Abstract
Background
Epiphyseal fusion (EF) marks the completion of longitudinal bone growth, a critical milestone monitored during treatment of skeletal growth and/or developmental disorders. Recently, a trend toward accelerated skeletal maturation in children has been documented. Because current methods for assessing skeletal maturation include children in their reference populations born as early as the 1930s, the timing of EF events in contemporary patients may differ substantially from those standards.
Questions/purposes
(1) Do children today initiate the process of EF in the hand and wrist earlier than past generations on which maturity standards are based? (2) Do children today complete EF in the hand and wrist earlier than past generations on which maturity standards are based?
Methods
A total of 1292 children (665 males, 627 females) participating in the Fels Longitudinal Study, born between 1915 and 2006, were included in this retrospective, observational study. Each participant had between one and 39 serial left hand-wrist radiographs during childhood obtained specifically for research purposes. Main outcomes were the chronological age at the first sign of EF initiation (EF-I) and the first chronological age when EF was complete (EF-C) in the radius and ulna, and metacarpals and phalanges of the first, third, and fifth rays according to criteria of the Fels method. EF is a reliable metric with an average κ agreement statistic of 0.91. Penalized B-splines were used to model the changes in EF-I and EF-C ages and to identify changes across continuous birth years with major comparisons between children born in 1935 and 1995.
Results
Approximately half of the epiphyses of the hand and wrist examined exhibited earlier EF-I and/or earlier EF-C in children born in 1995 compared with those born in 1935. The age at each milestone (EF-I and EF-C) decreased by as much as 6.7 and 6.8 months in males and 9.8 and 9.7 months in females, respectively. This change occurred gradually over the past century. The more proximal traits (EF of the distal radius, distal ulna, and metacarpals) were more likely to experience a shift in timing, whereas timing of EF in the phalanges remained relatively stable across birth years.
Conclusions
A trend has occurred over the past century in the timing of EF, in both initiation and completion of the process, for many of the bones of the hand and wrist. Earlier EF reflects modern population advances in both skeletal and sexual maturation. Shifts in the timing of EF have the potential to influence treatment strategies for skeletal growth and/or developmental disorders such as scoliosis or leg length inequality, moving treatment windows to earlier ages. Earlier EF-I and EF-C identified in this study signals a need to reevaluate the timing of maturational milestones and current standards for skeletal assessment.
Level of Evidence
Level II, prognostic study.
Introduction
The timing of epiphyseal fusion (EF) in the bones of the hand and wrist corresponds to late stages of skeletal maturation and signals the end of longitudinal growth. As such, assessments of EF status provide a simple means for insight into skeletal development status when evaluating children with disorders such as idiopathic scoliosis [3, 6, 17, 23, 31–33], leg length inequality [26, 36], or constitutional growth delay [35]. Current standards indicate that EF in the bones of the hand and wrist begins during adolescence [16, 33, 38]. The precise age at which EF begins is variable [5]. Initial assessment of EF in the distal-most traits of the hand and wrist is recommended at approximately 10 years of age in females and 12 years of age in males [29]. Signs of EF should be clear by 13 or 15 years of age in females or males, respectively [16]. However, notable population-level shifts in the timing of puberty, menarche, and skeletal maturation have been identified [2, 4, 11, 13, 19, 21, 22, 27] suggesting that the average age for EF may also be changing. If modern children are maturing earlier [4, 11] than previous reference standards, this may impact decisions of optimal timing for surgical correction of specific skeletal conditions.
A trend toward a more rapidly maturing skeleton in contemporary youth [2, 4, 11, 22, 27] raises questions regarding the anticipated timing of EF events used for treatment planning. Previous work by our group [11] documented accelerated bone development in overall skeletal maturity as well as in individual maturation indicators including EF. The present study extends that analysis by examining initiation and completion of EF as discrete events that can vary independently. These are analyzed relative to continuous birth years from 1915 to 2006. This approach provides an estimate of potential shifts in the timing of EF initiation (EF-I) and completion (EF-C).
We, therefore, sought to answer the following questions: (1) Do children today initiate the process of EF in the hand and wrist earlier than past generations on which maturity standards are based? (2) Do children today complete EF in the hand and wrist earlier than past generations on which maturity standards are based?
