Abstract
Choline has been recognized as an essential nutrient by the Food and Nutrition Board of the National Academies of Medicine since 1998. Its metabolites have structural, metabolic, and regulatory roles within the body. Humans can endogenously produce small amounts of choline via the hepatic phosphatidylethanolamine N-methyltransferase pathway. However, the nutrient must be consumed exogenously to prevent signs of deficiency. The Adequate Intake (AI) for choline was calculated at a time when dietary intakes across the population were unknown for the nutrient. Unlike the traditional National Academy of Medicine approach of calculating an AI based on observed or experimentally determined approximations or estimates of intake by a group (or groups) of healthy individuals, calculation of the AI for choline was informed in part by a depletion-repletion study in adult men who, upon becoming deficient, developed signs of liver damage. The AI for other gender and life-stage groups was calculated based on standard reference weights, except for infants 0 to 6 months, whose AI reflects the observed mean intake from consuming human breast milk. Recent analyses indicate that large portions of the population (ie, approximately 90% of Americans), including most pregnant and lactating women, are well below the AI for choline. Moreover, the food patterns recommended by the 2015–2020 Dietary Guidelines for Americans are currently insufficient to meet the AI for choline in most age-sex groups. An individual’s requirement for choline is dependent on common genetic variants in genes required for choline, folate, and 1-carbon metabolism, potentially increasing more than one-third of the population’s susceptibly to organ dysfunction. The American Medical Association and American Academy of Pediatrics have both recently reaffirmed the importance of choline during pregnancy and lactation. New and emerging evidence suggests that maternal choline intake during pregnancy, and possibly lactation, has lasting beneficial neurocognitive effects on the offspring. Because choline is found predominantly in animal-derived foods, vegetarians and vegans may have a greater risk for inadequacy. With the 2020–2025 Dietary Guidelines for Americans recommending expansion of dietary information for pregnant women, and the inclusion of recommendations for infants and toddlers 0 to 2 years, better communication of the role that choline plays, particularly in the area of neurocognitive development, is critical. This narrative review summarizes the peer-reviewed literature and discussions from the 2018 Choline Science Summit, held in Washington, DC, in February 2018.
Choline is an essential nutrient needed for proper liver, muscle, and brain functions; lipid metabolism; and cellular membrane composition and repair.1–4 Humans can produce small amounts of choline via the hepatic phosphatidylethanolamine N-methyltransferase pathway; however, most individuals must consume this nutrient through the diet to supplement the endogenously produced amount to prevent deficiency.1
The name choline is derived from the Greek term for bile (ie, chole) because it was first isolated from ox bile in 1862. Its nutritional importance was not recognized until the 1930s, when deficiency was demonstrated to cause fatty liver disease in dogs and rats, which resolved when choline was reintroduced to the diet. Over the following decade, consensus emerged around the general essentiality of choline to prevent liver damage in several mammalian species, including the rat, dog, chicken, pig, rhesus monkey, and baboon.5 Recognition that choline is an essential nutrient for humans was further advanced in the 1980s by studies in men and women on parenteral nutrition who developed liver damage in its absence.5,6 A dietary requirement for choline was first demonstrated in healthy men participating in a depletion-repletion metabolic study.7 The Adequate Intake (AI) for choline was established by the Food and Nutrition Board of the National Academy of Medicine (NAM) (formerly the Institute of Medicine) in 1998, at a time when dietary intakes across the population were unknown for the nutrient. Traditionally, the AI reflects an observed or experimentally determined approximation or estimate of intake by a group (or groups) of healthy individuals.1,5 Adequate Intakes have been used when data to calculate an estimated average requirement (EAR) and recommended dietary allowance (RDA) are not available. Unlike this typical NAM approach, the development of the AI for choline was informed by the previously mentioned depletion-repletion study in adult men, in which deficiency resulted in liver damage.1,5 The AI for adults was calculated as 7 mg/kg times the reference weight of a man (76 kg) or woman (61 kg), with rounding based on prevention of liver damage. Upward adjustments during pregnancy and lactation were made based on the amount of choline accretion by the fetus and placenta and the amount secreted in human breast milk. For infants aged 0 to 6 months, the AI was set to reflect the observed mean intake of choline from consuming human breast milk (note: this value does not take into consideration the higher content of the colostrum) (Table 1).1 The dietary requirement for choline has not been revisited by NAM since 1998, despite a growing body of scientific literature. There is a need for dose-response studies across various populations to inform revision of the current Dietary Reference Intakes (DRIs) to include an EAR and RDA, so that accurate assessment of the amounts of individuals who are inadequate can be determined.
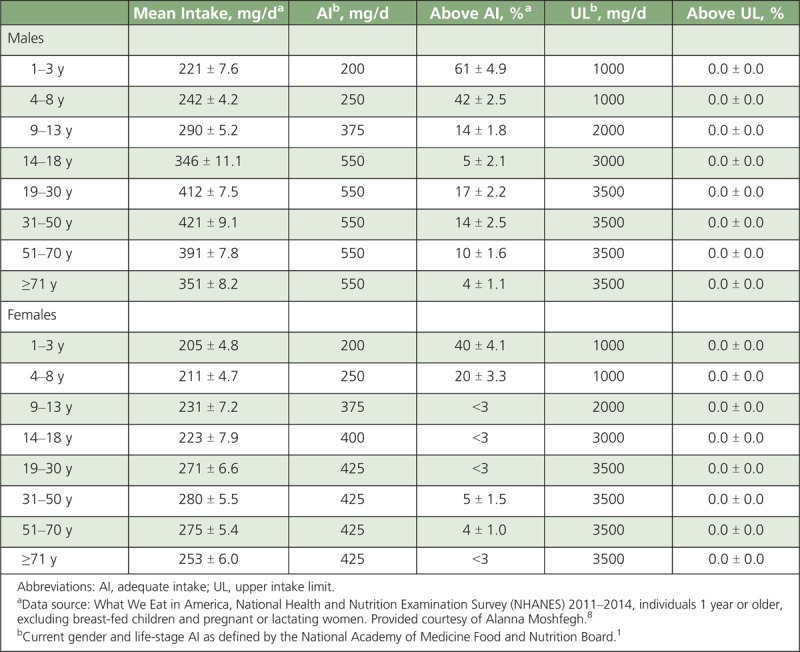
TABLE 1.
