Abstract
Background
Above-knee amputation (AKA) is a morbid procedure and is performed for a number of conditions. Although AKA is usually performed for dysvascular disease, trauma, and malignancy, AKA is also considered in patients who have failed multiple salvage attempts at treating periprosthetic joint infection (PJI) of TKA. Although aggressive measures are being taken to treat PJI, the huge volume of TKAs might result in a large number of AKAs being performed for PJI in the United States. However, the national trends in the incidence of AKAs from different etiologies and the relative contribution of different etiologies to AKA are yet to be studied.
Questions/purposes
(1) What are the temporal trends in the incidence of AKAs (from all causes) in the US population from 1998 to 2013? (2) What are the temporal trends in the incidence of AKAs by etiology (dysvascular disease, trauma, malignancy, and PJI)? (3) What are the temporal trends in the relative contribution of different etiologies to AKA?
Methods
Using the Nationwide Inpatient Sample (NIS) from 1998 to 2013, AKAs were identified using International Classification of Diseases, 9th Revision (ICD-9) procedure code 84.17. The NIS database is the largest all-payer database in the United States containing information on approximately 20% of all the hospital admissions in the country. As a result of its sampling design, it allows for estimation of procedural volumes at the national level. All AKAs were grouped into one of the following five etiologies in a sequential manner using ICD-9 diagnosis codes: malignancy, PJI, trauma, dysvascular disease (peripheral vascular disease, diabetic, or a combination), and others. All of the numbers were converted to national estimates using sampling weights provided by the NIS, and the national incidence of AKAs resulting from various etiologies was calculated using the US population as the denominator. Poisson and linear regression analyses were used to analyze the annual trends.
Results
From 1998 to 2013, the incidence of AKAs decreased by 47% from 174 to 92 AKAs per 1 million adults (incidence rate ratio [IRR]; change in the number of AKAs per 1 million adults per year; 0.96; 95% confidence interval [CI], 0.96-0.96; p < 0.001). The incidence of AKAs resulting from PJI increased by 263% (IRR, 1.07; 95% CI, 1.06-1.07; p < 0.001). An increase was also observed for AKAs from malignancy (IRR, 1.01; 95% CI, 1.00-1.02; p = 0.007), although to a smaller extent. AKAs from dysvascular causes (IRR, 0.96; 95% CI, 0.95-0.96; p < 0.001) and other etiologies (IRR, 0.97; 95% CI, 0.96-0.97; p < 0.001) decreased. There was no change in the incidence of AKAs related to trauma (IRR, 1.00; 95% CI, 0.99-1.00; p = 0.088). The proportion of AKAs resulting from PJI increased by 589% from 1998 to 2013 (coefficient = 0.18; 95% CI, 0.15-0.22; p < 0.001). The proportion of AKAs resulting from dysvascular causes decreased (coefficient = 0.18; 95% CI, 0.15-0.22; p < 0.001), whereas that resulting from malignancy (coefficient = 0.04; 95% CI, 0.03-0.05; p < 0.001) and trauma (coefficient = 0.13; 95% CI, 0.09-0.18; p < 0.001) increased.
Conclusions
The incidence of AKAs has decreased in the United States. AKAs related to dysvascular disease and other etiologies such as trauma and malignancy have either substantially decreased or remained fairly constant, whereas that resulting from PJI more than tripled. Given the increased resource utilization associated with limb loss, the results of this study suggest that national efforts to reduce disability should prioritize PJI. Further studies are required to evaluate the risk factors for AKA from PJI and to formulate better strategies to manage PJI.
Level of Evidence
Level III, therapeutic study.
Introduction
Above-knee amputation (AKA) is a morbid procedure associated with substantial impairment of patients’ quality of life [4, 6, 9, 14]. Peripheral vascular disease with or without diabetes, trauma, and malignancy have been historically considered the leading causes of AKA [5, 7, 24, 38, 44]. Periprosthetic joint infection (PJI) is another important cause of AKA. Despite the advancements in TKA and measures to prevent infection, PJI continues to be a challenging complication of TKA [12, 22, 28]. Although the incidence of PJI after primary TKA is as low as 1%, the success of treatment of PJI is only approximately 60% to 80% [3, 28, 34, 37, 40]. The success of treatment of PJI decreases further with each subsequent revision, and AKA is sometimes considered in some patients who have failed multiple salvage attempts at treating PJI [13, 41].
