Abstract
Background
Orthopaedic wear particles activate the NLRP3 inflammasome to produce active interleukin 1β (IL1β). However, the NLRP3 inflammasome must be primed before it can be activated, and it is unknown whether wear particles induce priming. Toll-like receptors (TLRs) are thought to mediate particle bioactivity. It remains controversial whether pathogen-associated molecular patterns (PAMPs) and/or alarmins are responsible for TLR activation by wear particles.
Questions/purposes
(1) Does priming of the NLRP3 inflammasome by wear particles depend on adherent PAMPs? (2) Does priming of the NLRP3 inflammasome by wear particles depend on TLRs and TIRAP/Mal? (3) Does priming of the NLRP3 inflammasome by wear particles depend on cognate TLRs? (4) Does activation of the NLRP3 inflammasome by wear particles depend on adherent PAMPs?
Methods
Immortalized murine macrophages were stimulated by as-received titanium particles with adherent bacterial debris, endotoxin-free titanium particles, or titanium particles with adherent ultrapure lipopolysaccharide. To study priming, NLRP3 and IL1β mRNA and IL1β protein levels were assessed in wild-type, TLR4-/-, TLR2-/-, and TIRAP/Mal-/- macrophages. To study activation, IL1β protein secretion was assessed in wild-type macrophages preprimed with ultrapure lipopolysaccharide.
Results
Compared with titanium particles with adherent bacterial debris, endotoxin-free titanium particles induced 86% less NLRP3 mRNA (0.05 ± 0.03 versus 0.35 ± 0.01 NLRP3/GAPDH, p < 0.001) and 91% less IL1β mRNA (0.02 ± 0.01 versus 0.22 ± 0.03 IL1β/GAPDH, p < 0.001). ProIL1β protein level was robustly increased in wild-type macrophages stimulated by particles with adherent PAMPs but was not detectably produced in macrophages stimulated by endotoxin-free particles. Adherence of ultrapure lipopolysaccharide to endotoxin-free particles reconstituted stimulation of NLRP3 and IL1β mRNA. Particles with adherent bacterial debris induced 79% less NLRP3 mRNA (0.09 ± 0.004 versus 0.43 ± 0.13 NLRP3/GAPDH, p < 0.001) and 40% less IL1β mRNA (0.09 ± 0.04 versus 0.15 ± 0.03 IL1β/GAPDH, p = 0.005) in TLR4-/- macrophages than in wild-type. Similarly, those particles induced 49% less NLRP3 mRNA (0.22 ± 0.10 versus 0.43 ± 0.13 NLRP3/GAPDH, p = 0.004) and 47% less IL1β mRNA (0.08 ± 0.02 versus 0.15 ± 0.03 IL1β/GAPDH, p = 0.012) in TIRAP/Mal-/- macrophages than in wild-type. Particles with adherent ultrapure lipopolysaccharide induced 96% less NLRP3 mRNA (0.012 ± 0.001 versus 0.27 ± 0.05 NLRP3/GAPDH, p = 0.003) and 91% less IL1β mRNA (0.03 ± 0.01 versus 0.34 ± 0.07 IL1β/GAPDH, p < 0.001) expression in TLR4-/- macrophages than in wild-type. In contrast, those particles did not induce less NLRP3 and IL1β mRNA in TLR2-/- macrophages. IL1β protein secretion was equivalently induced by particles with adherent bacterial debris or by endotoxin-free particles in a time-dependent manner in wild-type macrophages. For example, particles with adherent bacterial debris induced 99% ± 2% of maximal IL1β secretion after 12 hours, whereas endotoxin-free particles induced 92% ± 11% (p > 0.5).
Conclusions
This cell culture study showed that adherent PAMPs are required for priming of the NLRP3 inflammasome by wear particles and this process is dependent on their cognate TLRs and TIRAP/Mal. In contrast, activation of the NLRP3 inflammasome by titanium particles is not dependent on adherent PAMPs. Animal and implant retrieval studies are needed to determine whether wear particles have similar effects on the NLRP3 inflammasome in vivo.
Clinical Relevance
Our findings, together with recent findings that aseptic loosening associates with polymorphisms in the TIRAP/Mal locus, support that adherent PAMPs may contribute to aseptic loosening in patients undergoing arthroplasty.
Introduction
Aseptic loosening caused by polymeric and metallic wear particles [7] is the most common long-term reason for revision surgery after total joint arthroplasty despite the advent of crosslinked polyethylene [5]. Particles stimulate macrophages to release proinflammatory cytokines, which induce osteoclast differentiation and osteolysis [30, 33, 40, 57]. Interleukin 1β (IL1β; Table 1), along with IL1α, IL6, and TNFα, mediates particle-induced osteoclast differentiation in vitro [71]. IL1Ra, which antagonizes both IL1α and IL1β, reduces particle-induced osteolysis in mice [75] and a IL1Ra polymorphism is associated with aseptic loosening in patients [29], suggesting that IL1β plays a major role in aseptic loosening.
Table 1.
Abbreviations

The Nod-like-receptor-protein-3 (NLRP3, also known as NALP3) inflammasome processes inactive proIL1β to active IL1β [26]. The inflammasome consists of a sensor protein (NLRP3), an adaptor (apoptosis-associated-speck-like-protein [ASC]), and an effector protease (caspase-1). The NLRP3 inflammasome is regulated by a two-checkpoint system known as priming and activation (Fig. 1). Priming involves transcription and translation of NLRP3 and proIL1β. Priming can be induced by pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide and lipoteichoic acid and other inflammatory mediators that also stimulate the NF-κB signaling pathway [8, 12, 26, 44, 52, 58]. Activation of the NLRP3 inflammasome involves assembly of the complex and processing of proIL1β to IL1β. Activation can be induced by ATP, nonorthopaedic particles, and numerous other stimuli [8, 12, 26, 39, 44, 52, 67]. Caspase-1, the effector protease of all known inflammasomes, contributes to particle-induced osteolysis [13]. The NLRP3 inflammasome processes IL1β in response to orthopaedic particles [3, 13-15, 42, 49, 50, 63, 68]. However, those studies focused on activation of the NLRP3 inflammasome in preprimed macrophages and did not examine whether the particles can prime the NLRP3 inflammasome, which is important because priming is a prerequisite for activation [8, 12, 26, 44, 52, 58].
Fig. 1.
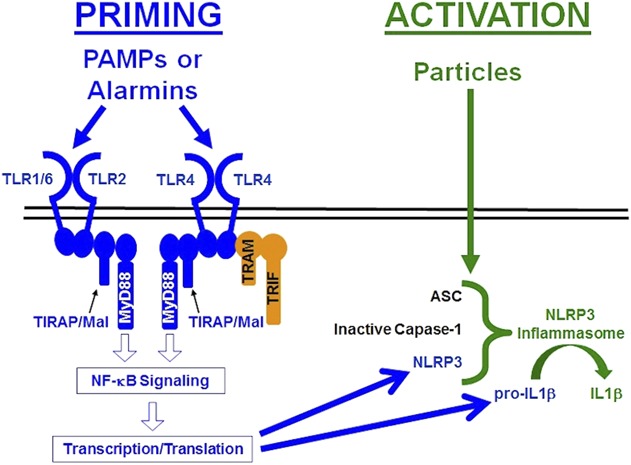
This diagram illustrates the pathways responsible for priming (blue) and activation (green) of the NLRP3 inflammasome. See text for details.
