Supplemental digital content is available in the text.
Key Words: MAAT, Amputation, Mobility, Comorbidity, Prosthetics
Abstract
Objective
The aim of the study was to determine the impact of comorbidities on mobility in patients with lower limb prostheses.
Design
Cohort database chart review was conducted to examine mobility in lower limb prosthesis users grouped according to comorbidities. Regression models were used to determine significant predictor comorbidities for mobility. General linear univariate models were implemented to investigate differences in mobility among cohorts (N = 596).
Results
Patient age and history of stroke, peripheral vascular disease, and anxiety/panic disorders were predictors of decreased mobility. After adjusting for covariates, the differences in mobility reported by patients older than 65 yrs was compared with those younger than 65 yrs; in addition, we compared those with a history of peripheral vascular disease with those without. The comparative analyses for both categories did not satisfy the minimal clinically important difference. There were no significant differences when comparing overall comorbid health after adjusting for covariates.
Conclusions
Clinicians should consider patient age and history of stroke, peripheral vascular disease, or anxiety/panic disorders when optimizing a lower limb prosthesis users' mobility because these variables may be predictive of modest but clinically meaningful decreased prosthetic mobility. By contrast, common comorbid health conditions such as arthritis, chronic obstructive pulmonary disease, congestive heart failure, and diabetes do not seem predictive of decreased mobility among lower limb prosthesis users.
The prevalence of lower limb amputation in the United States continues to rise with a projected population of 3.6 million affected individuals by 2050, up from 1.6 million in 2005.1 Accompanying the rise in patients with a lower limb amputation is the need for improved resource allocation to assure that those patients who will benefit from prosthetic rehabilitation have access to proper prosthetic technologies and appropriate care. Paramount to this is the identification and characterization of patients with lower limb amputation and their expected function with a lower limb prosthesis.
Previous studies have investigated the impact of co-morbidities and age for patients with lower limb prostheses.2–9 Comorbidities represent additional health aspects of individuals that may lend themselves to better identify potential successful prosthesis users. Improved identification of successful prosthesis users could assure that resources that are expended for prosthetic rehabilitation (e.g., rehabilitation professionals' time, monetary costs of devices, caregiver and patient time for travel to fittings and therapy appointments, etc.) are best allocated. In this way, individuals that would benefit from such resources are guaranteed access whereas those who would not benefit from prosthetic rehabilitation could instead have a more successful plan of care put implemented as a primary plan rather than a fallback. However, some of these studies have limited their investigation of mobility to a dichotomous fashion (e.g., reports limited to the use or abandonment of a prosthesis7,8). The results from such dichotomous investigations have shown phantom limb pain does not hinder the ability to independently ambulate.7,8 In the older patient (>60 yrs old), Hamamura et al.6 reported a significant difference between successful and unsuccessful prosthesis users with regard to the number of comorbidities. That study, however, defined success based on the ability to ambulate more than 100 meters, a criterion that would categorize an entire Medicare functional classification level of prosthesis users (i.e., Medicare functional classification level K1) as “unsuccessful.”10 An alternate approach was adopted by Webster et al.9 who reported on age and several other demographic variables in a cohort of 87 patients with lower limb amputation using hours of wear time as a proxy for a range of successful prosthetic outcomes.
Other studies that have investigated comorbidities in patients with lower limb amputation using comorbidity indices such as the Charlson Index and Elixhauser Index (e.g., see references7–9). These indices were developed with an emphasis on mortality and thus may not be best suited when mortality is not being considered.11,12 By contrast, the Functional Comorbidity Index (FCI) was developed to investigate the impact of comorbidities on a patient's function13 and as such may be more relevant to an understanding of the relationship between comorbid health and expected mobility among users of lower limb prostheses.
