Abstract
Background
Organisms may persist on polymethylmethacrylate (PMMA) spacer surfaces, and subclinical infection is postulated to be a source of infection recurrence. Several small patient series have shown a high proportion of positive sonication cultures on PMMA spacers at the second stage of a two-stage revision. However, the association between a positive sonication culture and the risk for recurrence of infection after two-stage exchange is not fully elucidated.
Questions/purposes
Are cultures derived from sonication of antibiotic spacers associated with infection control or recurrence after two-stage revision for prosthetic joint infection (PJI)?
Methods
Between September 2013 and April 2016, we treated 67 patients with PJI with two-stage revisions. At the second stage, all cement spacers were explanted and sonicated. A total of`10 (15%) patients were lost to followup or failed to reach 1-year followup during the study period, and another 16 (24%) were excluded for prespecified reasons, leaving 41 patients for analysis in this study. Of the 41 patients included in this study, there were 25 TKAs, 15 THAs, and one distal femoral replacement. All patients met the Musculoskeletal Infection Society criteria for PJI at Stage 1 of the two-stage revision. The most common infecting organisms prompting two-stage revision were methicillin-sensitive Staphylococcus aureus and coagulase-negative staphylococci. PMMA spacers were most frequently loaded with gentamicin or gentamicin/vancomycin. Standard 6-week intravenous antibiotic courses were used for index infections and postreimplantation suppression was used for 3 months in all patients as determined by cultures and sensitivities. Patients were assessed for recurrence of infection at postoperative clinic visits completed at standard intervals. The average length of followup was 1.9 years with a range of 1 to 3.3 years.
Results
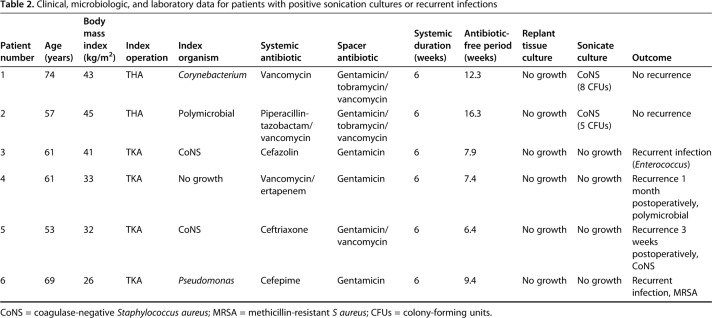
Sonication cultures that reached a threshold of 5 colony-forming units for positive culture had poor screening utility for subclinical persistent infection (sensitivity: 0%; confidence interval [CI], 0%–60%), but reasonable use for ruling in successful two-stage revision (specificity: 95%; 95% CI, 82%–99%). Positive sonication culture results in the two of 41 (4.9%) explanted spacers yielded coagulase-negative staphylococci, different from primary prosthesis cultures in both patients (Corynebacterium and Proteus mirabilis), and did not alter antibiotic choice. Neither of the patients has developed a reinfection at followup of 1.2 and 1.9 years. Of the 39 two-stage revisions with negative spacer sonication cultures, four developed reinfections.
Conclusions
Positive sonication fluid culture of PMMA spacers during reimplantation surgery was not associated with persistent or recurrent infection at minimum followup of 1 year. We do not recommend routine sonication of explanted PMMA spacers in the absence of clinical evidence suggesting persistent infection. Multicenter, prospective studies with long-term followup are needed to determine if sonication of PMMA spacers can predict persistent or recurrent infection.
Level of Evidence
Level III, diagnostic study.
Introduction
The causes of persistent and recurrent infection after treatment of periprosthetic joint infection (PJI) with two-stage revision hip and knee arthroplasty likely are multifactorial. One issue is the accurate identification of the infecting organism, which is critical for selecting targeted postexplantation antibiotic therapy. This can be technically challenging, because the sensitivity of both aspirate and tissue cultures is below 70% [20], which closely resembles that of other studies [22]. It is postulated that biofilm-producing bacteria are adherent to prosthesis surfaces, making traditional culture methods unreliable [2, 3]. Sonication disrupts bacterial biofilms on the infected prosthesis, allowing bacteria to be cultured. Sonicated implant cultures from explanted primary prostheses have improved the sensitivity of intraoperative cultures when compared with intraoperative tissue and synovial fluid cultures, especially in patients who received antibiotics within several weeks of explantation [20, 22, 23].