Priming the NLRP3 inflammasome can be induced by stimulation of toll-like receptors (TLRs) [8, 12, 26, 44, 52, 58], which are also associated with aseptic loosening [30, 45, 70]. Polymorphisms in the TLR pathway associate with aseptic loosening [48] and TLR-positive macrophages are increased in periprosthetic tissue of patients with aseptic loosening [45]. TLRs can be activated by exogenous PAMPs [1] or endogenous danger signals known as alarmins or danger-associated molecular patterns [11, 51]. For example, particle-induced osteolysis in mice is dependent on adherent PAMPs, their cognate TLRs (TLR2 and TLR4), and Toll-interleukin 1 receptor domain-containing adapter protein/Myd88 adapter-like protein (TIRAP/Mal), a member of the MyD88 family of adaptor proteins that is uniquely specific to TLR2 and TLR4 [9, 10, 32]. The requirement for a PAMP-TLR cognate pair supports the conclusion that alarmins are not sufficient and therefore adherent PAMPs are needed to induce TLR activation in cell culture and mouse models of particle-induced osteolysis [9, 32]. Consistent with that conclusion, the idea that bacterial PAMPs contribute to aseptic loosening has received considerable interest [30, 31, 38, 47, 56, 69, 74] but remains controversial [30, 45, 61, 69, 70, 74]. It is unknown whether adherent PAMPs are needed for orthopaedic particles to prime and/or activate the NLRP3 inflammasome, because the previous studies on wear particles and the NLRP3 inflammasome did not compare the effects of particles with and without adherent PAMPs [3, 13, 14, 42, 49, 50, 61, 63, 68].
This study was therefore designed to determine whether orthopaedic particles can prime the NLRP3 inflammasome and whether priming and/or activation of the NLRP3 inflammasome by orthopaedic particles depend on adherent PAMPs, their cognate TLRs, and TIRAP/Mal. Specifically we asked: (1) Does priming of the NLRP3 inflammasome by wear particles depend on adherent PAMPs? (2) Does priming of the NLRP3 inflammasome by wear particles depend on TLRs and TIRAP/Mal? (3) Does priming of the NLRP3 inflammasome by wear particles depend on cognate TLRs? (4) Does activation of the NLRP3 inflammasome by wear particles depend on adherent PAMPs?
Materials and Methods
Study Design
We first validated the current cell culture model system by determining whether it reproduces previous studies showing that stimulation of IL1β secretion by wear particles depends on the NLRP3 inflammasome and on adherent PAMPs. To address the first study question, we compared priming in response to titanium particles with adherent bacterial debris and endotoxin-free titanium particles (Fig. 2). To address the second question, we compared priming by wild-type, TLR2-/-, TLR4-/-, and TIRAP/Mal-/- macrophages in response to titanium particles with adherent bacterial debris (Fig. 2). To address the third question, we compared priming in response to titanium particles with adherent ultrapure lipopolysaccharide by wild-type, TLR2-/-, and TLR4-/- macrophages (Fig. 2). To address the final question, we compared activation by preprimed cells in response to titanium particles with adherent bacterial debris and endotoxin-free titanium particles (Fig. 2).
Fig. 2.
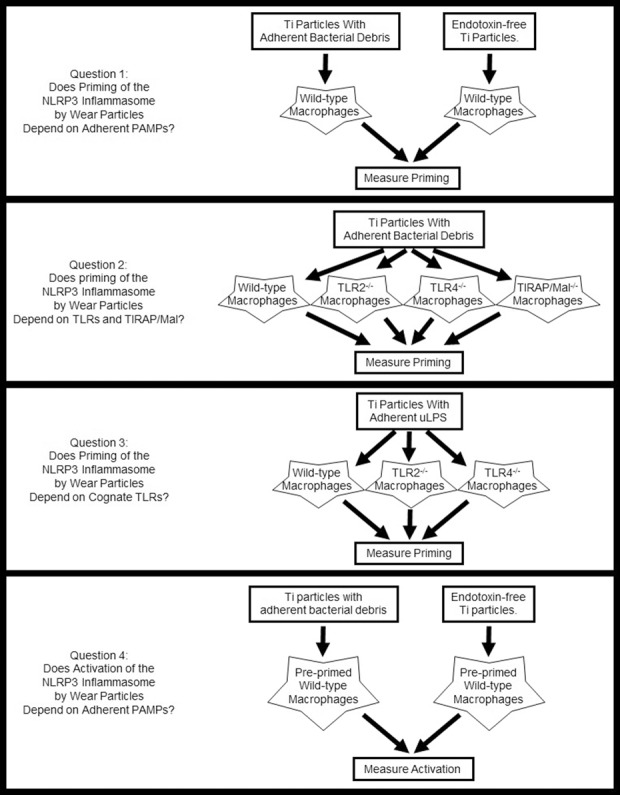
This diagram illustrates the overall study design. See text for details.
Titanium Particles
As-received titanium particles (catalog number 00681, lot F06Q16; Johnson Matthey, Royston, UK) have substantial adherent bacterial debris (34 EU/109 particles) [32, 59]. Endotoxin-free titanium particles (< 0.3 EU/109 particles) were prepared by removing > 99% of the endotoxin from the as-received particles as we previously described [59]. Titanium particles with adherent lipopolysaccharide (33 EU/109 particles) were prepared by incubating endotoxin-free titanium particles with 50 μg/mL ultrapure lipopolysaccharide (tlrl-eblps; InvivoGen, San Diego, CA, USA) for 4 days in phosphate-buffered saline (PBS) containing 1.1 mM calcium chloride [4, 32]. Those particles were then washed 10 times in PBS with calcium chloride to remove any unbound, soluble lipopolysaccharide. The final wash was confirmed to be endotoxin-free, documenting removal of unbound lipopolysaccharide. Endotoxin in the particle suspensions was measured using a chromogenic Limulus amebocyte lysate assay (50-647U; Lonza, Basel, Switzerland) as we previously described [55]. Thus, false-negatives resulting from assay inhibition were eliminated by assaying particle suspensions spiked with known amounts of endotoxin [55]. β-glucan blocker (Lonza) was used to eliminate false-positives resulting from β-glucan [55]. As-received particles, endotoxin-free particles, and particles with adherent ultrapure lipopolysaccharide were used for all experiments.
Cell Culture
Immortalized wild-type, NLRP3-/-, and TIRAP/Mal-/- macrophages were a gift from Dr Katherine Fitzgerald (University of Massachusetts Medical School). Immortalized TLR2-/- and TLR4-/- macrophages were obtained from BEI Resources (Manassas, VA, USA). The macrophage lines were immortalized as previously described and accurately reflect the phenotype of freshly isolated macrophages [9, 35, 54]. Cells were maintained in Minimal Essential Medium (Hyclone, South Logan, UT, USA) with 10% heat-inactivated fetal bovine serum (Hyclone), nonessential amino acids (Mediatech, Manassas, VA, USA), L-glutamine (Mediatech), streptomycin (Mediatech), and penicillin (Mediatech). Macrophages were plated at 2.5 x 105 cells/cm2 in 24-well culture plates (Falcon, Tewksbury, MA, USA) for mRNA and enzyme-linked immunosorbent assay (ELISA) experiments or in six-well plates (Falcon) for Western blot experiments.
To study priming of the NLRP3 inflammasome, cells were stimulated with 1 x 108 particles/cm2 of titanium particles with adherent bacterial debris, titanium particles with adherent ultrapure lipopolysaccharide, endotoxin-free titanium particles, 4 μg/mL ultrapure lipopolysaccharide as a positive control, or a negative control (culture medium supplemented as previously described). Priming the NLRP3 inflammasome was determined by measuring NLRP3 and IL1β mRNA by real-time polymerase chain reaction and proIL1β protein by Western blot (described subsequently).
