ABSTRACT
The human T cell receptor is capable of distinguishing between normal and post-translationally modified peptides. Because aberrant phosphorylation of cellular proteins is a hallmark of malignant transformation, the expression of the phosphorylated epitope could be an ideal antigen to combat cancer without damaging normal tissues. p53 activates transcription factors to suppress tumors by upregulating growth arrest and apoptosis-related genes. In response to DNA damage, p53 is phosphorylated at multiple sites including Ser33 and Ser37. Here, we identified phosphorylated peptide epitopes from p53 that could elicit effective T helper responses. These epitope peptides, p5322-41/Phospho-S33 and p5322-41/Phospho-S37, induced T helper responses against tumor cells expressing the phosphorylated p53 protein. Moreover, chemotherapeutic agents augmented the responses of such CD4 T cells via upregulation of phosphorylated p53. The upregulation of phosphorylated p53 expression by chemotherapy was confirmed in in vitro and xenograft models. We evaluated phosphorylated p53 expression in the clinical samples of oropharyngeal squamous cell carcinoma and revealed that 13/24 cases (54%) were positive for phosphorylated p53. Importantly, the lymphocytes specific for the phosphorylated p53 peptide epitopes were observed in the head and neck squamous cell cancer (HNSCC) patients. These results reveal that a combination of phosphorylated p53 peptides and chemotherapy could be a novel immunologic approach to treat HNSCC patients.
KEYWORDS: CD4 T cell, epitope, head and neck squamous cell carcinoma, immunotherapy, p53, phosphorylation, post-translational modification
Introduction
Despite recent advances in treatment, the survival rate of head and neck squamous cell carcinoma (HNSCC) patients has not improved for decades. Because advanced stages are initially detected in most patients, their prognosis is poor. The clinical use of nivolumab, an immune checkpoint inhibitor, revealed that the immunotherapy could be a novel treatment for HNSCC patients. However, the biomarker for select responders has not yet been found, and the expensive cost of the reagents hinder the broad application of immune checkpoint inhibitors.1–3
While some of the researchers have considered peptide vaccines as an old concept, optimal peptide conformation, immune adjuvants, and route of administration eliminated cancer in preclinical studies.4 Moreover, peptide vaccines have several advantages over other therapies such as cost-effectiveness, ease of synthesis, and a broad acceptance in clinical practice.5 Because CD4 helper T lymphocytes (HTLs) play a key role in direct killing of tumor cells in addition to their helper function for CD8 cytotoxic T lymphocytes (CTLs),6 the identification of a helper epitope from a tumor-associated antigen (TAA) is necessary to develop an effective peptide vaccine. Post-translational modifications, such as phosphorylation and acetylation, are unique targets in selecting a TAA.7 Andersen et al has described that CTLs can recognize the phosphorylated peptide as a completely different antigen compared to the non-phosphorylated peptide.8 In addition to CTLs, it has been reported that the T cell receptor (TCR) of HTLs has an ability to recognize a phosphorylated peptide derived from melanoma antigen (MART-1) recognized by T cells.9 Because phosphorylation is a hallmark of malignant transformation,10 phosphorylated peptide-targeted immunotherapy can be a novel approach to attack cancers without damaging normal tissues that weakly express TAA.7 The normal tissues that only express non-phosphorylated TAA might be spared in the phosphorylated antigen-targeted immunotherapy. Because MART-1 is only expressed in melanoma, the design of a phosphorylated peptide using a ubiquitous TAA would be valuable for the treatment of many types of cancer. p53 is an activator of proapoptotic signaling and widely expressed in tumors. More than 50% of human malignancies have a p53 mutation, and most of them are phosphorylated and acetylated.11–13 Accordingly, p53 is a candidate TAA to identify phosphorylated helper epitope peptides. Recently, we hypothesized that malignancy associated post-translated modifications could have a high immunogenicity and showed that acetylated p53 proteins served as the target of anti-tumor immunity.7 Not only hyperacetylation but also hyperphosphorylation have been observed in malignancies, and they occur at various stages of oncogenesis.
In the present study, we show that peptides p5322-41/Phospho-S33 and p5322-41/Phospho-S37 (referred as p-p53S33 and p-p53S37) were able to elicit antigen-specific, tumor-reactive HTL responses. The treatment of tumor cells with chemotherapeutic reagents enhanced these responses. These results suggest that the tumor-associated phosphorylated peptide vaccines with chemotherapy could be a promising approach in the HNSCC immunotherapy.
Results
Expression of phosphorylated p53 in oropharyngeal squamous cell carcinoma specimens
We first analyzed the expression of p53 and phosphorylated p53 in surgical specimens of oropharyngeal squamous cell carcinoma (OPSCC) by immunohistochemistry. The clinical parameters of 24 OPSCC patients are described in Supplemental Table 1. As shown in Fig. 1A, p53 was expressed in 18/24 cases (75%) of OPSCC. Phospho-p53S33 and phospho-p53S37 were expressed in 13/24 cases (54%) and 10/24 cases (42%), respectively (Fig. 1B and C). Ten cases (42%) of OPSCC were double positive for phospho-p53S33 and phospho-p53S37, indicating that the multiple serine residues of p53 are simultaneously modified in OPSCC. The expressions of HLA-DR and p16 (a surrogate marker of human papilloma virus infection) were found in 7/24 cases (29%) and 14/24 cases (58%), respectively (Supplemental Table 1). Phosphorylated p53 expression was not related to other parameters such as tumor stage, p16 expression, or HLA-DR expression (Supplemental Table 2). These results provide a rational basis for the development of immunotherapy to treat HNSCC by targeting phosphorylated p53 proteins.
Table 1.