This study is the second within a series of studies designed to investigate lower limb prosthesis user mobility.14 The purpose of this study was to (1) determine significant predictor comorbidities for mobility in the lower limb prosthesis user, (2) determine the impact of any noted significant predictor comorbidities on mobility for the lower limb prosthesis user, and (3) determine any difference in mobility with an increasing number of comorbidities. It was hypothesized that peripheral vascular disease (PVD)1 and history of stroke15–18 would be significant predictors and subsequently patients with these conditions would have reduced mobility. It was further hypothesized that a patient's mobility would decrease with increasing comorbidities.2
MATERIALS AND METHODS
Study Design
We performed a retrospective cohort study based on the review of an outcomes database and associated demographic data collected within multiple prosthetics clinics across the United States spanning multiple regions including the Northwest, Southwest, Rocky Mountains, Midwest, Southeast, Northeast, and East. It was important to have representation covering the entire continental United States to minimize regional bias and influence. A convenience sample of the most recent 1000 patients seen within a 1-yr time frame (April 1, 2016–May 1, 2017) at participating clinics was extracted for analysis. Importantly, as a standard practice, comorbidities were only reviewed with patients at evaluation type appointments. Subsequently, it was expected that only approximately 50% of the extracted patient charts would have documented comorbidities attached to their outcomes, achieving a sample size of 500 patients. For patients with multiple mobility outcomes, only the highest mobility score was extracted because this is considered to represent the patient's best mobility to date given their comorbid health. This database review was approved and deemed exempt from patient consent by Western Investigational Review Board (Protocol #20170059). This study conforms to all STROBE guidelines and reports the required information accordingly (see Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/A617).
Participants
Inclusion criteria were set as follows: (1) unilateral or bilateral lower limb amputation; (2) 18 yrs or older; (3) amputation level of ankle disarticulation, transtibial, knee disarticulation, or transfemoral; (4) presenting at a prosthetic clinic to initiate replacement of an existing prosthesis or obtain adjustments to an existing prosthesis; and (5) have the ability to read and understand English or Spanish. Inclusion criteria were set by the established limitations of the Prosthetic Limb Users Survey of Mobility (PLUS-M) outcomes instrument,19,20 limitations of outcomes instrument language availability, and the nature of the convenience sampling from prosthetic clinics. Specifically, the PLUS-M is not validated for individuals that have never used a prosthesis, and as such, these individuals were excluded. There were no restrictions with regard to prosthetic device or Medicare functional classification level. Individuals were, however, excluded if they had incomplete outcomes and/or comorbidities data.
Procedure
As part of the routine standard of care in participating clinics, patients are asked to complete the 12-item PLUS-M. The PLUS-M is a validated, objective patient-reported outcomes instrument, which reports on a patient's mobility.21–25 It is composed of 12 questions that ask a patient to rate their level of difficulty in performing 12 various tasks with responses on a five-point ordinal scale. The response categories were “unable to do,” “with much difficulty,” “with some difficulty,” “with a little difficulty,” and “without any difficulty.”26 Each of the 12 responses has a graded score and their sums comprise the raw score. The raw score is ultimately translated to a T-Score, whereby a score of 50 represents the population average and ± 10 points comprises one standard deviation above and below that mean. A higher score represents greater mobility. For cases that were missing a response, the raw score and subsequent T-Score were calculated as outlined by the instrument developers.26 The PLUS-M has been validated and reported to have good reliability with a minimal clinically important difference of greater than four points for the T-Score.22
At the start of an episode of care, initiating the procurement of a new prosthesis, socket, knee, or foot, clinicians review with the patient all comorbidities comprising the FCI (Table 1),13 with exception of obesity, which is noted from patient height and weight. During the course of patient history taking, other health issues beyond the comorbidities included in the FCI, hypertension, and hypercholesterolemia may be introduced by the patient but are not recorded in the outcomes database. Comorbidities included in the FCI were obtained through patient, caregiver report, and when possible medical record, a process similar to that used in the validation of the FCI.13 For purposes of FCI, obesity is defined as a body mass index of greater than 30.0. Body mass index was calculated using previously published algorithms based on patient height and weight accounting for specific missing anatomy.19,20 In addition, although not included within the FCI, clinicians obtain patient history for hypercholesterolemia and hypertension. Although hypercholesterolemia and hypertension were excluded from FCI calculation, they were entered as separate variables within the regression model along with the 18 comorbidities of the FCI and age. Importantly, examination of level of amputation and status of unilateral or bilateral amputation were not considered co-morbidities, thus were beyond the scope of this study, and were excluded from the analysis.
TABLE 1.
Functional comorbidities index

Analysis- Significant Predictor Comorbidities
It was anticipated that it would be necessary to extract 1000 patients older than 18 yrs to afford at least a sample size of 500 after exclusion of ineligible patients. Patients who did not meet the inclusion/exclusion criteria were excluded, and any patients who did not have either PLUS-M or comorbidities data were also excluded. The PLUS-M T-Score for each person was calculated from the highest raw score recorded for each individual on the 12-item PLUS-M short form signifying the patient's greatest mobility achieved given their comorbid health. A stepwise linear regression model was implemented with the 18 co-morbidities identified within the FCI (Table 1), hypertension, and hypercholesterolemia all entered as categorical predictors along with a single continuous predictor, patient age, with a single dependent variable for mobility (i.e., PLUS-M T-Score). For the regression model, a Bonferroni correction for multiple comparisons was implemented for an adjusted α level of 0.05/21 = 0.0024.