However, despite our improved ability to identify the infecting organism with sonication, persistent or recurrent infection after two-stage exchange still occurs. Infection persistence or recurrence after two-stage revision may be caused by the adherence of bacteria to implanted antibiotic polymethylmethacrylate (PMMA) spacers and subsequent biofilm formation, leading to a continued subclinical infection. Sonication of explanted PMMA spacers during two-stage revision arthroplasty for infection was first published by Borens et al. in 2011 [5]. In this initial report of 30 patients without outcomes data, replantation tissue cultures were negative in all patients, but one spacer sonicate was positive with 550 colony-forming units (CFUs) of Propionibacterium acnes [5]. Since this initial report, several authors have collected sonication data from explanted PMMA spacers; positive sonicate culture rates varied widely from 14.5% to 50% with some authors advocating for early débridement of all sonicate-positive patients [8, 15, 17, 21]. There is no consensus on the utility of sonicating explanted spacers and the association of a positive result with persistent or recurrent infection.
Therefore, in this study, we asked: Are cultures derived from sonication of antibiotic spacers associated with infection control or recurrence after two-stage revision for PJI?
Patients and Methods
A retrospective study was performed using an institutional review board-approved Joints Outcomes Registry Database of 67 patients who underwent two-stage revision of primary hip or knee arthroplasty for infection with antibiotic spacer sonication at the University of Pittsburgh Medical Center (urban, academic) hospitals in Pittsburgh, PA, USA, between September 2013 and April 2016. All antibiotic spacers explanted from patients were sonicated per departmental protocol. Exclusion criteria, which included insufficient data to satisfy Musculoskeletal Infection Society (MSIS) consensus criteria for prosthetic joint infection [19], followup of < 1 year, fungal-only cultures, the absence of reported sonication culture CFUs, and patients with multiply revised joints during the study period, left 41 patients for analysis in this study (Fig. 1). A total of seven patients (10%) were lost to followup and three patients (4.5%) did not reach 1-year followup at the time of data collection. Another 13 patients (20%) had insufficient data to satisfy MSIS criteria for PJI at initial presentation.
Fig. 1.
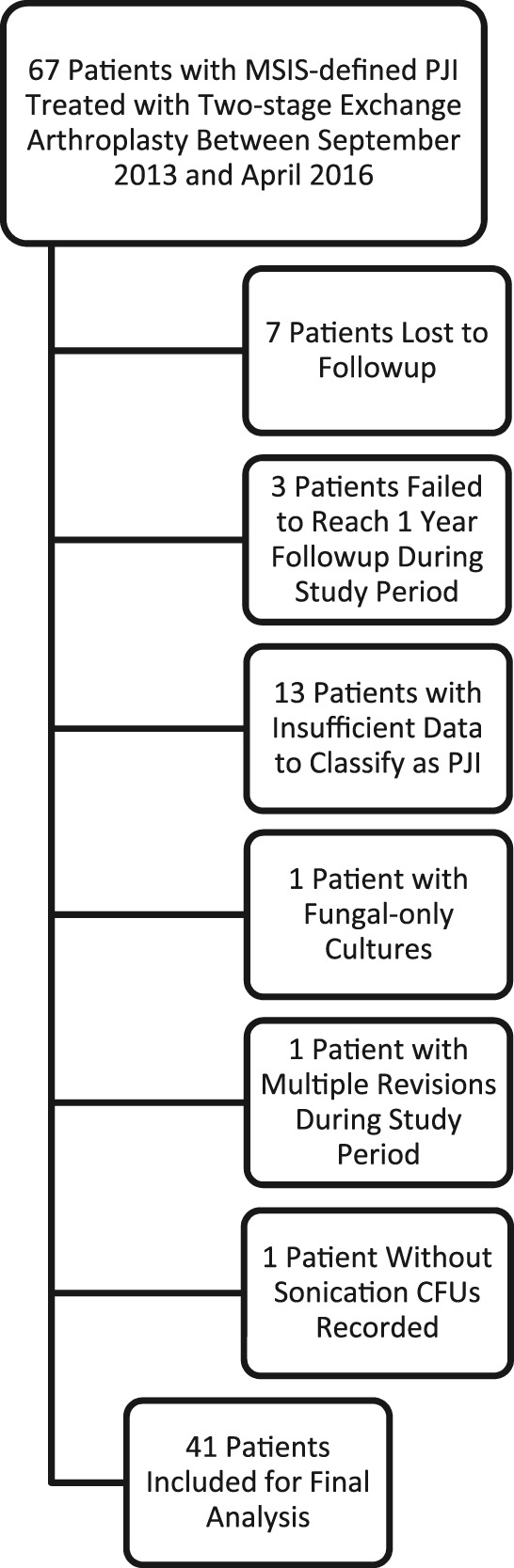
Flow diagram outlining inclusion and exclusion criteria.