In a study using the Medicare database, Son et al. [42] reported that surgeons are more aggressive in managing PJI because they found a decrease in the risk of AKA after an infected TKA. However, their study did not evaluate the incidence of AKAs from PJI in the US population. It is estimated that > 650,000 TKAs are performed each year in the United States with > 5 million people living with one [1, 32]. With such high volumes of TKAs, the number of AKAs performed for PJI is expected to be large even when the incidence of AKA after TKA is very low. Given the economic, health, and social implications of AKAs, various measures have been successfully implemented to reduce the number of AKAs, especially those from dysvascular disease [18, 20, 39]. Because future measures to reduce AKAs depend on the etiologies of AKAs, it is important to understand the reasons for AKA in the nation. However, there is limited literature about the national trends in etiologies of AKAs.
Study Questions
(1) What are the temporal trends in the incidence of AKAs (from all causes) in the United States from 1998 to 2013? (2) What are the temporal trends in the incidence of AKAs by etiology (dysvascular disease, trauma, malignancy, and PJI)? (3) What are the temporal trends in the relative contribution of different etiologies to AKA?
Materials and Methods
The Nationwide Inpatient Sample (NIS) from 1998 to 2013 was utilized for this study [19]. Our institutional review board deemed this study exempt from approval because it used nonidentifiable information obtained from a public source. Diagnosis and procedure information was captured using International Classification of Diseases, 9th Revision, Clinical Modification codes. The NIS is a stratified probability sample designed to approximate 20% of all community, nonfederal, short-term hospitals in the United States [19]. It is the largest all-payer database and contains information about inpatient hospital admissions such as patient demographics, International Classification of Disease, 9th Revision (ICD-9) procedure and diagnosis codes, insurance information, hospital data, length of stay, discharge dispositions, and total charges. As a result of its sampling design, it allows for estimation of procedural volumes at the national level, which is unique to this database.
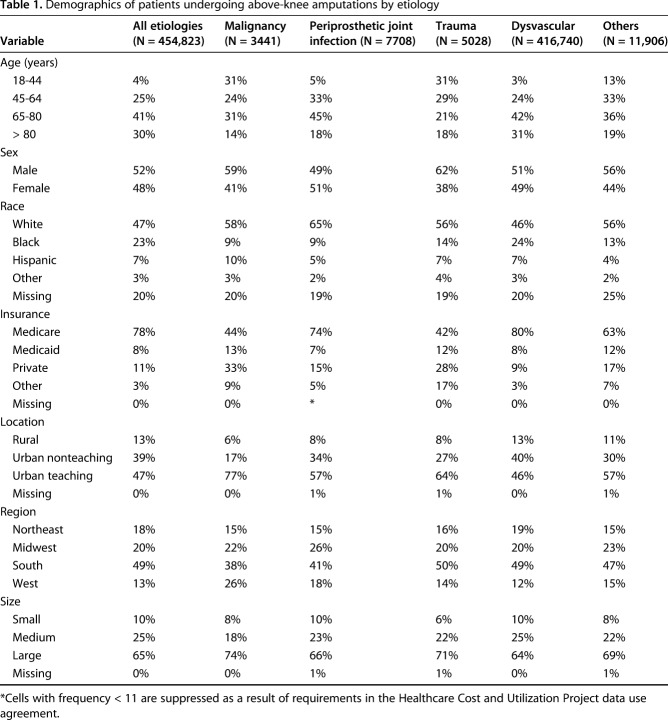
Selection of the study population followed an algorithm based on previously published studies (Fig. 1) [7, 13, 47]. At first, all adult patients (18 years and older) discharged with a primary or secondary procedure code for AKA were identified using ICD-9 procedure code 84.17. Then, all AKAs were grouped into one of the following five etiologies in a sequential manner using ICD-9 diagnosis codes: malignancy, PJI, trauma, dysvascular disease (peripheral vascular disease, diabetic, or a combination), and other. Such a sequential manner was utilized because patients can have multiple diagnosis codes. For example, a patient undergoing AKA for PJI can have diabetes or peripheral vascular disease as a comorbid condition. The order of the etiologies was determined based on the approximate likelihood of one being the primary reason for AKA if multiple diagnoses are simultaneously present. Therefore, malignancy of the lower limb, which is unlikely to be a secondary diagnosis, was chosen first and so on. AKAs resulting from PJI were identified using diagnosis code 996.66. Because specific ICD-9 procedure/diagnosis codes for TKA-related AKA or PJI of the knee were not available, the AKAs with a diagnosis code for PJI were assumed to be from an infection-related complication of TKA. Demographic and hospital-related information was recorded for all AKAs (Table 1).
Fig. 1.
