Abstract
Background
There is no consensus on the appropriate marker to use when deciding to perform reimplantation after two-stage exchange arthroplasty for periprosthetic joint infection (PJI).
Questions/purposes
What tests provide acceptable diagnostic value to guide appropriate timing of reimplantation in two-stage exchange arthroplasty for PJI?
Methods
A search of online databases (MEDLINE, EMBASE, OVID, and Cochrane database) was performed containing articles that provided sensitivity and specificity values for accuracy for predicting reimplantation of the hip and/or knee. Twelve articles were included for final analysis, which included data from 1047 patients. Data that described the diagnostic accuracy of markers for reimplantation were evaluated and categorized into four main entities according to diagnostic method (serologic, synovial, tissue, and diagnostic imaging). Twelve parameters were examined, including serum erythrocyte sedimentation (ESR) rate, serum C-reactive protein (CRP), serum white blood cell (WBC) count, synovial fluid Gram stain, synovial fluid culture, synovial fluid sonication culture, synovial fluid WBC, synovial fluid polymorphonucleocyte percentage (PMN%), tissue Gram stain, tissue culture, positron emission tomography scan, and leukocyte scan. Each of the included articles was independently analyzed for risk of bias and applicability by using QUADAS-2. Statistical heterogeneity was calculated by using the Cochran Q test, and an α of 0.10 was considered significant for heterogeneity.
Results
Tissue culture (sensitivity 0.82 [0.72-0.90], specificity 0.91 [0.89-0.95], diagnostic odds ratio (DOR) 46.87 [95% confidence interval {CI}, 22.03-99.69], synovial fluid PMN% (sensitivity 0.77 [0.46-0.95], specificity 0.74 [0.67-0.81], DOR 11.27 [95% CI, 2.89-43.61]), and synovial fluid culture (sensitivity 0.64 [0.52-0.74], specificity 0.96 [0.93-0.98], DOR 27.07 [95% CI, 2.55-288.00]) showed relatively high diagnostic performance. Other parameters had poorer diagnostic accuracy: ESR (sensitivity 0.56 [0.40-0.72], specificity 0.60 [0.53-0.66], DOR 2.41 [95% CI, 0.60-9.72), CRP (sensitivity 0.53 [0.39-0.67], specificity 0.72 [0.66-0.78], DOR 2.25 [95% CI, 0.09-4.63), and synovial fluid WBC count (sensitivity 0.37 [0.19-0.58], specificity 0.49 [0.41-0.57], DOR 0.94 [95% CI, 0.06-14.74). However, interpretation is limited, because only two to three studies were available for each pooled analysis. Both risks of bias and applicability concerns were low in the four domains assessed in QUADAS-2.
Conclusions
This meta-analysis suggests that no single marker was superior to all the others, and none (when used alone) is likely sufficient to confirm control of infection after the first stage of a two-stage protocol for PJI. Therefore, the current approach using multiple tools rather than a single marker is essential. Additionally, further studies must be conducted so that pooled analysis can be performed using multiple studies to determine ideal markers for reimplantation.
Level of Evidence
Level III, diagnostic study.
Introduction
Despite tremendous advances in the prevention, diagnosis, and treatment of periprosthetic joint infection (PJI), it remains a leading cause of morbidity and revision surgery after total joint arthroplasty (TJA) [12, 24]. Several approaches are used to treat PJI, including irrigation and débridement, one-stage exchange arthroplasty, and two-stage arthroplasty with subsequent reimplantation [21]. Among these methods, the accepted treatment in many countries for patients with a chronically infected TJA is two-stage exchange arthroplasty [24]. Insall et al. originally proposed the two-stage revision protocol for infected TKA [16, 17]. This typically consists of resection of index implants, thorough synovectomy and débridement of the infected tissue, implantation of either a static or articulating antibiotic-impregnated cement spacer for local delivery of high concentrations of antibiotic, and administration of systemic antibiotics followed by reimplantation of a total joint prosthesis. Although this approach is widely used [1-4, 8-10], two-stage exchange arthroplasty still is associated with a risk of reinfection as high as 33% [13, 18].
