Abstract
Background
The quantitative accuracy of MRI in predicting the intraosseous extent of primary sarcoma of bone has not been definitively confirmed, although MRI is widely accepted as an accurate tool to plan limb salvage resections. Because inaccuracies in MRI determination of tumor extent could affect the ability of a tumor surgeon to achieve negative margins and avoid local recurrence, we thought it important to assess the accuracy of MR-determined tumor extent to the actual extent observed pathologically from resected specimens in pediatric patients treated for primary sarcomas of bone.
Questions/purposes
(1) Does the quantitative pathologic bony margin correlate with that measured on preoperative MRI? (2) Are T1- or T2-weighted MRIs most accurate in determining a margin? (3) Is there a difference in predicting tumor extent between MRI obtained before or after neoadjuvant chemotherapy and which is most accurate?
Methods
We retrospectively studied a population of 211 potentially eligible patients who were treated with limb salvage surgery between August 1999 and July 2015 by a single surgeon at a single institution for primary sarcoma of bone. Of 131 patients (62%) with disease involving the femur or tibia, 107 (51%) were classified with Ewing’s sarcoma or osteosarcoma. Records were available for review in our online database for 79 eligible patients (37%). Twenty-six patients (12%) were excluded because of insufficient or unavailable clinical or pathology data and 17 patients (8%) were excluded as a result of inadequate or incomplete MR imaging, leaving 55 eligible participants (26%) in the final cohort. The length of the resected specimen was superimposed on preresection MRI sequences to compare the margin measured by MRI with the margin measured by histopathology. Arithmetic mean differences and Pearson r correlations were used to assess quantitative accuracy (size of the margin).
Results
All MR imaging types were positively associated with final histopathologic margin. T1-weighted MRI after neoadjuvant chemotherapy and final histopathologic margin had the strongest positive correlation of all MR imaging and time point comparisons (r = 0.846, p < 0.001). Mean differences existed between the normal marrow margin on T1-weighted MRI before neoadjuvant chemotherapy (t = 8.363; mean, 18.883 mm; 95% confidence interval [CI], 14.327-23.441; p < 0.001), T2-weighted MRI before neoadjuvant chemotherapy (t = 8.194; mean, 17.204 mm; 95% CI, 12.970-21.439; p < 0.001), T1-weighted after neoadjuvant chemotherapy (t = 10.808; mean, 22.178 mm; 95% CI, 18.042-26.313; p < 0.001), T2-weighted after neoadjuvant chemotherapy (t = 10.702; mean, 20.778 mm; 95% CI, 16.865-24.691; p < 0.001), and the final histopathologic margin. T1-weighted MRI after neoadjuvant chemotherapy compared with the final histopathologic margin had the smallest mean difference in MRI-measured versus histopathologic margin size (mean, 5.9 mm; SD = 4.5 mm).
Conclusions
T1 MRI after neoadjuvant chemotherapy exhibited the strongest positive correlation and smallest mean difference compared with histopathologic margin. When planning surgical resections based on MRI obtained after neoadjuvant chemotherapy, for safety, one should account for a potential difference between the apparent margin of a tumor on an MRI and the actual pathologic margin of that tumor of up to 1 cm.
Level of Evidence
Level III, diagnostic study.
Introduction
In the treatment of primary osseous sarcoma, positive margins have been independently associated with worse oncologic outcomes [5, 6, 15, 18, 23] including an increased risk of local recurrence [2, 5, 21]. If local recurrence does occur, it is associated with an increased risk of distant recurrence and decreased survival [16, 21, 23, 24, 26]. Despite the results of few conflicting studies, which may question the importance of the surgical margin [7, 21], and the observation that additional factors also affect oncologic outcomes [1, 6, 7, 9, 10, 12, 13, 18, 20,21,22], an adequate margin remains the goal of every resection. Although attempts have been made to define an adequate margin [15], there is growing evidence that a close but negative margin may be acceptable [3, 5, 8, 14, 20].