Assessment of T-cell resopnses to the phosphorylated p53 peptides in HNSCC patients and healthy donors
| No. | Sex | Age (y) | Pathological stage | Primary site | No antigen |
p-p53S33 |
anti-HLA-DR mAb |
p-p53S37 |
anti-HLA-DR mAb |
TT830-843 |
|---|---|---|---|---|---|---|---|---|---|---|
| (IFN-g, pg/ml) | ||||||||||
| 1 | M | 69 | T2N0M0 | Larynx | < | 124 ± 73 | < | 67 ± 22 | < | 195 ± 40 |
| 2 | M | 71 | T2N0M0 | Larynx | < | 216 ± 34 | < | 41 ± 20 | < | 305 ± 157 |
| 3 | M | 75 | T3N2cM0 | Oropharynx | < | 155 ± 90 | < | 57 ± 20 | < | 167 ± 101 |
| 4 | M | 47 | T2N0M0 | Oropharynx | < | 198 ± 89 | < | 92 ± 39 | < | 266 ± 163 |
| 5 | F | 79 | T2N0M0 | Hypopharynx | < | 236 ± 95 | < | 97 ± 32 | < | 388 ± 86 |
| 6 | M | 37 | Normal Volunteer | < | 47 ± 5 | < | 45 ± 7 | < | 414 ± 296 | |
| 7 | M | 34 | Normal Volunteer | < | 56 ± 7 | < | 43 ± 3 | < | 721 ± 401 | |
| 8 | F | 52 | Normal Volunteer | < | 43 ± 5 | < | 41 ± 11 | < | 325 ± 133 | |
<: less than the lower limit of detection (mean <30░pg/ml). No. 1–5 are HNSCC patients and No. 6–8 are healthy donors.
Figure 1.
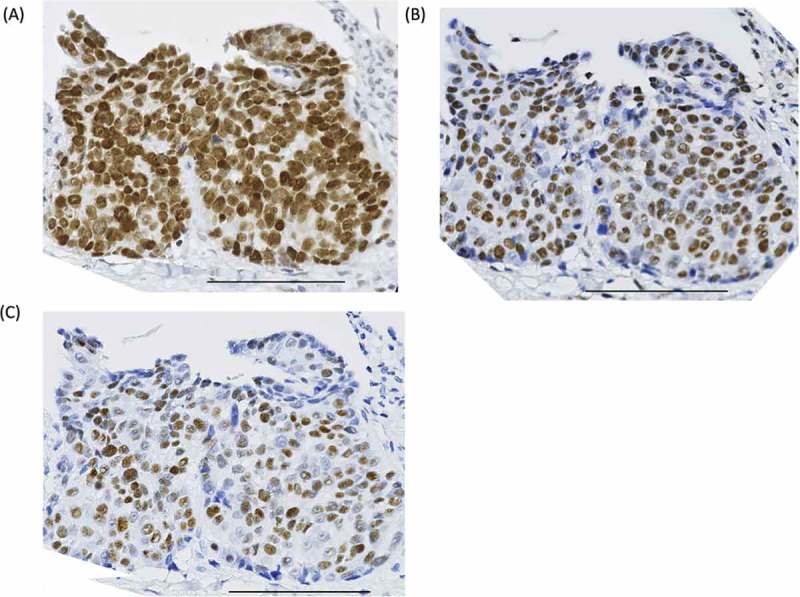
Expression of total p53 and phosphorylated p53 on oropharyngeal squamous cell carcinoma (OPSCC) specimens. (A) Total p53, (B) phospho-p53S33, and (C) phospho-p53S37 expression in formalin-fixed, paraffin-embedded OPSCC samples. Scale bar: 100 μm.
In vitro induction of p5322-41/Phospho-S33 and S37-specific CD4 T cell responses
The wild-type (wt) sequence peptide p5325-35 was previously reported as a naturally processed epitope recognized by human HTLs.14 To create peptides containing the phosphorylated serine residues at position 33 or 37 in the p53 transactivation domain,15 the elongated peptide (p5322-41) was assessed for its ability to elicit phosphorylated peptide-specific HTL responses.
As shown in Fig. 2, the dual-phosphorylated peptide p5322-41/Phospho-S33 and S37 (referred to as p-p53S33 and S37) could induce phosphorylated peptide-specific HTL responses from two individuals. These HTLs only responded to p-p53S33 and S37 and p-p53S33 in a concentration-dependent manner but not to p-p53S37 and non-phosphorylated wt p5322-41, suggesting that the TCR of these HTLs is specific to phosphorylated Ser33. These responses were restricted to HLA-DR molecules (Fig. 2B), indicating that the phosphorylated peptide was sufficient to allow binding to MHC class II molecules. To determine which HLA class II molecules presented the phosphorylated p53 peptides to the HTLs, a panel of mouse fibroblasts expressing single HLA-DR molecules was used as antigen presenting cells (APCs). As shown in Fig. 2C, HTLs J5 and J16 recognized the phosphorylated p53 peptide in the context of HLA-DR1, whereas the response of T20 was restricted by HLA-DR9. These findings demonstrate that the phosphorylated p53 peptides can bind to multiple HLA-DR molecules that cover a broad population of cancer patients. Taken together, these results revealed that HTL precursors that react to p-p53S33 and S37 exist in human PBMCs, and phosphorylated Ser33 might be more immunogenic than phosphorylated Ser37.
Figure 2.
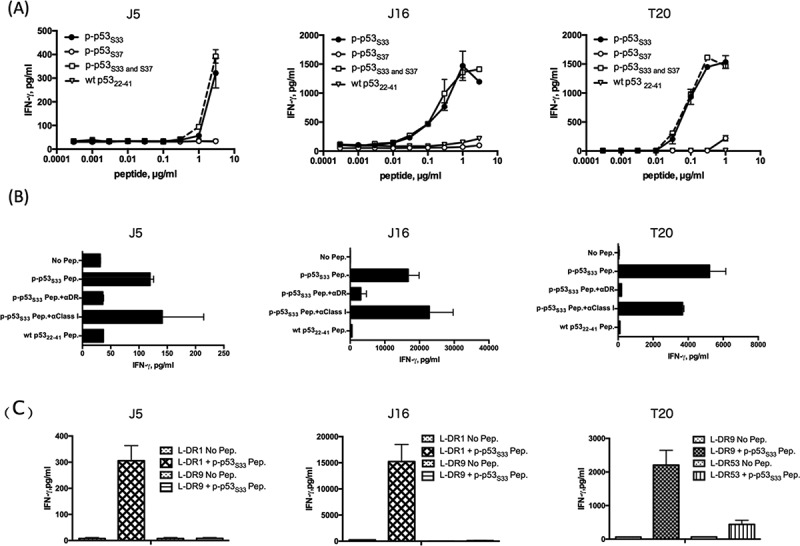
Induction of CD4 T cell responses using the dual-phosphorylated p53 peptide. (A) The dual-phosphorylated peptide p-p53S33 and S37-reactive CD4 T cells (J5, J16, and T20) were assessed for their ability to recognize various concentrations of dual-phosphorylated, mono-phosphorylated, and wild type (wt) peptides. Autologous PBMCs were used as antigen presenting cells (APCs). (B) MHC restriction analysis of phosphorylated p53-reactive CD4 T cells. Peptide-induced T cell responses of p-p53S33 and S37-reactive CD4 T cells (J5, J16, and T20) in the presence of anti-HLA DR mAb L243 or anti-HLA class I mAb W6/32 (negative control) were evaluated. Autologous PBMCs were used as APCs. (C) CD4 T cell responses to phosphorylated p53 peptides (J5, J16 and T20) were measured using L-cells transfected with individual HLA-DR genes as APCs to determine the restricting HLA class II molecules.