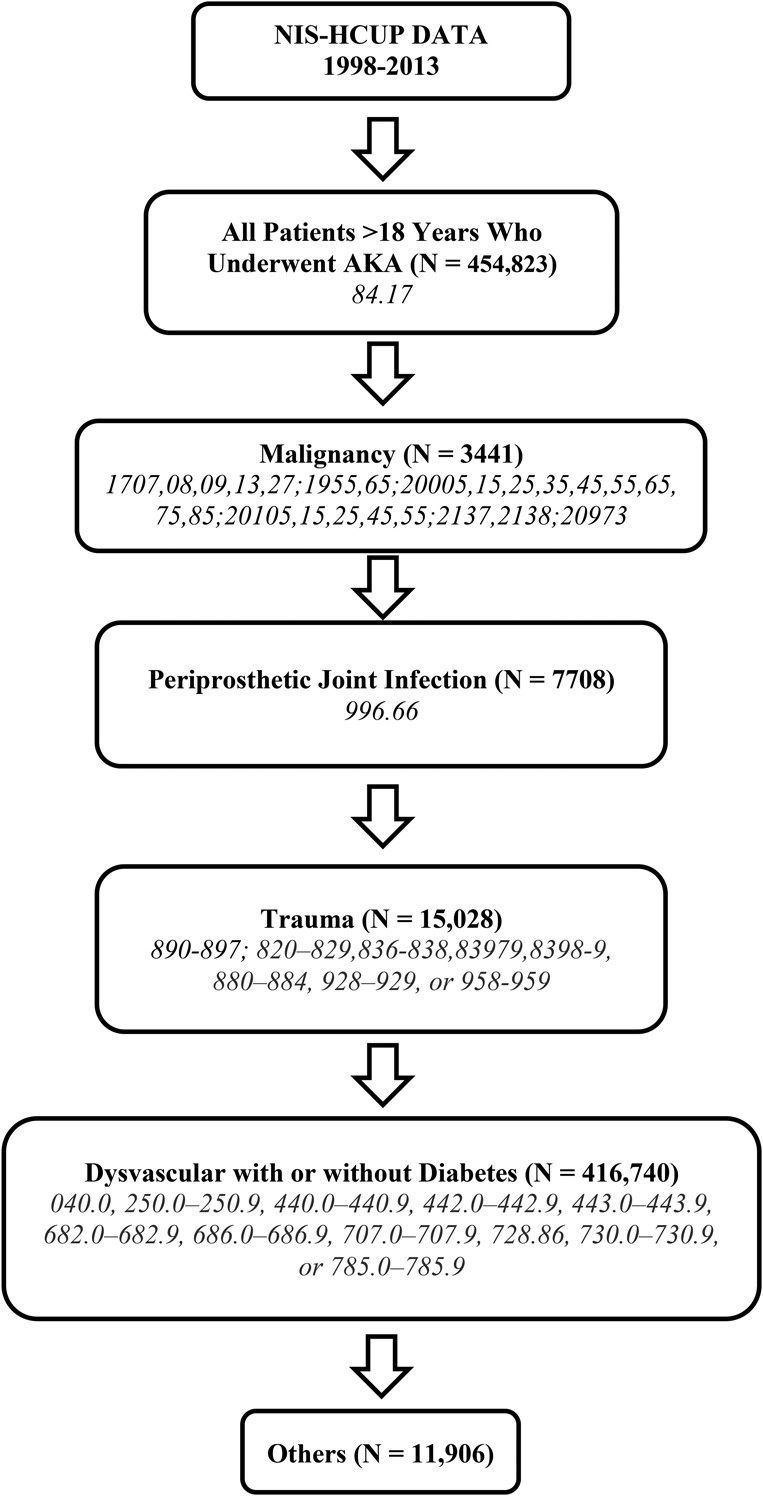
The figure shows the algorithm used to identify AKAs from different etiologies along with the ICD-9 codes used. HCUP = Healthcare Cost and Utilization Project.
Table 1.
Demographics of patients undergoing above-knee amputations by etiology
The incidences of AKAs were obtained by dividing the number of AKAs by the annual US adult population (18 years and older) obtained from the US Census Bureau [46]. The proportion of AKAs resulting from each etiology was also evaluated for each year by dividing the number of AKAs resulting from each etiology by the total number of AKAs.