To study NLRP3 inflammasome activation, cells were preprimed with 4 μg/mL ultrapure lipopolysaccharide for 4 hours. Cells were washed three times with PBS and subsequently stimulated with titanium particles with adherent bacterial debris, endotoxin-free titanium particles, 5 mM ATP as a positive control, or a negative control (culture medium supplemented as described previously). Activation of the NLRP3 inflammasome was determined by measuring secreted IL1β protein by ELISA.
Measurement of Specific mRNAs
Total RNA was isolated using the Promega SV Total RNA Isolation System and measured spectrophotometrically (Nanodrop™; Thermo Fisher Scientific, Waltham, MA, USA). Two hundred nanograms of total RNA were converted to cDNA using the Superscript First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Real-time polymerase chain reaction (PCR) was performed using SYBR™ Green (BioRad, Hercules, CA, USA) with the following primers: NLRP3 forward 5′-gcaacctccagaaactgtggt-3′, reverse 5′-tgggtccttcatcttttcaca-3′; IL-1β forward 5′-gaccccaaaagatgaaggg-3′, reverse 5′- aggtgctcatgtcctcatcc-3′; GAPDH forward 5′-atgggaagctggtcatcaac-3′, reverse 5′-gtggttcacacccatcacaa-3′. To exclude false-positives from genomic DNA, 3′ termini of the PCR primers overlap exon-exon junctions [16], except for the GAPDH amplicon, which is encoded within a single exon. All primers were validated by sequencing of PCR amplicons. Gene expression was determined from a standard curve [18] and normalized to GAPDH levels. Specificity of each reaction was verified by melt curve analysis and agarose gel electrophoresis.
Measurement of IL-1β Proteins
Culture supernatants were harvested, and cells were lysed with RIPA buffer (Thermo Fisher, Waltham, MA, USA) and both were stored at -80° C. IL1β ELISAs were performed on supernatants (Biolegend, San Diego, CA, USA). For Western blots, proteins of cell lysates were separated on 15% polyacrylamide gels (Lonza) and transferred onto polyvinylidene fluoride membranes (BioRad) at 24 V for 1 hour at 4° C. The membranes were then blocked with 5% nonfat milk in Tris-buffered-saline for 1 hour at room temperature and incubated with primary antibody overnight at 4° C followed by a secondary antibody for 1 hour at room temperature. Thereafter, membranes were incubated with chemoluminescent solution (Prime-ECL™; GE Healthcare, Chicago, IL, USA). Membranes were washed with Tris-buffered saline with 0.1% Tween (Sigma, St Louis, MO, USA) before administration of a secondary antibody and chemoluminescent solution. Membranes were imaged digitally (Kodak Image Station In-Vivo FX, Rochester, NY, USA). All Western blots are representative images of three independent experiments.
Primary antibodies were murine antihuman IL1β (3ZD, dilution-1:40,000; National Institutes of Health, Bethesda, MD, USA) and goat antimurine actin (Santa Cruz Biotechnology, Dallas, TX, USA; sc-1615, dilution-1:5000). Secondary antibodies conjugated to horseradish peroxidase were goat antimurine IgG and donkey antigoat IgG (Santa Cruz Biotechnology; sc-2005 and sc-2020, dilution-1:5000).
Statistics
All quantitative data represent means ± SD from n = 3 to 5 independent experiments. Each experiment included triplicate cell culture wells per group, each assayed in triplicate. Because data passed normality as assessed by the Shapiro-Wilk test and equal variance as assessed by the Brown Forsythe test, analysis was by two-way analysis of variance with one-sided post hoc Bonferroni corrections (Prism; GraphPad Software, La Jolla, CA, USA). Significance is denoted by *p < 0.05, **p < 0.01, *** p < 0.001. Error bars represent SD.
Results
Validation of Cell Culture Model System for Investigation of Effects of Wear Particles on the NLRP3 Inflammasome
Compared with the vehicle control, particles with adherent bacterial debris stimulated robust secretion of IL1β by wild-type macrophages (Fig. 3; mean difference 97.6% of maximal secretion [95% confidence interval {CI}, 96.6-98.6], p < 0.001). In contrast, NLRP3-/- macrophages secreted 93% less IL1β in response to particles with adherent bacterial debris (Fig. 3 [95% CI, 85-100], 7.4% ± 4.6% versus 100% of maximal secretion, p < 0.001). Moreover, endotoxin-free particles induced 94% less IL1β secretion by wild-type macrophages than particles with adherent bacterial debris (Fig. 3 [95% CI, 91-96], 6.3% ± 1.7% versus 100% of maximal secretion, p < 0.001). These results are consistent with previous studies showing that secretion of mature IL1β in response to wear particles depends on the NLRP3 inflammasome [3, 13-15, 42, 49, 50, 63, 68] and on adherent PAMPs [2, 9, 10, 17, 19] and therefore validated our cell culture model system for investigation of priming and activation of the NLRP3 inflammasome by orthopaedic wear particles.
Fig. 3.
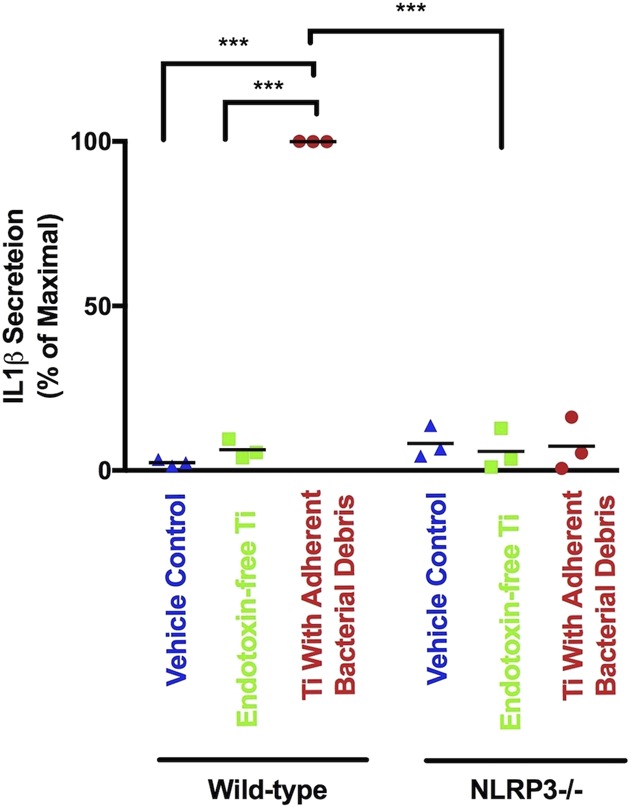
IL1β protein secretion depends on the NLRP3 inflammasome and adherent PAMPs. Wild-type and NLRP3-/- macrophages were treated with a vehicle control (blue triangles), endotoxin-free titanium particles (green squares), or titanium particles with adherent bacterial debris (red circles) for 8 hours. IL1β was measured in supernatant using ELISA. All values are shown as percent of maximal IL1β secretion in that experiment, which are the wild-type macrophages stimulated by titanium particles with adherent bacterial debris. N = 3 independent experiments were performed. Each experiment included triplicate cell culture wells per group, each assayed in triplicate. Error bars represent SD. Statistical significance was determined by one-sided analysis of variance. ***p < 0.001. Ti = titanium; NLRP3 = Nod-like-receptor-protein-3.