Patients and Methods
The present study includes 1292 participants (665 males; 627 females) from the Fels Longitudinal Study of southwest Ohio, the world’s largest and longest running study of normal growth, development, and body composition change across the lifespan [28, 34]. Participants were predominantly of European ancestry born between 1915 and 2006 and ranged in age from birth to 22.7 years. At each examination, anthropometric measures and radiographs of the left hand-wrist were obtained. For the present study, inclusion required at least one radiograph documenting EF-I or EF-C in at least one of the examined traits in the hand and wrist (Fig. 1). Additional inclusion criteria were, for each trait: (1) before the first documentation of EF-I, each participant was required to have a preceding radiograph, taken no more than 15 months prior, that exhibited no EF; and (2) before the first documentation of EF-C, each participant was required to have a preceding radiograph, taken no more than 15 months prior, that did not exhibit EF-C. Although each participant is represented by a single radiograph for EF-I and a single radiograph for EF-C, longitudinal data were required to determine the first observed instance of EF-I and EF-C. The median number of radiographs examined per person was 15 (range, 1-39). Children in this study were similar in height and weight to national norms [10] with a moderate increase in body weight between those born in the 1930s (mean weight in our participants of 64.8 kg in males and 52.8 kg in females) and those born in the 1990s (mean weight in our participants of 73.3 kg in males and 64.4 kg in females).
Fig. 1.
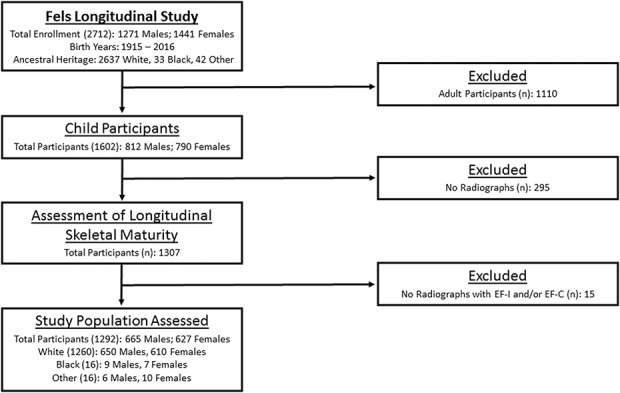
This STROBE diagram shows the distribution of participants in the Fels Longitudinal Study and the inclusion in the present study based on inclusion and exclusion criteria as well as the final participant group used in the current analysis.
The primary outcome measure for this study is chronological age at the first sign of EF-I and EF-C for participants across all continuous birth years between 1915 and 2006. Secondary outcomes are differences in EF-I and EF-C ages between children born in 1935 and 1995.
The University of Missouri institutional review board approved all procedures used in this study.
Assessment of Epiphyseal Fusion
EF-I and EF-C were assessed according to criteria outlined in the Fels method [29]. EF stage was assessed in 13 bones of the hand and wrist, including the radius and ulna as well as the metacarpals and phalanges of the first, third, and fifth rays. EF-I was defined as the first sign of bony union between the epiphysis and the metaphysis and EF-C was defined when no radiolucent space remained at the epiphyseal-metaphyseal junction. Note, the distal radius is divided into thirds and scored separately and the ulna is scored only for EF-C. Interobserver agreement for EF is > 90% [29]. There were three primary assessors for the present study (CC, KL, SL). Intraobserver agreement of EF grading was consistently > 90% for all traits measured with a κ statistic that ranged from 0.86 to 0.95.
Bias
Participants selected for inclusion in the present study are representative of the greater Fels Longitudinal Study population in terms of sex and race/ethnicity (Fig. 1). However, the Fels Study, and therefore the present analysis, is primarily composed of people of European ancestry. Although blacks and other minority groups are included, their numbers are not reflective of the distribution of these groups in the United States. Thus, results from the present study should be used with caution in nonwhite contexts.
Statistical Analysis
Because of known sex differences in the pattern and timing of skeletal maturity, males and females were examined separately. The chronological age of each EF trait was examined using participant birth year as a continuous variable for males (Table 1) and females (Table 2). All data were evaluated for internal consistency and outliers; any discrepancies were reevaluated and corrected before statistical analyses.
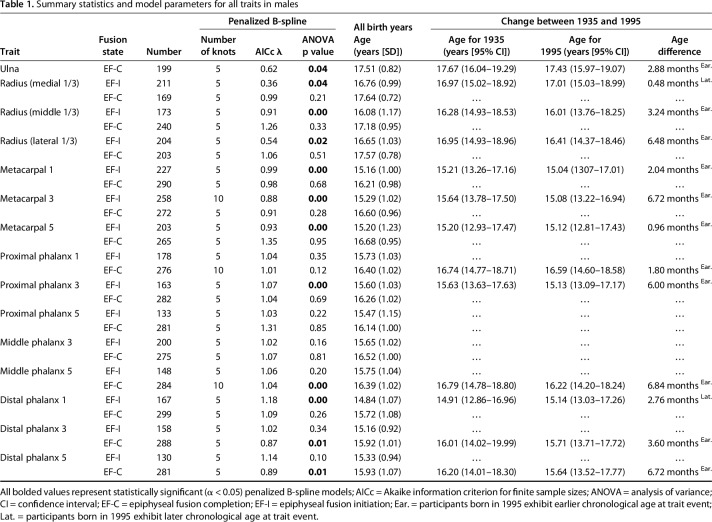
Table 1.