Mean Intakes of Choline From Foods and Beverages in the United States, 2011–2014
The American Academy of Pediatrics (AAP) (1985) and the US Food and Drug Administration recommend that infant formulas contain at least 7 mg of choline per 100 kcal.5,9 The AAP recently stated that “although all nutrients are necessary for brain growth, key nutrients that support neurodevelopment include protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6 and B12 and long-chain polyunsaturated fatty acids. Failure to provide these key nutrients during this critical period of brain development may result in lifelong deficits in brain function despite subsequent nutrient repletion.”9 The American Medical Association has also recently reinforced the importance of choline for fetal and infant development by recommending the nutrient as a component of all prenatal vitamin supplements.10
In February 2018, a second Choline Science Summit was held in Washington, DC, bringing together government, academic, and industry scientists along with leaders of several nongovernmental associations. The symposium addressed the latest science on choline that had accumulated since the 2009 Choline Science Summit4 and gathered insights for helping raise awareness and intake of this essential nutrient, particularly among vulnerable subpopulations. It included discussions on the role of choline in human health and development throughout the lifecycle, the role of genetics in influencing the dietary requirement, and a closer look at the gaps in choline requirements compared with actual dietary intakes in the US population. Participants also discussed future research needs and strategies for accurately communicating the importance of choline in future public health education efforts. The highlights of the 2018 Choline Science Summit, including call-to-action items, are presented in this manuscript.
CHOLINE CONTENT OF FOODS
The most common forms of choline in foods are fat-soluble phosphatidylcholine and sphingomyelin, as well as water-soluble phosphocholine, glycerophosphocholine, and free choline11 (Figure 1). Animal-derived products typically contain higher amounts of choline than certain plant foods do. Foods naturally containing choline include chicken liver (3 oz; 247 mg), salmon (3 oz; 187 mg), eggs (1 large egg with yolk; 147 mg), shitake mushrooms (1/2 cup; 58 mg), chicken broilers or fryers (3 oz; 56 mg), beef grass-fed strip steak (3 oz; 55 mg), wheat germ (1 oz toasted; 51 mg), milk (8 oz; 38 mg), Brussels sprouts (1/2 cup; 32 mg), and almonds (1 oz; 15 mg). Certain plant foods like cruciferous vegetables and certain beans are good sources of choline and contribute approximately 10% of the daily requirement.2
FIGURE 1.
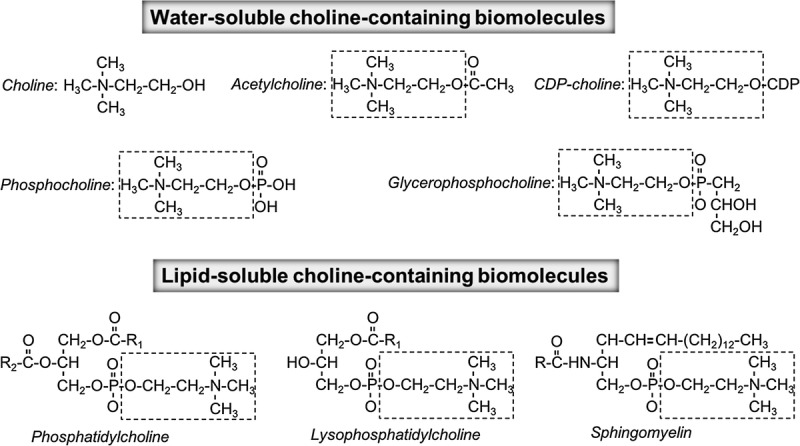
Water-soluble and lipid-soluble choline-containing molecules found in food. *Adapted with permission from Jiang and others (2013).12
CURRENT DIETARY INTAKES OF CHOLINE IN THE UNITED STATES
When the AI for choline was established by the NAM Food and Nutrition Board in 1998, dietary intakes of choline at the population level were unknown. A depletion-repletion metabolic study assessing liver damage in healthy men7 significantly informed the development of the current AIs.1,2 However, since then, studies showing significant variations in the dietary requirement for choline due to common genetic polymorphisms have become available. It is now generally accepted that choline has an impact on in cell structure, neurotransmitter synthesis, atherosclerosis, and possibly neurological disorders. It has shown to be potentially critical during pregnancy, lactation, and early childhood.2–4
In 2004, the US Department of Agriculture (USDA), in collaboration with the University of North Carolina, released the first provisional USDA Database for the Choline Content of Common Foods. This database was updated in 2008 and it can be used to estimate the choline content of more than 630 food items.11 Using this USDA database along with consumption data from the National Health and Nutrition Examination Survey, researchers have estimated usual choline intakes in the United States to be just over 300 mg/d for nonpregnant, nonlactating individuals.8,13,14 Only about 10% of Americans and 8% of pregnant women currently meet their gender- and life-stage-specific AI for choline8,13,14; again, those cutoffs are based on prevention of liver damage (ie, it is not based on the typical mean intake of the healthy population like the AI for other nutrients). Table 1 shows the average usual intakes, as well as the percentage of the US population above the AI and upper intake level (UL) for choline, grouped by gender and life stage. Young children are more likely to meet the AI for choline, although the DRIs for this population are extrapolated downward from studies in adults based on body mass. Premenopausal adult women are the least likely gender and life-stage subpopulation to meet the AI: the average usual intake of choline in this subpopulation is 319 ± 6 mg/d, which is only approximately 71% of the AI. The 25th percentile in this subpopulation has a usual intake of 254 ± 11 mg/d, which is roughly 60% of the AI.14 Vegetarians have the lowest intakes among the US population, estimated at 192 ± 7 mg/d.15 Choline intakes have been shown to be driven by egg intake and, secondarily, protein foods (ie, meat, poultry, and seafood) intake.14 Incorporating 1 to 2 eggs per day as a substitution for processed and/or red meat in the Healthy US-Style 2000 Kcal Pattern increases choline intakes to current recommended levels, without altering other essential nutrients and maintaining cholesterol intakes within a safe range for healthy individuals.14 Dietary supplements provide less than 5% of dietary choline in relation to recommended intakes because choline salts are bulky and vastly increase the size of the supplemental product.14 Current intakes cannot be deemed inadequate based upon the AI value alone. Although AIs may be useful in guiding individual dietary plans, by definition, they are established when the evidence is insufficient to calculate an EAR. Therefore, it is not possible to conclusively assess the risk of inadequacy in a population.16 Future research will be critical for establishing an EAR to assess the risk for inadequacy within subgroups of the population.