Our final study population included 25 total knee revisions, 15 total hip revisions, and one distal femoral replacement revision. Average age of patients was 62 ± 11 years. There were 21 men and 20 women. Average body mass index was 34 ± 8.0 kg/m2. A total of eight patients (20%) were diabetic and there were nine smokers (22%). Diagnosis, laterality, procedure, and surgeon were also recorded as well as variables such as inflammatory markers, culture results, spacer and postexplant antibiotics, and the presence/absence of sinus tracts. In all patients, postexplant intravenous antibiosis lasted 6 weeks with an antibiotic-free period of 12 ± 8.3 weeks. The most common infecting organisms were coagulase-negative staphylococci (17%), methicillin-sensitive Staphylococcus aureus (17%), and β-hemolytic streptococci (12%). The standard at our institution is 3 months of postreplantation oral suppression in all patients.
Tissue/Synovial Culture Method
Tissues were processed using a disposable tissue homogenizer in Trypticase soy broth (BD Microbiology Systems, Sparks, MD, USA) in an approximately 10% weight/volume suspension. For this study, 0.1-mL aliquots of either homogenized tissue or synovial fluid were plated on blood agar, colistin nalidixic acid agar, MacConkey agar, and chocolate agar (BD Microbiology Systems). Aerobic cultures were incubated for 48 hours at 37° C. If aerobic cultures did not grow at 48 hours, chocolate agar plates were incubated for an additional 24 hours before cultures were pronounced negative. Anaerobic cultures were plated on prereduced anaerobically sterilized Brucella, phenylethyl alcohol, and kanamycin vancomycin laked blood agars (Hardy Diagnostics, Santa Maria, CA, USA) and incubated in anaerobic jars at 35° C for 48 hours (14 days when P acnes was requested). Organisms were identified using matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
Sonication Procedure
The sonication procedure for our institution was described in detail by Rothenberg et al. [20]. A positive sonication culture was defined as greater than or equal to 5 CFUs. In the operating room, explanted antibiotic-impregnated PMMA spacers were placed in sterile Nalgene jars containing 400 mL lactated Ringer’s solution. The jars were sonicated in a degassed sonication water bath for 5 minutes (40 ± 2 kHz, 022 W/cm2). The jars were allowed to sit for 1 minute to allow any aerosol to settle and were vortexed for 30 seconds. They were transferred to a biologic safety cabinet, where the jars/lids were wiped with alcohol-soaked gauze and the contents of the jar were decanted into 50-mL conical centrifuge tubes. The tubes were centrifuged at 4° C at 3150 x g for 5 minutes; supernatant was decanted and all fluid except for the last 1 mL was discarded. Tubes were vortexed to resuspend sediment in 1 mL supernatant before combining all fluid and centrifuging for 5 minutes. Supernatant was discarded except for the last 4 mL; this was resuspended. For aerobic culture, 0.1-mL aliquots were plated onto blood agar and chocolate agar and incubated in 5% CO2 for 4 days. For the anaerobic sonicate cultures, aliquots were plated on anaerobically sterilized Brucella and phenylethyl alcohol agar and were incubated in anaerobic boxes at 35° C for 14 days. Organisms were identified in a manner identical to that of tissue/synovial cultures.