Priming of the NLRP3 Inflammasome by Wear Particles Depends on Adherent PAMPs
NLRP3 and IL1β mRNAs were robustly expressed in macrophages stimulated by particles with adherent bacterial debris, but not in macrophages stimulated by endotoxin-free titanium particles (Fig. 4A-B). For example, compared with titanium particles with adherent bacterial debris at the 2-hour time point, endotoxin-free titanium particles induced 86% less NLRP3 mRNA (Fig. 4A; 0.05 ± 0.03 versus 0.35 ± 0.01 NLRP3/GAPDH, mean difference 0.30 [95% CI, 0.21-0.40], p < 0.001) and 91% less IL1β mRNA (Fig. 4B; 0.02 ± 0.01 versus 0.22 ± 0.03 IL1β/GAPDH, mean difference 0.20 [95% CI, 0.11-0.30], p < 0.001). ProIL1β protein level was robustly increased in wild-type macrophages stimulated by particles with adherent PAMPs but was not detectably produced in macrophages stimulated by endotoxin-free particles (Fig. 4C). Adherence of ultrapure lipopolysaccharide to endotoxin-free particles reconstituted stimulation of NLRP3 mRNA (Fig. 5A; 0.27 ± 0.05 versus 0.02 ± 0.001 NLRP3/GAPDH, mean difference 0.25 [95% CI, 0.21-0.30], p < 0.001) and IL1β mRNA (Fig. 5B; 0.34 ± 0.07 versus 0.05 ± 0.004 IL1β/GAPDH, mean difference 0.29 [95% CI, 0.22-0.36], p < 0.001). Together, these results show that priming of the NLRP3 inflammasome by wear particles depends on adherent PAMPs.
Fig. 4 A-C.
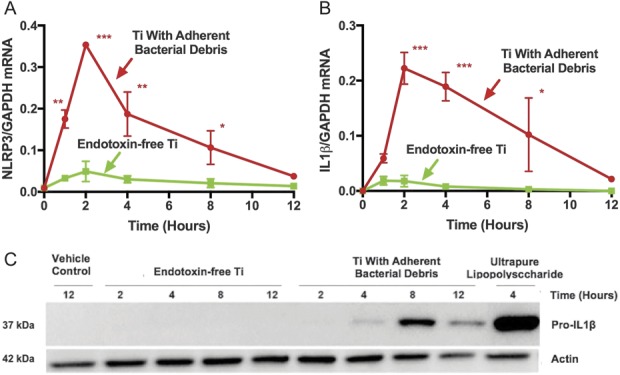
Priming of the NLRP3 inflammasome depends on adherent PAMPs at the mRNA (A-B) and protein (C) levels. Wild-type macrophages were treated with either endotoxin-free titanium particles (A-B, green squares) or titanium particles with adherent bacterial debris (A-B, red circles). Markers of priming (NLRP3 and IL1β mRNA and pro-IL1β protein) were measured in cell lysates at indicated time points and the mRNA values were normalized to GAPDH mRNA. N = 3 independent experiments were performed (A-B). Each experiment included triplicate cell culture wells per group, each assayed in triplicate. Error bars represent SD. Statistical significance was determined by one-sided analysis of variance. *p < 0.05, **p < 0.01, ***p < 0.001 with comparisons made between titanium particles with adherent bacterial debris and endotoxin-free titanium particles at each indicated time point. Pro-IL1β protein was detected by Western blot (C). The pictured gel is representative of three independent experiments. Ti = titanium; NLRP3 = Nod-like-receptor-protein-3; mRNA = messenger RNA; GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Fig. 5 A-B.
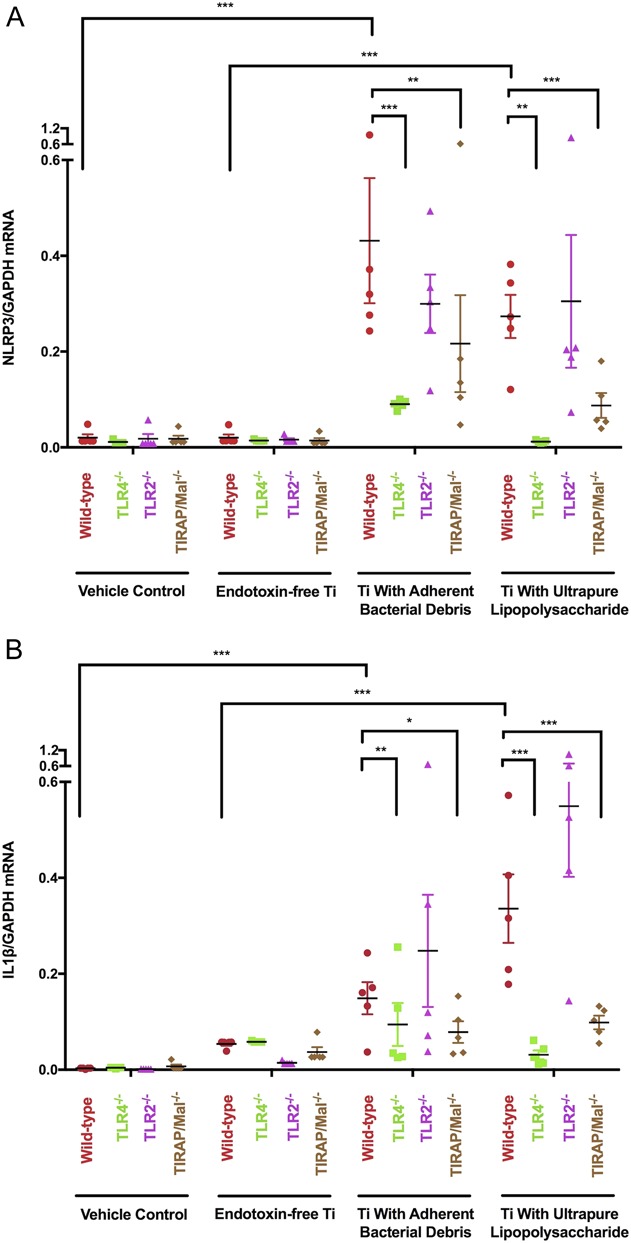
Priming of the NLRP3 inflammasome depends on adherent PAMPs, their cognate TLRs, and TIRAP/Mal. Wild-type (red circle), TLR4-/- (green square), TLR2-/- (purple triangle), and TIRAP/Mal-/- (brown diamond) macrophages were treated with vehicle control, endotoxin-free titanium particles, titanium particles with adherent bacterial debris, or titanium particles with adherent uLPS for 2 hours. NLRP3 and IL1β mRNA were measured in cell lysates and the mRNA values were normalized to GAPDH mRNA. N = 5 independent experiments were performed. Each experiment included triplicate cell culture wells per group, each assayed in triplicate. Error bars represent SD. Statistical significance was determined by one-sided analysis of variance. *p < 0.05, ** p < 0.01, ***p < 0.001.