Statistical Analysis
Discharge weights are provided in the NIS, which allow estimation of national trends. The number of AKAs in the entire nation is obtained by totalling the weights provided with each discharge and accounting for the complex survey design of NIS. Poisson regression analysis was used to analyze whether there was an annual increase in the incidence of the AKAs as a result of the count nature of the dependent variable. The population was used as an offset term in the regression model. The changes in the incidence of AKAs are represented using incidence rate ratios (IRRs) with IRR >1 denoting an increase in the procedural volume. The IRR denotes the change in the number of AKAs per 1 million adults per year (incidence of AKA in 1 year divided by the incidence in the preceding year). Linear regression analysis was used to study the annual changes in the proportion of AKAs resulting from different etiologies including PJI. Because the number of AKAs resulting from different etiologies differed in magnitude, normalization was performed when plotting the annual changes in the number of AKAs for better visualization. Statistical analyses were performed with SAS software version 9.3 (Cary, NC, USA). Ninety-five percent confidence intervals (CIs) were calculated. All of the p values were two-tailed, and a value of < 0.05 was considered statistically significant.
Results
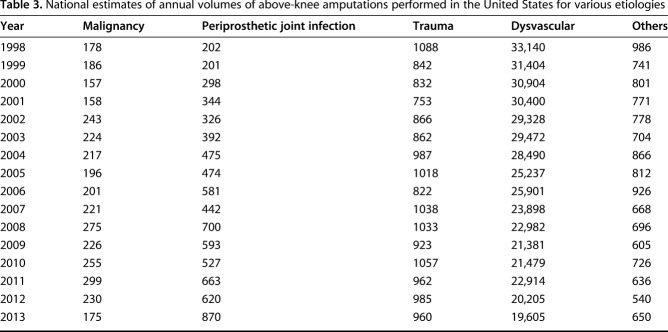
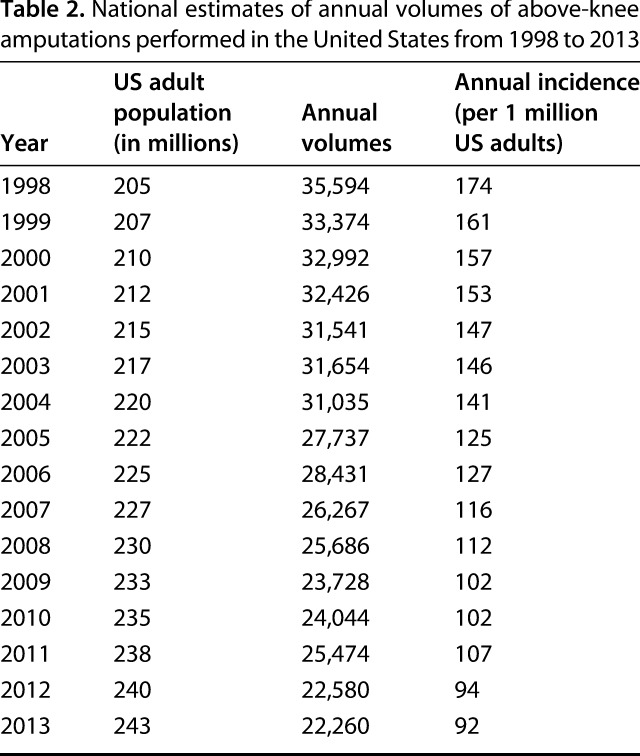
From 1998 to 2013, the incidence of AKAs decreased by 47% from 174 to 92 AKAs per 1 million adults (IRR [ie, change in the number of AKAs per 1 million adults per year], 0.96; 95% CI, 0.96-0.96; p < 0.001). There were 454,823 AKAs performed in the United States between 1998 and 2013. The annual number of AKAs decreased from 35,594 AKAs in 1998 to 22,260 AKAs in 2013 (Table 2).
Table 2.
National estimates of annual volumes of above-knee amputations performed in the United States from 1998 to 2013
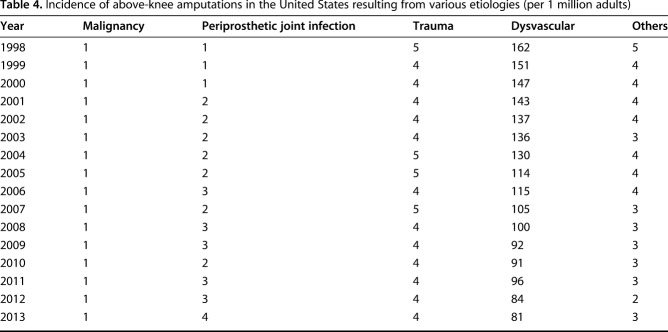
From 1998 to 2013, the annual incidence of AKAs resulting from PJI (IRR, 1.07; 95% CI, 1.06-1.07; p < 0.001; Tables 3, 4) and malignancy (IRR, 1.01; 95% CI, 1.00-1.02; p = 0.007) increased; there was no change in the incidence of AKAs related to trauma (IRR, 1.00; 95% CI, 0.99-1.00; p = 0.088), whereas those from dysvascular causes (IRR, 0.96; 95% CI, 0.95-0.96; p < 0.001) and other causes (IRR, 0.97; 95% CI, 0.96-0.97; p < 0.001) decreased (Fig. 2). Compared with 1998, the incidence of AKAs from PJI (in the population) was higher in 2013 (by 263%), whereas that resulting from dysvascular diseases (by 50%), malignancy (by 17%), trauma (by 26%), and other etiologies (by 44%) was lower in 2013 (Fig. 3).