Summary statistics and model parameters for all traits in males
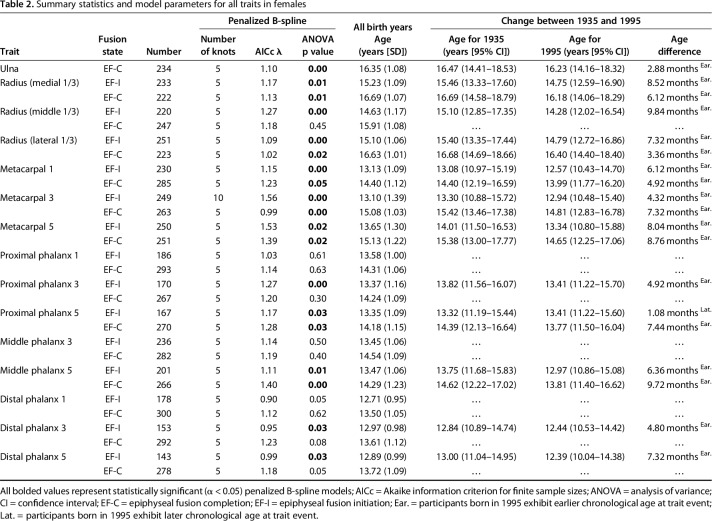
Table 2.
Summary statistics and model parameters for all traits in females
Each of the 29 EF traits was modeled across continuous birth years using a penalized B-spline model implemented [12] in the statistical package SAS, Version 9.4, for Windows (SAS Institute, Inc, Cary, NC, USA). A penalized B-spline is a nonlinear function based on a series of piecewise polynomials separated by internal “knots,” which typically indicate an inflection point or a change in slope. The data were originally fit with 100 such knots and resulted in a mean of five (range, 1-8) and six (range, 2-14) inflection points, or primary knots, per trait for males and females, respectively. To avoid model overfitting, the number of knots for each trait-specific model was subsequently reduced using the myoptic algorithm [30] based on the penalized smoothing parameter lambda (λ) and the Akaike information criterion [1] for finite sample sizes. The results from this study are, therefore, based on trait-specific models that were fit with either five or 10 interior knots (shown in Table 1 for males and Table 2 for females). The penalized B-spline procedure is robust, particularly when the number of internal knots is considerably smaller than the sample size; thus, the 1292 participants in the present analysis allowed for adequate power to detect changes in EF-I and EF-C ages across birth years.
A univariate analysis of variance was used to determine if the observed trend in the timing of EF-I and EF-C for each trait was significantly different (α < 0.05) across continuous birth years. All reported p values are based on the model-specific F-statistic, which includes parameters of both the degrees of freedom and error degrees of freedom. Statistically significant penalized B-splines were further characterized by the age in years for each trait during 1935 and 1995 to avoid undue influence of the individuals at the edge of our birth year range. However, the overall shape of the penalized B-spline includes participants in all birth years. The difference in age, measured in months, between participants born in 1935 and 1995 was also calculated to quantify how much earlier (or later) EF-I and EF-C are occurring.
Results
Initiation of Epiphyseal Fusion
The age at which the process of EF is initiated (EF-I) is variable among bones and among children, and EF-I in contemporary children is occurring at earlier ages in some bones than current skeletal maturity standards indicate. Since the 1930s, the age at which EF-I occurs has shifted earlier in six of 14 (50%) traits observed in males and in nine of 14 (79%) traits observed in females. The mean difference in age at EF-I between children born in 1935 versus those born in 1995 varied among the traits assessed. Males born in 1995 experienced EF-I as much as 6.7 months earlier (for the third metacarpal; Table 1). Females born in 1995 experienced EF-I as much as 9.8 months earlier (for the middle third of the distal radius; Table 2).