Analysis- Impact of Specific Comorbidities
Those comorbidities, which were identified as significant predictors from the regression model, were then selected for further investigation. Individuals identifying the presence of the comorbidities that were found to be significant predictors were grouped and then compared with individuals from the sample who had no comorbidities and also with those individuals who had comorbidities but not the specific condition being investigated. Group differences were tested under separate fixed-effects general linear univariate models. When testing each significant predictor, the other significant predictor comorbidities were then entered into the model as covariates to parse out the influence of these factors. This was done because of high likelihood of overlap of presence of multiple significant predictor comorbidities, allowing for investigation of each individual significant predictor while eliminating influence of the remaining predictors in individuals with multiple comorbidities.
Analysis- Overall Comorbid Health
A separate analysis was then conducted to determine the difference in mobility with an increasing number of comorbidities. To accomplish this analysis, all subjects were again divided into cohorts, this time based on their FCI. Functional comorbidity index was calculated by summing each factor comprising the FCI with an equally weighted value of one.13 Individuals were grouped into cohorts based on their summed FCI scores, with the final cohort consisting of those individuals with FCI of seven or higher to ensure that all groups retained a minimum of 30 subjects. Functional comorbidity index cohorts were compared using a general linear univariate model with Sidek confidence level adjustment for post hoc comparisons following significant main effect. Any significant predictor comorbidities identified from the regression analysis were then entered as categorical covariates to remove any influence of these specific comorbidities. This was done as the influence of specific predictor comorbidities was investigated separately as noted in previous paragraph.
RESULTS
Participants
Of the most recent 1000 patients seen at clinics for which outcomes were submitted, 596 were found to have verified comorbidities data in their record as well as meeting outlined inclusion/exclusion criteria (Table 2). Two individuals were excluded because of their amputation level (hip disarticulation) and the remaining 402 individuals were excluded because of lack of verification of comorbidities. On average, individuals were 10.5 ± 13.9 yrs after receipt of first prosthesis. Patient mobility as assessed through the PLUS-M T-Score generally followed a uniform, normal distribution consistent with the PLUS-M design with a mean of 47.8 and standard deviation of 11.7.
TABLE 2.
Patient demographics
Significant Predictor Comorbidities
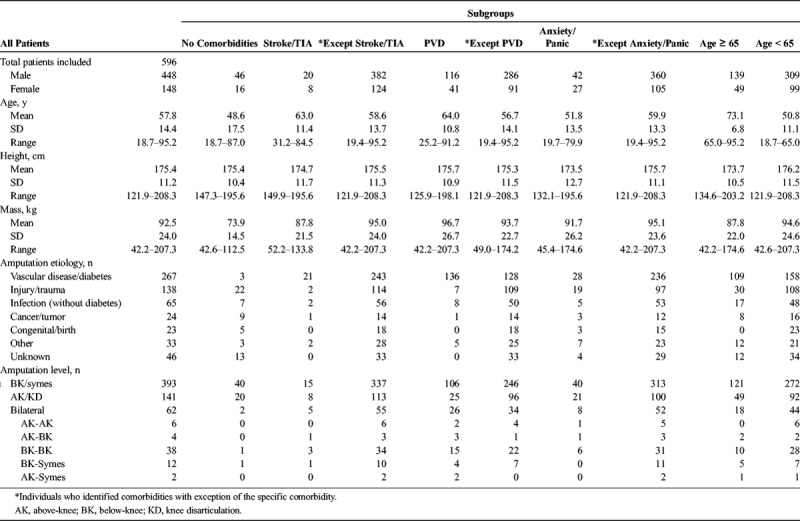
After stepwise linear regression, the significant predictor variables were age, history of stroke, PVD, and anxiety/panic disorders (R = 0.388; Table 3).
TABLE 3.
Stepwise regression results (dependent: PLUS-M T-Score)
Impact of Specific Comorbidities
Based on the results of the regression model, the impact of the predictor variables was further tested for differences in mobility via separate general linear univariate models with the other three variables input as covariates to remove their effects. When testing history of stroke, there was a significant main effect (F2,590 = 6.58, P = 0.001). Individuals with a history of stroke had significantly reduced mobility (n = 28; estimated marginal mean = 40.44; standard error of measurement [SEM] = 2.09; 95% confidence interval [CI] = 36.33–44.55) compared with individuals without any reported comorbidities (n = 62; estimated marginal mean = 49.06; SEM = 1.44; 95% CI = 46.23–51.89; P = 0.003) and individuals with reported comorbidities except stroke (n = 506; estimated marginal mean: 48.05; SEM: 0.48; 95% CI: 47.10, 49.00; P = 0.001). This was after removing effects of age, history of PVD, and history of anxiety/panic disorders.