Making matters more complex, recurrence may occur even without clinical symptoms and despite the absence of growth on synovial or intraoperative cultures [5, 25-27], suggesting that even the most reliable tests are inadequate to identify persistent, subclinical infection after the first stage of a two-stage revision procedure [26]. Given the ambiguities and apparent disagreements in the evidence [14], a meta-analysis might be helpful to guide the decision of which marker or markers might be most informative in determining the timing of reimplantation.
Therefore, we asked: What tests provide acceptable diagnostic value to guide appropriate timing of reimplantation in two-stage exchange arthroplasty for PJI?
Materials and Methods
Search Strategy
This study conformed to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines [22]. In phase 1 of the PRISMA search process, selected databases, including MEDLINE, EMBASE, OVID, and the Cochrane Library, were searched on May 5, 2016. By using a Boolean strategy, we used all of the following field search terms: (((((hip joint) OR hip)) OR ((knee) OR knee joint))) AND (((((infection) OR prosthetic joint infection) OR periprosthetic infection)) AND (((((two stage reconstruction) OR two stage revision) OR second stage revision) OR two stage reimplantation) OR exchange arthroplasty)). A hand search was performed on the reference lists from the selected articles for any additional references that might have been missed in the electronic search. In phase 2, abstracts and titles were screened to assess their relevance in relation to the study question. Titles and abstracts were screened by two independent reviewers (YSL, HV) based on the predefined inclusion criteria. The inclusion criteria of studies in this systematic review and meta-analysis were: (1) they should describe the diagnostic marker performed for two-stage reimplantation; and (2) they should include sensitivity and specificity values of the markers for accuracy. If adequacy of inclusion could not be determined based on the title and abstract, the full article was reviewed. Only articles with full-text studies were included for review. Most studies from the 584 initial studies were excluded after screening of titles and abstracts because they only reported clinical and radiologic results after two-stage implantation without reporting on diagnostic markers with sensitivity and specificity values. In phase 3, the full text of selected studies was reviewed to assess for the inclusion criteria and ability of the study to answer the predetermined question; in this phase, we also assessed study quality and risk of bias. Twelve articles were excluded because they did not contain a description of diagnostic accuracy; and another five articles evaluating for conditions other than reimplantation were also excluded. Additionally, three articles were excluded because they did not report sensitivity and specificity values. Finally, in phase 4, 12 studies were included in this systematic review and meta-analysis. Because this was a systematic review and meta-analysis of previous studies, institutional review board approval was waived.
Eligibility Criteria
True diagnostic accuracy studies that provided sensitivity and specificity values for accuracy were included for predicting reimplantation of the hip and/or knee. The minimum followup period was not a selection criterion for inclusion into this study, because this study did not evaluate radiologic and clinical outcomes, but evaluated the sensitivity and sensitivity of diagnostic parameters to confirm control of infection after the first stage of a two-stage protocol for PJI. We only included studies in English. Studies that evaluated conditions other than reimplantation such as those that simply diagnosed PJI were excluded. The reference standard used was the PJI criteria established by the Musculoskeletal Infection Society (MSIS) [27]. Studies that did not include sensitivity and specificity values were also excluded (Fig. 1).
Fig. 1.
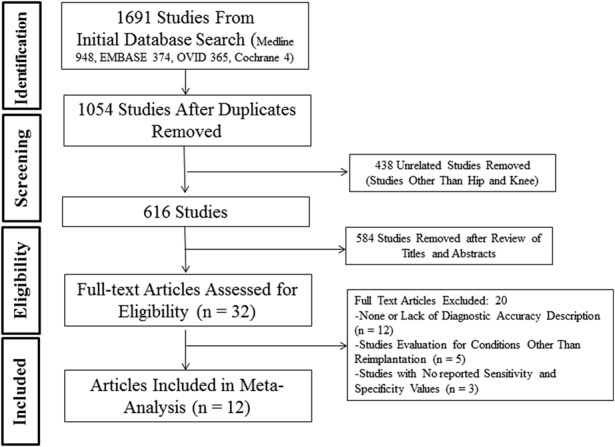
The PRISMA flow diagram is presented.