Assessment of Endpoints
Patients were assessed at postoperative clinic visits completed at standard 2-week, 6-week, 3-month, 6-month, 1-year, and 2-year appointments (when possible). Additional time points beyond 2 years were included when available. Persistent or recurrent infection, defined using the MSIS consensus criteria for PJI, reoperation of any type, and need for chronic antibiotic suppression were recorded as clinical outcomes. Average length of followup was 1.9 years with a range of 1 to 3.3 years. A minimum of 1-year followup was considered to be acceptable given that only two of 41 patients (4.8%) had positive sonication fluid cultures to correlate to long-term followup data. This low rate of positive cultures is the more clinically relevant finding in the context of the existing published literature.
Statistical Analysis
Descriptive statistics including sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated using two-by-two tables and 95% confidence intervals were provided as indicated.
Results
Sensitivity, Specificity, Predictive Value, and Accuracy
Sonication cultures with a 5-CFU threshold for positive culture had poor screening utility for subclinical persistent infection (sensitivity: 0% [0%–60%]; Table 1) and poor positive predictive value (0% [0%–80%], but there was reasonable specificity for ruling in effective two-stage exchange (95% [82%–99%]), negative predictive value (90% [75%–97%]), and overall accuracy (85% [71%–94%]). Two (4.9%) positive sonication cultures were obtained during this study. The overall rate of infection recurrence was 10% (four of 41 patients). The four patients who had recurrent infections had negative results with spacer sonication and tissue culture at reimplantation (Table 2).
Table 1.
Diagnostic test characteristics of sonication of polymethylmethacrylate spacers for patients treated with two-stage reimplantation
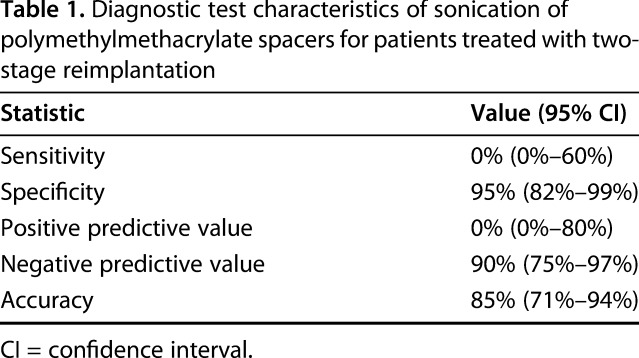
Table 2.
Clinical, microbiologic, and laboratory data for patients with positive sonication cultures or recurrent infections
Other Relevant Findings
The organism isolated in both patients with positive sonication fluid culture was coagulase-negative staphylococci, which was different from the primary prosthesis cultures (Corynebacterium and Proteus mirabilis) in both patients. Postreplant antibiotic suppression, which at our institution lasts 3 months, was not altered based on sonication results in either patient given these findings. Both have remained free of infection recurrence at followup of 1.2 and 1.9 years.
Discussion
Reinfection rates after two-stage revision arthroplasty for PJI are as high as 35%, despite explantation/débridement, targeted antibiosis, and prereplantation screening with cultures and serologic/synovial markers [4, 9, 10, 13, 16, 25]. Although some recurrences, particularly those occurring long after reimplantation, may be the result of bacterial invasion by traditional mechanisms, some may also be the result of bacterial adherence to and biofilm formation on antibiotic-loaded PMMA spacers used during the revision procedure [1, 6, 7, 11, 12, 14, 18, 24]. Sonication, a procedure by which bacterial biofilms on primary implant surfaces are disrupted, has shown increased sensitivity over both tissue and synovial cultures [20, 22]. The use of this procedure on antibiotic spacers in the setting of PJI has been used in several small studies with variable results [5, 15, 17, 21]. Therefore, we aimed to determine whether cultures derived from sonication of antibiotic spacers were associated with infection control or recurrence after two-stage revision arthroplasty for PJI.