For history of PVD, there was a significant main effect (F2,590 = 7.03, P = 0.001). Individuals with a history of PVD had significantly reduced mobility (n = 157; estimated marginal mean = 44.89; SEM = 0.90; 95% CI = 43.13–46.66) compared with individuals without any reported comorbidities (n = 62; estimated marginal mean = 49.70; SEM = 1.43; 95% CI = 46.90–52.51; P = 0.017) and individuals with reported co-morbidities except PVD (n = 377; estimated marginal mean = 48.69; SEM = 0.56; 95% CI = 47.59–49.79; P = 0.001). This was after removing effects of age, history of stroke, and history of anxiety/panic disorders.
For history of anxiety/panic disorders, there was a significant main effect (F2,590 = 4.90, P = 0.008). Individuals with a history of anxiety/panic disorders had significantly reduced mobility (n = 69; estimated marginal mean = 44.00; SEM = 1.33; 95% CI = 41.39–46.60) compared with individuals without any reported comorbidities (n = 62; estimated marginal mean = 49.19; SEM = 1.42; 95% CI = 46.39–52.98; P = 0.022) and individuals with reported comorbidities except anxiety/panic disorders (n = 465; estimated marginal mean = 48.17; SEM = 0.51; 95% CI = 47.18–49.17; P = 0.011). This was after removing effects of age, history of stroke, and history of PVD.
To further understand age, the total sample of 596 individuals was again divided into two cohorts with a cutoff of 65 yrs based on Medicare guidelines. Individuals 65 yrs or older had significantly reduced mobility (n = 188; estimated marginal mean = 45.27; SEM = 0.82; 95% CI = 43.65–46.88) compared with individuals younger than 65 yrs (n = 408; estimated marginal mean = 48.96; SEM = 0.55; 95% CI = 47.87–50.05; P < 0.001).
Overall Comorbid Health
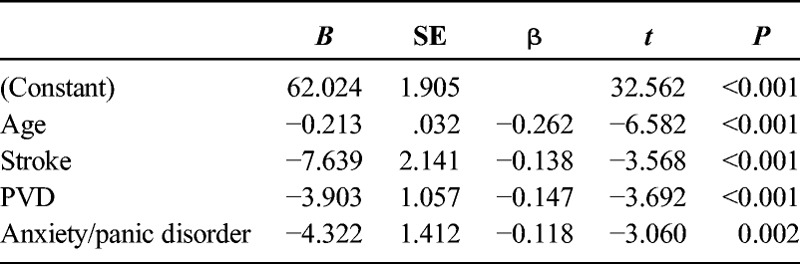
On average, the sample of 596 patients had an FCI of 2.68 ± 2.14 (range = 0–12). When comparing FCI cohorts without any adjustments for covariates, there was a significant difference for main effect (F7,588 = 5.932, P < 0.001), with multiple post hoc significant differences between groups (Table 4). However, after adjusting for the identified covariates (i.e., age and history of stroke, PVD, and anxiety/panic disorders), there were no significant differences in PLUS-M T-Scores between any of the groups (F7,584 = 0.430, P = 0.884) (Table 5, Fig. 1).
TABLE 4.
Functional comorbidity index cohorts comparisons - not adjusted for covariates
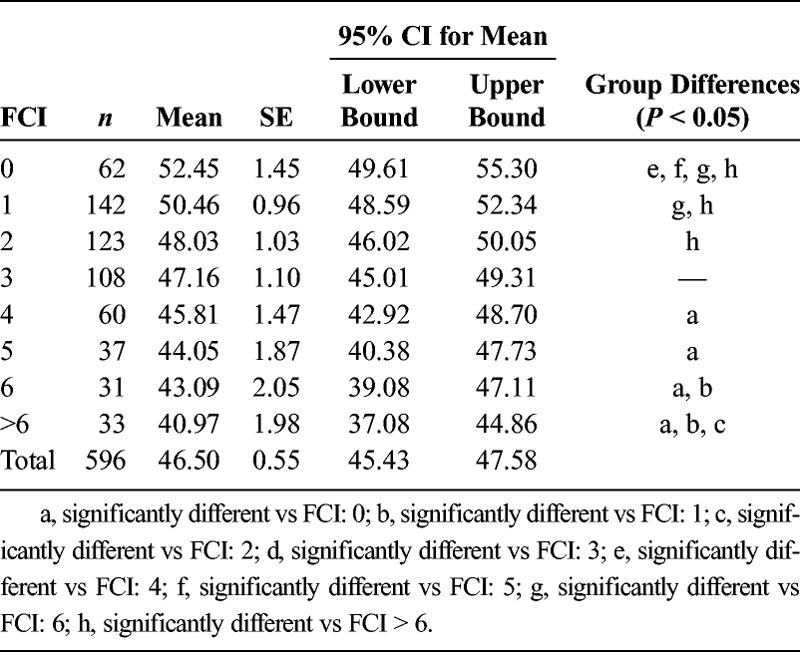
TABLE 5.