Search
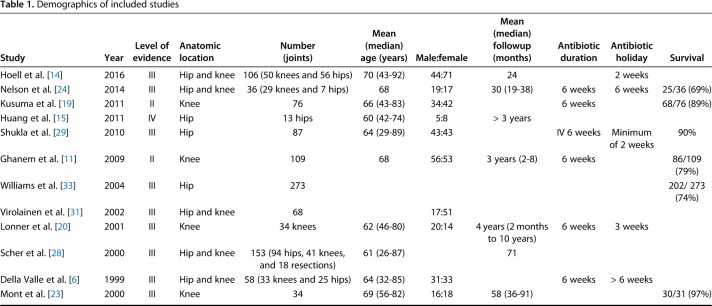
Twelve articles reported on the parameters [6, 11, 14, 15, 19, 20, 23, 24, 28, 29, 31, 33], which included data from 1047 patients. There were 10 diagnostic studies [6, 14, 19, 20, 23, 24, 28, 29, 31, 33] and two therapeutic studies [11, 15]. Three studies were level II [11, 19, 23], nine were level III [6, 14, 20, 23, 24, 28, 29, 31, 33], and one was level IV [15]. This level IV study was excluded from the study meta-analysis of our study and included only in the systematic review as a result of a low quality of evidence. Five studies provided data on both the hip and knee [6, 14, 24, 28, 31]; three studies were on hips [15, 29, 33] and four studies were on knees [11, 19, 20, 23] (Table 1). The diagnostic accuracy of all the included study is listed (Appendix, Supplemental Digital Content 1).
Table 1.
Demographics of included studies
Data Extraction
Each of the selected studies was evaluated by two independent authors (YSL, HV) for methodological quality. Data were extracted using the following standardized protocol: study design, level of evidence, involved part, patients/cases enrolled, age, sex ratio, followup, antibiotics used, antibiotic holiday, reimplantation guideline (serology, joint fluid aspiration, tissue, positron emission tomography [PET], and leukocyte scan), infection-free survival, and endpoint analysis, which integrated the results at the last followup in all included studies. Twelve parameters were examined, including serum erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP), serum white blood cell (WBC) count, synovial fluid Gram stain, synovial fluid culture, synovial fluid sonication culture, synovial fluid WBC, synovial fluid percentage polymorphonuclear cell (PMN%), tissue Gram stain, tissue culture, PET scan, and leukocyte scan. The extracted data were then crosschecked for accuracy, and any disagreement was settled by a third author (NF). Data were also categorized into four main entities including serum markers, synovial fluid markers, tissue studies, and imaging studies. Data regarding serum markers were extracted in five studies [11, 14, 19, 29, 31], synovial fluid markers in six studies [14, 19, 24, 29, 31, 33], tissue studies in four studies [6, 23, 31, 33], and imaging studies in three studies [15, 28, 31].
Quality Assessment
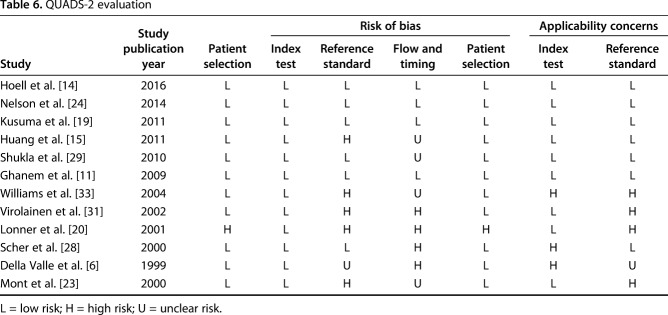
Each of the included articles was independently analyzed for risk of bias and applicability by using QUADAS-2 [7, 32], which consists of four key domains that cover patient selection, index test, reference standard, and enrollment flow of patients in the study as well as the timing of the index tests and reference standard (“flow and timing”). Bias was considered when study shortcomings influenced the results. Index tests included the 12 mentioned parameters. Both risks of bias and applicability concerns were low in the four domains assessed in QUADAS-2 (Table 2).
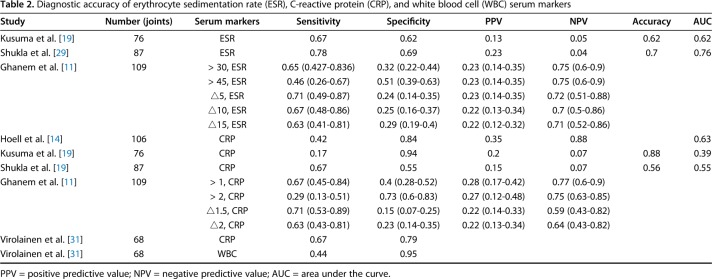
Table 2.