Only about 10% of Americans and 8% of pregnant women currently meet their gender- and life-stage-specific AI for choline.
CONSEQUENCES OF DEFICIENCY AND EXCESS
The risk of deficiency and excess intake of choline varies depending on a number of modifiable and nonmodifiable factors; current DRIs for nutrients take into account the impact of sex, age, and reproductive life-stage on nutrient needs and tolerable upper limits. For choline, it is worth noting that, in addition to the variables considered by the DRIs, significant additional variation in nutrient needs still remains due in part to genetic variants. For dietary choline, a large and growing body of research has supported the notion that common genetic variants in genes required for choline, folate, and 1-carbon metabolism influence dietary choline requirements.2,3,5,17 Several single-nucleotide polymorphisms have been shown to predict the likelihood of developing signs of choline deficiency in controlled laboratory settings where dietary intake is low, and they also influence the metabolic fate of dietary choline when intakes are adequate, as recently reviewed by Ganz et al.17 While further research is needed to define the precise contribution of genetic variants to dietary choline needs, clinicians working with patients/clients should remain aware of this evidence, particularly as the market for genotype-based diets continues to grow and questions will likely arise.
Deficiency
Choline deficiency causes clinically evident disease in humans. Healthy men and women with normal folate and vitamin B12 status who were fed a choline-deficient diet develop fatty liver disease, characterized by elevated liver enzymes in the blood, as well as muscle damage as indicated by increased circulating creatine phosphokinase concentrations, which resolve when choline is restored to the diet.7,18 Phosphatidylcholine, which can be derived from the diet and synthesized in small quantities by the body, is essential for normal assembly and secretion of very-low-density lipoproteins that transport triglycerides out of the liver; hepatic steatosis during deficiency results in the accumulation of triglycerides and resultant liver damage.19 The susceptibility to develop liver damage has been shown to be related to polymorphisms of the gene phosphatidylethanolamine N-methyltransferase,20 as well as polymorphisms in other enzymes involved in choline metabolism.21,22 Only about 44% of premenopausal women develop liver damage when they are choline deficient.21 The remaining 56% of premenopausal women who do not have these genetic polymorphisms produce endogenous choline sufficient to evade signs of liver damage. Choline production is enhanced by increased estrogen production. During pregnancy, estrogen concentrations increase dramatically (~60 times) at term23,24; despite the potential enhanced capacity for the body to synthesize choline, data from animal models suggest that fetal and infant demand is so high that maternal stores are depleted during pregnancy and lactation.2 Low maternal choline intakes during pregnancy have been shown to increase the risk of both neural tube defects and cleft palates.25–27 This risk is related to common genetic variations that alter one’s dietary choline requirement.28 A very common genetic variant in folate metabolism that has been linked to an increase risk of neural tube defects is the 5, 10-methylenetetrahydrofolate dehydrogenase (MTHFD1) 1958A allele (rs2236225), and premenopausal women carrying this allele have additionally been shown to be 15-times more likely to develop signs of clinical deficiency on a low choline diet.29
Toxicity
High intakes of choline are associated with a fishy body odor, vomiting, excessive sweating and salivation, hypotension, and liver toxicity.1 The NAM defined a 3500 mg choline per day tolerable UL for adults based on a study in 7 patients with Alzheimer’s disease, where oral administration of 7.5 g/d of choline resulted in a hypotensive effect accompanied by nausea and diarrhea.30 Similar gastrointestinal effects and a fishy body odor were observed in studies administering doses of 8 to 20 g/d of choline.31–33 The NAM considered 7.5 g/d of choline as the Lowest Observed Adverse Effect Level and, after application of an uncertainty factor of 2 and rounding, set a UL of 3.5 g/d for adults. The NAM was unable to establish ULs for infants because of the lack of data on adverse effects in this age group and the ULs for children were derived from the adult value by scaling according to reference body weight1 (Table 1). The European Food Safety Authority Panel on Dietetic Products, Nutrition, and Allergies did not define a UL for choline in 201634
Consumption of choline has been shown to increase the production of trimethylamine N-oxide (TMAO), a gut-derived metabolite, which has recently emerged as a candidate risk factor for cardiovascular diseases (CVDs).35,36 The choline-TMAO connection is not yet established. Microbes metabolize choline, betaine, and carnitine to trimethylamine (TMA), which is oxidized to TMAO in the host liver by the insulin-regulated enzyme flavin-containing monooxygenase 3. Significant preclinical evidence has been generated suggesting that high levels of TMAO exhibit proatherogenic and prothrombotic effects. Supporting this is a growing body of observational evidence associating high fasting TMAO levels with CVD.37 This evolving body of literature has suggested that dietary choline, betaine, and carnitine may increase the risk of CVDs. However, randomized clinical trial evidence to assess the relationship of TMAO to CVD in humans is limited, and ethical considerations preclude the likelihood of such studies. Current epidemiological studies do not show a link between dietary choline intakes and CVD.38 Of note, not all animal models have supported the relationship between TMAO and atherogenesis,39 and the evidence amassed from epidemiological cohorts has not adequately addressed the potential for reverse causality and/or confounding (ie, high TMAO levels could result from the impact of disease on kidney function and the microbiome). The available evidence at present leaves substantial uncertainty regarding the impact of efforts to reduce dietary choline to reduce TMAO levels and cardiovascular outcomes. Efforts to modify circulating TMAO concentrations with diet may additionally have unintended consequences, as choline exhibits pleiotropic effects beyond its impact on circulating TMAO levels (such as choline’s role in supporting hepatic function), and foods that contain additional cardioprotective nutrition, such as omega-3 fatty acid-rich fish, contain substantial quantities of preformed TMAs that can be converted to TMAO in the liver. A recent crossover feeding trial in healthy young men consuming meals containing TMAO (fish, cod), its dietary precursors, choline (eggs), and carnitine (beef) versus a fruit control showed that fish consumption yielded higher blood and urinary concentrations of TMAO (42–62 times) than did eggs, beef, or the fruit control.40 High TMAO producers were found to have a higher ratio of the microbial phyla Firmicutes to Bacteroidetes and a less diverse gut microbiome40; this is consistent with previous reports that suggest that TMAO is produced by Firmicutes but not Bacteroidetes.41 Notably, a greater ratio of Firmicutes to Bacteroidetes has previously been associated with an increased risk of obesity and metabolic syndrome.42 This research highlights the important role of gut microbial composition in determining TMAO production and bolsters the notion that higher circulating concentrations of TMAO in a diseased versus nondiseased state may reflect differences in gut microbe composition owing to the disease itself or other lifestyle factors, rather than indicating a causative role of TMAO itself in the disease process.40,43
The choline-TMAO connection is not yet established.