A sex-specific pattern was observed in EF-I along the proximodistal gradient. In males, five of six proximal traits (middle and lateral third of the distal radius and first, third, and fifth metacarpals) and one of eight distal traits (proximal phalanx of the third digit) exhibited an earlier age at EF-I for participants born in 1995 than when compared with those born in 1935. One proximal trait (medial third of the distal radius) and one distal trait (distal phalanx of the first digit) in males exhibited a later age at EF-I in participants born in 1995. In females, all proximal traits (all three components of the distal radius and the first, third, and fifth metacarpals) and four of eight distal traits (proximal phalanx of the third digit, middle phalanx of the fifth digit, and the distal phalanx of the third and fifth digits) exhibited earlier EF-I. One distal trait (proximal phalanx of the fifth digit) exhibited later EF-I in females born in 1995. For traits exhibiting an earlier age at EF-I, the mean magnitude of change in age in males was 4.2 months with a maximum change of 6.7 months for the third metacarpal (age in 1935 was 15.64 with 95% confidence interval [CI], 13.78-17.50; age in 1995 was 15.08 with 95% CI 13.22-16.94). Traits with later ages at EF-I in males born in 1995 had a mean magnitude of 1.6 months with a maximum of 2.8 months in the first distal phalanx (age in 1935 was 14.91 with 95% CI 12.86-16.96; age in 1995 was 15.14 with 95% CI 13.03-17.26). In females, the mean difference in age for traits with earlier EF-I was 6.8 months with a maximum change of 9.8 months for the middle third of the distal radius (age in 1935 was 15.1 with 95% CI 12.85-17.35; age in 1995 was 14.28 with 95% CI 12.02-16.54). The single trait (fifth proximal phalanx) with a later age at EF-I had a magnitude of 1 month (age in 1935 was 13.32 with 95% CI 11.19-15.44; age in 1995 was 13.41 with 95% CI 11.22-15.60).
Completion of Epiphyseal Fusion
The age at which EF is complete (EF-C) is occurring earlier in contemporary children than those from past generations. Since 1935, the mean age at which EF-C occurs has shifted earlier in five of 15 (33%) traits observed in males and in eight of 15 (53%) traits observed in females. The mean difference in age at EF-C between children born in 1935 and those born in 1995 varied among the traits assessed and was found to be as much as 6.8 months earlier in males for the middle phalanx of the fifth digit and as much as 9.7 months earlier in females for the middle phalanx of the fifth digit in participants born in 1995.
A sex-specific pattern was also observed in age at EF-C along the proximodistal gradient. In males, one of seven proximal traits (ulna) and four of eight distal traits (distal phalanx of the third and fifth digits, middle phalanx of the fifth digit, and the proximal phalanx of the first digit) exhibited earlier EF-C in those born in 1995 when compared with participants born in 1935. In females, six of seven proximal traits (ulna, medial, and lateral thirds of the distal radius and metacarpals of the first, third, and fifth digits) and two of eight distal traits (middle and proximal phalanges of the fifth digit) exhibited earlier EF-C in those born in 1995. For traits with an earlier age at EF-C, the mean change in age was 4.4 months with a maximum change in age of 6.8 months for the fifth middle phalanx (age in 1935 was 16.79 with 95% CI 14.78-18.80; age in 1995 was 16.62 with 95% CI 14.20-18.24) in males. In females, the mean change in age was 6.3 months with a maximum change in age of 9.7 months for the fifth middle phalanx (age in 1935 was 14.62 with 95% CI 12.22-17.02; age in 1995 was 13.81 with 95% CI 11.40-16.62).
For most traits examined, the change in age for EF-I and EF-C has occurred gradually over the past century in males (Fig. 2) and females (Fig. 3). An anomalous result was observed in the third metacarpal, which appears to have experienced a temporary large reduction of approximately 16 months in the age at EF-I in both males and females with a subsequent increase in age in later years.
Fig. 2.