p53 Ser33 and Ser37 are immunogenic phosphorylation sites that induce CD4 T cell responses
To confirm the immunogenicity of phosphorylated p53 peptides, we used the mono-phosphorylated peptides (p-p53S33 or p-p53S37) to elicit peptide-specific HTLs. As shown in Fig. 3 A, we successfully isolated HTLs specific for phosphorylated peptide p53 Ser33 (K2 and J44 from donor 3 and donor1, respectively) and p53 Ser37 (H2 from donor 4) but not the non-phosphorylated wt p5322-41 peptide. HLA-DR molecules restricted the reaction of these HTLs (Fig. 3B). To define the restricting HLA class II molecules, L-cells transfected with individual HLA-DRs were used as APCs. As shown in Fig. 3C, p-p53S33 peptide-specific HTLs K2 and J44 recognized the phosphorylated p53 peptide in the context of HLA-DR1, whereas the response of p-p53S37 peptide-specific HTLs H2 was restricted by HLA-DR9.
Figure 3.
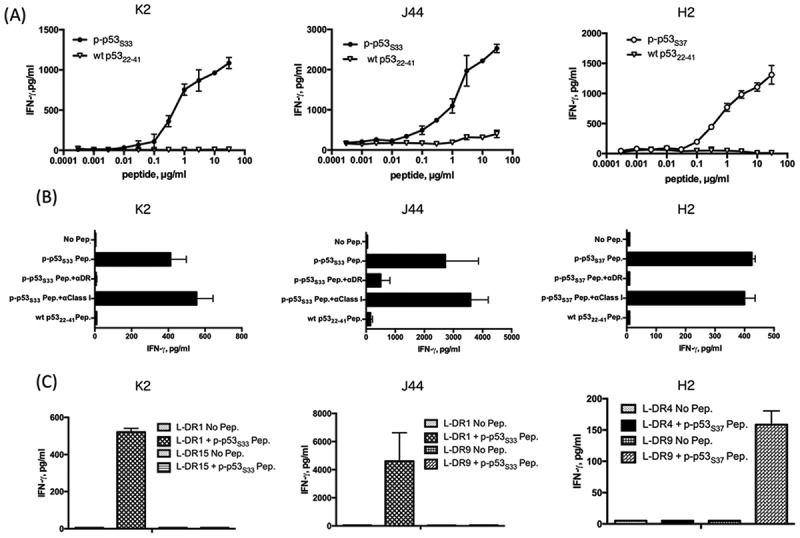
Induction of CD4 T cell responses using the mono-phosphorylated p53 peptide. (A) The phosphorylated peptide p-p53S33-reactive CD4 T cells (K2 and J44) and p-p53S37-reactive CD4 T cells (H2) were assessed for their ability to recognize various concentrations of phosphorylated peptides. Autologous PBMCs were used as APCs. (B) MHC restriction analysis of phosphorylated p53-reactive CD4 T cells. Peptide-induced T cell responses of p-p53S33-reactive (K2 and J44) and p-p53S37 -reactive cells (H2) in the presence of anti-HLA DR mAb L243 or anti-HLA class I mAb W6/32 (negative control) were evaluated. Autologous PBMCs were used as APCs. (C) CD4 T cell responses to p-p53S33-reactive (K2 and J44) and p-p53S37-reactive cells (H2) were assessed using L-cells transfected with individual HLA-DR genes as APCs to determine the restricting HLA class II molecules.
An increase in phosphorylated p53 was induced by treatment with chemotherapeutic reagents in vitro and in murine xenograft models
To test the ability of phosphorylated p53 peptide-reactive HTLs to directly recognize tumors, we first examined the expression of phosphorylated p53 in tumor cells. Western blot analysis showed that phosphorylated p53 was expressed in several tumor cell lines, including HNSCC (Fig. 4A-C). EBC1 (lung SCC) and SAS (HNSCC) cells expressed neither wt nor phosphorylated p53. Because DNA damage induces the phosphorylation of p53, tumor cells were treated with chemotherapeutic reagents. As previously reported,16–18 phosphorylated p53 expression was augmented by the addition of doxorubicin and cisplatin, a key drug for HNSCC treatment (Fig. 4C).
Figure 4.
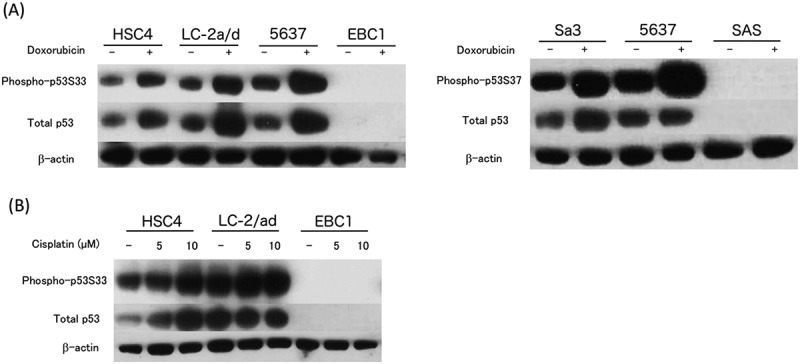
Expression of total p53 protein and p53 carrying phosphorylated Ser33 or Ser37 in tumor cells. (A-C) The expressions of total p53 protein and phosphorylated p53 (phosphorylated Ser33 or Ser37) were examined by Western blotting. The tumor cell lines were treated with or without doxorubicin (1 μM) or cisplatin (5 or 10 μM) for 24 hours before assessment. HNSCC: HSC4, SAS, and Sa-3. Lung adenocarcinoma: LC-2 a/d. Lung squamous cell carcinoma: EBC1. Bladder cancer: 5637.