Functional comorbidity index cohorts comparisons after covariate adjustments
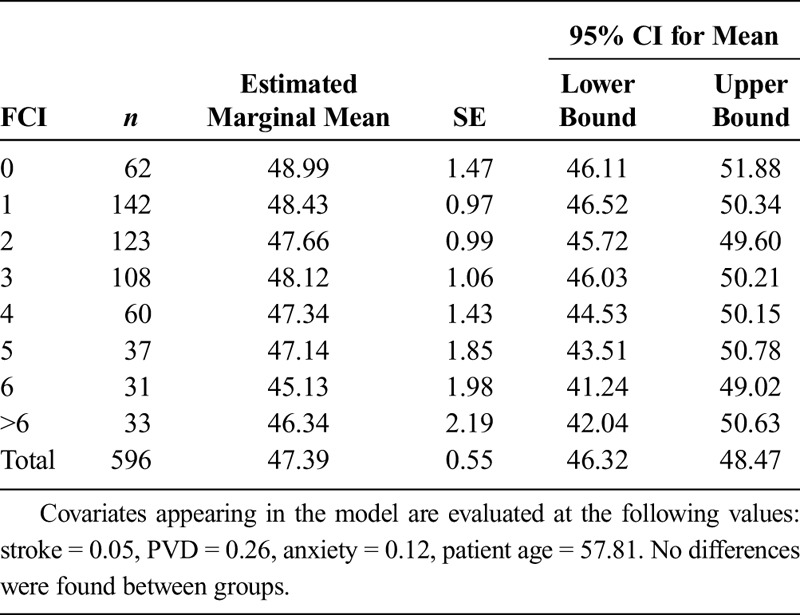
FIGURE 1.
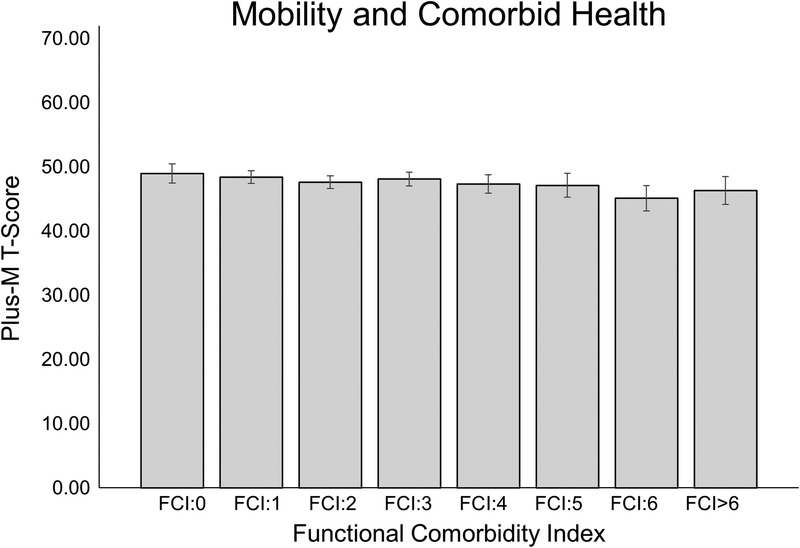
Group estimated marginal means for each FCI cohort after removing effects of covariates. There was a lack of significant differences between groups.