Table 3.
National estimates of annual volumes of above-knee amputations performed in the United States for various etiologies
Table 4.
Incidence of above-knee amputations in the United States resulting from various etiologies (per 1 million adults)
Fig. 2.
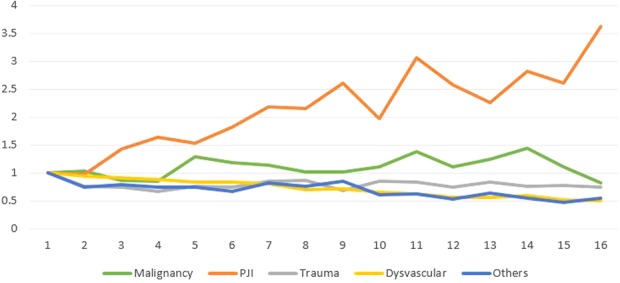
The figure shows the annual trends in the incidence of AKAs resulting from various etiologies. The volumes have been normalized to 1998 values for comparison across etiologies.
Fig. 3.
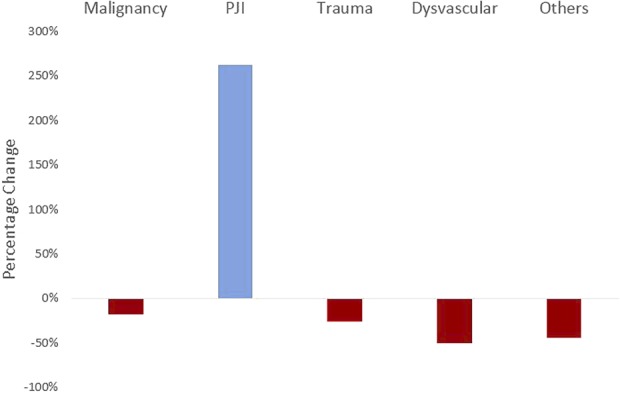
The figure shows the percentage change in the incidence of AKAs from 1998 to 2013 resulting from various etiologies.
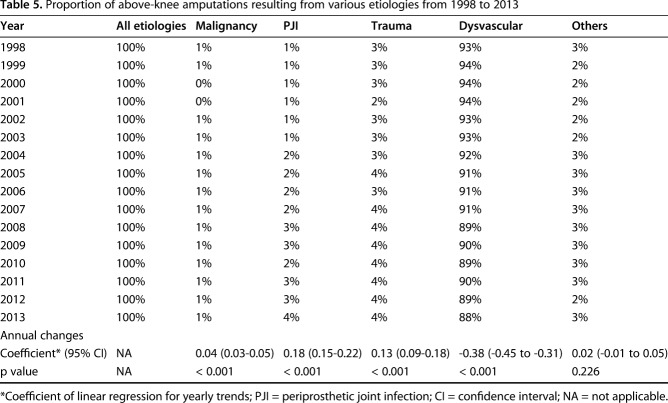
PJI (coefficient = 0.18; 95% CI, 0.15-0.22; p < 0.001), trauma (coefficient = 0.13; 95% CI, 0.09-0.18; p < 0.001), and malignancy (coefficient = 0.04; 95% CI, 0.03-0.05; p < 0.001) increased in relative contributing etiologies to AKA, whereas dysvascular disorders decreased (coefficient = 0.18; 95% CI, 0.15-0.22; p < 0.001) (Table 5). There was no change in the relative contribution from other etiologies (coefficient = 0.02; 95% CI, -0.01 to 0.05; p < 0.226). Of the 35,594 AKAs performed in 1998, 33,140 (93%) were the result of dysvascular causes, 1088 (3%) were the result of trauma, 202 (0.6%) were the result of PJI, and 178 (0.5%) were the result of malignancy. Of the 22,260 AKAs performed in 2013, 19,605 (88%) were the result of dysvascular causes, 960 (4%) were the result of trauma, 870 (4%) were the result of PJI, and 175 (0.8%) were the result of malignancy (Table 3).
Table 5.