In clinical settings, it is important to have an increase in phosphorylated p53 in tumors in vivo. Consistent with western blot findings, we observed that the positive staining of tumor cells for phosphorylated p53 (Fig. 5A-D), and total p53 levels (Supplemental Fig. 1A-D) were increased in murine xenograft tumors from cisplatin or doxorubicin treated mice. It is noteworthy that the chemotherapy induced a diffuse expression of phosphorylated p53 in the tumor. These findings suggest that such anti-cancer drugs enable not only an induction of immunogenic cell death (ICD)19,20 but also enhancement of tumor antigens.
Figure 5.
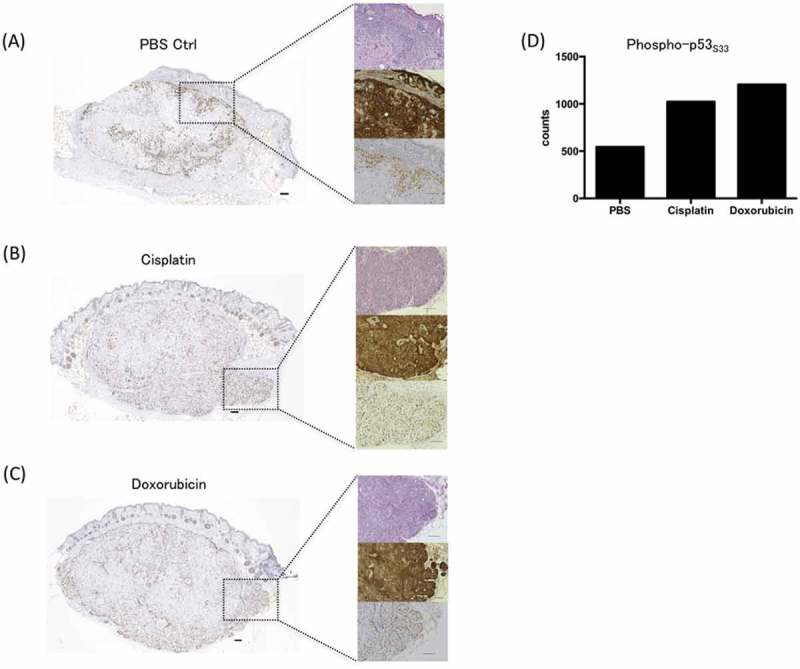
Immunohistochemical analysis of phosphorylated p53 expression in a xenograft model. BALB/c-nu/nu mice were inoculated with HSC4 cells (human HNSCC). PBS (negative control), cisplatin, or doxorubicin treatment was applied when the tumor reached 5 mm. Twenty-four hours after the treatment, mice were sacrificed and the expressions phosphorylated p53 in FFPE tumor samples were examined and representative immunohistochemical images were shown. Phospho-p53S33 expression with PBS (control) (A), cisplatin (B), or doxorubicin treatment (C). Cell counts of phospho-p53S33 positive cells per unit area (mm2) (D). Left boxes: The representative Fig. of the serial-cut section (4-µm thick). Right upper boxes: Hematoxylin-eosin staining. Right middle boxes: cytokeratin 5/6 staining. Right lower boxes: phosphorylated p53 staining. The expression of cytokeratin 5/6 showed that tumor cells consist of SCC. Scale bar is 100 μm.
Direct recognition of phospho-p53 positive tumor cells by phosphorylated peptide-reactive CD4 T cells
Next, we evaluated whether phosphorylated p53-reactive HTLs could directly recognize phosphorylated p53 expressing tumor cells. The peptide-reactive HTLs and HLA-DR-matched phosphorylated p53-positive tumor cells were cocultured, and IFN production in the culture supernatant was measured. As shown in Fig. 6A, all phosphorylated p53-specific HTLs (K2, J44, and H2) could directly recognize the HLA-DR-matched phosphorylated p53-positive tumor cells but not HLA-DR-matched phosphorylated p53-negative tumor cells in an HLA-DR dependent manner. These results indicate that the phosphorylation of p53 is maintained through the antigen processing machinery and the phosphorylated peptides in tumor MHC class II molecules are naturally presented to HTLs.
Figure 6.
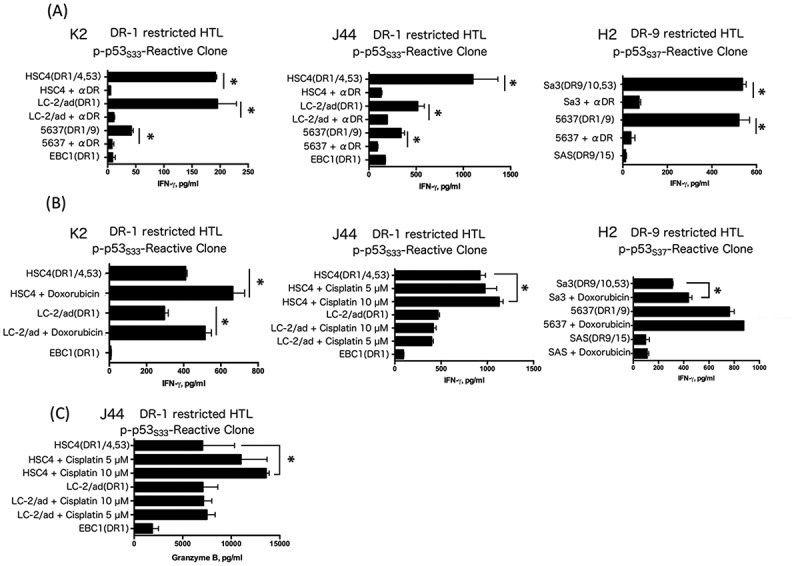
Direct tumor recognition by phosphorylated peptide-specific CD4 T-cells. Direct tumor recognition of naturally processed p53 antigen expressed in tumor cells by p-p53S33-reactive clones (K2 and J44) and the p-p53S37-reactive clone (H2). (A) p-p53S33-reactive T cells (K2 and J44, DR-1-restricted) were tested for their capacity to recognize HLA-DR1+/p53 positive (HSC4, LC-2/ad and 5637) or DR1+/p53 negative (EBC1) tumor cells, which was used as a negative control. The p-p53S37-reactive clone (H2) was tested for its capacity to recognize antigens directly on HLA-DR9+/p53 positive tumor cells (Sa3, 5637) or DR9+/p53 negative (SAS) tumor cells. (B) Tumor cells were treated with doxorubicin or cisplatin 24 hours before coculture with T cells (K2, J44 and H2). (C) Granzyme B production by the p-p53S33-reactive clone (J44) during coculture with tumor cells.