For many, MRI is the imaging modality of choice for planning oncologic resections. An updated assessment of the ability of MRI to accurately identify the osseous extent of tumor may allow the surgeon to better plan and consistently achieve margin-negative resections while potentially avoiding unnecessary morbidity from an overly extensive resection. Prior studies of MRI utilizing synthetic models have determined a high degree of spatial accuracy [19], and studies in mice have demonstrated that MRIs of tumor postresection were as accurate as the histopathologic assessment of the margin status in controlled study conditions [4]. However, the only known published data in humans, Sundaram et al. [25] reported only qualitative evidence that MRI was the most accurate imaging modality for predicting the intramedullary extent of tumor. Unfortunately, no quantitative measures of accuracy were reported. The accuracy of computer-navigated resections utilizing fused CT and MRI with custom cutting templates has also been demonstrated in a small number of patients [3, 11, 17, 28]. In these studies, despite the ability to precisely reproduce the preoperative plan to within approximately 2 mm, an “error in safe margin” of approximately 8 mm was observed, leaving in question the accuracy with which the osseous extent of tumor can be identified on preoperative imaging. However, no prior studies have quantitatively compared the accuracy of T1- versus T2-weighted MRI in identifying the osseous extent of tumor nor whether MRIs obtained before or after neoadjuvant chemotherapy are most accurate in assessing the true extent of the tumor. The surgeon’s ability to accurately identify the tumor extent on prechemotherapy MRI may be significantly hindered by the presence of inflammation and edema that is often absent postchemotherapy.
In pediatric patients treated with limb salvage for primary osseous sarcoma, we therefore assessed whether MRI accurately reflects the bony extent of tumor compared with the corresponding pathologic specimen of a given resection length. We arbitrarily chose a pediatric population because often there is a desire to preserve more host bone in the very young, but we suspect this question could be assessed in either adult or pediatric patients. We asked: (1) Does the quantitative pathologic bony margin correlate with that measured on MRI? (2) Are T1- or T2-weighted MRIs most accurate in determining a margin? (3) Is there a difference in predicting tumor extent between MRI obtained before or after neoadjuvant chemotherapy and which is more accurate?
Patients and Methods
This is a retrospective study of pediatric patients treated with limb salvage surgery for primary osteosarcoma or Ewing’s sarcoma of the lower extremity. Two hundred eleven potentially eligible pediatric patients treated with limb salvage surgery between August 1999 and July 2015 by a single surgeon (EUC) at a single institution for primary sarcoma of bone were identified. Of 131 patients (62%) with disease involving the femur or tibia, 107 (51%) were classified with Ewing’s sarcoma or osteosarcoma. Records were available for review in our online database for 79 eligible patient (37%). Twenty-six patients (12%) were excluded because of insufficient or unavailable pathology data and 17 patients (8%) were excluded as a result of inadequate or incomplete MR imaging, leaving 55 eligible participants (26%) in the final cohort.
After institutional review board approval, clinical data were collected using the institution's clinical tracking tool and medical record. All study participants underwent standard imaging studies including MRI of the affected extremity at presentation. Postchemotherapy MRI scans were obtained before surgical treatment approximately 10 weeks after initial biopsy. Patients were treated with osseous resection and reconstruction incorporating an intercalary allograft or oncologic implant depending on the location and extent of the primary tumor.
Resected specimens were routinely evaluated by the pathology department and were photographed and catalogued at the time of resection. We defined the pathology margin as the closest distance between the pathologically confirmed tumor and the diaphyseal extent of the specimen. This was obtained from the final pathology report generated at the time of initial resection. Resection specimen length was retrospectively confirmed utilizing the scaled images of the bisected osseous specimens (Fig. 1A). In patients with intercalary resections, the residual juxtaarticular segment was measured and added to the resection length to identify the distance from the adjacent joint (a reliable reference point available on all preoperative imaging) to the diaphyseal cut.
Fig. 1A-C.