CHOLINE IN HUMAN HEALTH
Choline’s role in human health begins prenatally and extends into adulthood and old age. Its functions are complex and include but are not limited to neurotransmitter synthesis (acetylcholine), cell membrane signaling (phospholipids), lipid transport (lipoproteins), and methyl-group metabolism (homocysteine conversion to methionine). Lactation increases the maternal demand for choline because human milk is rich in choline. More detailed reviews of the health effects of choline across the lifespan have been published elsewhere.2,3,44 The following is an overview of new research that was presented at the 2018 Choline Science Summit.
Placental Health
Increasing evidence demonstrates that the placenta is also sensitive to choline supply throughout pregnancy. Optimal development and functioning of the placenta result in both maternal and fetal health, and impairments result in maternal disease, such as preeclampsia, and undesirable fetal outcomes, including growth restriction. In a recent randomized, controlled feeding trial of 480 versus 930 mg/d of choline intakes, placentas from third-trimester pregnant women consuming higher amounts of dietary choline exhibited significant changes in the expression of genes regulating placental vascularization, angiogenesis, and stress reactivity. Higher maternal choline intakes decrease placental expression of the antiangiogenic factor Fms-like tyrosine kinase-1, a preeclampsia risk factor,45 and infant plasma cortisol,46 which may reduce the risk of stress-related diseases in later life. In vitro studies have demonstrated that lower choline availability induces inflammation47 and that this may impair early placental development and the arterial remodeling required for placental perfusion and nutrient transfer to the fetus. These effects in cultured cells have been largely recapitulated in rodent models, whereby higher dietary choline intakes influence markers of inflammation and angiogenesis and help to facilitate transport of nutrients, including docosahexaenoic acid, across the placenta to the fetus.48,49
Infant and Child Neurocognitive Development
High choline intake during the perinatal period has been demonstrated to have a lasting neuroprotective effect in both animal and human studies. Caudill and others (2017) most recently reported the beneficial effects of a higher third-trimester maternal choline intake (930 vs 480 mg/d) on infant processing speed at 4, 7, 10, and 13 months of age (n = 24). Intriguingly, in the lower maternal choline intake group (ie, 480 mg/d), infants born to mothers who were enrolled for a greater duration of pregnancy consuming the study diet exhibited faster reaction times, suggesting that even modest increases in prenatal choline intake (usually between 300 and 350 mg/d), in the context of a nutritionally complete diet, may produce cognitive benefits in humans.50 In humans, the hippocampus continues to develop after birth, resembling the adult structure by 4 years of age. To evaluate the persistence of these cognitive benefits, Strupp and colleagues recently conducted a follow-up study of the 24 children enrolled in the Caudill et al 2017 cohort at 7 years of age. Results of the cognitive assessment revealed lasting benefits of the higher maternal choline intake on child attention, memory, and problem solving, providing the first randomized controlled trial evidence that higher maternal choline intake has benefits that last into the school-age years.51 Although these findings require confirmation in a larger sample, they provide compelling evidence that maternal choline intake during pregnancy has lasting effects on a child’s cognitive function. Data from observational studies are somewhat mixed but tend to also support that higher choline intakes during pregnancy enhance cognitive outcomes of the offspring.52–55 Consistent with the findings from the Caudill and Strupp laboratories, observational data obtained from Project Viva in Massachusetts demonstrated that maternal choline intake within the AI range during pregnancy was associated with better memory function in children 7 years of age as compared with children of mothers whose consumption was approximately 50% of the AI.53 A narrative review by McCann and others (2006) highlighted 34 rodent studies that examined the relationship of choline during early development and suggested that supplementation during pregnancy may strongly contribute to changes in neurological function in the fetus, as well as improvement in postnatal cognitive behavioral tests across the lifespan.56
Adult Neurocognitive Function and Protection
The mechanisms and clinical evidence with regard to the neurodevelopmental and neuroprotective actions of choline have recently been extensively reviewed by Blusztajn and others57 and Wallace.3 High choline intakes during gestation and early childhood have been shown to enhance cognition across the lifespan in multiple animal models,2,3,57 but this has yet to be confirmed in human studies. Several challenge studies in both younger and older adults provide mixed results.3 The most compelling short-duration challenge study showed supplementation with choline bitartrate to decrease pupil size, a widely accepted biomarker of cholinergic function, within 70 minutes. Healthy younger adults treated with choline bitartrate in this study had greater precision at rapidly hitting centers of targets as compared with those on the placebo (note: participant baseline choline intake or blood status not assessed).58 Human observational studies assessing protection from cognitive decline in older adults also report inconsistent results. However, studies in rodents provide evidence of lasting effects of increased maternal intakes that become more pronounced with aging.3
A recent Cochrane systematic review and meta-analysis of cytidinediphosphocholine-choline supplementation reported improvements in memory and behavior deficits among elderly subjects with chronic cerebral disorders, although only 1 study lasted longer than 3 months. Evidence on global impression was also strong but limited by the duration of studies.59 Higher choline intake has been inversely correlated with white-matter hyperintensity volume among participants in the Framingham Offspring Cohort (average baseline intake, ~320 mg/d),60 a fairly well-accepted marker for Alzheimer’s disease and age-related cognitive decline. The strongest evidence for neuroprotection in humans stems from a case control study of individuals with diagnosed cognitive decline, in which patients with Alzheimer’s disease were shown to exhibit lower levels of 8 choline-containing phospholipids (and 2 non-choline-containing species) as compared with healthy controls. It should be noted that other causes may have been responsible for the disease state, and this study was not designed to assess causality. These 10 lipids derived from peripheral blood have been validated to predict mild cognitive impairment or Alzheimer’s disease within a 2- to 3-year timeframe with over 90% accuracy.61 Although no animal model of age-related cognitive decline fully imitates the human disease state, supplementation has been shown to influence Alzheimer’s disease through modulating the accumulation of amyloid plaques in mice.57
THERE IS A CRITICAL NEED TO INCREASE AWARENESS AND INTAKE OF CHOLINE THROUGH PUBLIC AND PROFESSIONAL EDUCATION
With only an estimated 10% of the US population achieving the AIs and no indication of excessive intakes above the ULs that were set in 1998, coupled with compelling evidence of negative health outcomes associated with lower choline intakes and the absence of harm, the summit participants agreed that there is a need to increase public and health professional awareness of choline by providing education on foods rich in choline. Among health professionals, including registered dietitians and physicians, awareness and knowledge of the importance of choline remain low. Choline has been shown to be ranked last among common nutrients as a nutrient to recommend for a healthy diet, and only about 10% of health professionals indicate moderate familiarity with choline. Among obstetricians and gynecologists, only 6% report they are likely to recommend choline-rich foods to pregnant women.62 Enlisting the support of professional societies to increase the number of feature articles in journals, e-blasts, magazines, tool-kits, webinars, conference presentations, and other continuing education programs will likely help increase the awareness of the importance of the importance of choline in human health. Targeted education to the obstetrics and gynecology communities is a good start; however, all health professionals need to be aware of food sources of choline.