Priming of the NLRP3 Inflammasome by Wear Particles Depends on TLR4 and TIRAP/Mal
Particles with adherent bacterial debris induced 79% less NLRP3 mRNA (Fig. 5A; 0.09 ± 0.004 versus 0.43 ± 0.13 NLRP3/GAPDH, mean difference 0.34 [95% CI, 0.17-0.51], p < 0.001) and 40% less IL1β mRNA (Fig. 5B; 0.09 ± 0.04 versus 0.15 ± 0.03 IL1β/GAPDH, mean difference 0.06 [95% CI, 0.02-0.10], p = 0.005) in TLR4-/- macrophages than in wild-type macrophages. Similarly, those particles induced 49% less NLRP3 mRNA (Fig. 5A; 0.22 ± 0.10 versus 0.43 ± 0.13 NLRP3/GAPDH, mean difference 0.21 [95% CI, 0.05-0.38], p = 0.004) and 47% less IL1β mRNA (Fig. 5B; 0.08 ± 0.02 versus 0.15 ± 0.03 IL1β/GAPDH, mean difference 0.07 [95% CI, 0.03-0.11], p = 0.012) in TIRAP/Mal-/- macrophages than in wild-type macrophages. In contrast, deletion of TLR2 did not detectably reduce NLRP3 expression (Fig. 5A; 0.30 ± 0.06 versus 0.43 ± 0.13 NLRP3/GAPDH, mean difference 0.13 [95% CI, -0.06 to 0.33], p = 0.13) or IL1β expression (Fig. 5B; 0.25 ± 0.12 versus 0.15 ± 0.03 IL1β/GAPDH, mean difference 0.10 [95% CI, -0.04 to 0.24], p = 0.13) in response to particles with adherent bacterial debris. Together, these results show that priming of the NLRP3 inflammasome by wear particles depends on TLR4 and TIRAP/Mal.
Priming of the NLRP3 Inflammasome by Wear Particles Depends on Cognate TLRs
Particles with adherent ultrapure liposaccharide induced 96% less NLRP3 mRNA (Fig. 5A; 0.012 ± 0.001 versus 0.27 ± 0.05 NLRP3/GAPDH, mean difference 0.26 [95% CI, 0.06-0.46], p = 0.003) and 91% less IL1β mRNA (Fig. 5B; 0.03 ± 0.01 versus 0.34 ± 0.07 IL1β/GAPDH, mean difference 0.31 [95% CI, 0.16-0.44], p < 0.001) expression in TLR4-/- macrophages than in wild-type macrophages. In contrast, those particles did not induce less NLRP3 mRNA (Fig. 5A; 0.31 ± 0.14 versus 0.27 ± 0.05 NLRP3/GAPDH, nonsignificant [NS]) or IL1β mRNA (Fig. 5B; 0.55 ± 0.15 versus 0.34 ± 0.07 IL1β/GAPDH, NS) in TLR2-/- macrophages when compared with wild-type macrophages. Together, these results show that priming of the NLRP3 inflammasome by particles with adherent uLPS depends on the cognate TLR4, but not on the noncognate TLR2.
Activation of the NLRP3 Inflammasome by Wear Particles Does Not Depend on Adherent PAMPs
IL1β protein secretion was equivalently induced by particles with adherent bacterial debris or by endotoxin-free particles in a time-dependent manner in wild-type macrophages that had been preprimed with lipopolysaccharide (Fig. 6). For example, particles with adherent bacterial debris induced 99% ± 2% of maximal IL1β secretion after 12 hours, whereas endotoxin-free particles induced 92% ± 11% (mean difference 7 [95% CI, -36 to 23], p > 0.5 at all time points). Activation induced by the endotoxin-free particles is unlikely the result of residual lipopolysaccharide from the prepriming step because the vehicle control group did not induce detectable activation (Fig. 6; 0.83 ± 0.03 versus 99% ± 2% of maximal IL1β secretion after 12 hours, mean difference 98 [95% CI, 95-101], p < 0.001). Together, these results show that activation of the NLRP3 inflammasome by wear particles does not depend on adherent PAMPs.
Fig. 6.
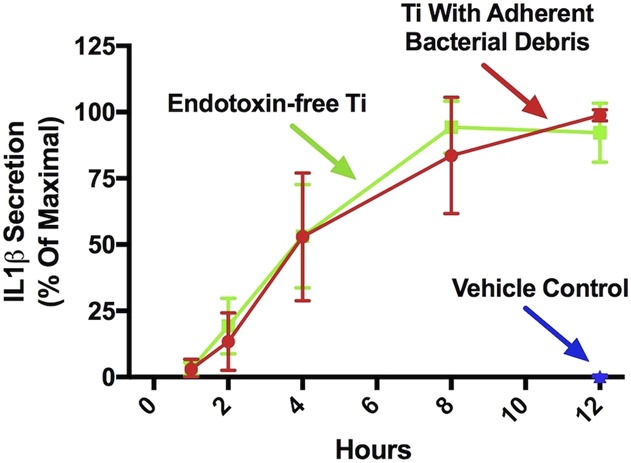
Activation of the NLRP3 inflammasome does not depend on adherent PAMPs. Wild-type macrophages were primed with uLPS for 4 hours and subsequently treated with a vehicle control (blue triangle), endotoxin-free titanium particles (green squares), or titanium particles with adherent bacterial debris (red circles). IL1β protein was measured in culture supernatants at the indicated time points using ELISA. All values are shown as percent of maximal IL1β secretion in that experiment. N = 3 independent experiments were performed. Each experiment included triplicate cell culture wells per group, each assayed in triplicate. Error bars represent SD. Statistical significance was determined by one-sided analysis of variance. p > 0.5 at all time points when comparing titanium particle with adherent bacterial debris and endotoxin-free titanium particles at each indicated time point. Ti = titanium.
Discussion
Previous studies showed that orthopaedic wear particles can activate the NLRP3 inflammasome to process proIL1β to bioactive IL1β but did not examine whether the particles can prime the NLRP3 inflammasome, which is a prerequisite for activation [8, 12, 26, 44, 52, 58]. Moreover, it was unknown whether adherent PAMPs are needed for orthopaedic particles to prime and/or activate the NLRP3 inflammasome, because the previous studies did not compare the effects of particles with and without adherent PAMPs [3, 13-15, 42, 49, 50, 63, 68]. Our aim was therefore to evaluate whether orthopaedic particles prime the NLRP3 inflammasome and whether priming and/or activation are dependent on adherent PAMPs. Our results document that adherent PAMPs are required for particles to prime the NLRP3 inflammasome and that this process is dependent on their cognate TLRs and TIRAP/Mal (Fig. 7). In contrast, our results document that activation of the NLRP3 inflammasome by particles is not dependent on adherent PAMPs (Fig. 7).
Fig. 7.
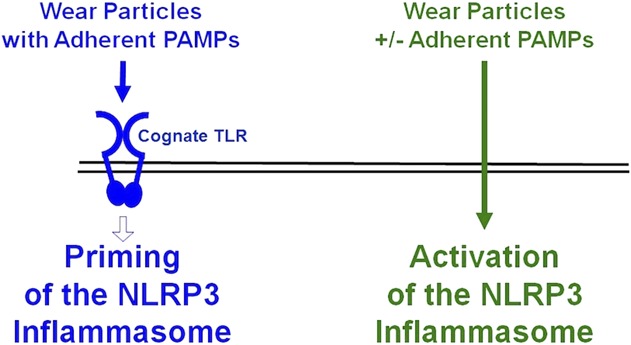
This diagram illustrates the current working model. Priming of the NLRP3 inflammasome by wear particles depends on adherent PAMPs and their cognate TLRs (blue). Activation of the NLRP3 inflammasome by wear particles does not depend on adherent PAMPs (green). See text for details.