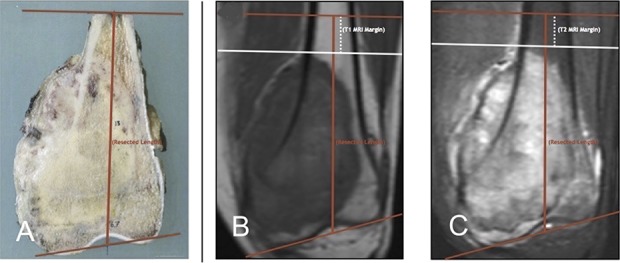
(A) The resection length was measured on the catalogued scaled image grid for each specimen. (B) On the corresponding T1 MRI, the diaphyseal extent of the tumor was then marked perpendicular to the anatomic axis of the involved bone (white line). The resection length was then superimposed on the MR image (red line). The “MRI margin” was measured from the diaphyseal extent of the tumor to the diaphyseal resection level. (C) The process described in B was repeated on the corresponding T2 MRI.
MR images were analyzed using standard clinical imaging software (Centricity Enterprise Web V3.0, 2006; GE Medical Systems, Barrington, IL, USA). Pre- and postchemotherapy coronal MR images were evaluated retrospectively on both T1- (Fig. 1B) and T2- (Fig. 1C) weighted sequences. For each image sequence at each time point, the apparent diaphyseal extent of the osseous tumor was identified. This was marked with a line perpendicular to the anatomic axis of the bone. The measured length of the pathologic specimen was then plotted directly onto the coronal MR image, measured from the corresponding articular surface marker (verified with each individual scaled photograph). This measurement was performed along the long axis of the bone in single millimeter increments. The difference between the plotted diaphyseal tumor extent and the plotted diaphyseal bony cut was then measured along the anatomic axis; we defined this as the MRI margin. For each MRI sequence at each time point, the diaphyseal extent of tumor and corresponding measurements were determined by consensus between two observers (MJT, CNJ). The difference between the MRI margin and the pathology margin was then calculated for each sequence at each time point. The primary study outcome was the difference between the MRI margin for each sequence at each time point and the pathology margin for each specimen.
Statistical Analysis
The IBM Statistical Package for Social Sciences Version 24.0 for Mac (IBM Corp, Armonk, NY, USA) was used for statistical analyses of a priori hypotheses. Initially all variables had relative distributions determined as well as the normality of the data and the accuracy of data entry assessed. Participants with missing data were excluded from final analyses.
To investigate the study questions, Student’s t-tests and Pearson’s r correlations were used to compare the diaphyseal pathology margin with the diaphyseal MRI margin on T1 MRI before chemotherapy (T1-MRI PRE), T1 MRI after chemotherapy (T1-MRI POST), T2 MRI before chemotherapy (T2-MRI PRE), and T2 MRI after chemotherapy (T2-MRI POST). For all analyses, the α level was set at 0.05 with a confidence interval at the 95% level.
Results
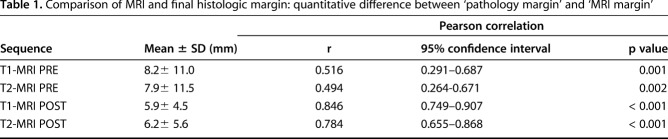
Both MR imaging types (T1-weighted and T2-weighted MRI) at both time points were positively associated with final pathologic margin. T1-weighted MRI before neoadjuvant chemotherapy (r = 0.516, p = 0.001), T2-weighted MRI before neoadjuvant chemotherapy (r = 0.494, p = 0.002), T1-weighted MRI after neoadjuvant chemotherapy (r = 0.846, p < 0.001), and T2-weighted MRI after neoadjuvant chemotherapy (r = 0.784, p < 0.001) correlated with final pathologic margin. T1-weighted MRI after neoadjuvant chemotherapy and final pathologic margin had the strongest positive correlation of all MR imaging and time point comparisons (r = 0.846, p < 0.001; Table 1).
Table 1.