Because the Western blot analysis presented in Fig. 4 showed that p53 phosphorylated at Ser33 or Ser37 and total p53 protein expression levels in tumor cells were enhanced by the treatment with doxorubicin or cisplatin, tumor cells were pretreated with these reagents before coculturing with HTLs. As shown in Fig. 6B, the responses of p-p53S33 or p-p53S37-reactive HTLs against tumor cells were augmented by treatment of the tumor cells with doxorubicin or cisplatin. Most importantly, granzyme B production from HTL clone J44 against tumor cells was observed with cisplatin treatment, indicating that the HTL had a cytotoxic effect on tumor cells (Fig. 6C). These results suggest that the accumulation of DNA damage by chemotherapy can act as an adjuvant to amplify expression of phosphorylated p53 and augment the efficacy of phosphorylated p53-targeted peptide vaccines.
Recognition of phosphorylated p53 peptides by PBMCs from cancer patients
The existence of precursor T cells specific for the phosphorylated peptides in the PBMCs of HNSCC patients is a prerequisite for the application of a phosphorylated p53 peptide vaccine in the clinic. To evaluate the responses of T cells from HNSCC patients to p-p53S33 and p-p53S37, we carried out a short-term culture using peptide-stimulated PBMC from five HNSCC patients. Since the volumes of blood obtained from these patients were small, we could not establish T cell lines to perform more detailed studies or HLA restriction analyses. A tetanus toxoid peptide (TT830-843) was used as a positive control because this peptide generates robust HTL responses regardless of the HLA-DR alleles.21 As shown in Table 1, substantial T cell responses to phosphorylated p53 peptides were observed in HNSCC patients but not in healthy donors, suggesting that the precursor of phosphorylated p53-reactive HTLs exists in HNSCC patients. Moreover, HTL responses to p-p53S33 was higher than that to p-p53S37. This result indicates that phosphorylated Ser33 might be more immunogenic than phosphorylated Ser37, which is consistent with the results in Fig. 2A.
Discussion
In this study, we demonstrated that the phosphorylated peptides from p53 tumor suppressor proteins are candidates for a helper peptide vaccine to treat HNSCC. Since MDM2 tightly regulates the amount of p53 under normal conditions, the amount of p53 is maintained at a very low level. On the other hand, tumors frequently express an aberrant amount of p53.22 Consequently, it appears that self p53-reactive T cells, which have a low affinity for TCR, might only react to tumor (high burden of p53) but not to normal tissues (low burden of p53). Because only lymphocytes from HNSCC patients but not from healthy donors reacted to phosphorylated p53 peptides in a short-term culture (Table 1), the increased amount of p53 in tumors may spontaneously increase the number of p53-reactive HTLs. In response to DNA damaging reagents or ultraviolet irradiation, the phosphorylation of p53 at multiple sites including transactivation domain (Ser33 and Ser37) increases the stability and activity of p53 by decreasing its ability to bind MDM2 in tumors.13,23,24 Because T cells have been reported to precisely recognize phosphorylated epitopes and post-translationally modified epitopes on MHC molecules,25–27 the phosphorylated epitope peptides from p53 would be an ideal antitumor vaccine to combine with conventional therapies. Phosphorylated p53 peptides bound to multiple HLA-DR alleles (Fig. 2C), indicating that these peptides can be applied to a broad population.
There are multiple phosphorylated serine residues in p53, including Ser6, Ser9, Ser15, Ser18, Ser20, Ser33, Ser37, Ser46, Ser372, and Ser392.11,28 We targeted Ser33 and Ser37 because they are located close to the sequence previously reported for eliciting HTL responses against wt p53 peptides.14 There is an overlap between the peptide sequence that we selected and the p5325-35 peptide previously defined as a CTL epitope, indicating that this peptide has a capacity to activate p53-reactive CTLs in addition to p53-reactive HTLs.29 Although the phosphorylation of p53 might induce phosphorylated peptide-specific CTL responses,8 it is also possible that the phosphorylation of the p5325-35 peptide might attenuate the stability of the MHC-non-phosphorylated peptide-TCR complex to stimulate CTLs. Further research is required to examine whether our peptide has an ability to elicit phosphorylated p53-reactive CTLs. The stability of phosphorylated peptides in vivo also remains to be elucidated.
While T-cell based immunotherapy mainly focuses on CTLs, HTLs have cytotoxic activity against tumor cells in addition to their helper function.6 In addition to the pMART-1-specific HTL response,9 this report opens the avenue to utilize phosphorylated serine bearing peptides as helper epitope peptides. To induce robust responses to peptide vaccines in vivo, the appropriate selection of adjuvants is indispensable. Toll-like receptor (TLR) 3 or 7 ligand is one of the desirable adjuvants for an antitumor peptide vaccine. TLR3 and TLR7 ligands induce the expression of MHC Class II on DCs,30 which plays an indispensable role in activating HTLs through the presentation of peptide. Interestingly, imiquimod, a TLR7 ligand, induced apoptosis through p53 activation.31 However, p53 signaling increases the expression of TLR3,32,33 indicating that the TLR3 or 7 ligand elicits p53 expression in a positive feedback loop. Thus, these adjuvants may work in two different ways: 1) activation of APCs or 2) the upregulation of antigens (p53). Because we previously reported that the EGFR inhibition augments the expression of MHC Class II on HNSCC cells,34 an EGFR inhibitor that has been currently available as a treatment option in the patients with HNSCC could be an interesting adjuvant to combine with the phosphorylated p53 peptide vaccine.