Health professionals need to be aware of food sources of choline.
Continuing to highlight government tools such as MyPlate that assist Americans in selecting nutrient-dense foods that are within daily calorie goals is essential to helping increase intakes of all shortfall nutrients, including choline. Data indicate that increasing consumption of plant foods may offer health benefits; however, this means that there is a need to include more plant foods in the diet, but not necessarily eliminate nutrient-dense animal-derived foods such as eggs, lean meat, and milk products that contain choline. Some participants urged that the National Institutes of Health sponsor a workshop to encourage health professionals outside of the nutrition space to educate themselves on choline. The food industry will be instrumental in developing new product innovations and formulations that are targeted to the needs of the individual consumer. To facilitate these changes, specific recommendations in future iterations of the Dietary Guidelines for Americans for pregnant and lactating women to increase consumption of choline-rich foods with supplementation to fill any dietary gaps are an important step toward bringing attention and awareness for this essential nutrient. Tables 2 to 5 provide menu models for helping patients meet the AIs for choline, based on a 2000-calorie diet and the dietary patterns based on recommendations from the 2015–2020 Dietary Guidelines for Americans.63
TABLE 2.
Menu Model for a Choline-Focused Mediterranean-Style Dieta
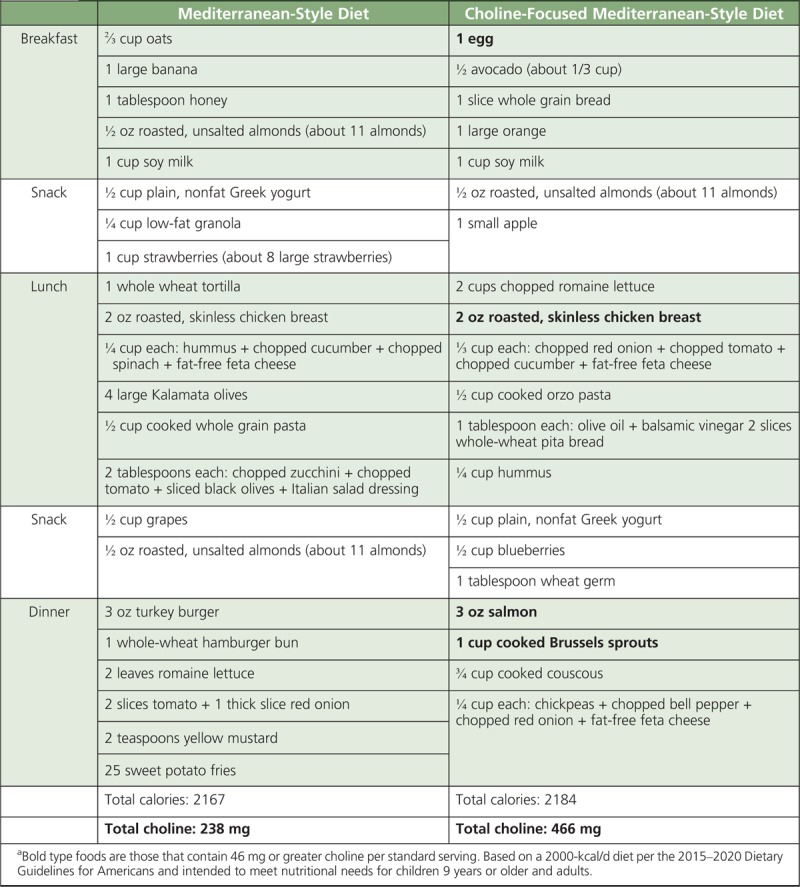
TABLE 5.
Menu Model for a Choline-Focused Pregnancy Eating Patterna
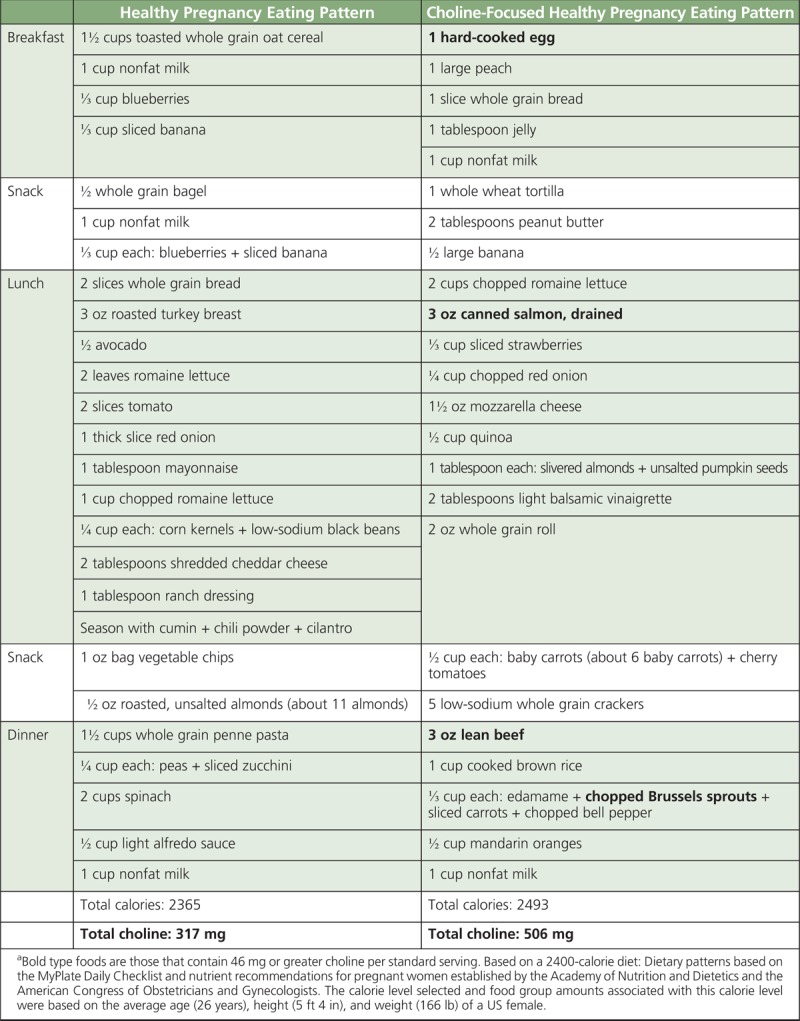
TABLE 3.