Comparison of MRI and final histologic margin: quantitative difference between ‘pathology margin’ and ‘MRI margin’
Discussion
Preoperative tools available to plan margin-negative resections are critical in determining safe tumor margins and should be critically evaluated. In the setting of primary bone sarcomas such as osteosarcoma and Ewing’s sarcoma of the extremity, we are not aware of any published quantitative data regarding the accuracy with which MRI defines the osseous extent of tumor. To our knowledge no prior studies address either the relative accuracy of T1 versus T2 sequences or pre- versus postneoadjuvant chemotherapy MRI for this purpose. In this study, we confirmed that the pathologic osseous surgical extent correlated with both MR imaging techniques at both time points and found that T1-weighted MRI after neoadjuvant chemotherapy and final pathologic margin had the strongest positive correlation of all MR imaging and time point comparisons. Additionally, T1-weighted MR imaging after neoadjuvant chemotherapy had the smallest mean difference as compared with the final pathology margin.
This study has several limitations. Inclusion of only those patients with available imaging at both time points and pathology may insert selection bias; however, these elements are necessary to answer the study question. The ability of the surgeon to reproduce measurements from preoperative cross-sectional imaging at the time of resection has not been assessed in this study. We have not studied inter- and intraobserver reliability in measuring margins on MR images. Measurement errors almost certainly occur whenever one tries to measure something on an image and we have not assessed measurement error in this study. To address this limitation, considerable effort was devoted to ensuring that the coronal MR image chosen to perform measurements matched the pathologic specimen grid and that the extent of tumor and all corresponding measurements were recorded based on consensus evaluation of two observers. Images were crossreferenced to verify that the anatomic landmarks utilized to perform individual measurements were consistent between the pathology specimen and the MR image. Despite these efforts, it is possible that minor differences in the coronal plane of the MRI compared with the coronal plane of the bisected specimen may affect measurements to a minor degree. This is felt to parallel everyday practice, and the effects of specimen orientation should be routinely considered when planning resections with an appropriate margin of safety. Because the pathology margin was obtained from the final pathology report, this study does not account for potential variations in the assessment and/or measurement of the pathologic margin among pathologists. This parallels clinical practice and should be considered in the interpretation of these results. It was not feasible to determine the specific MRI equipment utilized to obtain all images within the data set. The potential effects of improved technology over the time period in which study data were generated may also be important. It is possible that MRI accuracy was better during the latter portion of this study; however, this was not assessed. It may be useful to perform a similar study in which the same MRI machine, magnet strength, and imaging protocols are utilized; however, this would represent an experimental model not reflective of our standard clinical practice. Possible variations in MRI technology should be considered when planning surgical margins. We did not assess osteosarcoma and Ewing’s sarcoma separately. There may be differences in how these tumors respond to therapy, which could affect margin measurements, but because we used the same principles for resection in both tumor types, we did not believe separate analysis was necessary and we had insufficient numbers to study them individually.
We observed that the bony margin predicted by MRI correlated with the pathologic margin and shows that there is, on average, approximately a 6- to 10-mm margin of error that should be considered when planning surgical resections based on MRI. These findings appear consistent with the navigation-assisted study, which reported an average quantitative “error in safe margin” of 8 mm [28]. However, the observed range of differences may warrant a larger safety factor and special circumstances may warrant additional consideration when planning the bony cut. The spatial accuracy of MRI in general has been described [4, 19] and a small number of computer-navigated limb-sparing resections utilizing fused CT and MRI models supplemented with intraoperative cutting templates have been studied [3, 11, 17, 28]. In one study that quantitatively assessed margin status, it is important to consider that only nine of 26 cut planes of the pelvis were included in the margin analysis [28]. Despite this limitation, these techniques yielded accurate cuts within 2 mm of the planned resection and an average “error in safe margin” (the difference between the planned and achieved margin) of 8 mm [28]. Thus, in that study, the accuracy with which preoperative imaging identified the osseous extent of tumor appears to nearly parallel the accuracy observed in the present study. Although navigation is felt to improve the accuracy with which the planned resection is executed, not all surgeons are trained to use or have routine access to these technologies [29]. Furthermore, under circumstances that allow precise reproduction of planned resections, a negative margin remains dependent on the accurate identification of the bony extent of tumor on preoperative imaging. Whether surgeons are planning a freehand or navigation-guided resection, they must be able to consider the accuracy with which preoperative imaging identifies the tumor extent to plan within an appropriate margin of safety. One prior study of primary sarcoma of bone assessed the accuracy of MRI in assessing the bony extent of tumor by comparing imaging with corresponding pathology specimens. In 1986, Sundaram et al. [25] prospectively assessed 16 consecutive patients with various osseous sarcomas and found that, compared with alternative imaging modalities, MRI was the most accurate in determining the intramedullary extent of tumor. They compared measurements of the longitudinal extent of tumor on coronal MRI (slice thickness of 10-15 mm) with those made on sectioned gross specimens. Although they state that MRI defined the intramedullary extent of the tumor, no quantitative measurements were reported and no comparison was made to examine the relative difference between T1- and T2-weighted MRI sequences.