Proportion of above-knee amputations resulting from various etiologies from 1998 to 2013
Discussion
AKA is associated with severe impairment in quality of life and high mortality [38]. PJI is a serious complication of TKA, which may lead to AKA when infection cannot be controlled and/or severe bone or soft tissue loss precludes limb salvage treatments [14, 41]. Historically, the majority of AKAs were considered to be the result of vascular disease, diabetes, trauma, and malignancy with PJI not usually considered a major cause of AKA [47]. Previous studies have found that the risk of AKA after an infected TKA is decreasing [42]. However, given the substantial increase in the volumes of TKA, the national trends in the incidence of AKAs from PJI in the population are unclear. The present study used the NIS database and found that the overall number of AKAs in the United States decreased by 47% from 1998 to 2013. The incidence of AKAs from dysvascular disease has substantially decreased, whereas those from trauma and malignancy have remained fairly constant. However, the incidence of AKAs related to PJI almost quadrupled since 1998. As a result, the proportion of AKAs resulting from PJI has increased approximately six times, resulting in the emergence of PJI as a noteworthy cause of AKAs in the United States. Therefore, despite the findings of a decrease in the risk of AKA after a failed TKA by Son et al. [42], the present study found an increase in the overall number of AKAs performed for PJI in the country.
This study has some notable limitations. AKAs resulting from PJI and other etiologies were identified using ICD-9 procedure and diagnosis codes and might be subject to coding errors [15, 31]. For example, a patient could require AKA for vascular disease and a severe diabetic foot infection with seeding of a prosthetic joint other than the knee or of a knee prosthesis on the contralateral side. Although rare, such instances are possible and might have been erroneously classified as AKAs from PJI in this study. Although national joint registries in countries such as the United Kingdom, Canada, and Australia allow the monitoring of outcomes of all TKAs at a national level, a national registry was not available in the United States [2, 21, 36]. Therefore, an administrative database like NIS was used for estimating national trends in line with other investigators [11, 25, 27]. Although coding errors can be present in the NIS, it is the largest available national database and provides reliable estimates of the national-level incidence of various procedures or conditions. Because patients undergoing AKA can have multiple etiologies, a sequential method was used to classify the patients into different etiologies, and so some patients might have been misclassified into a wrong category. However, because the approach was similar for all years, and because the study evaluated the yearly changes in etiologies, this is unlikely to affect the conclusions of the study.
The incidence of AKAs from all causes in the United States is declining. The declining trends of AKAs have been previously reported [17, 23, 33]. In a study of Medicare beneficiaries between 1996 and 2011, Goodney et al. [17] found that the incidence of AKAs declined from 91 to 47 AKAs per 100,000 Medicare patients. The incidence of AKAs (from all causes) reported in our study is higher than that reported in their study, probably because their study included only Medicare patients, whereas our study included all adults in the United States. However, an earlier study, which evaluated the trends in major and minor limb amputations from 1988 to 1996 using the NIS database, showed an increasing incidence of amputations, including AKAs [7]. Although our study did not analyze data before 1998, it is possible that the decline in the incidence of AKAs started in the late 1990s [10]. Similar to other studies, the majority of the AKAs in this study were a result of dysvascular causes [17, 23, 33]. Because a substantial decrease in the incidence of dysvascular amputations was found in our study and previous studies, it is not surprising to see a decrease in the overall incidence of AKAs [17, 23, 33].
AKAs resulting from most etiologies such as vascular disease, trauma, and malignancy have decreased or remained constant during the study period, whereas a considerable increase in AKAs resulting from PJI was observed. A number of factors might be responsible for the declining rates of dysvascular amputations. Increasing awareness about amputations along with an emphasis on early screening and detection of vascular disease in patients at risk for amputation may be partly responsible for the decline in amputation rates [16, 30]. Additionally, improvements in revascularization procedures and aggressiveness of reconstructive surgeries might have also contributed to the declining trends of vascular-related amputations in recent years [8, 16]. Similarly, with the implementation of simple and effective interventions such as diabetes self-management education and targeted foot screening programs, the rate of lower extremity amputation in persons with diabetes declined by 47% from 2000 to 2010 [35, 43, 45]. Unfortunately, the declining trends of other etiologies of amputations were accompanied by rising numbers of AKAs resulting from PJI, probably related to the rising number of TKAs and the stable revision burden [26, 28].