Diagnostic accuracy of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell (WBC) serum markers
Analysis
Pooled analysis was possible for the following parameters: serum ESR, serum CRP, synovial fluid WBC count, synovial fluid PMN%, synovial fluid culture, and tissue culture. Alpha defensin could not undergo pooled analysis because there were no eligible trials that could be included in this reimplantation study. For the parameters that underwent pooled analysis, coupled forest plots for sensitivity and specificity were presented for each test, and a summary receiver operating characteristic was drawn to observe overall results.
Statistical Analysis
MetaDiSc (Version 1.4, downloaded form; http://www.hrc.es/investigacion/metadisc_en.htm) for Windows and Review Manager 5.3 statistical software were used for statistical analysis. Statistical heterogeneity was calculated using the Cochran Q test based on inverse variance weights, which also has the I2 index. An α of 0.10 was considered to be significant for heterogeneity, because the number of studies included was small. The random-effects model was used to calculate the effect size rather than the fixed-effects model to manage heterogeneity. The following indices of test accuracy were calculated for each study: sensitivity, specificity, positive likelihood ratio (how a positive result changes the likelihood of a test detecting the condition), negative likelihood ratio (how a negative result changes the likelihood of a test detecting the condition), diagnostic odds ratio (a ratio that measures the effectiveness of a diagnostic test), and summary receiver operating characteristic curve (a graphic plot that illustrates the ability of a test to discriminate the diagnostic ability of a test).
Serum Markers
For the usefulness of serologic markers to successfully detect infection control before reimplantation, three studies provided data on serum ESR [11, 19, 29], five studies on serum CRP [11, 14, 19, 29, 31], and one study on serum WBC count [31] (Table 3).
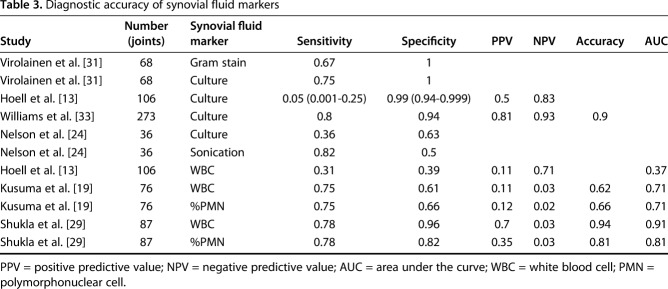
Table 3.
Diagnostic accuracy of synovial fluid markers
Synovial Fluid Markers
For the role of synovial fluid in reimplantation, Gram stain was evaluated in one study [31], synovial fluid culture in four studies [14, 24, 31, 33], synovial fluid WBC count in three studies [14, 19, 29], and synovial fluid PMN% in two studies [19, 29] (Table 4).
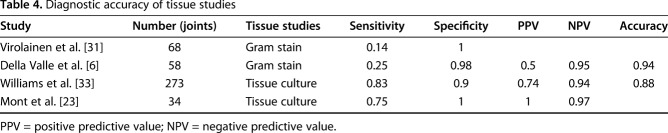
Table 4.
Diagnostic accuracy of tissue studies
Tissue Studies
Gram stain was evaluated in two studies [6, 31] and tissue culture was examined in two studies [23, 33] (Table 5).
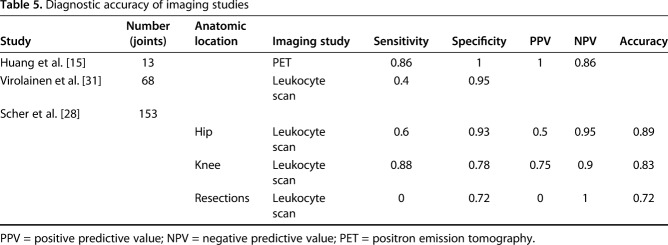
Table 5.
Diagnostic accuracy of imaging studies
Imaging Markers
Three studies assessed the usefulness of nuclear imaging (technetium 99/indium and FDG-PET) [15, 28, 31] (Table 6).
Table 6.
QUADS-2 evaluation
Results
Tissue culture (307 patients), synovial fluid PMN% (163 patients), and synovial fluid culture (483 patients) showed relatively high diagnostic performance in terms of sensitivity and specificity. Tissue culture, synovial PMN%, and synovial fluid culture were not different in terms of sensitivity. However, in terms of specificity, synovial fluid culture and tissue culture were more specific than synovial fluid PMN% (Fig. 2).