DISCUSSION
The continued increase in prevalence of lower limb amputation1 has created an increased need for a better understanding of the implications of comorbid health on the mobility achieved by those with lower limb amputation. Improved clinical standards of care that track outcomes and monitor patient mobility in a quantified, objective manner are providing new opportunities for further understanding of comorbid health in patients with lower limb amputation. A regression model was implemented to investigate the role of noted comorbidities comprising the FCI as predictors for lower limb prosthesis users' mobility. The patient's comorbid health was quantified using the FCI, a comorbidity index developed specifically for looking at the impact of comorbidities on a person's overall function.13 Our results showed age and histories of stroke, PVD, or anxiety/panic disorders are all significant predictor variables for modest but clinically meaningful declines lower limb prosthesis users' mobility. When these four variables are removed from consideration, there was no significant differences between cohorts without any comorbid health conditions (i.e., FCI: 0) and all remaining cohorts with their increased prevalence of comorbid health concerns (FCI: 1-FCI > 6).
Impact of Specific Comorbidities
There are certain comorbidities whose prevalence in lower limb prosthesis users has subsequently lent themselves to perceptions of inherently compromised outcomes.2,6,20,27–29 In addition to age, history of stroke, PVD, and anxiety/panic disorders were found to be significant predictors in the model. History of stroke would seem to be most impactful on lower limb prosthesis users' mobility. This is consistent with previous reports of reduced mobility in amputees with a history of a co-morbid stroke.15–18 However, it should be noted that of the 28 individuals with reported history of stroke, only 3 (10.7%) scored 21.8 on the PLUS-M, which translates to being unable to do any of the tasks with their prosthesis, indicating that although the mobility of those with comorbid stroke is limited, its complete absence seems rare among those with a prosthesis. As a noted limitation, the observation of history of stroke did not reflect whether the impaired side was contralateral or ipsilateral of amputation, which would also likely impact mobility. The significance of PVD is important given the high incidence and prevalence within the lower limb prosthesis user population.1 Thus, it was relevant to further examine the influence of PVD while accounting for other comorbidities. The patient with PVD will likely have lower mobility; however, the presence of PVD should not be considered independent of patient's age, history of stroke, and anxiety/panic disorders as part of the clinical assessment. In particular, when these variables were removed from the model, the difference in group mobility means between individuals without PVD and individuals with PVD is reduced nearly 36% (5.91 vs 3.8 points), again dropping below the minimal clinically important difference of four points. Age was examined more thoroughly by dividing the sample into cohorts based on Medicare age guidelines (65 yrs). From this, it was possible to discern that although age was significant, once the influence of stroke, PVD, and anxiety/panic disorders is removed, the mean mobility difference between individuals younger than 65 yrs and older than 65 yrs fell below the four-point minimal threshold for a clinically important difference in mobility.22 As a result, whereas clinicians working with Medicare age patients should be aware of age, other factors are more likely to be impactful.
Perhaps more interesting than the significant comorbidities were those that were not found to be significant, for example, diabetes (n = 288) was not a significant factor. It is possible that the low prevalence of certain less frequent comorbidities such as osteoporosis (n = 14), asthma (n = 25), angina (n = 18), and neurological conditions such multiple sclerosis and Parkinson's (n = 9) precluded significance. However, comorbidities such as arthritis (n = 172), chronic obstructive pulmonary disease (n = 35), congestive heart failure (n = 48), heart attack (n = 48), hypertension (n = 182), hypercholesterolemia (n = 115), upper gastrointestinal disease (n = 55), depression (n = 94), visual impairment (n = 78), hearing impairment (n = 41), degenerative disc disease (n = 76), and obesity (n = 341) all failed to reach significance.
Impact of Multiple Comorbidities
The FCI was calculated for patients based on their report of presence and history of the noted comorbidities (Table 1). Although there were significant group differences without any adjustments for covariates, once the influence of age and history of stroke, PVD, and anxiety/panic disorders was removed, there were no longer any significant differences between groups. These results are interesting because the FCI is designed to inform patient's physical function13 with a negative relationship. In the case of patients with lower limb amputation, when the influence of the four noted covariates is removed, there was no significant difference in mobility regardless of the absence, presence, or number of comorbidities. The FCI was developed using the patient-reported outcome instrument Short-Form 36 survey, focusing on physical function subscale, which may explain why the predictor variables were different for the PLUS-M instrument.13 However, the FCI was only used as a guide for which comorbidities needed to be recorded. The results of this regression analysis would indicate that for the purpose of mobility, age and history of PVD, stroke, and anxiety/panic disorders are the only pertinent variables.
Limitations
There are certain limitations with this study. This is a retrospective analysis, which prevents reporting on causation. In addition, comorbidities are recorded primarily through patient report, in some instances they have been verified through medical record by the patient's clinician. It should be noted that this is the procedure used in the validation of the FCI13 as co-morbidities are primarily obtained through patient history.
There is a potential for selection bias as this retrospective chart review would have precluded individuals that received a prosthesis and never used it or have fully abandoned prosthesis use.