This study found that an increasing proportion of AKAs performed in the United States are the result of PJI. Using the NIS, Ziegler-Graham et al. [47] estimated the prevalence of major lower extremity amputations in the United States was approximately 600,000 in 2005 with vascular disease and diabetes responsible for approximately 80% of the major lower limb amputations followed by trauma and cancer-related amputations. All other etiologies including congenital anomalies and orthopaedic implant failures contributed to < 3% of all the amputations. This is consistent with the results of the current study, which estimated AKAs resulting from PJI were < 3% for year 2005. However, given the projected increase in TKA, PJI can become a major cause of limb loss in the coming years [27]. PJI, which was responsible for only < 1% of the AKAs in 1998, was accountable for 4% of the AKAs in 2013, a more than six times increase during the 16-year period. If these trends continue unaltered, and if this increase is projected to the coming years, more than one-fifth of AKAs in 2030 could be the result of PJI. Because previous studies have projected a substantial increase in the volume of infected TKAs, the number of AKAs related to PJI is also expected to increase [27, 29].
In summary, we found that the incidence of AKAs has declined in the United States. AKAs related to dysvascular disease decreased by half, whereas those resulting from etiologies such as trauma and malignancy have remained fairly constant. However, AKAs performed as a result of PJI more than tripled since 1998. Dysvascular disease is the leading cause of AKA, but PJI appears to be emerging as an important etiology of AKAs in the United States. Given the increased resource utilization associated with limb loss, the results of this study suggest that national efforts to reduce disability should prioritize PJI. Because the demand for TKA is expected to further increase, PJI could emerge as an important reason for limb loss. Given the increased resource utilization associated with limb loss, further studies are required to evaluate the risk factors for AKA from PJI and to formulate better strategies to manage PJI.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Cleveland Clinic, Cleveland, OH, USA.
References
- 1.Agency for Healthcare Research & Quality. HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. 2017. Available at: http://hcupnet.ahrq.gov/. Accessed July 23, 2017.
- 2.Bohm ER, Dunbar MJ, Bourne R. The Canadian Joint Replacement Registry–what have we learned? Acta Orthop. 2010;81:119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buller LT, Sabry FY, Easton RW, Klika AK, Barsoum WK. The preoperative prediction of success following irrigation and débridement with polyethylene exchange for hip and knee prosthetic joint infections. J Arthroplasty. 2012;27:857–864.4. [DOI] [PubMed] [Google Scholar]
- 4.Burger H, Marincek C, Isakov E. Mobility of persons after traumatic lower limb amputation. Disabil Rehabil. 1997;19:272–277. [DOI] [PubMed] [Google Scholar]
- 5.Cutson TM, Bongiorni DR. Rehabilitation of the older lower limb amputee: a brief review. J Am Geriatr Soc. 1996;44:1388–1393. [DOI] [PubMed] [Google Scholar]
- 6.Davies B, Datta D. Mobility outcome following unilateral lower limb amputation. Prosthet Orthot Int. 2003;27:186–190. [DOI] [PubMed] [Google Scholar]
- 7.Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95:875–883. [DOI] [PubMed] [Google Scholar]
- 8.Egorova NN, Guillerme S, Gelijns A, Morrissey N, Dayal R, McKinsey JF, Nowygrod R. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51:878–885, 885.e1. [DOI] [PubMed] [Google Scholar]
- 9.Fedorka CJ, Chen AF, McGarry WM, Parvizi J, Klatt BA. Functional ability after above-the-knee amputation for infected total knee arthroplasty. Clin Orthop Relat Res. 2011;469:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinglass J, Brown JL, LoSasso A, Sohn MW, Manheim LM, Shah SJ, Pearce WH. Rates of lower-extremity amputation and arterial reconstruction in the United States, 1979 to 1996. Am J Public Health. 1999;89:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankowski JJ, Watkins-Castillo S. Primary Total Knee and Hip Arthroplasty Projections for the US Population to the Year 2030. Rosemont, IL, USA: American Academy of Orthopedic Surgeons; 2002. [Google Scholar]
- 12.George J, Klika AK, Higuera CA. Use of chlorhexidine preparations in total joint arthroplasty. J Bone Joint Infect. 2017;2:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George J, Navale SM, Schiltz NK, Siccha M, Klika AK, Higuera CA. Racial disparities in above-knee amputations after TKA: a national database study. Clin Orthop Relat Res. 2017;475:1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George J, Newman JM, Caravella JW, Klika AK, Barsoum WK, Higuera CA. Predicting functional outcomes after above knee amputation for infected total knee arthroplasty. J Arthroplasty. 2017;32:532–536. [DOI] [PubMed] [Google Scholar]