Fig. 2.
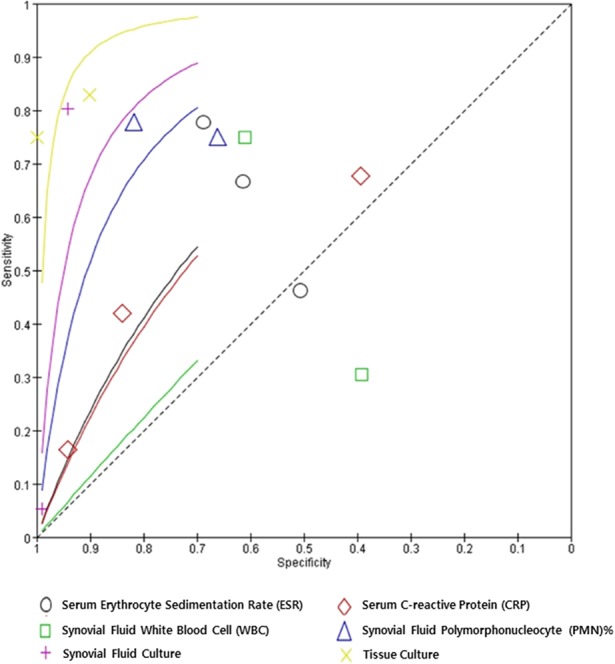
The summary receiver operating characteristic plots for each marker are presented.
Regarding tissue culture, Mont et al. [23] reported a sensitivity of 0.75 and a specificity of 1.00, whereas Williams et al. [33] reported a sensitivity of 0.83 and a specificity of 0.90. Tissue culture (Fig. 3) had a pooled sensitivity of 0.82 (0.72-0.90) with heterogeneity I2 = 0% (p = 0.709) and pooled specificity of 0.91 (0.89-0.95) with heterogeneity I2 = 83% (p = 0.021). The positive likelihood ratio of tissue culture was 10.40 (95% confidence interval [CI], 3.53-30.68), the negative likelihood ratio was 0.20 (95% CI, 0.13-0.33), and the diagnostic odds ratio was 46.87 (95% CI, 22.03-99.69).
Fig. 3.
The forest plot of tissue culture is shown. TP = true-positive; FP = false-positive; FN = false-negative; TN = true-negative.
For synovial fluid PMN%, Kusuma et al. [19] reported a sensitivity of 0.75 and a specificity of 0.66; Shukla et al. [29] reported a sensitivity of 0.78 and a specificity of 0.82. Synovial fluid PMN% (Fig. 4) had a pooled sensitivity of 0.77 (0.46-0.95) with heterogeneity I2 = 0% (p = 0.913). Synovial fluid PMN% had a pooled specificity of 0.74 (0.67-0.81) with I2 = 79% (p = 0.029). The positive likelihood ratio of synovial fluid PMN% was 3.13 (95% CI, 1.64-5.98), negative likelihood ratio was 0.30 (95% CI, 0.11-0.82), and diagnostic odds ratio was 11.23 (95% CI, 2.90-43.61).
Fig. 4.
The forest plot of synovial fluid PMN% is shown. TP = true-positive; FP = false-positive; FN = false-negative; TN = true-negative.