In this study, data are captured in clinics through patient-reported outcomes. The PLUS-M has been validated22 and demonstrated reliability in various administrative formats24 including settings where outcomes are administered and not controlled. To minimize error and improve consistency in outcomes data reported to the database, all clinics participating have undergone training in face-to-face classroom format. Similarly, potential errors in patient-reported outcomes may also exist in a patient's ability to accurately report comorbidities. This places strong emphasis on a patient's ability to provide their medical history. However, this reliance is done routinely in the clinical setting where physicians are making critical plan of care decisions based on medical history report. We offset this potential limitation by having a large sample size with at least 30 subjects per comorbid health cohort.
There is an additional limitation with regard to choosing the study outcome of mobility. By choosing mobility as an outcome, the study lends itself to the misperception that this is the only goal of lower limb prosthetic rehabilitation, which fails to account for many successful prosthetic users that rely on their prosthesis for effective transfers. The K1 prosthesis user is defined as the individual that “has the ability or potential to use a prosthesis for transfers or ambulation on level surfaces at fixed cadence.”10 The importance of provision of lower limb prostheses for ease of transfer should not be lost in any efforts designed to identify successful prosthesis users given the importance independent transfers have with regard to patient independence and reducing caregiver burden.30 Future studies and outcomes assessments should include the ability to include prosthesis users that define success through independence gained with the ability to transfer.
CONCLUSIONS
In conclusion, our results showed only age and history of stroke, PVD, and anxiety/panic disorders to be significant predictors for lower limb prosthesis users' mobility. However, further examination of these specific comorbidities showed that when removing influence of the other predictor variables, patients with PVD and those older than 65 yrs retained group mobility levels within the minimal clinically significant difference compared with those without these predictive health states. Patients with a history of stroke should be examined with care as part of the clinical care plan for optimizing their future mobility. This is similar for those identifying history of anxiety/panic disorders. Surprisingly, diabetes was not a significant factor for lower limb prosthesis users' mobility. In general, a patient's overall comorbid health is not a factor for the patient's mobility with a lower limb prosthesis.
Supplementary Material
Footnotes
The study was partially supported by a Small Grant Award (EB-043016) from the American Orthotics and Prosthetics Association.
The results of this study were recently presented as part of a symposium on outcomes at the American Congress of Rehabilitation Medicine in October 2017.
All authors contributed equally to the preparation of this study and manuscript write-up.
Sponsor funding was used to offset costs of research assistants hired by the investigators. The sponsor had no contribution to study design, data collection, data interpretation, decision to publish, or manuscript drafting.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
REFERENCES
- 1.Ziegler-Graham K, MacKenzie E, Ephraim P, et al. : Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008;89:422–9 [DOI] [PubMed] [Google Scholar]
- 2.Kahle J, Highsmith MJ, Schaepper H, et al. : Predicting walking ability following lower limb amputation: an updated systematic literature review. Technol Innov 2016;18:125–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sansam K, Neumann V, O'Connor R, et al. : Predicting walking ability following lower limb amputation: a systematic review of the literature. J Rehabil Med 2009;41:593–603 [DOI] [PubMed] [Google Scholar]
- 4.Raya M, Gailey RS, Fiebert IM, et al. : Impairment variables predicting activity limitation in individuals with lower limb amputation. Prosthet Orthot Int 2010;341:73–84 [DOI] [PubMed] [Google Scholar]
- 5.Shah SK, Bena JF, Allemang MT, et al. : Lower extremity amputations: factors associated with mortality or contralateral amputation. Vasc Endovascular Surg 2013;47:608–13 [DOI] [PubMed] [Google Scholar]
- 6.Hamamura S, Chin T, Kuroda R, et al. : Factors affecting prosthetic rehabilitation outcomes in amputees of age 60 years and over. J Int Med Res 2009;37:1921–7 [DOI] [PubMed] [Google Scholar]