During the malignant transformation, post-translational modifications play a pivotal role.35 A number of tumor cell types tend to accumulate the p53 protein, which mainly exhibits phosphorylation and acetylation.11 Because the DNA-damaging reagents such as cisplatin and doxorubicin augmented the expression of phosphorylated p53, the recognition of the tumor was augmented by phosphorylated p53-specific HTLs (Fig. 4, 6B, and 6C). Having been first-line treatments for HNSCC patients,36 platinum-based drugs are acceptable to use as an adjuvant. Cisplatin has been reported to upregulate the expression of antigen processing machinery components.37 Since the induction of ICD has been appreciated in doxorubicin, the effect of cisplatin in inducing ICD might be inferior to its derivative oxaliplatin.20 However, it is still possible that cisplatin stimulates APCs by inducing HMGB1 from dead tumor cells.19 Interestingly, p53 activation might also play a role in stimulating innate immunity via the induction of HMGB1 and ATP from tumor cells.38,39 Thus, chemotherapeutic reagents activate APCs and increase the amount as well as the presentation of antigens in tumors. Moreover, the production of IFN from T cells can counteract platinum-resistance by altering the metabolism in cancer-associated fibroblasts, indicating that the cisplatin-induced infiltration of T cells renders tumors sensitive to chemotherapy. Collectively, non-lymphoablative chemotherapy and T-cell-targeted immunotherapy could attack tumors by complementing each other.
In conclusion, we have identified novel helper epitopes from phosphorylated tumor protein p53 (p-p53S33 and p-p53S37) that can induce antigen-specific HTL responses. The variant of amino acid sequences of these peptides has not been reported,40 suggesting that these peptides are well-conserved. Furthermore, cisplatin and doxorubicin may work as adjuvants for immunotherapy by upregulating phosphorylated p53. This approach could be a desirable therapy to treat HNSCC with chemotherapy, which expresses a high level of phosphorylated p53.
Materials and Methods
Cell lines
Mouse fibroblast cell lines (L-cells) expressing individual human HLA-DR molecules (DR1, DR4, DR9, DR15, and DR53) were kindly provided by Dr. Robert W. Karr (Karr Pharma, St. Louis, MO) and Dr. T. Sasazuki (International Medical Center of Japan, Tokyo, Japan). The HNSCC cell lines HSC4 (tongue SCC), Sa-3 (gingival SCC), and the lung cancer cell lines LC-2/ad (adenocarcinoma) and EBC1 (squamous cell carcinoma) were supplied by the RIKEN Bio-Resource Center (Tsukuba, Japan). The HNSCC cell line SAS (tongue SCC) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The bladder cancer cell line 5637 was obtained from Cell Resource Center for Biomedical Research Institute of Development (Aging and Cancer, Tohoku University, Sendai, Japan). HSC4, LC-2 a/d and 5637 cells possess mutant p53R248Q, p53S241 C and p53R280 T protein, respectively.41 EBC1 and SAS cells lack any detectable p53 protein. All cell lines were maintained in a tissue culture medium as recommended by the supplier and incubated at 37℃ in a humidified atmosphere with 5% CO2.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) samples were prepared from pretreatment biopsy tissues of oropharyngeal squamous cell carcinoma (OPSCC) patients or the subcutaneous tumors of BALB/c-nu/nu mice inoculated with HSC4 cells. An indirect immunoperoxidase technique (the streptavidin-biotin method) was performed. Polyclonal rabbit anti-human phospho-p53S33, phospho-p53S37 (1:100, CST, #2526 and #9289, Denver, CO), mouse anti-human p53 mAb (1:50, Santa Cruz, sc-126, DO-1, CA), monoclonal mouse anti-human HLA-DR chain (1:1000, Dako, M0746, TAL.1B5, Denmark), mouse anti-human p16INK4A mAb (Ready to Use antibody, Roche Diagnostics, 518111915, E6H4, Swiss), and mouse anti-human CK5/6 (1:10, Dako, M7237, D5/16/B4, Denmark) served as primary antibodies.
The p53 staining patterns were characterized into three categories: (a) ++: overexpression (at least 50% of the tumor cells express nuclear staining); (b) +: normal expression (no more than 49% of the tumor cells express nuclear staining); and (c) -: negative when there was a complete absence of staining in the tumor. The p53 expression in neighboring normal tissues served as an internal positive control. In order to quantify the number of DAB staining cells, we used a BZ-X710 microscope (Keyence, Tokyo, Japan) with the BZ-H3░C hybrid cell count software (Keyence).
Synthetic peptides
We selected peptide epitopes from the previously described sequence.14 Synthetic peptides were purchased from GenScript (Tokyo, Japan). The wild-type (wt) p5322-41 (LWKLLPENNVLSPLPSQAMD), p5322-41/Phospho-S33 and S37, p5322-41/Phospho-S33, and p5322-41/Phospho-S37 (referred as p-p53S33 and S37, p-p53S33, p-p53S37) peptides were used throughout this work. The tetanus toxoid (TT830-843; QYIKANSKFIGITE) peptide was used as a positive control that could bind to multiple HLA class II molecules.21
In vitro induction of peptide-reactive CD4 helper T cells
The procedure utilized for the generation of peptide-reactive HTL lines from peripheral blood mononuclear cells (PBMCs) of healthy donors (donor 1: HLA-DR1/9, DR53, donor 2: HLA-DR9/14, 53, donor 3: HLA-DR1/15, 51, and donor 4: HLA-DR4/9, 53) was described in detail.42 In short, HTLs purified by CD4 MACS microbeads (Miltenyi Biotech, Auburn, CA) were stimulated with peptide-pulsed autologous dendritic cells (DCs) and followed by 2–3 cycles of stimulations with γ-irradiated autologous PBMCs and peptides (3 μg/mL). Peptide-reactive HTLs that showed at least a three-fold increase in cytokine production in response to peptide stimulation compared to the non-peptide control were expanded. HTL clones were isolated by limiting dilution. All blood samples from human healthy donors were collected after oral and signed informed consent (the institutional ethics committee gave approval number #16040).
Measurement of antigen-specific responses with CD4 T cells
An evaluation of HTL responses to diverse antigens was performed as previously described.34 The tumor cells were treated with IFN-γ (500 IU/ml, PeproTech, Rocky Hill, NJ) for 48 hours to induce the expression of HLA-DR before culturing with HTLs. To enhance phosphorylation of p53 in tumor cells, the tumor cells were treated with or without 1 μM doxorubicin (CST, Denver, CO), or cisplatin (5 or 10 μM; R&D Systems, Minneapolis, MN) during the last 24 hours of IFN-γ treatment.