Menu Model for a Choline-Focused Vegetarian Dieta
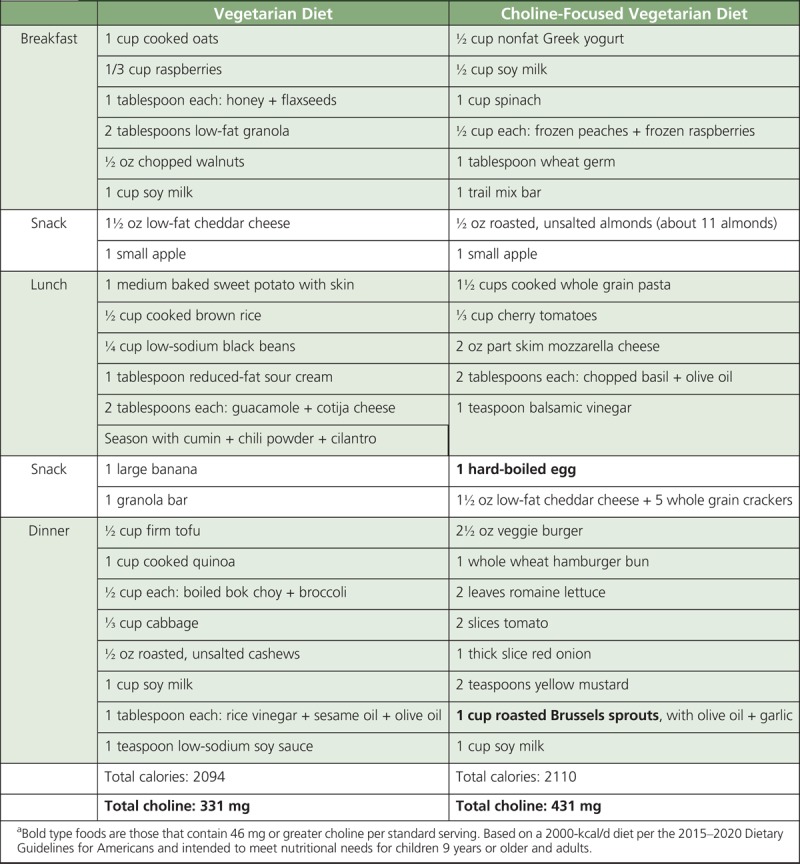
TABLE 4.
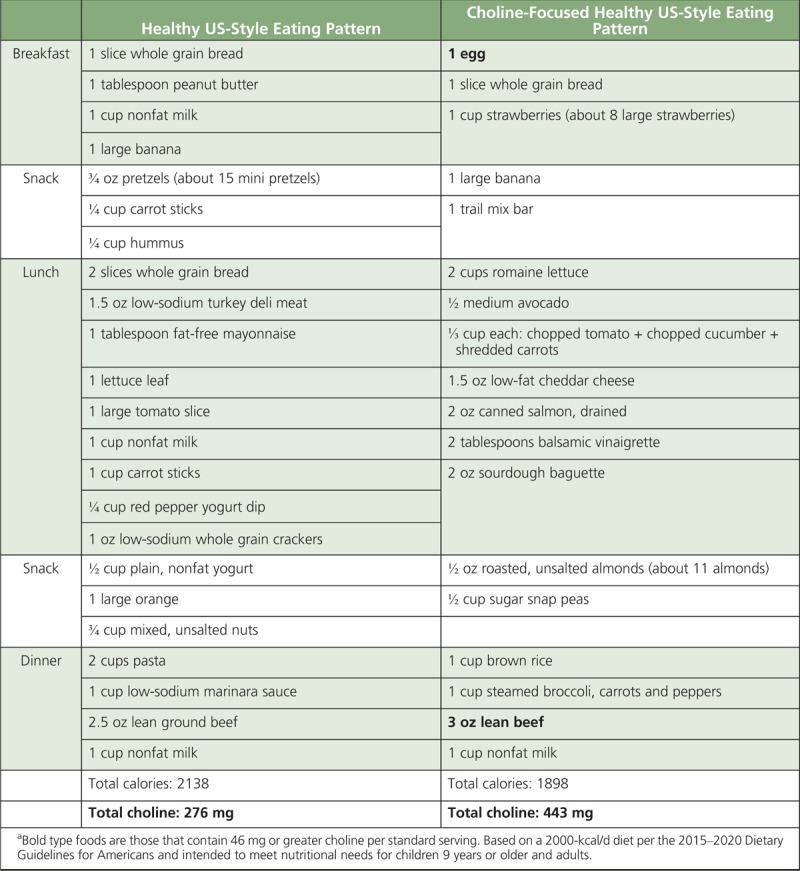
Menu Model for a Choline-Focused Healthy US-Style Eating Patterna
CALL TO ACTION: CONCLUSIONS FROM THE 2018 CHOLINE SCIENCE SUMMIT
Our knowledge and understanding of choline have increased exponentially from the time when the DRIs were developed in 19981 and since the previous 2009 Choline Science Summit.4 Recognition of the growing evidence relating inadequate intakes to health consequences, coupled to evidence of suboptimal intakes in high-risk populations, warrants a need for improved public health recommendations for choline. Choline seems to be a critical nutrient involved with neurocognitive development during gestation and lactation, with lasting effects in children, although studies elucidating the dietary requirement for choline or whether it has lasting effects into adulthood are still largely absent from the scientific literature. The majority of the US population is not consuming sufficient choline to meet the AI. The AI (unlike an EAR) cannot be used to estimate the prevalence of inadequacy,8,13–16 and more research is needed. Additional dose response data by genotype in adults is greatly needed to fully elucidate dietary requirements for choline before EARs and RDAs can be developed. Lack of an EAR severely limits the interpretation of the population intake data because it is not possible to assess whether an intake below the AI results in suboptimal health status. Observational data will likely play a critical role in the revision of the DRIs, particularly for calculating an EAR, since ethical barriers prohibit clinical deprivation of any essential nutrient in humans. Cognition data may facilitate calculation of an EAR during gestation and lactation; however, markers of liver (serum ALT and AST) and muscle function (serum creatine kinase) are also needed and likely the strongest indicators of the choline requirement in healthy nonpregnant, nonlactating adults. Participants attending the roundtable agreed that these dose-response data are needed before identifying choline as a “nutrient of public health concern” in US nutrition policy.