- 7.van Eijk MS, van der Linde H, Buijck B, et al. : Predicting prosthetic use in elderly patients after major lower limb amputation. Prosthet Orthot Int 2012;36:45–52 [DOI] [PubMed] [Google Scholar]
- 8.Eijk MS, van der Linde H, Buijck BI, et al. : Geriatric rehabilitation of lower limb amputees: a multicenter study. Disabil Rehabil 2012;34:145–50 [DOI] [PubMed] [Google Scholar]
- 9.Webster JB, Hakimi KN, Williams RM, et al. : Prosthetic fitting, use, and satisfaction following lower-limb amputation: a prospective study. J Rehabil Res Dev 2012;49:1493–504 [DOI] [PubMed] [Google Scholar]
- 10.DMERC, “Local Coverage Determinations,” 2016. Available at: https://www.cms.gov/medicare-coverage-database/details/lcddetails. aspx?LCDId=33787&ContrId=140&ver=9&ContrVer=2&CntrctrSelected=140*2 &Cntrctr=140&name=CGS+Administrators%2c+LLC+(18003%2c+DME+MAC)&Doc Type=Active&LCntrctr=140*2&bc=AgACAAQAAAAAAA%3d%3d&. Accessed December 21, 2016
- 11.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83 [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 13.Groll D, To T, Bombardier C, et al. : The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595–602 [DOI] [PubMed] [Google Scholar]
- 14.Wurdeman SR, Stevens PM, Campbell JH: Mobility Analysis of AmpuTees (MAAT I): quality of life and satisfaction are strongly related to mobility for patients with a lower limb prosthesis. Prosthet Orthot Int 2018;42:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert JS, Payne MW, Wolfe DL, et al. : Comorbidities in amputation: a systematic review of hemiplegia and lower limb amputation. Disabil Rehabil 2012;34:1943–9 [DOI] [PubMed] [Google Scholar]
- 16.Prvu-Bettger JA, Bates BE, Bidelspach DE, et al. : Short- and long-term prognosis among veterans with neurological disorders and subsequent lower-extremity amputation. Neuroepidemiology 2009;32:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu CC, Chen CE, Wang TG, et al. : Influencing factors and ambulation outcomes in patients with dual disabilities of hemiplegia and amputation. Arch Phys Med Rehabil 2000;81:14–7 [DOI] [PubMed] [Google Scholar]
- 18.Brunelli S, Averna T, Porcacchia P, et al. : Functional status and factors influencing the rehabilitation outcome of people affected by above-knee amputation and hemiparesis. Arch Phys Med Rehabil 2006;87:995–1000 [DOI] [PubMed] [Google Scholar]
- 19.Tzamaloukas AH, Patron A, Malhotra D: Body mass index in amputees. JPEN J Parenter Enteral Nutr 1994;18:355–8 [DOI] [PubMed] [Google Scholar]
- 20.Tzamaloukas A, Leger A, Hill J, et al. : Body mass index in patients with amputations on peritoneal dialysis: error of uncorrected estimates and proposed correction. Adv Perit Dial 2000;16:138–42 [PubMed] [Google Scholar]
- 21.Amtmann D, Kim J, Chung H, et al. : Comparison of computerized adaptive testing and fixed-length short forms of the Prosthetic Limb Users Survey of Mobility (Plus-M). Qual Life Res 2015;24:142–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafner BJ, Gaunaurd IA, Morgan SJ, et al. : Construct validity of the Prosthetic Limb Users Survey of Mobility (PLUS-M) in adults with lower limb amputation. Arch Phys Med Rehabil 2017;98:277–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafner BJ, Morgan SJ, Abrahamson DC, et al. : Characterizing mobility from the prosthetic limb user's perspective: use of focus groups to guide development of the Prosthetic Limb Users Survey of Mobility. Prosthet Orthot Int 2016;40:582–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafner BJ, Morgan SJ, Askew RL, et al. : Psychometric evaluation of self-report outcome measures for prosthetic applications. J Rehabil Res Dev 2016;53:797–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan SJ, Amtmann D, Abrahamson DC, et al. : Use of cognitive interviews in the development of the PLUS-M item bank. Qual Life Res 2014;23:1767–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UWCORR: Prosthetic Limb Users Survey. 2013. Available at: http://www.plusm.org. Accessed December 20, 2016
- 27.Finch DR, Macdougal M, Tibbs DJ, et al. : Amputation for vascular disease: the experience of a peripheral vascular unit. Br J Surg 1980;67:233–7 [DOI] [PubMed] [Google Scholar]
- 28.Norvell DC, Turner AP, Williams RM, et al. : Defining successful mobility after lower extremity amputation for complications of peripheral vascular disease and diabetes. J Vasc Surg 2011;2:412–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penn-Barwell JG: Outcomes in lower limb amputation following trauma: a systematic review and meta-analysis. Injury 2011;42:1474–9 [DOI] [PubMed] [Google Scholar]
- 30.Oldenkamp M, Hagedoorn M, Slaets J, et al. : Subjective burden among spousal and adult-child informal caregivers of older adults: results from a longitudinal cohort study. BMC Geriatr 2016;16:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