Granzyme B ELISA
To evaluate the cytotoxic activity of HTL clones, the supernatant from antigen-specific responses by CD4 T cells were collected and assessed for Granzyme B by ELISA (MABTECH, Stockholm, Sweden) according to manufacturer’s instructions.
Western blot analyses
Expression of p53 was assessed by Western blotting after the treatment of tumor cell lines with or without 1 μM doxorubicin or cisplatin (5 or 10 μM) for 24 hours. The tumor cell lines were lysed in LDS sample buffer (Invitrogen, #NP0008, Carlsbad, CA), and the lysates were submitted to electrophoresis in NuPAGE bi-Tris SDS-PAGE gels (Invitrogen, #NP0322PK2, Carlsbad, CA). Antibodies used to detect specific protein expression were as follows: mouse anti-human p53 mAb (1: 1,000, Santa Cruz, sc-126, DO-1, CA), rabbit anti-human phospho-p53S33 mAb (1: 1,000, CST, #2526, Denver, CO), rabbit anti-human phospho-p53S37 mAb (1: 1,000, CST, #9289, Denver, CO), and mouse anti-actin (1:1,000, Santa Cruz, sc-47778, C4, Santa Cruz, CA).
Analysis of wild-type p53 and phosphorylated p53 expression in a xenograft model
Six-week-old female BALB/c-nu/nu mice were obtained from Charles River Japan (Yokohama, Japan) and were maintained in accordance with the animal facility at the Asahikawa Medical University. Five mice were injected subcutaneously with 2 × 106 HSC4 cells in a flank. When the tumor reached 5 mm, PBS (control), cisplatin (7.5 mg/kg), or doxorubicin (5 mg/kg) was injected into the peritoneal cavity. Twenty-four hours later, mice were sacrificed, and tumor samples were collected for evaluating the p53 expression by immunohistochemistry. All animal experiments were approved by the Institutional Animal Care and Use Committee of Asahikawa Medical University.
Measurement of peptide-specific responses in cancer patients
Peripheral blood lymphocytes were isolated from fresh heparinized whole blood of HNSCC patients by LymphoprepTM (Axis-Shield PoC AS, Oslo, Norway)-centrifugation, and cultured with peptides (10 μg/mL) in 96-well plates as described previously.7,43 The lymphocytes were stimulated for 2 cycles (7 days/cycle). Seven days after the second peptide stimulation, production of IFN-γ or GM-CSF was assessed by ELISA (BD PharMingen, #555142). The institutional ethics committee approved this study protocol (approval number #16040).
Statistical analysis
The data were analyzed using Student’s t-test and Chi-squared test. p < 0.05 indicates statistical significance. Error bars in the data indicate the standard error of the mean (SEM). GraphPad Prism 6 was used for the analyses.
Disclosure of Potential Conflicts of Interest
Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). The other authors declare no conflict of interest.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number 17K16884 and 16K20223, 18H02948, and the grant from the Akiyama life science foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. doi: 10.1056/NEJMoa071028 PMID:17960012 [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–67. PMID:27718784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491 PMID:23297039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumai T, Lee S, Cho HI, Sultan H, Kobayashi H, Harabuchi Y, Celis E.. Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses. Cancer Immunol Res. 2017;5:72–83. doi: 10.1158/2326-6066 PMID:27941004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumai T, H Kobayashi, Harabuchi Y, Celis E.. Peptide vaccines in cancer-old concept revisited. Curr Opin Immunol. 2016;45:1–7. doi: 10.1016/j.coi.2016.11.001 CIR-16-0194. PMID:27940327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishibashi K, Kumai T, Ohkuri T, Kosaka A, Nagato T, Hirata Y, Ohara K, Oikawa K, Aoki N, Akiyama N, et al. Epigenetic modification augments the immunogenicity of human leukocyte antigen G serving as a tumor antigen for T cell-based immunotherapy. Oncoimmunology. 2016;5:e1169356. doi: 10.1080/2162402X.2016.1169356 PMID:27471649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumai T, Ishibashi K, Oikawa K, Matsuda Y, Aoki N, Kimura S, Hayashi S, Kitada M, Harabuchi Y, Celis E, et al. Induction of tumor-reactive T helper responses by a posttranslational modified epitope from tumor protein p53. Cancer Immunol Immunother. 2014;63:469–78. doi: 10.1007/s00262-014-1533-z PMID:24633296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen MH, Bonfill JE, Neisig A, Arsequell G, Sondergaard I, Valencia G,, Neefjes J, Zeuthen J, Elliott T, Haurum JS, et al. Phosphorylated peptides can be transported by TAP molecules, presented by class I MHC molecules, and recognized by phosphopeptide-specific CTL. J Immunol. 1999;163:3812–8. PMID:10490979 [PubMed] [Google Scholar]
- 9.Depontieu FR, Qian J, Zarling AL, McMiller TL, Salay TM, Norris A, English AM, Shabanowitz J, Engelhard VH, Hunt DF, et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. PNAS. 2009;106:12073–8. doi: 10.1073/pnas.0903852106 PMID:19581576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, Engelhard VH, Hunt DF.. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. PNAS. 2006;103:14889–94. doi: 10.1073/pnas.0604045103 PMID:17001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minamoto T, Buschmann T, Habelhah H, Matusevich E, Tahara H, Boerresen-Dale AL, Harris C, Sidransky D, Ronai Z.. Distinct pattern of p53 phosphorylation in human tumors. Oncogene. 2001;20:3341–7. doi: 10.1038/sj.onc.1204458 PMID:11423984 [DOI] [PubMed] [Google Scholar]
- 12.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–61. doi: 10.1056/NEJMoa073770 PMID:18094376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/S0092-8674(00)81871-1 PMID:9039259 [DOI] [PubMed] [Google Scholar]
- 14.Ito D, Albers A, Zhao YX, Visus C, Appella E, Whiteside TL, DeLeo AB.. The wild-type sequence (wt) p53(25-35) peptide induces HLA-DR7 and HLA-DR11-restricted CD4+ Th cells capable of enhancing the ex vivo expansion and function of anti-wt p53(264-272) peptide CD8+ T cells. J Immunol. 2006;177:6795–803. doi: 10.4049/jimmunol.177.10.6795 PMID:17082593 [DOI] [PubMed] [Google Scholar]