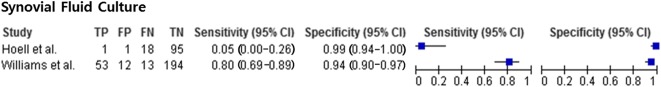
Regarding synovial fluid culture, studies [14, 24, 31, 33] reported a sensitivity of 0.36 to 0.80 and a specificity of 0.63 to 1. For the synovial fluid culture, pooled analysis was performed only using two of the four studies on synovial fluid culture [14, 33] because there was no information on SD in two studies [24, 31]. Synovial fluid culture (Fig. 5) had a pooled sensitivity of 0.64 (0.52-0.74) with heterogeneity I2 = 97% (p < 0.001). Synovial fluid culture had a pooled specificity of 0.96 (0.93-0.98) with heterogeneity I2 = 78% (p = 0.032). The positive likelihood ratio of synovial fluid culture was 13.23 (95% CI, 7.63-22.95), the negative likelihood ratio was 0.45 (95% CI, 0.01-25.90), and the diagnostic odds ratio was 27.07 (95% CI, 2.55-288.00). Other parameters were less useful. For serum ESR, studies [11, 19, 29] reported a sensitivity of 0.46 to 0.78 and a specificity of 0.51 to 0.69. Serum ESR (Fig. 6) had a pooled sensitivity of 0.56 (0.40-0.72) with heterogeneity I2 = 37% (p = 0.206). Serum ESR also had a pooled specificity of 0.60 (0.53-0.66) with heterogeneity I2 = 64.5% (p = 0.060). The positive likelihood ratio for ESR was 1.58 (95% CI, 0.82-3.06), the negative likelihood ratio was 0.67 (95% CI, 0.30-1.50), and the diagnostic odds ratio was 2.41 (95% CI, 0.60-9.72).
Fig. 5.
The forest plot of synovial fluid culture is shown. TP = true-positive; FP = false-positive; FN = false-negative; TN = true-negative.
Fig. 6.
The forest plot of serum ESR is shown. TP = true-positive, FP = false-positive; FN = false-negative; TN = true-negative.
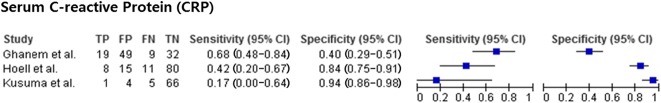
For serum CRP, studies [11, 14, 19, 29, 31] reported a sensitivity of 0.17 to 0.71 and a specificity of 0.15 to 0.94. Serum CRP (Fig. 7) had a pooled sensitivity of 0.53 (0.39-0.67) with heterogeneity I2 = 71% (p = 0.032). Serum CRP had a pooled specificity of 0.72 (0.66-0.78) with heterogeneity I2 = 97.1% (p < 0.001). For serum CRP, pooled analysis was performed using three of the five studies on serum CRP [11, 14, 19] because there was no information about SD in two studies [29, 31]. The positive likelihood ratio was 1.73 (95% CI, 0.82-3.64), the negative likelihood ratio was 0.79 (95% CI, 0.62-1.01), and the diagnostic odds ratio was 2.25 (95% CI, 0.09-4.63).
Fig. 7.
The forest plot of serum CRP is shown. TP = true-positive; FP = false-positive; FN = false-negative; TN = true-negative.
Regarding synovial fluid WBC count, the studies [14, 19, 29] reported the sensitivity as 0.31 to 0.78 and the specificity as 0.39 to 0.96. Synovial fluid WBC count (Fig. 8) had a pooled sensitivity of 0.37 (0.19-0.58) with heterogeneity I2 = 65% (p = 0.093). Synovial fluid WBC count had a pooled specificity of 0.49 (0.41-0.57) with heterogeneity I2 = 87% (p = 0.005). Additionally, it had a positive likelihood ratio of 0.98 (95% CI, 0.21-4.54), negative likelihood ratio of 1.04 (95% CI, 0.23-4.80), and diagnostic odds ratio of 0.94 (95% CI, 0.06-14.74). Intraoperative Gram stains showed very poor sensitivity (0.14 and 0.25) despite its high specificities (1 and 0.98) in two studies [6, 31]. Imaging markers demonstrated variable sensitivities (range, 0-0.88), but generally high specificity (range, 0.72-1; Table 6).
Fig. 8.
The forest plot of synovial fluid WBC is shown. TP = true-positive; FP = false-positive; FN = false-negative; TN = true-negative.
Discussion
The International Consensus on PJI established a complex algorithm to achieve reliable diagnostic accuracy for PJI and has shown that local proinflammatory cytokines have favorable diagnostic properties for PJI [27]. However, assessment of infection control is more difficult after component explantation, because prolonged antibiotic therapy may confound results and the presence of an antibiotic-impregnated cement spacer may act as a scaffold to which biofilms may attach and lead to reinfection [30]. Therefore, we aimed to evaluate parameters that may provide guidance for appropriate timing of reimplantation.