There was general consensus from the more than 40 experts present at the roundtable meeting that the USDA Birth to 24-Months and Pregnant Women Program and the 2020–2025 Dietary Guidelines Advisory Committee should consider the recommendations from the American Medical Association and AAP,9,10 as well as the recent scientific literature presented at the roundtable meeting and summarized in this article. Roundtable attendees offered the following call to action for policy-makers and the nutrition science community:
There is compelling evidence from nationally representative surveys and cohort studies that demonstrate that most of the US population does not meet the current AIs and that excessive intakes above the ULs are absent; establishment of an EAR is critical for determining the point where low intakes result in adverse health outcomes. The choline requirement of an individual depends on one’s genotype and more should be done to educate consumers and health professionals on the importance of choline-rich foods in the diet. Choline must be integrated into the prenatal supplement regimen.
Current research suggests that failing to achieve the AI is likely detrimental to health, particularly in regard to liver and muscle function in healthy adults, as well as cognitive function in the developing fetus and infant. Adverse neurological consequences due to suboptimal maternal choline intakes may be identified in future human clinical research. We must focus on providing resources to all health professionals on the potential consequences of choline inadequacy.
At present, advocating for dietary guidance that helps individuals meet the AI is needed. This is particularly needed for pregnant/lactating women and infants.
Research foundations, the industry, and government agencies such as the National Institutes of Health should consider future studies that have the potential to provide data that could inform revision of the DRIs for choline to include an EAR and RDA as well as support other nutrition policies and programs.

No caption available

No caption available
Footnotes
Funding for this manuscript was provided through an unrestricted educational grant from Balchem, the Egg Nutrition Center, and the Beef Checkoff, a contractor to the National Cattleman’s Beef Association. The funding bodies had no influence or role in the writing of the manuscript and the decision to submit it for publication.
The authors have received research funding, travel reimbursement, and speaker honoraria from Balchem, the Egg Nutrition Center, and the Beef Checkoff, a contractor to the National Cattlemen’s Beef Association.
REFERENCES
- 1.National Academies of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press; 1998. [PubMed] [Google Scholar]
- 2.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace TC. A comprehensive review of eggs, choline, and lutein on cognition across the lifespan. J Am Coll Nutr. 2018;37:269–285. [DOI] [PubMed] [Google Scholar]
- 4.Caudill MA, da Costa K-A, Zeisel S, Hornick B. Elevating awareness and intake of choline: an essential nutrient for public health. Nutrition Today. 2011;46(5):235–241. [Google Scholar]
- 5.Caudill MA, Miller JW, Gregory JF, Shane B. Folate, choline, vitamin B12, and vitamin B6 (chapter 25). In: Stipanuk MH, Caudill MA, eds. Biochemical, Physiological, and Molecular Aspects of Human Nutrition. : 3rd ed 2013:565–608. [Google Scholar]
- 6.Buchman AL, Ament ME, Sohel M, et al. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. JPEN J Parenter Enteral Nutr. 2001;25(5):260–268. [DOI] [PubMed] [Google Scholar]
- 7.Zeisel SH, Da Costa KA, Franklin PD, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 8.Moshfegh AJ. Choline intake in the US. Presented at: 2018 Choline Summit. February 21, 2018; Washington, DC.
- 9.Schwarzenberg SJ, Georgieff MK. Committee on Nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. 2018;141(2): e20173716. [DOI] [PubMed] [Google Scholar]
- 10.AMA Wire. AMA backs global health experts in calling infertility a disease. 2017. https://wire.ama-assn.org/ama-news/ama-backs-global-health-experts-calling-infertility-disease. Accessed February 24, 2018).
- 11.US Department of Agriculture. USDA Database for the Choline Content of Common Foods: Release Two. Washington, DC; 2008. https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Choline/Choln02.pdf. Accessed February 23, 2018. [Google Scholar]
- 12.Jiang X, Yan J, Caudill MA. Choline. In: Zemplini J, Stover PJ, Gregory JF., III, Suttie K, eds. Handbook of Vitamins. Philadelphia, PA: Taylor and Francis; 2013. [Google Scholar]
- 13.Wallace TC, Fulgoni VL., 3rd Assessment of total choline intakes in the United States. J Am Coll Nutr. 2016;35(2):108–112. [DOI] [PubMed] [Google Scholar]
- 14.Wallace TC, Fulgoni VL. Usual choline intakes are associated with egg and protein food consumption in the United States. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace TC. Choline in foods, functional foods and dietary supplements. Presented at: Institute of Food Technologists Annual Meeting; July 12, 2015.