Although both sequences at both time points correlated positively with the pathologic margin size, the disparity between the MRI and pathology margin assessed by mean differences was greater for images obtained before neoadjuvant chemotherapy. This may be even more for Ewing’s sarcoma because changes may be more dramatic on postchemotherapy images as shown in a limited number of patients with Ewing’s sarcoma, but we did not do a comparison between patients with Ewing’s sarcoma and those with osteosarcoma so we cannot confirm that supposition. This is likely the result of few tumors in this cohort exhibiting a significant volumetric response to neoadjuvant chemotherapy. A potential reduction in the extent of peritumoral edema resulting from chemotherapy may also allow for easier identification of the tumor margin. The same consideration may explain why patients have a large discrepancy between T1- and T2-weighted imaging postchemotherapy in that the identification of the tumor margin may be more obscured by residual peritumoral edema on fluid-sensitive sequences. In this cohort, postneoadjuvant chemotherapy T1-weighted MRI had the smallest mean difference when compared with the pathologic assessment of the surgical margin.
A close but negative margin may represent adequate local control in the treatment of primary osseous sarcoma [3, 5, 8, 14, 20]. Although this may offer a degree of reassurance in planning close margin resections, caution is recommended. The surgeon should consider the potential disparity between the apparent extent of tumor on MRI and the actual extent of tumor on pathology and should understand that this disparity is separate from the concept of “location accuracy”--the execution of a planned cut. It is possible that improving technology allows the precision with which the surgeon is able to recreate a surgical plan better than the accuracy with which the desired level of resection can be identified on preoperative imaging. Resection level should account for the acceptable margin, as determined by the surgeon in the context of the other prognostic disease parameter, plus a margin of safety. Preoperative MRI and fluid-sensitive sequences may show a larger discrepancy. When planning surgical resections based on MRI obtained after neoadjuvant chemotherapy, for safety, the surgeon should account for a potential difference between the apparent margin of a tumor on an MRI and the actual pathologic margin of that tumor of at least 6 to 10 mm, on average, and larger discrepancies may be observed.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283–292. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Longhi A, Ferrari S, Mercuri M, Versari M, Bertoni F. Prognostic factors in non-metastatic Ewing's sarcoma tumor of bone: an analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol. 2006;45:469–475. [DOI] [PubMed] [Google Scholar]
- 3.Bellanova L, Paul L, Docquier PL. Surgical guides (patient-specific instruments) for pediatric tibial bone sarcoma resection and allograft reconstruction. Sarcoma. 2013;2013:787653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellanova L, Schubert T, Cartiaux O, Lecouvet F, Galant C, Banse X, Docquier PL. MRI-based assessment of safe margins in tumor surgery. Sarcoma. 2014;2014:686790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand TE, Cruz A, Binitie O, Cheong D, Letson GD. Do surgical margins affect local recurrence and survival in extremity, nonmetastatic, high-grade osteosarcoma? Clin Orthop Relat Res. 2016;474:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. [DOI] [PubMed] [Google Scholar]
- 7.Bispo Junior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byerly S, Chopra S, Nassif NA, Chen P, Sener SF, Eisenberg BL, Tseng WW. The role of margins in extremity soft tissue sarcoma. J Surg Oncol. 2016;113:333–338. [DOI] [PubMed] [Google Scholar]