The present study has certain limitations. First, the publication times were widely distributed and some of the data was acquired from old articles that could provide variable results. Surgical techniques, antibiotic availability, infection control methods, and diagnostic equipment precision likely improved over time, which may have reduced the likelihood of reinfection and could favor more recent studies. However, laboratory tests have remained the same throughout the years, allowing the aggregation of multiple studies including older studies. Second, the quality of studies was widely variable, as observed in the QUADAS-2 assessment. This study contained some studies with high risk of bias regarding the reference standard according to the MSIS PJI criteria [27], because only certain parameters were evaluated and we could not evaluate other potentially useful parameters such as alpha defensin and leukocyte esterase test because these studies were not in our inclusion criteria [34]. However, orthopaedic surgeons most commonly use the parameters listed in this study when treating patients with PJI. Third, heterogeneity for each parameter was variable. However, it was inevitable because the number of studies included in each parameter analysis was too small. Instead of the interpretation of heterogeneity, detailed ranges of each index were added to strengthen the systematic review and to allow for more accurate interpretation of data. Fourth, some included studies had a relatively small number of cases, and only a small amount of data was available for some tests. However, by aggregating the studies together, the data provided may be more robust than individual studies. Furthermore, direct comparison of all data was not impossible. Thus, we placed these data in systematic review instead of meta-analysis. The aggregated data can still help shape clinical decision-making. Finally, most of parameters except for CRP and ESR had only two studies in their analysis. Therefore, the meta-analysis portion of this study could not conclude with strong findings.
Tissue culture, synovial fluid PMN%, and synovial fluid culture showed the most promise for guiding reimplantation, but because few studies were available on each, we could not provide a firm recommendation regarding the superiority of any one of those tests over the others. All three of those tests, however, were more sensitive and specific than serum ESR, serum CRP, and serum WBC count. Although many clinicians evaluate serial serum inflammatory markers such as ESR and CRP when an antibiotic cement spacer is present, these tests demonstrated low sensitivity in our analysis (range, 0.29-0.78). In this analysis, synovial WBC, PMN%, Gram stain, and culture results were evaluated and showed low and variable sensitivity (range, 0.05-0.82); this is consistent with a report that preoperative aspiration is associated with a high risk of false-negative results [29]. It was interesting to note that sonication improved the sensitivity of culture from 0.63 to 0.82, which demonstrated the highest sensitivity from synovial fluid diagnosis [24]. However, studies evaluating these synovial fluid biomarkers such as synovial WBC, PMN%, Gram stain, and culture are lacking in patients undergoing reimplantation.
In this study, Gram stain in two studies [6, 31] and culture in two studies [23, 33] were evaluated in tissue samples. Overall, intraoperative Gram stains showed very poor sensitivity (0.14 and 0.25) despite high specificities (1 and 0.98) [6, 31]. Williams et al. [33] reported that the sensitivity and specificity were 0.8 and 0.94 for aspiration and 0.83 and 0.9 for tissue biopsy, respectively. They concluded that a more invasive tissue biopsy offers no advantage over aspiration in terms of diagnosis of bacterial colonization and results in more false-positive results. However, tissue culture showed relatively higher sensitivity compared with others (range, 0.75-0.83) [23, 33].
Several studies have examined the usefulness of technetium/indium-labeled leukocyte imaging, gallium imaging, FDG-PET scan, and technetium Tc-99 bone marrow imaging in the primary diagnosis of PJI of both the hip and knee [15, 28, 31]. Given the substantial variability in statistical data and methodological flaws, the American Academy of Orthopaedic Surgeons offered a “weak” recommendation for their use in the diagnosis of PJI in select cases of equivocal laboratory investigation [5]. The MSIS criteria did not incorporate nuclear imaging as a reliable method of diagnosis [27].
This meta-analysis suggests that no single marker was superior to all others, and no marker (when used alone) is sufficient to confirm control of infection after the first stage of a two-stage protocol for PJI. Therefore, the current approach using multiple tools rather than a single marker is essential. Additionally, further studies should be conducted so that pooled analysis can be performed.
Acknowledgments
We thank and acknowledge O-Sung Lee and Seung Hoon Lee at Seoul National University Bundang Hospital for their contributions to revising the manuscript.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Rothman Institute at Thomas Jefferson University, Philadelphia, PA, USA.