- 15.George J, Newman JM, Ramanathan D, Klika AK, Higuera CA, Barsoum WK. Administrative databases can yield false conclusions–an example of obesity in total joint arthroplasty. J Arthroplasty. 2017;32(Suppl):S86–S90. [DOI] [PubMed] [Google Scholar]
- 16.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. [DOI] [PubMed] [Google Scholar]
- 17.Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among Medicare patients. JAMA Surg. 2015;150:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouzien A, de Vignemont F, Touillet A, Martinet N, De Graaf J, Jarrassé N, Roby-Brami A. Reachability and the sense of embodiment in amputees using prostheses. Sci Rep. 2017;7:4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HCUP-Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD, USA: Agency for Healthcare Research and Quality; 2015. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 1, 2015. [Google Scholar]
- 20.Horgan O, MacLachlan M. Psychosocial adjustment to lower-limb amputation: a review. Disabil Rehabil. 26:837–850. [DOI] [PubMed] [Google Scholar]
- 21.Huang T, Wang W, George D, Mao X, Graves S. What can we learn from AOANJRR 2014 annual report? Ann Transl Med. 2015;3:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jämsen E, Furnes O, Engesaeter LB, Konttinen YT, Odgaard A, Stefánsdóttir A, Lidgren L. Prevention of deep infection in joint replacement surgery. Acta Orthop. 2010;81:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from US Medicare 2000-2008. J Am Coll Cardiol. 2012;60:2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kald A, Carlsson R, Nilsson E. Major amputation in a defined population: incidence, mortality and results of treatment. Br J Surg. 1989;76:308–310. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–2254. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. [DOI] [PubMed] [Google Scholar]
- 29.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61–65.e1. [DOI] [PubMed] [Google Scholar]
- 30.Larsson J, Apelqvist J. Towards less amputations in diabetic patients. Incidence, causes, cost, treatment, and prevention–a review. Acta Orthop Scand. 1995;66:181–192. [DOI] [PubMed] [Google Scholar]
- 31.Losina E, Barrett J, Baron JA, Katz JN. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. J Clin Epidemiol. 2003;56:515–519. [DOI] [PubMed] [Google Scholar]
- 32.Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res. Dev. 2000;37:23–30. [PubMed] [Google Scholar]
- 34.Mortazavi SMJ, Molligan J, Austin MS, Purtill JJ, Hozack WJ, Parvizi J. Failure following revision total knee arthroplasty: infection is the major cause. Int Orthop. 2011;35:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. Healthy people 2010: final review. Government Printing Office; 2011. Available at: http://www.cdc.gov/nchs/healthy_people/hp2010/hp2010_final_review.htm. Accessed January 1, 2016.
- 36.National Joint Registry for England and Wales. 12th annual report. 2015. Available at: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th annual report/NJR Online Annual Report 2015.pdf. Accessed May 5, 2017.
- 37.Peel TN, Cheng AC, Buising KL, Choong PFM. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56:2386–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pell JP, Donnan PT, Fowkes FG, Ruckley C V. Quality of life following lower limb amputation for peripheral arterial disease. Eur J Vasc Surg. 1993;7:448–451. [DOI] [PubMed] [Google Scholar]
- 39.Ragnarson Tennvall G, Apelqvist J. Health-economic consequences of diabetic foot lesions. Clin Infect Dis. 2004;39:S132–S139. [DOI] [PubMed] [Google Scholar]
- 40.Sabry FY, Buller L, Ahmed S, Klika AK, Barsoum WK. Preoperative prediction of failure following two-stage revision for knee prosthetic joint infections. J Arthroplasty. 2014;29:115–121. [DOI] [PubMed] [Google Scholar]
- 41.Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85:1000–1004. [DOI] [PubMed] [Google Scholar]
- 42.Son M-S, Lau E, Parvizi J, Mont MA, Bozic KJ, Kurtz S. What are the frequency, associated factors, and mortality of amputation and arthrodesis after a failed infected TKA? Clin Orthop Relat Res. 2017;475:2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strine TW, Okoro CA, Chapman DP, Beckles GLA, Balluz L, Mokdad AH. The impact of formal diabetes education on the preventive health practices and behaviors of persons with type 2 diabetes. Prev Med (Baltimore). 2005;41:79–84. [DOI] [PubMed] [Google Scholar]
- 44.Traugh GH, Corcoran PJ, Reyes RL. Energy expenditure of ambulation in patients with above-knee amputations. Arch Phys Med Rehabil. 1975;56:67–71. [PubMed] [Google Scholar]
- 45.Tseng C-L, Rajan M, Miller DR, Lafrance J-P, Pogach L. Trends in initial lower extremity amputation rates among Veterans Health Administration health care dystem users from 2000 to 2004. Diabetes Care. 2011;34:1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Census Bureau. National population estimates. 2016. Available at: https://www.census.gov/data/tables/2016/demo/popest/nation-detail.html. Accessed May 5, 2017.
- 47.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. [DOI] [PubMed] [Google Scholar]