One limitation is that any cell culture study may not reflect the in vivo situation. For example, the NLRP3 inflammasome might be primed in vivo in a subset of macrophages by alarmins released either constitutively or in response to particle-induced tissue damage [51]. Wear particles would then be expected to activate the NLRP3 inflammasome in those macrophages even in the absence of PAMPs. Such a mechanism may account for our previous results that endotoxin-free titanium particles induce approximately 50% as much osteolysis in murine calvaria as is induced by titanium particles with adherent bacterial debris [32]. However, osteolysis induced by endotoxin-free particles is not affected by deletion of TLR2 or TLR4, either alone or together [32]. Thus, if alarmins contribute to particle-induced osteolysis in murine calvaria, they do so independently of TLR2 and TLR4. A second limitation is that the PAMP removal process [59] may alter particle surface chemistry and thereby impair cellular responses [70]. However, no differences are detected in shape, size, or surface chemical composition [22, 59]. Moreover, changes in chemical properties (hydrophobicity, oxidation, ionization, charge, etc) would likely be reversible during the extensive PBS washes that follow PAMP removal [59]. In addition, other methods of PAMP removal that would be expected to have different effects on surface chemistry also substantially reduce particle bioactivity [17, 22, 64, 65, 73]. Finally, results that adherence of lipopolysaccharide [28, 32, 37, 64] or lipoteichoic acid [32, 53] reconstitutes the bioactivity and TLR dependence of particles strongly argues against an impaired response resulting from surface chemistry. Some authors have claimed that the particles with adherent lipopolysaccharide expose macrophages to levels of lipopolysaccharide found during sepsis [61, 62]. However, those authors did not note that most of the lipopolysaccharide is removed from the particle suspensions during the extensive PBS washes [59]. A third limitation is the sole use of titanium particles, because polyethylene is the predominant wear debris in most patients with aseptic loosening [7, 60]. Investigation of polyethylene particles in cell culture is not feasible because they float in culture media, preventing interactions with the macrophages. However, adherent PAMPs increase the bioactivity of all types of orthopaedic particles [30, 31, 37, 53, 61, 62], including polyethylene [30, 32, 76]. Moreover, titanium and polyethylene particles produce indistinguishable responses in the mouse calvarial model [43, 46, 72] and in patients with aseptic loosening [34].
The initial experiments in this study showed that IL1β secretion in response to titanium particles depends on the NLRP3 inflammasome and adherent PAMPs. Those results are consistent with previous studies that documented dependence of IL1β secretion in response to wear particles on the NLRP3 inflammasome [3, 13-15, 42, 49, 50, 63, 68] and on adherent PAMPs [2, 9, 10, 17, 19] and therefore validated our cell culture model system for investigation of priming and activation of the NLRP3 inflammasome by orthopaedic wear particles.
This study found that inflammasome priming by orthopaedic particles depends on adherent PAMPs, their cognate TLRs, and TIRAP/Mal. The following evidence documents that alarmins are insufficient to activate TLRs and prime the NLRP3 inflammasome and that this process is dependent on adherent PAMPs. First, we found that endotoxin-free particles, in contrast to particles with adherent bacterial debris, are unable to prime the NLRP3 inflammasome. Those results are consistent with previous findings that adherence of lipopolysaccharide [9, 28, 32, 37] or lipoteichoic acid [32, 53] substantially increases stimulation by particles of mRNAs encoding IL1β or NLRP3. Second, noncognate TLRs do not contribute to priming by particles with adherent PAMPs as would be expected if alarmins were sufficient for TLR-dependent priming [30]. For example, we found that priming by particles with adherent ultrapure lipopolysaccharide is not affected by deletion of the noncognate TLR2 but is potently inhibited by deletion of either the cognate TLR4 or TIRAP/Mal. Thus, if alarmins are released in response to the particles, they are not sufficient and adherent PAMPs are needed to activate TLRs during priming of the NLRP3 inflammasome. Similarly, alarmins are not sufficient to activate TLRs during particle-induced cytokine production or particle-induced osteolysis [9, 32]. Alarmins may nonetheless contribute to aseptic loosening either by acting together with PAMPs to activate TLRs or by mechanisms independent of TLRs [25, 30]. Our results may appear to conflict with reports that orthopaedic particles that lack adherent PAMPs can activate the NLRP3 inflammasome [3, 13-15, 42, 49, 50, 63, 68]. However, priming with soluble PAMPs was needed in those studies to allow activation by the particles. One study reported that cobalt-chrome particles activate the NLRP3 inflammasome in the absence of priming [61]. However, that study relied on macrophages pretreated with 12-O-tetradecanoylphorbol-13-acetate (TPA, also known as PMA) or thioglycolate, either of which potently prime the NLRP3 inflammasome [6, 23, 41].
The findings described in the previous paragraph that inflammasome priming by orthopaedic particles depends on adherent PAMPs, their cognate TLRs, and TIRAP/Mal further supports the controversial hypothesis that bacterial PAMPs contribute to aseptic loosening. Multiple other lines of evidence also support that hypothesis [30, 31, 38, 47, 56, 69, 74]. Clinically, antibiotics can reduce aseptic loosening [24, 30], and PAMPs are found in periprosthetic tissue from patients with aseptic loosening [36, 55]. Possible PAMP sources include bacterial flora in the gastrointestinal tract and oral cavity from which bacteria and PAMPs episodically translocate to the systemic circulation, potentially reaching the implant [30]. Subclinical, low-grade bacterial biofilms on implant surfaces are another possible source of PAMPs [20, 21, 30, 38, 47, 56, 66, 74], and the presence of biofilms strongly correlates with increased osteolysis [66]. Additionally, TLR pathway polymorphisms associated with aseptic loosening [48] and increased TLR-positive macrophages were reported in periprosthetic tissue of patients with aseptic loosening [45]. In cell culture and the murine calvarial model of particle-induced osteolysis, titanium particle-induced inflammation and osteolysis are partially dependent on TLR2 and TLR4 but only if their cognate PAMPS are adherent to the particles [32]. Our current results extend those previous findings to inflammasome processing of proIL1β and thereby further support the hypothesis that bacterial PAMPs contribute to aseptic loosening [30, 31, 38, 47, 56, 69, 74].
In contrast to priming, activation of the NLRP3 inflammasome does not depend on adherent PAMPs, as demonstrated by equivalent secretion of IL1β protein by preprimed macrophages subsequently stimulated by either endotoxin-free titanium particles or titanium particles with adherent bacterial debris. Thus, it is possible that particles with and without adherent PAMPs can work together by sequentially priming (PAMP-dependent) and then activating (PAMP-independent) the NLRP3 inflammasome. Of all the macrophage responses induced by wear particles that have been studied [9, 10, 22, 28, 30-32, 37, 38, 47, 53, 56, 69, 72, 74, 76], cell death [22, 64] and activation of the NLRP3 inflammasome are the only ones that are not increased by adherent PAMPs. Intriguingly, cell death can also activate the NLRP3 inflammasome [27]. Future studies should therefore determine whether cell death contributes to activation of the NLRP3 inflammasome by orthopaedic wear particles.
This cell culture study showed that orthopaedic wear particles with adherent PAMPs can prime the NLRP3 inflammasome and that the particles can then activate the NLRP3 inflammasome independently of adherent PAMPs. Future animal and implant retrieval studies are needed to determine whether wear particles have similar effects on the NLRP3 inflammasome in vivo. Nonetheless, the current results add further support to the controversial concept that bacterial PAMPs may contribute to aseptic loosening.
Acknowledgments
We thank Dr Katherine Fitzgerald for the wild-type, NLRP3-/-, and TIRAP/Mal-/- immortalized macrophages and Bryan Hausman for assisting with experiments.
Footnotes
One or more of the authors received funding from the Dudley P. Allen Fellowship (GWM); the National Institutes of Health TL1 TR000441 (BPF), T32 AR007505 (BPF), and R21 AR069785 (EMG); and the Harry E. Figgie III MD Professorship (EMG).