References
- 1.Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res. 2009;467:1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth RE, Jr, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60. [PubMed] [Google Scholar]
- 3.Borden LS, Gearen PF. Infected total knee arthroplasty. A protocol for management. J Arthroplasty. 1987;2:27–36. [DOI] [PubMed] [Google Scholar]
- 4.Cuckler JM. The infected total knee: management options. J Arthroplasty. 2005;20:33–36. [DOI] [PubMed] [Google Scholar]
- 5.Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93:1355–1357. [DOI] [PubMed] [Google Scholar]
- 6.Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684–689. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Ledezma C, Lamberton C, Lichstein P, Parvizi J. Diagnosis of periprosthetic joint infection: the role of nuclear medicine may be overestimated. J Arthroplasty. 2015;30:1044–1049. [DOI] [PubMed] [Google Scholar]
- 8.Durbhakula SM, Czajka J, Fuchs MD, Uhl RL. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768–774. [DOI] [PubMed] [Google Scholar]
- 9.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. [DOI] [PubMed] [Google Scholar]
- 10.Gehrke T, Alijanipour P, Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J. 2015;97:20–29. [DOI] [PubMed] [Google Scholar]
- 11.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez MM, Tan TL, Manrique J, Deirmengian GK, Parvizi J. The fate of spacers in the treatment of periprosthetic joint infection. J Bone Joint Surg Am. 2015;97:1495–1502. [DOI] [PubMed] [Google Scholar]
- 13.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. [DOI] [PubMed] [Google Scholar]
- 14.Hoell S, Moeller A, Gosheger G, Hardes J, Dieckmann R, Schulz D. Two-stage revision arthroplasty for periprosthetic joint infections: what is the value of cultures and white cell count in synovial fluid and CRP in serum before second stage reimplantation? Arch Orthop Trauma Surg. 2016;136:447–452. [DOI] [PubMed] [Google Scholar]
- 15.Huang MJ, Hsieh PH, Ueng SW, Ho KC, Yen TC, Lee MS. Use of positron emission tomography to detect infection around antibiotic-loaded cement spacers in patients with high C-reactive protein levels. Orthopedics. 2011;34:e605–609. [DOI] [PubMed] [Google Scholar]
- 16.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 17.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. 1983. J Bone Joint Surg Am. 2002;84-a:490. [DOI] [PubMed] [Google Scholar]
- 18.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468:2060–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469:1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop (Belle Mead NJ). 2001;30:305–309. [PubMed] [Google Scholar]
- 21.Mahmud T, Lyons MC, Naudie DD, Macdonald SJ, McCalden RW. Assessing the gold standard: a review of 253 two-stage revisions for infected TKA. Clin Orthop Relat Res. 2012;470:2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 23.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82-a:1552–1557. [DOI] [PubMed] [Google Scholar]
- 24.Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. 2014;472:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvizi J, Gehrke T. International consensus on periprosthetic joint infection: let cumulative wisdom be a guide. J Bone Joint Surg Am. 2014;96:441. [DOI] [PubMed] [Google Scholar]
- 26.Parvizi J, Heller S, Berend KR, Della Valle CJ, Springer BD. Periprosthetic joint infection: the algorithmic approach and emerging evidence. Instr Course Lect. 2015;64:51–60. [PubMed] [Google Scholar]
- 27.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scher DM, Pak K, Lonner JH, Finkel JE, Zuckerman JD, Di Cesare PE. The predictive value of indium-111 leukocyte scans in the diagnosis of infected total hip, knee, or resection arthroplasties. J Arthroplasty. 2000;15:295–300. [DOI] [PubMed] [Google Scholar]
- 29.Shukla SK, Ward JP, Jacofsky MC, Sporer SM, Paprosky WG, Della Valle CJ. Perioperative testing for persistent sepsis following resection arthroplasty of the hip for periprosthetic infection. J Arthroplasty. 2010;25:87–91. [DOI] [PubMed] [Google Scholar]
- 30.Vielgut I, Sadoghi P, Wolf M, Holzer L, Leithner A, Schwantzer G, Poolman R, Frankl B, Glehr M. Two-stage revision of prosthetic hip joint infections using antibiotic-loaded cement spacers: When is the best time to perform the second stage? Int Orthop. 2015;39:1731–1736. [DOI] [PubMed] [Google Scholar]
- 31.Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg. 2002;91:178–181. [DOI] [PubMed] [Google Scholar]
- 32.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 33.Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J Arthroplasty. 2004;19:582–586. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2016;98:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]