The current study has several limitations, including the relatively small sample size of our population. Although a much larger sample size would be ideal to detect differences among groups, we feel that given the high sensitivity and specificity of our sonication procedure for primary infections and that the reinfection rate in this group is similar to that typically reported, our results are clinically relevant. The practice at our institution has been to sonicate all antibiotic spacers utilized during two-stage revisions for infection; however, many patients were excluded from this study as a result of not meeting infection criteria as proposed by the MSIS, in some cases as a result of lack of patient data (Fig. 1). Given this, the overall reinfection rate during the followup period of this study was similar to that reflected in registry data from our institution and others [20]. Antibiotic-free periods after explantation and spacer placement were also variable in this study with a 6-week minimum but ranging from 6 to 16 weeks (Table 2). This protocol is similar to that of other groups, and longer antibiotic-free periods would likely result in a higher rate of positive reimplantation culture rates, which was not observed in this study. Members of the orthopaedic and infectious disease teams were not blinded to sonication or tissue culture results; however, no changes were made to the postreplantation antibiotic management based on these cultures. Choice of spacer antibiotic was surgeon-dependent and may have resulted in selection bias. Like with most retrospective studies, a number of patients in the original cohort were lost to followup and may have potentially affected our results. Finally, both host and intraoperative factors (operative time, for example) likely play roles in all studies of PJI and could only be controlled for in larger study populations.
Sonication has limited use in identifying persistent or recurrent infection after revision arthroplasty for PJI. Several studies, including a registry review at our institution of primary PJI meeting MSIS criteria, have demonstrated high sensitivity of primary prosthesis sonication cultures, especially in the setting of antibiotic use, when compared with traditional synovial and intraoperative tissue cultures [20, 22]. Despite strong evidence supporting the use of sonication in primary infections, the role of this procedure in the revision setting is less clear. Several small series have explored the role of PMMA spacer sonication in the revision setting [5, 15, 17, 21]. These studies, although informative, varied by inclusion criteria, infection definition, antibiotic management, culture acquisition, CFU threshold, and treatment of positive sonication cultures. The overall rate of positive sonication culture also varied in these studies, ranging from 3.3% to 50%, despite relative consensus on the sonication procedure as proposed by the Mayo Clinic [22]. In the only published study of which we are aware, using the MSIS definition of PJI, Nelson et al. [17] reported a 50% positive sonication rate in a cohort of 36 patients with minimum followup of 2 years; however, sonication detected persistent infection missed by tissue cultures in three of 11 patients, and 50% of patients with positive sonication cultures did not become reinfected at last followup. Differences in our results and those previously published, despite relatively constant sonication procedures, highlight the need for larger studies in which factors such as host characteristics, surgical timing, regional bioburden, and antibiotic protocol can be controlled for.
In addition to the debate about the usefulness of PMMA spacer sonication during revision arthroplasty, the treatment of positive sonication cultures in this setting is another topic that requires further exploration. Opinions differ regarding the treatment of different versus identical bacterial species when comparing sonication cultures with primary prosthesis infection. Some authors have advocated for aggressive treatment of same-species sonication cultures, assuming that eradication of the initial infection was incomplete [15], whereas others argue that different-species cultures represent either missed primary prosthesis polymicrobial infections or postexplant reinfection with virulent microorganisms [8]. Thus far, studies have lacked the sample size and followup required to provide clinically applicable insight into the handling of positive sonication cultures; this algorithm likely involves host characteristics, organism species/virulence, and timing, among other variables.
Biofilm-forming bacteria can adhere to and persist on antibiotic-loaded PMMA spacers used during revision hip and knee arthroplasty, but sonication appears to have limited ability to detect persistent infection before reimplantation and was not associated with recurrent infection. Our institution continues to perform sonication on all explanted antibiotic spacers during two-stage revisions; however, given these data, changes may be made to the currently accepted sonication procedure and CFU thresholds as they apply to antibiotic spacers. Large-scale prospective studies are needed to determine if there is any role for sonication in this setting and also to determine the optimal surgical and antibiotic management of positive cultures.
