided biopsy, and Yanbiao Fu MD (Department of Pathology, Second Affiliated Hospital of Zhejiang University School of Medicine) for pathology evaluation.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Nong Lin and Hengyuan Li contributed equally to this work.
References
- 1.Barrientos-Ruiz I, Ortiz-Cruz EJ, Peleteiro-Pensado M. Reconstruction after hemipelvectomy with the ice-cream cone prosthesis: what are the short-term clinical results? Clin Orthop Relat Res. 2017;475:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell RS, Davis AM, Wunder JS, Buconjic T, McGoveran B, Gross AE. Allograft reconstruction of the acetabulum after resection of stage-IIB sarcoma. Intermediate-term results. J Bone Joint Surg Am. 1997;79:1663–1674. [DOI] [PubMed] [Google Scholar]
- 3.Biau DJ, Thevenin F, Dumaine V, Babinet A, Tomeno B, Anract P. Ipsilateral femoral autograft reconstruction after resection of a pelvic tumor. J Bone Joint Surg Am. 2009;91:142–151. [DOI] [PubMed] [Google Scholar]
- 4.Bus MP, Szafranski A, Sellevold S, Goryn T, Jutte PC, Bramer JA, Fiocco M, Streitburger A, Kotrych D, van de Sande MA, Dijkstra PD. LUMiC(R) endoprosthetic reconstruction after periacetabular tumor resection: short-term results. Clin Orthop Relat Res. 2017;475:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campanacci D, Chacon S, Mondanelli N, Beltrami G, Scoccianti G, Caff G, Frenos F, Capanna R. Pelvic massive allograft reconstruction after bone tumour resection. Int Orthop. 2012;36:2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delloye C, Banse X, Brichard B, Docquier PL, Cornu O. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J Bone Joint Surg Am. 2007;89:579–587. [DOI] [PubMed] [Google Scholar]
- 7.Donati D, Di Bella C, Frisoni T, Cevolani L, DeGroot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin Orthop Relat Res. 2011;469:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enneking W, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 9.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746. [PubMed] [Google Scholar]
- 10.Guo W, Li D, Tang X, Ji T. Surgical treatment of pelvic chondrosarcoma involving periacetabulum. J Surg Oncol. 2010;101:160–165. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Li D, Tang X, Yang Y, Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res. 2007;461:180–188. [DOI] [PubMed] [Google Scholar]
- 12.Hipfl C, Stihsen C, Puchner S, Kaider A, Dominkus M, Funovics PT, Windhager R. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J. 2017;99:841–848. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann C, Gosheger G, Gebert C, Jürgens H, Winkelmann W. Functional results and quality of life after treatment of pelvic sarcomas involving the acetabulum. J Bone Joint Surg Am. 2006;88:575–582. [DOI] [PubMed] [Google Scholar]
- 14.Houdek M, Rose P, Bakri K, Wagner ER, Yaszemski MJ, Sim FH, Moran SL. Outcomes and complications of reconstruction with use of free vascularized fibular graft for spinal and pelvic defects following resection of a malignant tumor. J Bone Joint Surg Am. 2017;99:e69. [DOI] [PubMed] [Google Scholar]
- 15.Ji T, Guo W, Yang RL, Tang XD, Wang YF. Modular hemipelvic endoprosthesis reconstruction–experience in 100 patients with mid-term follow-up results. Eur J Surg Oncol. 2013;39:53–60. [DOI] [PubMed] [Google Scholar]
- 16.Lackman R, Crawford E, Hosalkar H, King J, Ogilvie C. Internal hemipelvectomy for pelvic sarcomas using a T-incision surgical approach. Clin Orthop Relat Res. 2009;467:2677–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laffosse JM, Pourcel A, Reina N, Tricoire JL, Bonnevialle P, Chiron P, Puget J. Primary tumor of the periacetabular region: resection and reconstruction using a segmental ipsilateral femur autograft. Orthop Traumatol Surg Res. 2012;98:309–318. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Jeon DG, Cho WH, Song WS, Kong CB. Comparison of pasteurized autograft-prosthesis composite reconstruction and resection hip arthroplasty for periacetabular tumors. Clin Orthop Surg. 2017;9:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puget J, Uthéza G. Reconstruction of the iliac bone using the homolateral femur after resection for pelvic tumor. Rev Chir Orthop Reparatrice Appar Mot. 1986;72:151–155. [PubMed] [Google Scholar]
- 20.Renard A, Veth R, Schreuder HW, Pruszczynski M, Keller A, van Hoesel Q, Bökkerink JP. The saddle prosthesis in pelvic primary and secondary musculoskeletal tumors: functional results at several postoperative intervals. Arch Orthop Trauma Surg. 2000;120:188–194. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz A, Kiatisevi P, Eilber F, Eilber F, Eckardt J. The Friedman-Eilber resection arthroplasty of the pelvis. Clin Orthop Relat Res. 2009;467:2825–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao Q, Yan X, Sun J, Xu T. Internal hemipelvectomy with reconstruction for primary pelvic neoplasm: a systematic review. ANZ J Surg. 2015;85:553–560. [DOI] [PubMed] [Google Scholar]
- 23.Sherman CE, O'Connor MI, Sim FH. Survival, local recurrence, and function after pelvic limb salvage at 23 to 38 years of followup. Clin Orthop Relat Res. 2012;470:712–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang X, Guo W, Yang R, Yan T, Tang S, Li D. Acetabular reconstruction with femoral head autograft after intraarticular resection of periacetabular tumors is durable at short-term followup. Clin Orthop Relat Res. 2017;475:3060–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traub F, Andreou D, Niethard M, Tiedke C, Werner M, Tunn PU. Biological reconstruction following the resection of malignant bone tumors of the pelvis. Sarcoma. 2013;2013:745360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wafa H, Grimer RJ, Jeys L, Abudu AT, Carter SR, Tillman RM. The use of extracorporeally irradiated autografts in pelvic reconstruction following tumour resection. Bone Joint J. 2014;96:1404–1410. [DOI] [PubMed] [Google Scholar]