- 15.Gu B, Zhu WG.. Surf the post-translational modification network of p53 regulation. Int J Biol Sci. 2012;8:672–84. doi: 10.7150/ijbs.4283 PMID:22606048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L.. p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem. 1998;273:11995–8. doi: 10.1074/jbc.273.20.11995 PMID:9575138 [DOI] [PubMed] [Google Scholar]
- 17.Appella E, Anderson CW.. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–72. doi: 10.1046/j.1432-1327.2001.02225.x PMID:11358490 [DOI] [PubMed] [Google Scholar]
- 18.Fraser M, Bai T, Tsang BK.. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int J Cancer. 2008;122:534–46. doi: 10.1002/ijc.23086 PMID:17918180 [DOI] [PubMed] [Google Scholar]
- 19.Beyranvand Nejad E, van der Sluis TC, van Duikeren S, Yagita H, Janssen GM, PA van Veelen, Melief CJ, van der Burg SH, Arens R.. Tumor Eradication by Cisplatin Is Sustained by CD80/86-Mediated Costimulation of CD8+ T Cells. Cancer Res. 2016;76:6017–29. doi: 10.1158/0008-5472.CAN-16-0881 PMID:27569212 [DOI] [PubMed] [Google Scholar]
- 20.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G.. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107 PMID:27748397 [DOI] [PubMed] [Google Scholar]
- 21.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A.. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–42. doi: 10.1002/eji.1830191209 PMID:2481588 [DOI] [PubMed] [Google Scholar]
- 22.Olivier M, Hollstein M, Hainaut P.. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008 PMID:20182602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichwan SJ, Yamada S, Sumrejkanchanakij P, Ibrahim-Auerkari E, Eto K, Ikeda MA.. Defect in serine 46 phosphorylation of p53 contributes to acquisition of p53 resistance in oral squamous cell carcinoma cells. Oncogene. 2006;25:1216–24. doi: 10.1038/sj.onc.1209158 PMID:16247456 [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E.. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–41. doi: 10.1101/gad.12.18.2831 PMID:9744860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen J, Purcell AW, Rossjohn J.. Post-translationally modified T cell epitopes: immune recognition and immunotherapy. J Mol Med. 2009;87:1045–51. doi: 10.1007/s00109-009-0526-4 PMID:19763524 [DOI] [PubMed] [Google Scholar]
- 26.Engelhard VH, Altrich-Vanlith M, Ostankovitch M, Zarling AL.. Post-translational modifications of naturally processed MHC-binding epitopes. Curr Opin Immunol. 2006;18:92–7. doi: 10.1016/j.coi.2005.11.015 PMID:16343885 [DOI] [PubMed] [Google Scholar]
- 27.Zarling AL, Ficarro SB, White FM, Shabanowitz J, Hunt DF, Engelhard VH.. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J Exp Med. 2000;192:1755–62. doi: 10.1084/jem.192.12.1755 PMID:11120772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai C, Gu W.. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–36. doi: 10.1016/j.molmed.2010.09.002 PMID:20932800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA.. Targeting p53 as a general tumor antigen. PNAS. 1995;92:11993–7. doi: 10.1073/pnas.92.26.11993 PMID:8618830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P.. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–4. doi: 10.1182/blood-2006-04-015719 PMID:17218388 [DOI] [PubMed] [Google Scholar]
- 31.Huang SW, Chang SH, SW Mu, Jiang HY, Wang ST, Kao JK, Huang JL, Wu CY, Chen YJ, Shieh JJ.. Imiquimod activates p53-dependent apoptosis in a human basal cell carcinoma cell line. Dermatol Sci J. 2016;81:182–91. doi: 10.1016/j.jdermsci.2015.12.011 PMID:26775629 [DOI] [PubMed] [Google Scholar]
- 32.Shatz M, Menendez D, Resnick MA.. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res. 2012;72:3948–57. doi: 10.1158/0008-5472.CAN-11-4134 PMID:22673234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taura M, Eguma A, Suico MA, Shuto T, Koga T, Komatsu K, Komune T, Sato T, Saya H, Li JD, et al. p53 regulates Toll-like receptor 3 expression and function in human epithelial cell lines. Mol Cell Biol 2008;28:6557–67. doi: 10.1128/MCB.01202-08 PMID:18779317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, Celis E, Kobayashi H.. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109:2155–66. doi: 10.1038/bjc.2013.577 PMID:24045666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueger KE, Srivastava S.. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics. 2006;5:1799–810. doi: 10.1074/mcp.R600009-MCP200 PMID:16844681 [DOI] [PubMed] [Google Scholar]
- 36.Haddad R, Colevas AD, Tishler R, Busse P, Goguen L, Sullivan C, Norris CM, Lake-Willcutt B, Case MA, Costello R, et al. Docetaxel, cisplatin, and 5-fluorouracil-based induction chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck: the Dana Farber Cancer Institute experience. Cancer. 2003;97:412–8. doi: 10.1002/cncr.11063 PMID:12518365 [DOI] [PubMed] [Google Scholar]
- 37.Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, Spielbauer K, Van Waes C, Schmitt NC.. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Carcinoma Squamous Cell. Res Cancer Immunol. 2017;5(12):1141–51. doi: 10.1158/2326-6066.CIR-17-0235 PMID:29097421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW.. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529 PMID:17251933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo G, Yu M, Xiao W, Celis E, Cui Y.. Local Activation of p53 in the Tumor Microenvironment Suppression Overcomes Immune and Immunity Enhances Antitumor. Res Cancer. 2017;77:2292–305. doi: 10.1158/0008-5472.CAN-16-2832 PMID:28280037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UniProt: the universal protein knowledgebase. Nucleic Acids Res 2017;45:D158–d69. doi: 10.1093/nar/gkw1099 PMID:27899622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, Olivier M.. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum Mutat. 2016;37:865–76. doi: 10.1002/humu.23035 PMID:27328919 [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi H, Wood M, Song Y, Appella E, Celis E.. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60:5228–36.PMID:11016652 [PubMed] [Google Scholar]
- 43.Kumai T, Ohkuri T, Nagato T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Celis E, Harabuchi Y, Kobayashi H, et al. Targeting HER-3 to elicit antitumor helper T cells against head and neck squamous cell carcinoma. Sci Rep. 2015;5:16280. doi: 10.1038/srep16280 PMID:26538233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