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85-95. [DOI] [PubMed] [Google Scholar]
- 2.Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res. 2002;59:507-515. [DOI] [PubMed] [Google Scholar]
- 3.Alippe Y, Wang C, Ricci B, Xiao J, Qu C, Zou W, Novack DV, Abu-Amer Y, Civitelli R, Mbalaviele G. Bone matrix components activate the NLRP3 inflammasome and promote osteoclast differentiation. Sci Rep. 2017;7:6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashwood P, Thompson RP, Powell JJ. Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Exp Biol Med (Maywood). 2007;232:107-117. [PubMed] [Google Scholar]
- 5.Australian Orthopaedic Association. National Joint Replacement Registry Hip, Knee & Shoulder Arthroplasty. Annual Report 2016. Available at: https://aoanjrr.sahmri.com/documents/10180/275066/Hip%2C%20Knee%20%26%20Shoulder%20Arthroplasty. Accessed June 1, 2016. [Google Scholar]
- 6.Ayna G, Krysko DV, Kaczmarek A, Petrovski G, Vandenabeele P, Fesus L. ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PLoS One. 2012;7:e40069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer TW. Particles and periimplant bone resorption. Clin Orthop Relat Res. 2002;405:138-143. [DOI] [PubMed] [Google Scholar]
- 8.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechtel CP, Gebhart JJ, Tatro JM, Kiss-Toth E, Wilkinson JM, Greenfield EM. Particle-induced osteolysis is mediated by TIRAP/Mal in vitro and in vivo: dependence on adherent pathogen-associated molecular patterns. J Bone Joint Surg Am. 2016;98:285-294. [DOI] [PubMed] [Google Scholar]
- 10.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082-2091. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1-5. [DOI] [PubMed] [Google Scholar]
- 12.Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455-464. [DOI] [PubMed] [Google Scholar]
- 13.Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, Sculco TP, Santambrogio L, Ross FP, Goldring SR, Purdue PE. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2013;31:73-80. [DOI] [PubMed] [Google Scholar]
- 14.Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res. 2009;27:847-854. [DOI] [PubMed] [Google Scholar]
- 15.Caicedo MS, Samelko L, McAllister K, Jacobs JJ, Hallab NJ. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. J Orthop Res. 2013;31:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Hausman BS, Luo G, Zhou G, Murakami S, Rubin J, Greenfield EM. Protein kinase inhibitor gamma reciprocally regulates osteoblast and adipocyte differentiation by downregulating leukemia inhibitory factor. Stem Cells. 2013;31:2789-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho DR, Shanbhag AS, Hong CY, Baran GR, Goldring SR. The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res. 2002;20:704-713. [DOI] [PubMed] [Google Scholar]
- 18.Dai JC, He P, Chen X, Greenfield EM. TNFalpha and PTH utilize distinct mechanisms to induce IL-6 and RANKL expression with markedly different kinetics. Bone. 2006;38:509-520. [DOI] [PubMed] [Google Scholar]
- 19.Daniels AU, Barnes FH, Charlebois SJ, Smith RA. Macrophage cytokine response to particles and lipopolysaccharide in vitro. J Biomed Mater Res. 2000;49:469-478. [DOI] [PubMed] [Google Scholar]
- 20.Dapunt U, Lehner B, Burckhardt I, Zimmermann S, Hansch GM, Ewerbeck V. Evaluation of implant sonication as a diagnostic tool in implant-associated infections. J Appl Biomater Funct Mater. 2014;12:135-140. [DOI] [PubMed] [Google Scholar]
- 21.Demeo PJ, Costerton JW, Ehrlich GD, Heinz W. Culture Negative Orthopedic Biofilm Infections . Heidelberg, Germany: Springer; 2014. [Google Scholar]
- 22.Ding H, Zhu Z, Tang T, Yu D, Yu B, Dai K. Comparison of the cytotoxic and inflammatory responses of titanium particles with different methods for endotoxin removal in RAW264.7 macrophages. J Mater Sci Mater Med. 2012;23:1055-1062. [DOI] [PubMed] [Google Scholar]
- 23.Ding Z, Liu S, Wang X, Theus S, Fan Y, Deng X, Mehta JL. LOX-1-dependent mitochondrial DNA damage and NLRP3 activation during systemic inflammation in mice. Biochem Biophys Res Commun. 2014;451:637-643. [DOI] [PubMed] [Google Scholar]
- 24.Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:644-651. [DOI] [PubMed] [Google Scholar]
- 25.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989-999. [DOI] [PubMed] [Google Scholar]
- 26.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaidt MM, Hornung V. The NLRP3 inflammasome renders cell death pro-inflammatory. J Mol Biol. 2018;430:133-141. [DOI] [PubMed] [Google Scholar]
- 28.Gordon A, Greenfield EM, Eastell R, Kiss-Toth E, Wilkinson JM. Individual susceptibility to periprosthetic osteolysis is associated with altered patterns of innate immune gene expression in response to pro-inflammatory stimuli. J Orthop Res. 2010;28:1127-1135. [DOI] [PubMed] [Google Scholar]
- 29.Gordon A, Kiss-Toth E, Stockley I, Eastell R, Wilkinson JM. Polymorphisms in the interleukin-1 receptor antagonist and interleukin-6 genes affect risk of osteolysis in patients with total hip arthroplasty. Arthritis Rheum. 2008;58:3157-3165. [DOI] [PubMed] [Google Scholar]
- 30.Greenfield EM. Do genetic susceptibility, Toll-like receptors, and pathogen-associated molecular patterns modulate the effects of wear? Clin Orthop Relat Res. 2014;472:3709-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenfield EM, Bechtold J; Implant Wear Symposium Biologic Work Group. What other biologic and mechanical factors might contribute to osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S56-62. [DOI] [PubMed] [Google Scholar]
- 32.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. J Biol Chem. 2010;285:32378-32384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Van De Motter RR. The role of osteoclast differentiation in aseptic loosening. J Orthop Res. 2002;20:1-8. [DOI] [PubMed] [Google Scholar]
- 34.Grosse S, Haugland HK, Lilleng P, Ellison P, Hallan G, Hol PJ. Wear particles and ions from cemented and uncemented titanium-based hip prostheses--a histological and chemical analysis of retrieval material. J Biomed Mater Res B Appl Biomater. 2015;103:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartmann ES, Kohler MI, Huber F, Redeker JI, Schmitt B, Schmitt-Sody M, Summer B, Fottner A, Jansson V, Mayer-Wagner S. Factors regulating bone remodeling processes in aseptic implant loosening. J Orthop Res. 2017;35:248-257. [DOI] [PubMed] [Google Scholar]
- 37.Hirayama T, Tamaki Y, Takakubo Y, Iwazaki K, Sasaki K, Ogino T, Goodman SB, Konttinen YT, Takagi M. Toll-like receptors and their adaptors are regulated in macrophages after phagocytosis of lipopolysaccharide-coated titanium particles. J Orthop Res. 2011;29:984-992. [DOI] [PubMed] [Google Scholar]
- 38.Hoenders CS, Harmsen MC, van Luyn MJ. The local inflammatory environment and microorganisms in 'aseptic' loosening of hip prostheses. J Biomed Mater Res B Appl Biomater. 2008;86:291-301. [DOI] [PubMed] [Google Scholar]
- 39.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271-1286. [DOI] [PubMed] [Google Scholar]