- 9.Eary JF, Conrad EU, O'Sullivan J, Hawkins DS, Schuetze SM, O'Sullivan F. Sarcoma mid-therapy [F-18]fluorodeoxyglucose positron emission tomography (FDG PET) and patient outcome. J Bone Joint Surg Am. 2014;96:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP; Pediatric Oncology Group. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. [DOI] [PubMed] [Google Scholar]
- 11.Gouin F, Paul L, Odri GA, Cartiaux O. Computer-assisted planning and patient-specific instruments for bone tumor resection within the pelvis: a series of 11 patients. Sarcoma. 2014;2014:842709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins DS, Conrad EU, Butrynski JE, Schuetze SM, Eary JF. [F-18]-Fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer. 2009;115:3519–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU, Eary JF. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. [DOI] [PubMed] [Google Scholar]
- 14.Kandel R, Coakley N, Werier J, Engel J, Ghert M, Verma S; Sarcoma Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. Surgical margins and handling of soft-tissue sarcoma in extremities: a clinical practice guideline. Curr Oncol. 2013;20:e247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165–172. [DOI] [PubMed] [Google Scholar]
- 16.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, Kevric M, Klingebiel T, Kotz R, Maas R, Schwarz R, Semik M, Treuner J, Zoubek A, Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23:559–568. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kang HG, Kim HS. MRI-guided navigation surgery with temporary implantable bone markers in limb salvage for sarcoma. Clin Orthop Relat Res. 2010;468:2211–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Shin KH, Kim HY, Cho YJ, Noh JK, Suh JS, Yang WI. Postoperative nomogram to predict the probability of metastasis in Enneking stage IIB extremity osteosarcoma. BMC Cancer. 2014;14:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koivukangas T, Katisko J, Koivukangas J. Detection of the spatial accuracy of a magnetic resonance and surgical computed tomography scanner in the region of surgical interest. J Med Imaging (Bellingham). 2014;1:015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Moretti VM, Ashana AO, Lackman RD. Impact of close surgical margin on local recurrence and survival in osteosarcoma. Int Orthop. 2012;36:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan SS, Gorlick R, Bukata S, Chou A, Morris CD, Boland PJ, Huvos AG, Meyers PA, Healey JH. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107:1607–1616. [DOI] [PubMed] [Google Scholar]
- 22.Reddy KI, Wafa H, Gaston CL, Grimer RJ, Abudu AT, Jeys LM, Carter SR, Tillman RM. Does amputation offer any survival benefit over limb salvage in osteosarcoma patients with poor chemonecrosis and close margins? Bone Joint J. 2015;97:115–120. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Galindo C, Shah N, McCarville MB, Bdillups CA, Neel MN, Rao BN, Daw NC. Outcome after local recurrence of osteosarcoma: the St Jude Children's Research Hospital experience (1970-2000). Cancer. 2004;100:1928–1935. [DOI] [PubMed] [Google Scholar]
- 24.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram M, McGuire MH, Herbold DR, Wolverson MK, Heiberg E. Magnetic resonance imaging in planning limb-salvage surgery for primary malignant tumors of bone. J Bone Joint Surg Am. 1986;68:809–819. [PubMed] [Google Scholar]
- 26.Takeuchi A, Lewis VO, Satcher RL, Moon BS, Lin PP. What are the factors that affect survival and relapse after local recurrence of osteosarcoma? Clin Orthop Relat Res. 2014;472:3188–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White LM, Wunder JS, Bell RS, O’Sullivan B, Catton C, Ferguson P, Blackstein M, Kandel RA. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1439–1445. [DOI] [PubMed] [Google Scholar]
- 28.Wong KC, Kumta SM. Joint-preserving tumor resection and reconstruction using image-guided computer navigation. Clin Orthop Relat Res. 2013;471:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong KC, Kumta SM. Use of computer navigation in orthopaedic oncology. Curr Surg Rep. 2014;2:47. [DOI] [PMC free article] [PubMed] [Google Scholar]