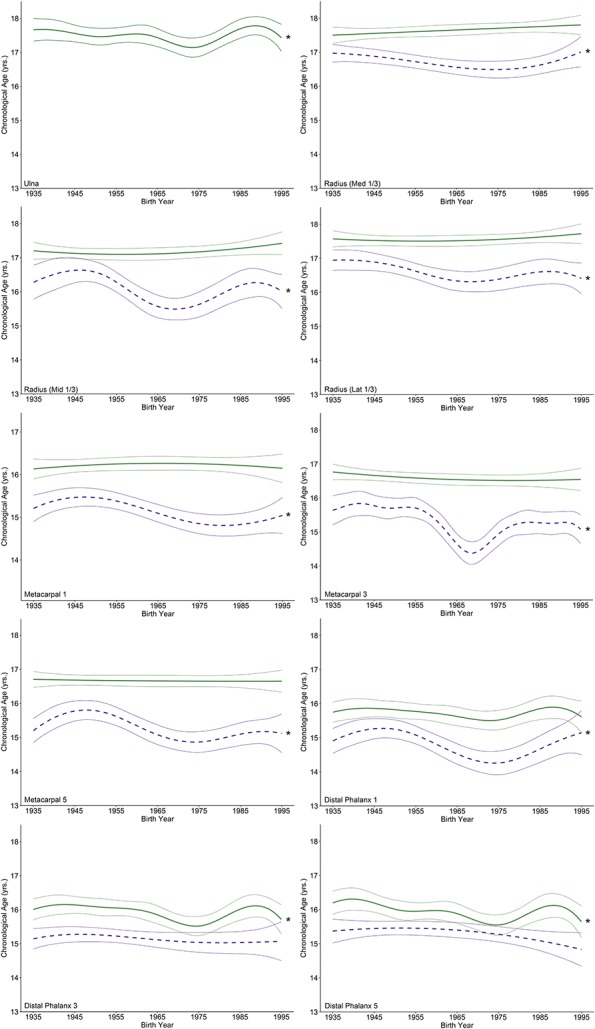
These graphs illustrate penalized B-spline curves for EF-I and EF-C traits in the distal ulna and radius as well as the metacarpals and distal phalanges of the first, third, and fifth rays in males born between 1935 and 1995. EF-I is represented by purple-dotted lines and EF-C is represented by green solid lines, each with 95% confidence bands. Models that were statistically significant are denoted by an asterisk.
Fig. 3.
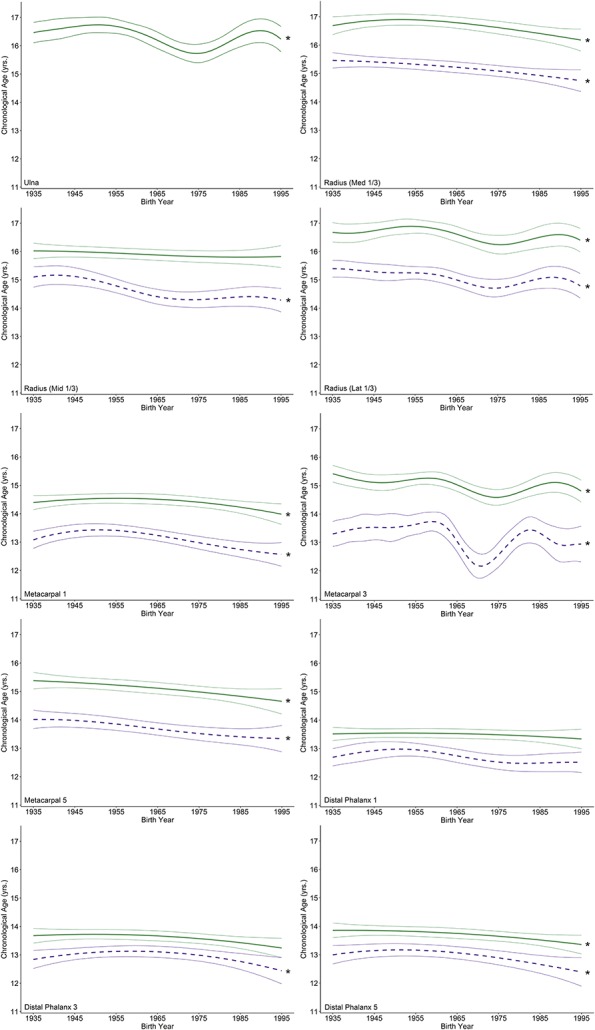
These graphs illustrate penalized B-spline curves for EF-I and EF-C traits in the distal ulna and radius as well as the metacarpals and distal phalanges of the first, third, and fifth rays in females born between 1935 and 1995. EF-I is represented by purple-dotted lines and EF-C is represented by green solid lines, each with 95% confidence bands. Models that were statistically significant are denoted by an asterisk.