- 41.Jhang JJ, Cheng YT, Ho CY, Yen GC. Monosodium urate crystals trigger Nrf2- and heme oxygenase-1-dependent inflammation in THP-1 cells. Cell Mol Immunol. 2015;12:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, Eisenbarth SC, Flavell RA. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A. 2011;108:14867-14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaar SG, Ragab AA, Kaye SJ, Kilic BA, Jinno T, Goldberg VM, Bi Y, Stewart MC, Carter JR, Greenfield EM. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. J Orthop Res. 2001;19:171-178. [DOI] [PubMed] [Google Scholar]
- 44.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611-7622. [DOI] [PubMed] [Google Scholar]
- 45.Lahdeoja T, Pajarinen J, Kouri VP, Sillat T, Salo J, Konttinen YT. Toll-like receptors and aseptic loosening of hip endoprosthesis-a potential to respond against danger signals? J Orthop Res. 2010;28:184-190. [DOI] [PubMed] [Google Scholar]
- 46.Langlois J, Zaoui A, Bichara DA, Nich C, Bensidhoum M, Petite H, Muratoglu OK, Hamadouche M. Biological reaction to polyethylene particles in a murine calvarial model is highly influenced by age. J Orthop Res. 2016;34:574-580. [DOI] [PubMed] [Google Scholar]
- 47.Lieder R, Petersen PH, Sigurjonsson OE. Endotoxins-the invisible companion in biomaterials research. Tissue Eng Part B Rev. 2013;19:391-402. [DOI] [PubMed] [Google Scholar]
- 48.MacInnes SJ, Del Vescovo E, Kiss-Toth E, Ollier WE, Kay PR, Gordon A, Greenfield EM, Wilkinson MJ. Genetic variation in inflammatory and bone turnover pathways and risk of osteolytic responses to prosthetic materials. J Orthop Res. 2015;33:193-198. [DOI] [PubMed] [Google Scholar]
- 49.Maitra R, Clement CC, Scharf B, Crisi GM, Chitta S, Paget D, Purdue PE, Cobelli N, Santambrogio L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol Immunol. 2009;47:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, Mehal WZ. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci U S A. 2011;108:20095-20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991-1045. [DOI] [PubMed] [Google Scholar]
- 52.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820-3826. [DOI] [PubMed] [Google Scholar]
- 53.Naganuma Y, Takakubo Y, Hirayama T, Tamaki Y, Pajarinen J, Sasaki K, Goodman SB, Takagi M. Lipoteichoic acid modulates inflammatory response in macrophages after phagocytosis of titanium particles through Toll-like receptor 2 cascade and inflammasomes. J Biomed Mater Res A. 2016;104:435-444. [DOI] [PubMed] [Google Scholar]
- 54.Nagpal K, Plantinga TS, Wong J, Monks BG, Gay NJ, Netea MG, Fitzgerald KA, Golenbock DT. A TIR domain variant of MyD88 adapter-like (Mal)/TIRAP results in loss of MyD88 binding and reduced TLR2/TLR4 signaling. J Biol Chem. 2009;284:25742-25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nalepka JL, Lee MJ, Kraay MJ, Marcus RE, Goldberg VM, Chen X, Greenfield EM. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res. 2006;451:229-235. [DOI] [PubMed] [Google Scholar]
- 56.Nelson CL, McLaren AC, McLaren SG, Johnson JW, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Relat Res. 2005;437:25-30. [DOI] [PubMed] [Google Scholar]
- 57.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, Konttinen YT, Goodman SB, Gallo J. Macrophages--key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101:3033-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol. 2003;171:6329-6333. [DOI] [PubMed] [Google Scholar]
- 59.Ragab AA, Van De Motter R, Lavish SA, Goldberg VM, Ninomiya JT, Carlin CR, Greenfield EM. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res. 1999;17:803-809. [DOI] [PubMed] [Google Scholar]
- 60.Revell PA, Weightman B, Freeman MA, Roberts BV. The production and biology of polyethylene wear debris. Arch Orthop Trauma Surg. 1978;91:167-181. [DOI] [PubMed] [Google Scholar]
- 61.Samelko L, Landgraeber S, McAllister K, Jacobs J, Hallab NJ. Cobalt alloy implant debris induces inflammation and bone loss primarily through danger signaling, not TLR4 sctivation: implications for DAMP-ening implant related inflammation. PLoS One. 2016;11:e0160141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samelko L, Landgraeber S, McAllister K, Jacobs J, Hallab NJ. TLR4 (not TLR2) dominate cognate TLR activity associated with CoCrMo implant particles. J Orthop Res. 2017;35:1007-1017. [DOI] [PubMed] [Google Scholar]
- 63.Scharf B, Clement CC, Wu XX, Morozova K, Zanolini D, Follenzi A, Larocca JN, Levon K, Sutterwala FS, Rand J, Cobelli N, Purdue E, Hajjar KA, Santambrogio L. Annexin A2 binds to endosomes following organelle destabilization by particulate wear debris. Nat Commun. 2012;3:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwab LP, Marlar J, Hasty KA, Smith RA. Macrophage response to high number of titanium particles is cytotoxic and COX-2 mediated and it is not affected by the particle's endotoxin content or the cleaning treatment. J Biomed Mater Res A. 2011;99:630-637. [DOI] [PubMed] [Google Scholar]
- 65.Schwab LP, Xing Z, Hasty KA, Smith RA. Titanium particles and surface-bound LPS activate different pathways in IC-21 macrophages. J Biomed Mater Res B Appl Biomater. 2006;79:66-73. [DOI] [PubMed] [Google Scholar]
- 66.Sierra JM, Garcia S, Martinez-Pastor JC, Tomas X, Gallart X, Vila J, Bori G, Macule F, Mensa J, Riba J, Soriano A. Relationship between the degree of osteolysis and cultures obtained by sonication of the prostheses in patients with aseptic loosening of a hip or knee arthroplasty. Arch Orthop Trauma Surg. 2011;131:1357-1361. [DOI] [PubMed] [Google Scholar]
- 67.So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol. 2017. [DOI] [PubMed] [Google Scholar]
- 68.St Pierre CA, Chan M, Iwakura Y, Ayers DC, Kurt-Jones EA, Finberg RW. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res. 2010;28:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177-197. [DOI] [PubMed] [Google Scholar]
- 70.Takagi M, Takakubo Y, Pajarinen J, Naganuma Y, Oki H, Maruyama M, Goodman SB. Danger of frustrated sensors: role of Toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements. J Orthop Translat. 2017;10:68-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taki N, Tatro JM, Lowe R, Goldberg VM, Greenfield EM. Comparison of the roles of IL-1, IL-6, and TNFalpha in cell culture and murine models of aseptic loosening. Bone. 2007;40:1276-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taki N, Tatro JM, Nalepka JL, Togawa D, Goldberg VM, Rimnac CM, Greenfield EM. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376-383. [DOI] [PubMed] [Google Scholar]
- 73.Theiss SM LW, Baker DG, Cuckler JM. The effect of endotoxin on the inflammatory response to particulate debris. Trans Orthop Res Soc 1993;18:269. [Google Scholar]
- 74.Wasko MK, Goodman SB. Emperor's new clothes: Is particle disease really infected particle disease? J Orthop Res. 2016;34:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH, Wooley PH. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther. 2004;11:483-491. [DOI] [PubMed] [Google Scholar]
- 76.Zaveri TD, Dolgova NV, Lewis JS, Hamaker K, Clare-Salzler MJ, Keselowsky BG. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials. 2017;115:128-140. [DOI] [PMC free article] [PubMed] [Google Scholar]