- 16.National Academy of Medicine, Subcommittee on Interpretation and Uses of Dietary Reference Intakes. Dietary Reference Intakes: Applications in Dietary Assessment. Washington, DC: National Academies Press; 2000. [Google Scholar]
- 17.Ganz AB, Klatt KC, Caudill MA. Common genetic variants alter metabolism and influence dietary choline requirements. Nutrients. 2017;9:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–170. [DOI] [PubMed] [Google Scholar]
- 19.Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta. 2012;1821(5):754–761. [DOI] [PubMed] [Google Scholar]
- 20.Song J, da Costa KA, Fischer LM, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005;19:1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21(10):2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resseguie ME, da Costa K-A, Galanko JA, Patel M, Davis IJ, Zeisel SH. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J Biol Chem. 2011;286:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarda IR, Gorwill RH. Hormonal studies in pregnancy, I: total unconjugated estrogens in maternal peripheral vein, cord vein, and cord artery serum at delivery. Am J Obstet Gynecol. 1976;124:234–238. [PubMed] [Google Scholar]
- 24.Adeyemo O, Jeyakumar H. Plasma progesterone, estradiol-17 beta and testosterone in maternal and cord blood, and maternal human chorionic gonadotropin at parturition. Afr J Med Sci. 1993;22(3):55–60. [PubMed] [Google Scholar]
- 25.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. [DOI] [PubMed] [Google Scholar]
- 26.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160(2):102–109. [DOI] [PubMed] [Google Scholar]
- 27.Carmichael SL, Yang W, Shaw GM. Periconceptional nutrient intakes and risks of neural tube defects in California. Birth Defects Res Part A Clin Mol Teratol. 2010;88:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills JL, Fan R, Brody LC, et al. Maternal choline concentrations during pregnancy and choline-related genetic variants as risk factors for neural tube defects. Am J Clin Nutr. 2014;100(4):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA. 2005;102(44):16025–16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd WD, Graham-White J, Blackwood G, Glen I, McQueen J. Clinical effects of choline in Alzheimer senile dementia. Lancet. 1977;2(8040):711. [DOI] [PubMed] [Google Scholar]
- 31.Growdon JH, Cohen EL, Wurtman RJ. Huntington’s disease: clinical and chemical effects of choline administration. Ann Neurol. 1977;1:418–422. [DOI] [PubMed] [Google Scholar]
- 32.Gelenberg AJ, Doller-Wojcik JC, Growdon JH. Choline and lecithin in the treatment of tardive dyskinesia: preliminary results from a pilot study. Am J Psychiatry. 1979;136:772–776. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence CM, Millac P, Stout GS, Ward JW. The use of choline chloride in ataxic disorders. J Neurol Neurosurg Psychiatry. 1980;43(5):452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Food Safety Authority Nutrition Dietetics and Allergies Panel. Scientific opinion on Dietary Reference Values for choline. EFSA J. 2016;14(8):4484, 70 pp. [Google Scholar]
- 35.Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Ann Rev Nutr. 2017;37:157–181. [DOI] [PubMed] [Google Scholar]
- 36.Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab. 2017;28(2):121–130. [DOI] [PubMed] [Google Scholar]
- 37.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer KA, Shea J. Dietary choline and betaine and risk of CVD: a systematic review and meta-analysis of prospective studies. Nutrients. 2017;9(7):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins HL, Drazul-Schrader D, Sulpizio AC, et al. l-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis. 2016;244:29–37. [DOI] [PubMed] [Google Scholar]
- 40.Cho CE, Taesuwan S, Malysheva OV, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324. [DOI] [PubMed] [Google Scholar]
- 41.Falony G, Vieira-Silva S, Raes J. Microbiology meets big data: the case of gut microbiota-derived trimethylamine. Ann Rev Microbiol. 2015;69:305–321. [DOI] [PubMed] [Google Scholar]
- 42.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klatt KC, Caudill MA. Pressing the trimethylamine N-oxide narrative. Am Med J. 2017;2:132. [Google Scholar]
- 44.Leermakers ETM, Moreira EM, Kiefte-de Jong JC, et al. Effects of choline on health across the life course: a systematic review. Nutr Rev. 2015;73(8):500–522. [DOI] [PubMed] [Google Scholar]
- 45.Jiang X, Bar HY, Yan J, et al. A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor Fms-like tyrosine kinase-1 (sFLT1). FASEB J. 2013;27:1245–1253. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X, Yan J, West AA, et al. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 2012;26:3563–3574. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Jones S, Andrew BY, et al. Choline inadequacy impairs trophoblast function and vascularization in cultured human placental trophoblasts. J Cell Physiol. 2014;229(8):1016–1027. [DOI] [PubMed] [Google Scholar]
- 48.Kwan STC, King JH, Yan J, et al. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta. 2017;53:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwan STC, King JH, Yan J, et al. Maternal choline supplementation modulates placental nutrient transport and metabolism in late gestation of mouse pregnancy. J Nutr. 2017;147(11):2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J. 2018;32(4):2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nevins JEH, Beckman KA, Bahnfleth CL, Drewes BM, Caudill MA, Strupp BJ, Canfield RL. Maternal choline supplementation during pregnancy improves executive functioning in children at age 7 y. Presented at: American Society for Nutrition Annual Meeting; Boston, MA; June 9–12, 2018.
- 52.Villamor E, Rifas-Shiman SL, Gillman MW, Oken E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol. 2012;26:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177:1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Signore C, Ueland PM, Troendle J, Mills JL. Choline concentrations in human maternal and cord blood and intelligence at 5 y of age. Am J Clin Nutr. 2008;87(4):896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu BT, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7(8):e43348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biohehav Rev. 2006;30(5):696–712. [DOI] [PubMed] [Google Scholar]
- 57.Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naber M, Hommel B, Colzato LS. Improved human visuomotor performance and pupil constriction after choline supplementation in a placebo-controlled double-blind study. Sci Rep. 2015;5:13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev. 2005;2:CD000269. [DOI] [PubMed] [Google Scholar]
- 60.Poly C, Massaro JM, Seshadri S, et al. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am J Clin Nutr. 2011;94:1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mapstone M, Cheema AK, Fiandaca MS, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20(4):415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.StrategyOne Health Professionals Survey. Online study among 252 health care professionals from Harris Interactive’s Physicians and Specialty Health Professionals Panels. Sponsored by American Egg Board/Egg Nutrition Center. March 2017. http://www.cholineinfo.org/healthcare_professionals/overview.asp. Accessed December 7, 2009).
- 63.Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed December 2015. https://health.gov/dietaryguidelines/2015/guidelines/. Accessed on April 17, 2018.