Discussion
EF is a primary trait used by pediatric orthopaedists when assessing the skeletal maturity status of a child, particularly when developing treatment plans for skeletal growth and/or developmental disorders. However, the current gold standard methods for determining skeletal maturity, including the ages at which EF should be achieved, are based on populations of children born 60 to 80 years in the past, and century-long population shifts in the timing of maturation have likely influenced the age at which EF occurs. The present study aimed to quantify changes in EF timing by leveraging a large-scale longitudinal sample that ranges in birth years from 1915 to 2006 and provides clinicians with a sense of the “new normal” for EF-I and EF-C in the bones of the hand and wrist. Our results suggest that children born more recently experience EF-I and EF-C at earlier ages than when compared with those born in the early part of the 20th century and used to develop the gold standard methods. For example, EF-I in the third metacarpal is reached approximately 6.7 months earlier in males born in 1995 than those born in 1935. Our findings are critical for clinical assessments of skeletal maturity because EF-I and EF-C are occurring at substantially younger ages in normally developing children than previously thought.
This study has limitations related to the geographic and familial heritage of the study participants. The participants in this study accurately represent the greater Fels Longitudinal Study (Fig. 1), most of whom are white and from southwest Ohio. Documented differences in the timing of maturation in nonwhite children may limit the generalizability of our results to children of different ethnic groups and possibly even white children from other regions within the United States. Extrapolating these results to other groups or populations should be done with caution. However, detecting trends over time in traits such as EF timing requires longitudinal data that span many decades in a single population. Dense longitudinal data of this kind are rare, and the Fels Longitudinal Study is an important resource for tracking such trends. Nevertheless, the patterns of earlier age at EF observed in this study may be similar to those experienced by children in other geographic areas. Additional studies are needed to confirm if these trends hold true for other ethnic groups. Examination of historic radiographic records of such groups would be essential for this type of confirmation. Another minor limitation of this study is the frequency of radiographic assessments. Fels Longitudinal Study participants were examined at 6-month intervals, thereby limiting the resolution of the exact timing of specific EF events. However, the 6-month interval used by the Fels Longitudinal Study is both consistent with clinical followup assessments and is narrower than many other longitudinal studies that assess participants on a yearly basis [7, 24, 37].
We previously reported a trend toward earlier skeletal maturity in US children born after 1965, including accelerated EF [11]. The present study examined EF-I and EF-C as separate events across continuous birth years, showing that the timing of EF has shifted in contemporary males and females. Assuming shifts in the timing of EF are truly independent of progression in other indicators of maturation such as shape changes, projections, and radiopaque densities, as shown previously [11], then maturity assessments that do not grade fusion separately (including Gruelich-Pyle [16]) may mask the influence of EF on bone age, particularly EF-I. The Fels [29] and Sanders [33] methods are more sensitive to EF events and should be utilized more readily in contexts in which linear growth is most important.
The changes in EF timing we observed over the past century have happened gradually and are consistent with trends toward earlier maturation in puberty and menarche [13, 19, 21]. The typical age of menarche, for example, has decreased by approximately 3 months in white females [15, 21] and 6 months in black females [15, 20, 21] between the early 1960s and the late 1990s in the United States. Similar trends have occurred worldwide (see Euling et al. [13] for a full review). It is not surprising that maturation in other systems such as the skeleton would follow and also exhibit advancement. In addition to trends identified in Fels Longitudinal Study participants, contemporary children from other studies in the United States also exhibit advanced skeletal maturation between 4 and 10 months when compared with Greulich-Pyle standards [16]. In South Africa, children born in 1990 showed advanced skeletal maturation relative to children born in the 1960s with South African blacks exhibiting an advancement of approximately 12 months on average and South African whites exhibiting an advancement of approximately 3 months [18]. These previous studies, however, do not report the timing of fusion separately, but rather overall skeletal age.
The mechanism underlying fusion events at earlier chronological ages may be systemic and related to the secular changes in puberty noted previously. Maturation of the skeleton is influenced by sex hormones, which itself can be influenced by adipose tissue. The Fels Longitudinal Study has documented a trend in the age of onset for puberty and menarche [9] and a modest increase in body mass index [10], contributing to earlier exposure to these hormones. Because estrogen, in particular, is critical for EF-I and EF-C during adolescence [8, 14, 25], active estrogen receptors on growth plate chondrocytes may underlie the noted trend toward advanced EF. Because estrogen accelerates the senescent decline of chondrocytes in the hypertrophic zone [39], earlier introduction to estrogen through environmental and dietary exposure may, therefore, lead to an earlier reduction in growth plate cartilage thickness in both males and females, contributing to growth plate closure. Although the exact mechanism behind the observed advancement in the timing of EF milestones remains unknown, it is essential to consider the sources of potential factors influencing EF timing when examining individual growth trajectories for diagnosis and the development of treatment plans. Earlier natural fusion of the physes in contemporary children alters the expectations of clinical observations compared with skeletal maturity standards. When monitoring a patient for anticipated growth-related manipulation, earlier interventions may be warranted.