Footnotes
Each author certifies that he has no commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arizono T, Oga M, Sugioka Y. Increased resistance of bacteria after adherence to polymethyl methacrylate. An in vitro study. Acta Orthop Scand. 1992;63:661–664. [DOI] [PubMed] [Google Scholar]
- 2.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. [DOI] [PubMed] [Google Scholar]
- 4.Biring GS, Kostamo T, Garbuz DS, Masri BA, Duncan CP. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study. J Bone Joint Surg Br. 2009;91:1431–1437. [DOI] [PubMed] [Google Scholar]
- 5.Borens O, Baalbaki R, Nussbaumer F, Clauss M, Trampuz A. Sonication of temporary devices (spacers and cement nails) inserted during a two-stage implant exchange in patients with prosthetic joint infection. J Bone Joint Surg Br. 2011;93:321.21357952 [Google Scholar]
- 6.Chang CC, Merritt K. Effect of Staphylococcus epidermidis on adherence of Pseudomonas aeruginosa and Proteus mirabilis to polymethyl methacrylate (PMMA) and gentamicin-containing PMMA. J Orthop Res. 1991;9:284–288. [DOI] [PubMed] [Google Scholar]
- 7.Chang CC, Merritt K. Microbial adherence on poly(methyl methacrylate) (PMMA) surfaces. J Biomed Mater Res. 1992;26:197–207. [DOI] [PubMed] [Google Scholar]
- 8.Esteban J, Gadea I, Perez-Jorge C, Sandoval E, Garcia-Canete J, Fernandez-Roblas R, Blanco A, Prieto-Borja L, Cordero-Ampuero J. Diagnosis of spacer-associated infection using quantitative cultures from sonicated antibiotics-loaded spacers: implications for the clinical outcome. Eur J Clin Microbiol Infect Dis. 2016;35:207–213. [DOI] [PubMed] [Google Scholar]
- 9.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. [DOI] [PubMed] [Google Scholar]
- 11.Kendall RW, Duncan CP, Beauchamp CP. Bacterial growth on antibiotic-loaded acrylic cement. A prospective in vivo retrieval study. J Arthroplasty. 1995;10:817–822. [DOI] [PubMed] [Google Scholar]
- 12.Kendall RW, Duncan CP, Smith JA, Ngui-Yen JH. Persistence of bacteria on antibiotic loaded acrylic depots. A reason for caution. Clin Orthop Relat Res. 1996;329:273–280. [DOI] [PubMed] [Google Scholar]
- 13.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468:2060–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma D, Shanks RMQ, Davis CM, Craft DW, Wood TK, Hamlin BR, Urish KL. Viable bacteria persist on antibiotic spacers following two-stage revision for periprosthetic joint infection. J Orthop Res. 2017. May 19. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariconda M, Ascione T, Balato G, Rotondo R, Smeraglia F, Costa GG, Conte M. Sonication of antibiotic-loaded cement spacers in a two-stage revision protocol for infected joint arthroplasty. BMC Musculoskelet Disord. 2013;14:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. [DOI] [PubMed] [Google Scholar]
- 17.Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. 2014;472:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neut D, van de Belt H, Stokroos I, van Horn JR, van der Mei HC, Busscher HJ. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother. 2001;47:885–891. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothenberg AC, Wilson AE, Hayes JP, O'Malley MJ, Klatt BA. Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res. 2017;475:1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorli LL, Puig TC, Gonzalez A, Alier A, Knobel H, Salvado M, Horcajada JP. The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacers and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis. J Bone Joint Surg Br. 2012;94:249–253. [DOI] [PubMed] [Google Scholar]
- 22.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. [DOI] [PubMed] [Google Scholar]
- 23.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br. 1998;80:568–572. [DOI] [PubMed] [Google Scholar]
- 24.Urish KL, DeMuth PW, Kwan BW, Craf DWt, Ma D, Haider H, Tuan RS, Wood TK, Davis CM. Antibiotic-tolerant Staphylococcus aureus biofilm persists on arthroplasty materials. Clin Orthop Relat Res. 2016;474:1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. [DOI] [PubMed] [Google Scholar]