A trend toward a more rapidly maturing skeleton in contemporary cohorts suggests that the timing of EF is likely changing; thus, the windows of treatment timing such as deformity correction or physeal manipulation may also be affected. Using nearly a century of longitudinal data, we showed a moderate shift toward earlier ages of EF-I and EF-C in 13 of 29 (45%) traits in males and in 19 of 29 (66%) traits in females. In general, EF-I traits exhibited greater changes in age than did EF-C traits with the magnitude of the observed trends appearing to be larger in females than in males. As the contemporary population shifts the timing of maturational milestones, expectations regarding the average age of occurrence for a maturational milestone, including EF, must shift as well.
Acknowledgments
We thank the participants of the Fels Longitudinal Study for their lifelong dedication to research. Thank you to the anonymous reviewers and the various faculty and staff involved in the Fels Study over the years, specifically Beverly Barry, Carol Cottom, Kimberly Lever, and Sharon Lawrence for data collection and Christina Holzhauser and Nicole Odom for assistance in skeletal phenotyping.
Footnotes
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases of the National Institutes of Health under Award Number R01AR055927 (DLD) with additional historical data being collected under R01HD012252.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 2.Boeyer M, Ousley S. Skeletal assessment and secular changes in knee development: a radiographic approach. Am J Phys Anthropol. 2017;162:229–240. [DOI] [PubMed] [Google Scholar]
- 3.Busscher I, Kingma I, de Bruin R, Wapstra F, Verkerke G, Veldhuizen A. Predicting the peak growth velocity in the individual child: validation of a new growth model. Eur Spine J. 2012;21:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calfee R, Sutter M, Steffen J, Goldfarb C. Skeletal and chronological ages in American adolescents: current findings in skeletal maturation. J Child Orthop. 2010;4:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron N. Can maturity indicators be used to estimate chronological age in children? Ann Hum Biol. 2015;42:302–307. [DOI] [PubMed] [Google Scholar]
- 6.Chazono M, Tanaka T, Marumo K, Kono K, Suzuki N. Significance of peak height velocity as a predictive factor for curve progression in patients with idiopathic scoliosis. Scoliosis. 2015;10:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole TJ, Rousham EK, Hawley NL, Cameron N, Norris SA, Pettifor JM. Ethnic and sex differences in skeletal maturation among the Birth to Twenty cohort in South Africa. Arch Dis Child. 2015;100:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler G. The role of estrogen in bone growth and maturation during childhood and adolescence. J Steriod Biochem Mol Biol. 1997;61:141–144. [PubMed] [Google Scholar]
- 9.Demerath E, Bradford T, Chumlea W, Sun S, Czerwinski S, Remsberg K, Siervogel R. Recent decline in age at menarche: the Fels Longitudinal Study. Am J Hum Biol. 2004;16:453–457. [DOI] [PubMed] [Google Scholar]
- 10.Demerath EW, Li J, Sun SS, Chumlea WC, Remsberg KE, Czerwinski SA, Towne B, Siervogel RM. Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. Am J Clin Nutr. 2004;80:441–446. [DOI] [PubMed] [Google Scholar]
- 11.Duren D, Nahhas R, Sherwood R. Do secular trends in skeletal maturity occur equally in both sexes? Clin Orthop Relat Res. 2015;473:2559–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eilers P, Marx B. Flexible smoothing with b-splines and penalties. Stat Sci. 1996;11:89–121. [Google Scholar]
- 13.Euling S, Herman-Giddens M, Lee P, Selevan S, Juul A, Sorensen T, Dunkel L, Himes J, Teilmann G, Swan S. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–191. [DOI] [PubMed] [Google Scholar]
- 14.Frank GR. Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol. 2003;41:217–221. [DOI] [PubMed] [Google Scholar]
- 15.Freedman D, Kahn L, Serdula M, Dietz W, Srinivasan S, Berenson G. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. [DOI] [PubMed] [Google Scholar]
- 16.Greulich W, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist. Stanford, CA, USA: Stanford University Press; 1959. [Google Scholar]
- 17.Hacquebord J, Leopold S. In Brief: The Risser classification: a classic tool for the clinician treating adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2012;470:2335–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley N, Rousham E, Norris S, Pettifor J, Cameron N. Secular trends in skeletal maturity in South Africa: 1962-2001. Ann Hum Biol. 2009;36:584–594. [DOI] [PubMed] [Google Scholar]
- 19.Herman-Giddens M. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. Int J Androl. 2006;29:241–246; discussion 286–290. [DOI] [PubMed] [Google Scholar]
- 20.Herman-Giddens M, Slora E, Wasserman R, Bourdony C, Bhapkar M, Koch G, Hasemeier C. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the pediatric research in office settings network. Pediatrics. 1997;99:505–512. [DOI] [PubMed] [Google Scholar]
- 21.Himes J. Examining the evidence for recent secular changes in the timing of puberty in US children in light of increases in the prevalence of obesity. Mol Cell Endocrinol. 2006;254–255:13–21. [DOI] [PubMed] [Google Scholar]
- 22.Lin N, Ranjitkar S, Macdonald R, Hughes T, Taylor J, Townsend G. New growth references for assessment of stature and skeletal maturation in Australians. Aust Orthod J. 2006;22:1–10. [PubMed] [Google Scholar]
- 23.Little D, Song K, Katz D, Herring J. Relationship of peak height velocity to other maturity indicators in idiopathic scholiosis in girls. J Bone Joint Surg Am. 2000;82:685–693. [DOI] [PubMed] [Google Scholar]
- 24.McCormack SE, Chesi A, Mitchell JA, Roy SM, Cousminer DL, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA. Relative skeletal maturation and population ancestry in nonobese children and adolescents. J Bone Miner Res. 2017;32:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson O, Chrysis D, Pajulo O, Boman A, Holst M, Rubinstein J, Ritzen E, Savendahl L. Localization of estrogen receptors-alpha and -beta and androgen receptors in the human growth plate at different pubertal stages. J Endocrinol. 2003;177:319–326. [DOI] [PubMed] [Google Scholar]
- 26.Paley D, Bhave A, Herzenberg J, Bowen J. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82:1432–1446. [DOI] [PubMed] [Google Scholar]
- 27.Ranjitkar S, Lin N, Macdonald R, Taylor J, Townsend G. Stature and skeletal maturation of two cohorts of Australian children and young adults over the past two decades. Aust Orthod J. 2006;22:47–58. [PubMed] [Google Scholar]
- 28.Roche A. Growth, Maturation, and Body Composition: The Fels Longitudinal Study 1929-1991. New York, NY, USA: Cambridge University Press; 1992. [Google Scholar]
- 29.Roche A, Chumlea W, Thissen D. Assessing the Skeletal Maturity of the Hand-wrist: FELS Method. Springfield, IL, USA: Charles C. Thomas; 1988. [DOI] [PubMed] [Google Scholar]
- 30.Ruppert D. Selecting the number of knots for penalized splines. J Comput Graph Stat. 2002;11:735–757. [Google Scholar]
- 31.Sanders J. Maturity indicators in spinal defomity. J Bone Joint Surg Am. 2007;89(Suppl 1):14–20. [DOI] [PubMed] [Google Scholar]
- 32.Sanders J, Browne R, McConnell S, Margraf S, Cooney T, Finegold D. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am. 2007;89:64–73. [DOI] [PubMed] [Google Scholar]
- 33.Sanders J, Khoury J, Kishan S, Browne R, Mooney J, 3rd, Arnold K, McConnell S, Bauman J, Finegold D. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90:540–553. [DOI] [PubMed] [Google Scholar]
- 34.Sherwood R, Duren D. Growth of a species, an association, a science: 80 years of growth and development research. Am J Phys Anthropol. 2013;150:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spadoni GL, Cianfarani S. Bone age assessment in the workup of children with endocrine disorders. Horm Res Paediatr. 2010;73:2–5. [DOI] [PubMed] [Google Scholar]
- 36.Stephens M, Hsu L, Leong J. Leg length descrepancy after femoral shaft fractures in children. J Bone Joint Surg Br. 1989;71:615–618. [DOI] [PubMed] [Google Scholar]
- 37.Suri S, Prasad C, Tompson B, Lou W. Longitudinal comparison of skeletal age determined by the Greulich and Pyle method and chronologic age in normally growing children, and clinical interpretations for orthodontics. Am J Orthod Dentofacial Orthop. 2013;143:50–60. [DOI] [PubMed] [Google Scholar]
- 38.Tanner J, Healy M, Goldstein H, Cameron N. Assessment of Skeletal Maturity and Prediction of Adult Height (TW3 method). 3rd ed. London, UK: Saunders Ltd; 2001. [Google Scholar]
- 39.Weise M, De-Levi S, Barnes K, Gafni R, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci U S A. 2001;98:6871–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]